Abstract
Background:
Pregnant women are at increased risk of severe disease with coronavirus disease 2019 (COVID-19). Despite strong recommendations from American College of Obstetricians and Gynecologists and Society for Maternal Fetal Medicine for vaccination, COVID-19 vaccination hesitancy persists. With this study, we aim to evaluate opinions about the COVID-19 vaccine in a cohort of high-risk pregnant patients.
Materials and Methods:
Institutional review board approval was obtained. Patients attending a regional Maternal–Fetal Medicine clinic in central New York were surveyed about the COVID-19 vaccine using a standardized questionnaire. Demographic, obstetrical, and medical information was abstracted using medical records. The vaccinated and unvaccinated groups were evaluated using chi-square tests and a Bayesian model.
Results:
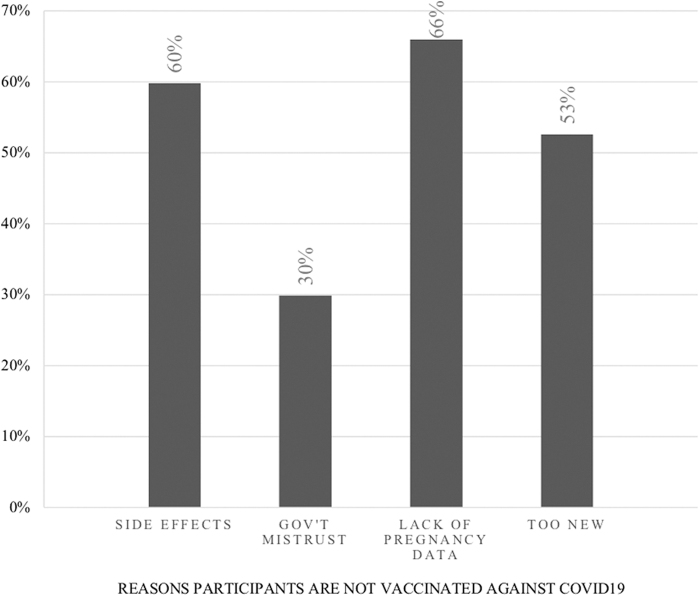
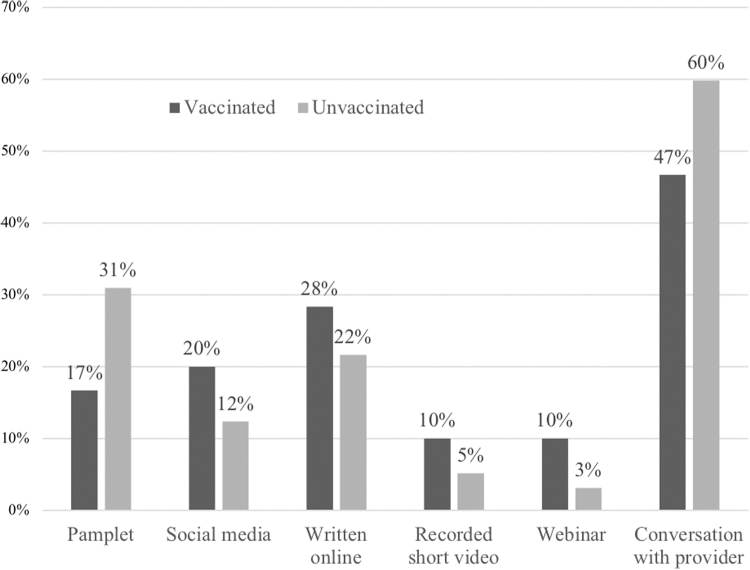
Among the 157 participants, 38.2% are vaccinated. There were no significant differences in race/ethnicity, living situation, marital status, employment status, insurance type, pregravid body mass index, history of recreational drug use, number of living children, or gestational age at the time of survey. Patients with less formal education are less likely to be vaccinated. There was no difference between influenza and tetanus diphtheria pertussis vaccination rates with COVID-19 vaccination rates. Unvaccinated patients cite lack of data in pregnancy (66%) as their primary concern. Most patients prefer to learn about vaccines via conversation with their doctor (46.7% for vaccinated and 59.8% for unvaccinated).
Conclusions:
The vaccination rate is low in our population. A provider-initiated conversation about COVID-19 vaccination included with routine prenatal care could increase the vaccination rate.
Keywords: COVID, pregnancy, vaccination
Introduction
Pregnant women are at increased risk of severe disease with infectious viruses.1 With coronavirus disease 2019 (COVID-19) infection, pregnant women are more likely to be admitted to an intensive care unit, require invasive ventilation, and die2,3 compared with nonpregnant subjects. Despite these statistics, there is widespread distrust with the vaccine and lack of vaccination in pregnant women. By May 2021, 46% of reproductive-aged women received one dose of the COVID-19 vaccine, whereas only 16% of pregnant women had done the same.4,5 The vaccination rate of pregnant women rose to only 33% by October 1, 2021 despite multiple efforts to improve access and information about the vaccination.6–14
Pregnant women were excluded from initial COVID-19 vaccination clinical trials.1,12 As a result, initial recommendations for the vaccines were mostly empiric for pregnant women, primarily based on observational real data and resulted in a great amount of confusion and concern (with the additive effect of politization).
The Centers for Disease Control (CDC) has an ongoing registry (V-Safe) to collect data regarding vaccination outcomes in pregnancy.13 Since the onset of V-safe, studies that compiled the results of the registry demonstrated safety in pregnancy.14 The American College of Obstetricians and Gynecologists (ACOG), Society for Maternal Fetal Medicine (SMFM), and CDC all published statements strongly in support of vaccination against COVID-19 in all women, including pregnant and lactating patients.15–30 The mistrust created by the early trial designs and exacerbated by social media leaves pregnant women and fetuses vulnerable during this pandemic.
COVID-19 infection has been shown to cause more severe disease in patients with existing comorbidities, such as obesity, hypertension, diabetes mellitus, and asthma.31,32 With the additional baseline risk of severe disease in pregnancy, there is an increasing risk of severe disease if infected with COVID-19 in pregnant patients. With this study, we aim to evaluate opinions about the COVID-19 vaccine in a sample of high-risk pregnant patients in central New York.
Materials and Methods
Institutional review board approval was obtained (IRB No. 1722484-1, approved June 6, 2021). All patients who presented for prenatal care at a central New York regional Maternal–Fetal Medicine clinic September to October 2021 while a research staff member was present were invited to participate. Participants were excluded if they met exclusion criteria: age <18 years, unable to understand English, or incarcerated (Fig. 1). Convenience sampling was used due to ease of logistics with limited resources.
FIG. 1.
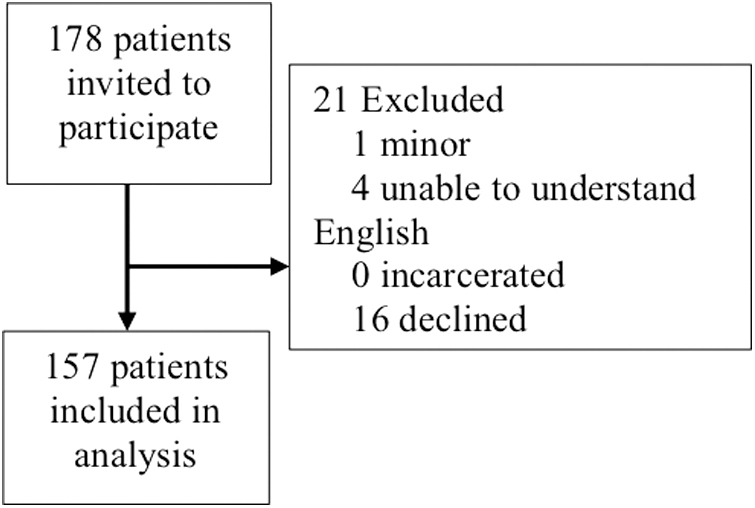
Prisma diagram.
The research staff member administered the survey to all available participants for four sequential weeks during all clinic hours except Fridays before noon. After informed consent was obtained, patients answered a standardized survey about the COVID-19 vaccine and demographic information. The survey was modeled after information gathered by Kaiser Family Foundation for national general population trends about COVID-19 vaccination hesitancy.33 Participants were given a tablet to self-administer the written survey while a research staff member was available for clarification questions.
The survey was available only in English. Further demographic, obstetrical, and medical information was abstracted using medical records. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted by SUNY Upstate Medical University.34,35 The COVID-19 vaccination status was used to identify the primary outcome. The vaccinated and unvaccinated groups were evaluated with a hierarchical Bayesian model. An effect size was generated via the Bayesian model; a credible interval that did not include zero was considered statistically significant.
Results
A total of 178 pregnant women were invited to participate in the study. Twenty-one participants were excluded; 16 declined to participate, 4 were unable to understand English, and 1 was <18 years old (Fig. 1). One hundred fifty-seven pregnant women were included in the analysis, for an 88% response rate (Table 1). All participants completed the survey provided. 38.2% reported that they received the COVID-19 vaccine. The most common reason for vaccine hesitancy was the lack of data about the vaccination in pregnancy (Fig. 2).
Table 1.
Categorical Abstracted Information, by Coronavirus Disease 2019 Vaccinated Versus Not Vaccinated Status
| Characteristics | Total (%) | Vaccinated, n (%) | Not vaccinated, n (%) | Bayesian model: effect size (95% CI)a |
|---|---|---|---|---|
| Total | 157 (100%) | 60 (38.2) | 97 (61.7) | n/a |
| Race and ethnicity | ||||
| Asian | 8 | 1 (12.5) | 7 (87.5) | −1.04 (−3.05 to 1.07) |
| Non-Hispanic White | 116 | 49 (42.4) | 67 (57.8) | 0.85 (−1.03 to 2.67) |
| Hispanic | 8 | 5 (62.5) | 3 (37.5) | 1.02 (−1.03 to 3.09) |
| Non-Hispanic Black | 17 | 2 (11.8) | 15 (88.2) | −0.33 (−2.38 to 1.69) |
| American Indian/Alaskan Native | 2 | 0 (0) | 2 (100) | −0.54 (−2.83 to 1.68) |
| Other | 5 | 2 (40) | 3 (60) | 0.24 (−1.68 to 2.24) |
| Living situation | ||||
| Own home | 66 | 32 (48.5) | 34 (51.5) | 0.12 (−1.81 to 1.94) |
| Rent home | 73 | 21 (28.8) | 52 (71.2) | −0.27 (−2.09 to 1.55) |
| Family household | 18 | 7 (38.9) | 11 (61.1) | −0.12 (−1.99 to 1.81) |
| Employment status | ||||
| Full time | 76 | 31 (40.8) | 45 (59.2) | −0.30 (−2.07 to 1.56) |
| Part time | 18 | 11 (61.1) | 7 (38.9) | 0.73 (−1.21 to 2.65) |
| Other | 11 | 3 (27.2) | 8 (72.7) | −0.98 (−2.92 to 0.94) |
| Unemployed | 44 | 13 (29.5) | 31 (70.5) | −0.28 (−2.11 to 1.54) |
| Disabled | 6 | 2 (33.3) | 4 (66.7) | 0.38 (−1.63 to 2.47) |
| Declined to state | 2 | 0 (0) | 2 (100) | −0.14 (−2.54 to 2.15) |
| Level of education | ||||
| <8th grade | 2 | 0 (0) | 2 (100) | −2.36 (−4.34 to −0.31) |
| 9–11th grade | 16 | 3 (18.8) | 13 (81.3) | −1.24 (−2.67 to 0.26) |
| High school or GED | 53 | 14 (26.4) | 39 (73.6) | −0.54 (−1.84 to 0.77) |
| Vocational or technical school | 7 | 1 (14.3) | 6 (85.7) | 0.03 (−1.19 to 1.28) |
| Associate degree or some college | 38 | 18 (47.4) | 20 (52.6) | 0.62 (−0.58 to 1.92) |
| Bachelor's degree | 22 | 13 (59.1) | 9 (40.9) | 1.38 (−0.05 to 2.86) |
| Insurance type | ||||
| Medicaid or Medicare | 75 | 19 (25.3) | 56 (74.7) | −0.36 (−2.00 to 1.39) |
| Private insurance | 82 | 40 (48.8) | 42 (51.2) | 0.05 (−1.63 to 1.78) |
| Substance use | ||||
| No drug use | 111 | 48 (43.2) | 63 (56.8) | 0.42 (−1.29 to 2.07) |
| Current drug use | 11 | 1 (9.1) | 10 (90.9) | −0.80 (−2.71 to 0.96) |
| Former drug use | 33 | 10 (30.3) | 23 (69.7) | −0.61 (−2.28 to 1.14) |
| Unknown drug use | 4 | 3 (75) | 1 (25) | 0.42 (−1.29 to 2.07) |
| Marital status | ||||
| Single | 66 | 20 (30.3) | 46 (69.7) | −0.22 (−2.09 to 1.64) |
| Married | 74 | 32 (43.2) | 42 (56.8) | −0.50 (−2.34 to 1.35) |
| Living as married | 11 | 6 (54.5) | 5 (45.5) | 0.52 (−1.43 to 2.50) |
| Divorced | 2 | 2 (100) | 0 (0) | 0.53 (−1.55 to 2.70) |
| Separated | 4 | 0 (0) | 4 (100) | −0.48 (−2.66 to 1.71) |
| Method to learn more | ||||
| Provider conversation | 86 | 28 (32.6) | 58 (67.4) | −0.79 (−1.55 to −0.08) |
| Pamphlet | 40 | 10 (25) | 30 (75) | −1.24 (−2.08 to −0.47) |
| Prerecorded videos | 11 | 6 (54.5) | 5 (45.4) | 0.42 (−0.73 to 1.59) |
| Social media | 24 | 12 (50) | 12 (50) | 0.55 (−0.38 to 1.56) |
| Webinar | 9 | 6 (66.7) | 3 (33.3) | 0.44 (−0.79 to 1.59) |
| Written on website | 38 | 17 (44.7) | 21 (55.3) | 0.12 (−0.69 to 0.98) |
| Infectious disease status | ||||
| Received flu vaccine | 30 | 15 (50) | 15 (50) | 0.18 (−0.59 to 0.95) |
| Received Tdap vaccine | 61 | 21 (34.4) | 40 (65.6) | 0.61 (−0.25 to 1.45) |
| Previous positive COVID test | 6 | 2 (33.3) | 4 (66.7) | 0.08 (−0.47 to 0.65) |
CI, credible interval; COVID-19, coronavirus disease 2019; Tdap, tetanus diphtheria pertussis.
Bayesian model included in Figure 3.
FIG. 2.
Reasons for lack of vaccination.
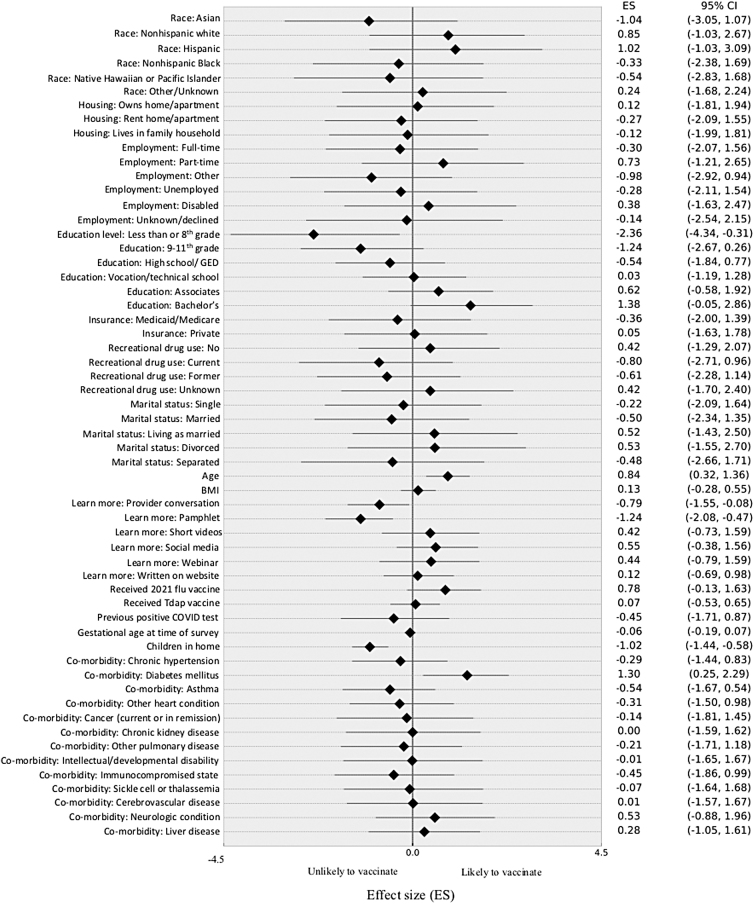
A hierarchical Bayesian logistic regression was performed to control for multiple variables simultaneously based on conditional probabilities (Fig. 3). This model suggests that vaccinated individuals were slightly older (effect size 0.84 [95% credible interval; CI: 0.32–1.36]) (Fig. 3 and Table 1).
FIG. 3.
Hierarchical logistic Bayesian regression.
There were no significant differences in race/ethnicity (Asian: −1.04 [95% CI: −3.05 to 1.07], non-Hispanic White: 0.85 [95% CI: −1.03 to 2.67], Hispanic: 1.02 [95% CI: −1.03 to 3.09], non-Hispanic Black: −0.33 [95% CI: −2.38 to 1.69], American Indian/Alaskan Native: −0.54 [95% CI: −2.83 to 1.68], other: 0.24 [95% CI: −1.68 to 2.24]); living situation (own home: 0.12 [95% CI: −1.81 to 1.94], rent home: −0.27 [95% CI: −2.09 to 1.55], live in family household: −0.12 [95% CI: −1.99 to 1.81]); marital status (single: −0.22 [95% CI: −2.09 to 1.64], married: −0.50 [95% CI: −2.34 to 1.35], living as married: 0.52 [95% CI: −1.43 to 2.50], divorced: 0.52 [95% CI: −1.43 to 2.50], separated: −0.48 [95% CI: −2.66 to 1.71]).
There was no difference in employment status (full time: −0.30 [95% CI: −2.07 to 1.56], part time: 0.73 [95% CI: −1.21 to 2.65], other: −0.98 [95% CI: −2.92 to 0.94], unemployed: −0.28 [95% CI: −2.11 to 1.54], disabled: 0.38 [95% CI: −1.63 to 2.47]); insurance type (Medicaid or Medicare: −0.36 [95% CI: −2.00 to 1.39], private insurance: 0.05 [95% CI: −1.63 to 1.78]); history of recreational drug use (no drug use: 0.42 [95% CI: −1.29 to 2.07], current drug use: −0.80 [95% CI: −2.71 to 0.96], former drug use: −0.61 [95% CI: −2.28 to 1.14], unknown drug use: 0.42 [95% CI: −1.29 to 2.07]) (Fig. 3 and Table 1).
There were no differences in pregravid body mass index (0.13 [95% CI: −0.28 to 0.55]), number of living children (−1.02 [95% CI: −1.44 to −0.58]), or gestational age at the time of survey (−0.06 [95% CI: −0.19 to 0.07]) between vaccinated and unvaccinated groups (Fig. 3 and Table 2).
Table 2.
Continuous Abstracted Information, by Coronavirus Disease 2019 Vaccinated Versus Not Vaccinated Status
| Characteristics | Vaccinated: mean (standard deviation) | Not vaccinated: mean (standard deviation) | Bayesian model: effect size (95% CI)a |
|---|---|---|---|
| Age, years | 30.6 (± 5.6) | 28.9 (± 5.8) | 0.84 (0.32 to 1.36) |
| Pregravid body mass index, kg/m2 | 30.9 (± 9.2) | 31.4 (± 9.0) | 0.13 (−0.28 to 0.55) |
| Gestational age range, weeks | 28 0/7–29 6/7b | 26 0/7–27 6/7b | −0.06 (−0.19 to 0.07) |
| No. of living children | 1 (± 1) | 2 (± 2) | −1.02 (−1.44 to −0.58) |
Bayesian model included in Figure 3.
Standard deviation unable to be calculated.
We identified a trend that patients with less education were less likely to be vaccinated (less than eighth grade completed: −2.36 [95% CI: −4.34 to −0.31], ninth to eleventh grade: −1.24 [95% CI: −2.67 to 0.26], high school or GED: −0.54 [95% CI: −1.84 to 0.77], vocational or technical school: 0.03 [95% CI: −1.19 to 1.28], associate degree or some college: 0.62 [95% CI: −0.58 to 1.92], and bachelor's degree: 1.38 [95% CI: −0.05 to 2.86]) (Fig. 3 and Table 1). Patients with an eighth-grade level education or less were the least likely to have been vaccinated. Vaccination rates increase with increasing level of education.
There was no correlation between COVID-19 vaccine rates and influenza (0.18 [95% CI: −0.59 to 0.95]) or tetanus diphtheria pertussis (Tdap) vaccination rates (0.61 [95% CI: −0.25 to 1.45]) (Fig. 3 and Table 1). Fifty percent of both the COVID-19 vaccinated and unvaccinated groups received the 2021 influenza vaccine. 65.6% of COVID-19 unvaccinated participants received the Tdap vaccine during pregnancy, whereas only 34.4% of COVID-19 vaccinated participants had. Six participants had a previous positive COVID-19 test on file; four of these participants remained unvaccinated at the time of survey (0.08 [95% CI: −0.47 to 0.65]) (Fig. 3 and Table 1). This was not statistically significant, likely because of the small number of participants with a positive test.
The high-risk clinic population was selected to capture pregnant patients with medical comorbidities. Conditions that qualified an individual for early vaccination during the stratified rollout in New York Status were evaluated. It was hypothesized that vaccination rates would be higher in these high-risk individuals. Diabetes was the only medical comorbidity associated with a higher vaccination rate (1.30 [95% CI: 0.25–2.29]) (Fig. 3 and Table 3).
Table 3.
Medical Comorbidities by Vaccinated Versus Unvaccinated Status, by Coronavirus Disease 2019 Vaccinated Versus Not Vaccinated Status
| Characteristics | Total (%) | Vaccinated, n (%) | Not vaccinated, n (%) | Bayesian model: effect size (95% CI)a |
|---|---|---|---|---|
| Hypertension | 20 | 6 (30.0) | 14 (70.0) | −0.29 (−1.44 to 0.83) |
| Diabetes mellitus | 27 | 16 (59.3) | 11 (40.7) | 1.30 (0.25 to 2.29) |
| Asthma | 40 | 12 (30.0) | 28 (70.0) | −0.54 (−1.67 to 0.54) |
| Other heart conditions | 9 | 2 (22.2) | 7 (77.8) | −0.31 (−1.50 to 0.98) |
| Cancer | 1 | 0 (0) | 1 (100) | −0.14 (−1.81 to 1.45) |
| Chronic kidney disease | 0 | 0 | 0 | 0.00 (−1.59 to 1.62) |
| Other pulmonary disease | 3 | 1 (33.3) | 2 (66.7) | −0.21 (−1.71 to 1.18) |
| Intellectual and developmental disabilities | 0 | 0 | 0 | −0.01 (−1.65 to 1.67) |
| Immunocompromised states | 6 | 2 (33.3) | 4 (66.7) | −0.45 (−1.86 to 0.99) |
| Sickle cell disease or thalassemia | 1 | 0 (0) | 1 (100) | −0.07 (−1.64 to 1.68) |
| Cerebrovascular disease | 0 | 0 | 0 | 0.01 (−1.57 to 1.67) |
| Neurological conditions | 4 | 2 (50.0) | 2 (50.0) | 0.53 (−0.88 to 1.96) |
| Liver disease | 7 | 3 (42.9) | 4 (57.1) | 0.28 (−1.05 to 1.61) |
Bayesian model included in Figure 3.
No other medical comorbidity correlated with COVID-19 vaccination status (hypertension: −0.29 [95% CI: −1.44 to 0.83], asthma: −0.54 [95% CI: −1.67 to 0.54], other heart conditions: −0.31 [95% CI: −1.50 to 0.98], cancer: −0.14 [95% CI: −1.81 to 1.45], chronic kidney disease: 0.00 [95% CI: −1.59 to 1.62], other pulmonary disease: −0.21 [95% CI: −1.71 to 1.18], intellectual and developmental disabilities: −0.01 [95% CI: −1.65 to 1.67], immunocompromised states: −0.45 [95% CI: −1.86 to 0.99], sickle cell disease or thalassemia: −0.07 [95% CI: −1.64 to 1.68], cerebrovascular disease: 0.01 [95% CI: −1.57 to 1.67], neurological conditions: 0.53 [95% CI: −0.88 to 1.96], and liver disease: 0.28 [95% CI: −1.05 to 1.61]) (Fig. 3 and Table 3).
When given different options to learn more about the vaccine, most patients in both groups preferred to learn more about the vaccine via a conversation with their doctor (Fig. 4).
FIG. 4.
Patients answered how they would prefer to learn more about vaccinations.
Discussion
The vaccination rate of our cohort is higher than the published national vaccination rates in pregnant women3 although is still low. A lack of data in pregnancy is the most common cited reason for being unvaccinated. Despite strong recommendations by the experts in the field and subsequent studies demonstrating safety in pregnancy,14,28–30 patients continue to express this concern. The exclusion of pregnant women in the first COVID-19 vaccination trials1,12 propagated a sentiment of fear and danger that persists. An element of politics also appeared to be influencing the decision to get vaccinated or not. However, this study was not designed to address this issue and currently cannot be quantified.
Less formal education correlates with a decrease in vaccination. This is consistent with international trends of COVID-19 vaccination hesitancy,36,37 but unlike vaccination trends seen with childhood vaccination.38 Increasing age is associated with increased COVID-19 vaccination rates. This may represent patients who have completed more formal education. It is unlikely that patients with a less formal education prioritize reading the latest scientific information about COVID-19 vaccination in pregnancy; this may impact the trend seen.
Influenza vaccination is recommended universally to pregnant patients due to concern for increased severe illness from influenza.39 Tdap vaccination is recommended to pregnant patients starting at 28 weeks of gestation to aid with immunity against pertussis in mother and neonate after delivery.39 Influenza and Tdap vaccination statuses are unrelated to COVID-19 vaccination status. This suggests that the fears and concerns surrounding COVID-19 vaccinations are not universal.
Patients with a history of diabetes mellitus are associated with an increase in vaccination. Unlike other comorbidities, patients with diabetes mellitus require more frequent visits with a provider throughout pregnancy independent of prepregnancy control of disease to adjust medications and perform adequate fetal monitoring.40 Perhaps the frequent visits with a provider can positively contribute to a patient's understanding and willingness to be vaccinated.
Most patients in both groups prefer to learn about the vaccination via a conversation with their provider. This outlines the importance of the providers' responsibility to include well-informed counseling about the COVID-19 vaccine in routine visits.
This study is limited by the small sample size. The timeframe of survey collection was limited due to research staff availability and as a result, the audience captured is small. Nevertheless, we believe that it reflects the true rates of COVID-19 vaccination in central New York. The nature of the survey was nonrandom as every patient who presented to the clinic while a research staff member was present was included. The survey was available only in English; the generalizability of these results is limited to those with English proficiency.
The survey was available only via tablet and may underrepresent participants who are uncomfortable with technology, although the goal of the staff member at time of survey administration was to attempt to reduce this. Although the staff member was blinded to the answers, the presence of the staff member may increasingly contribute to a social desirability bias. Recall bias is present as the survey was not administered at the time of potential COVID-19 vaccination. The high-risk maternal fetal medicine clinic was targeted to capture a higher-risk pregnant population, but the result is a Berkson bias.
Anecdotally, several patients included in this survey have been subsequently admitted while pregnant due to symptoms secondary to COVID-19 pneumonia. It would be of interest to follow up with patients to expand on number of provider visits in the past year, and if there is any information that is influencing their opinions about the vaccine since the initial survey. It would also be meaningful to evaluate if an intervention, such as provider education and talking points for provider-led conversations at routine visits, leads to a change in vaccine rates. COVID-19 vaccination opinions are dynamic throughout the natural history of the pandemic and future studies may be useful to re-evaluate patients' evolving views.
Abbreviations Used
- CDC
Centers for Disease Control
- CI
credible interval
- COVID-19
coronavirus disease 2019
- Tdap
tetanus diphtheria pertussis
Authors' Contributions
The authors confirm contribution to the article as follows: study conception and design by M.D.J., N.B., and D.M.; data collection by M.D.J.; analysis and interpretation of results by E.R., M.D.J., and D.M.; draft article preparation by M.D.J. and D.M. All authors reviewed the results and approved the final version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No external funding was received for this article.
Cite this article as: DesJardin M, Raff E, Baranco N, Mastrogiannis D (2022) Cross-sectional survey of high-risk pregnant women's opinions on COVID-19 vaccination, Women's Health Report 3:1, 608–616, DOI: 10.1089/whr.2022.0006.
References
- 1. Whitehead CL, Walker SP. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet 2020;395:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC. COVID-19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID-19. 2021. Available at: https://emergency.cdc.gov/han/2021/han00453.asp Accessed December 18, 2021.
- 4. ACOG: COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG Practice Advisory, 2020. Last updated 2021. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Accessed January 6, 2022.
- 5. CDC. Percent of pregnant people aged 18–49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Data-link. United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women Accessed December 18, 2021.
- 6. Shook LL, Kishkovich TP, Edlow AG. Countering COVID-19 vaccine hesitancy in pregnancy: The “4 Cs”. Am J Perinatol 2021. [Epub ahead of print]; DOI: 10.1055/a-1673-5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Razzaghi H, Meghani M, Pingali C, et al. COVID-19 vaccination coverage among pregnant women during pregnancy—Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diesel J, Sterrett N, Dasgupta S, et al. COVID-19 vaccination coverage among adults—United States, December 14, 2020-May 22, 2021. MMWR Morb Mortal Wkly Rep 2021;70:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Male V. Are COVID-19 vaccines safe in pregnancy? [published correction appears in Nat Rev Immunol 2021]. Nat Rev Immunol 2021;21:200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chervenak FA, McCullough LB, Grünebaum A. Reversing physician hesitancy to recommend COVID-19 vaccination for pregnant patients. Am J Obstet Gynecol 2021;S0002-9378(21)01210-2 [Epub ahead of print]; DOI: 10.1016/j.ajog.2021.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am J Obstet Gynecol 2021;226:236..e1–236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg I, Shekhar R, Sheikh AB, Pal S. COVID-19 vaccine in pregnant and lactating women: A review of existing evidence and practice guidelines. Infect Dis Rep 2021;13:685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. V-safe COVID-19 vaccine pregnancy registry. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html Accessed October 25, 2021.
- 14. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons [published correction appears in N Engl J Med 2021;385:1536]. N Engl J Med 2021;384:2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang PH, Lee WL, Yang ST, Tsui KH, Chang CC, Lee FK. The impact of COVID-19 in pregnancy: Part II. Vaccination to pregnant women. J Chin Med Assoc 2021;84:903–910. [DOI] [PubMed] [Google Scholar]
- 16. Iacobucci G. Covid-19 and pregnancy: Vaccine hesitancy and how to overcome it. BMJ 2021;375:n2862. [DOI] [PubMed] [Google Scholar]
- 17. Mohan S, Reagu S, Lindow S, Alabdulla M. COVID-19 vaccine hesitancy in perinatal women: A cross sectional survey. J Perinat Med 2021;49:678–685. [DOI] [PubMed] [Google Scholar]
- 18. Ceulemans M, Foulon V, Panchaud A, et al. Vaccine willingness and impact of the COVID-19 pandemic on women's perinatal experiences and practices—A multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res Public Health 2021;18:3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. CDC. COVID-19 vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total Accessed December 18, 2021.
- 20. Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020;222:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajewska A, Mikołajek-Bedner W, Lebdowicz-Knul J, Sokołowska M, Kwiatkowski S, Torbé A. COVID-19 and pregnancy—Where are we now? A review. J Perinat Med 2020;48:428–434. [DOI] [PubMed] [Google Scholar]
- 22. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019-nCoV infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang CL, Liu YY, Wu CH, Wang CY, Wang CH, Long CY. Impact of COVID-19 on pregnancy. Int J Med Sci 2021;18:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goncu Ayhan S, Oluklu D, Atalay A, et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet 2021;154:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirshberg JS, Huysman BC, Oakes MC, et al. Offering onsite COVID-19 vaccination to high-risk obstetrical patients: Initial findings. Am J Obstet Gynecol MFM 2021;3:100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Januszek SM, Faryniak-Zuzak A, Barnaś E, et al. The approach of pregnant women to vaccination based on a COVID-19 systematic review. Medicina (Kaunas) 2021;57:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayakawa S, Komine-Aizawa S, Takada K, Kimura T, Yamada H. Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan. J Obstet Gynaecol Res 2021;47:1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. COVID-19 vaccines for people who would like to have a baby. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/planning-for-pregnancy.html Accessed October 25, 2021.
- 29. ACOG: Statement of strong medical consensus for vaccination of pregnant individuals against COVID-19. American College of Obstetricians and Gynecologists: News Releases. Available at: https://www.acog.org/news/news-releases/2021/08/statement-of-strong-medical-consensus-for-vaccination-of-pregnant-individuals-against-covid-19 Accessed October 25, 2021.
- 30. SMFM: Provider considerations for engaging in COVID-19 vaccine counseling with pregnant and lactating patients. Available at: https://s3.amazonaws.com/cdn.smfm.org/media/3134/Provider_Considerations_for_Engaging_in_COVID_Vaccination_Considerations_10-1-21_%28final%29.pdf Accessed January 6, 2022.
- 31. Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health 2020;13:1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben-Shoshan M. COVID-19 and comorbidities: A systematic review and meta-analysis. Postgrad Med 2020;132:749–755. [DOI] [PubMed] [Google Scholar]
- 33. KFF. COVID-19 vaccine monitor: September 2021. Available at: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-september-2021/ Accessed January 6, 2022.
- 34. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 2021;6:e210–e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 2021;39:2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cunningham RM, Minard CG, Guffey D, Swaim LS, Opel DJ, Boom JA. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad Pediatr 2018;18:154–160. [DOI] [PubMed] [Google Scholar]
- 39. Maternal Immunization. Committee Opinion No. 741. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;131:e214–e217. [DOI] [PubMed] [Google Scholar]
- 40. Pregestational diabetes mellitus. ACOG Practice Bulletin No. 201. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;132:e228–e248. [DOI] [PubMed] [Google Scholar]