Abstract
We report the persistent circulation of third-generation cephalosporin resistant Salmonella Typhi in Mumbai, linked to the acquisition and maintenance of a previously characterized IncX3 plasmid carrying the ESBL gene blaSHV-12 and the fluoroquinolone resistance gene qnrB7 in the genetic context of a triple mutant also associated with fluoroquinolone resistance.
Keywords: typhoid fever, third-generation cephalosporins, extended-spectrum beta lactamases (ESBL), antimicrobial resistance (AMR), genomic epidemiology
Typhoid fever is a serious enteric disease spread through food and water contaminated with Salmonella enterica serovar Typhi (S. Typhi), which disproportionately affects populations in locations with limited sanitation and hygiene. There remains a significant burden of typhoid fever in India [1], where antimicrobial therapy continues to be the linchpin of treatment and control. However, populations of S. Typhi rapidly develop resistance whenever a new antimicrobial is introduced for the treatment of typhoid fever [2].
Increasing rates of resistance against fluoroquinolones linked to the expansion of genotype 4.3.2.1 (haplotype H58 lineage II) [3] have led to the adoption of cefixime and ceftriaxone (third generation cephalosporins) and azithromycin (macrolide) as the first-line treatments for typhoid fever in India [4]. This reliance on third generation cephalosporins may trigger the emergence of further resistance, as has been observed with extensively drug resistant (XDR) S. Typhi in Pakistan [5]. Third-generation cephalosporin resistant S. Typhi have been previously isolated in India and associated with the presence of the AmpC genes blaCMY-2 [6], blaACC-1 [7], blaDHA-1 [8], and ESBL gene blaSHV-12 [9]. As with other Gram-negative bacteria, ESBL and AmpC genes in S. Typhi are often located on plasmids, which commonly also carry the fluoroquinolone resistance gene qnr [5, 9].
Despite the increasing use of third generation cephalosporins [2, 10], the prevalence of resistance in S. Typhi in India remains low, ranging from 0 to 5% [10]. However, a recent study from Mumbai reported a rate of ceftriaxone resistance of 12.5% [11].
To better understand the genetic context of third-generation cephalosporin resistant S. Typhi in Mumbai, we characterized the antimicrobial susceptibility profiles and the genomes of 92 isolates from blood culture-confirmed patients with S. Typhi infections collected by a tertiary care hospital in Mumbai between January 2017 and December 2018. The demographic and clinical characteristics of the patients are described in Supplementary Table 1. The species identification and antimicrobial susceptibility testing were performed using the VITEK-2 compact system and minimum inhibitory concentration (MIC) values were interpreted according to the Clinical Laboratory Standards Institute 2019 guidelines. Azithromycin and chloramphenicol were not included in the standard panel of antibiotics of the VITEK-2 system but were tested separately by disc diffusion. All S. Typhi isolates were susceptible to piperacillin-tazobactam, ertapenem, imipenem, meropenem, colistin, chloramphenicol and azithromycin. The majority (83/92; 90.2%) of the isolates were nonsusceptible to ciprofloxacin. Twelve isolates (13.0%) were resistant to ceftriaxone, out of which 11/12 were also nonsusceptible to cefepime. All isolates exhibiting phenotypic resistance to third-generation cephalosporins were also resistant to ciprofloxacin. The patients were treated with the empiric combination therapy of ceftriaxone and azithromycin for suspected enteric fever. After drug susceptibility results, the patients with ceftriaxone-resistant S. Typhi were continued treatment with azithromycin for 14 days with resolution of signs and symptoms. Hospital records did not indicate that these patients had previously received treatment with third-generation cephalosporins.
Two of the ceftriaxone-resistant isolates were recovered in 2017, whereas the remaining 10 were recovered in 2018, mostly between January and July, suggesting a cluster of cases (Supplementary Figure 1). Out of 80 patients with clinical data, a higher proportion of patients with ceftriaxone-resistant S. Typhi were inpatients (8/11, 72.7%), compared to patients with ceftriaxone-susceptible S. Typhi (39/69, 56.2%), but this difference was not significant (Fisher exact test 0.51, P>.05).
To investigate the genetic relationship between the ceftriaxone-resistant isolates and to determine the molecular basis of resistance, we sequenced the 92 S. Typhi on Illumina HiSeq X10 with 150bp paired-end reads, resulting in 89 whole genome sequences after assembly, quality control and analysis using pipelines developed within the National Institute for Health Research Global Health Research Unit on Genomic Surveillance of AMR [12]. Sequence data were deposited at the European Nucleotide Archive under study accession PRJEB29740. AMR genes and point mutations were identified from sequence reads using ARIBA v2.14.4 [13] with the NCBI database [14] and the pointFinder database [15]. Genotype [16] and plasmid replicon type [17] information, as well as AMR determinants, were obtained from assemblies using Pathogenwatch [18]. Details of the methods are provided in the Supplementary materials.
Lineage 4.3.1 (haplotype H58) encompassed 87.6% of the genome sequences, with approximately three-quarters (67/89; 75.3%) of the genomes belonging to genotype 4.3.1.2, followed by 4.3.1.1 (6/89, 6.7%) and 4.3.1 (5/89, 5.6%). Resistance to ceftriaxone was associated with a blaSHV-12 ESBL gene in 11/12 isolates belonging to genotype 4.3.1.2. We did not identify any known mechanism of ceftriaxone resistance in the genome of the additional isolate, which was assigned to 4.3.1. This isolate was susceptible to ampicillin and the MIC for ceftriaxone was 4mg/L (the breakpoint for ceftriaxone resistance), which were reproduced upon retesting. All isolates carried at least one mutation in the quinolone-resistance determining region in gyrA, but we did not identify any of the described mutations or genes that confer resistance to azithromycin. Only 1 organism was predicted to be MDR (ie, resistant to ampicillin, chloramphenicol and sulfamethoxazole-trimethoprim), which belonged to genotype 4.3.1.1 and carried resistance genes typically located on an IncHI1 plasmid or in a composite transposon inserted into the chromosome [19] (Figure 1A). The lack of an IncHI1 replicon and further inspection of the genome assembly pointed to a yidA chromosomal insertion site, thus indicating the stable integration of the composite transposon into the genome.
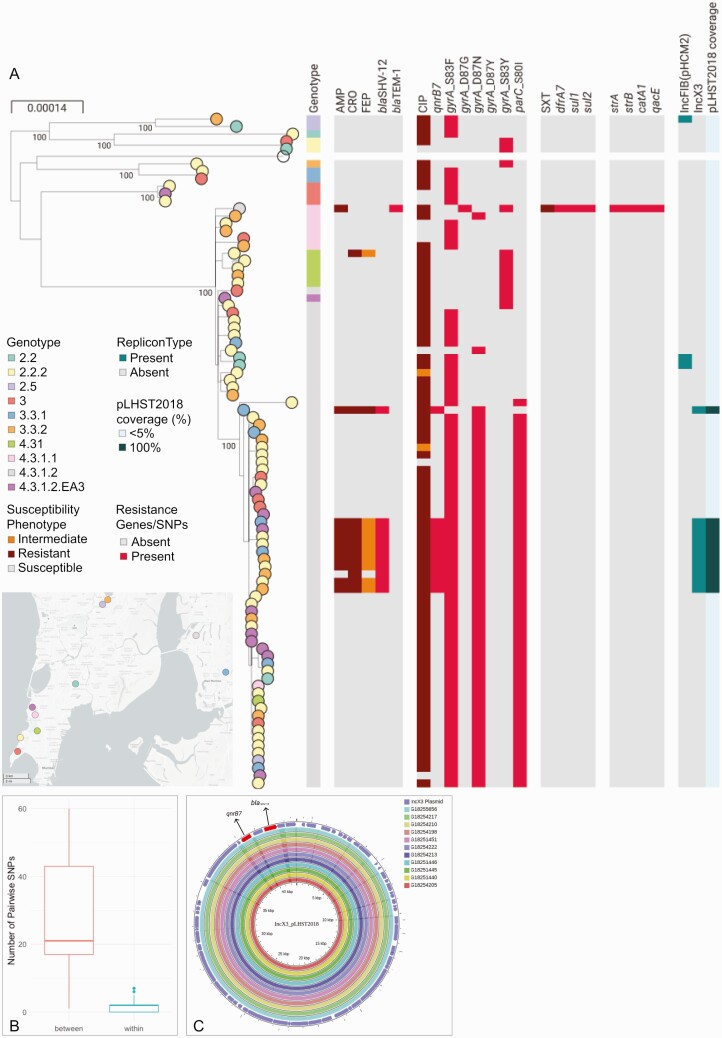
Figure 1.
Analysis of the S. Typhi genomes. A, Phylogenetic tree of 89 genomes with nodes colored by the neighborhood in Mumbai where the patient resided. Maximum-likelihood tree was inferred from 1496 SNP positions identified by mapping the sequence reads to the complete chromosome of strain CT18 (NC_003198) and masking regions corresponding to mobile genetic elements and recombination from the alignment of pseudogenomes. Tree is annotated with the genotype; the susceptibility phenotype to AMP, CRO, FEP, CIP, and SXT; the distribution of resistance genes and mutations conferring resistance to these antibiotics and others not tested; the distribution of plasmid replicon types identified in the genome assemblies with Pathogenwatch; the sequence coverage of the plasmid pLHST2018 determined by mapping sequence reads of each genome to the plasmid sequence. The data are available at https://microreact.org/project/S.Typhi_Mumbai_2017-2018/8713a72b. B, Boxplot showing the distribution of the pairwise SNP differences within the cluster of 10 isolates carrying blaSHV-12 (blue) or between the genomes in this cluster and other 4.3.1.2 genomes not in this cluster (red). The horizontal line indicates the median and the box indicates the interquartile range. C, Comparison of assembly contigs from 11 genomes in this study carrying blaSHV-12, qnrB7 and the IncX3 replicon type to the complete sequence of plasmid pLHST2018. Outermost circle shows the plasmid genes, with resistance genes shown in red. Abbreviations: AMP, ampicillin; CIP, ciprofloxacin; CRO, ceftriaxone; FEP, cefepime; SNP, single nucleotide polymorphism; SXT, sulfamethoxazole-trimethoprim.
A phylogenetic investigation of the 89 genome sequences determined that the eleven 4.3.1.2 isolates harboring blaSHV-12 were located in two independent branches of the tree (Figure 1A). One group was represented by a single isolate characterized by the presence of gyrA mutation D87N, the qnrB7 gene and the IncX3 replicon sequence. The second comprised ten isolates and also carried the qnrB7 gene and the IncX3 type replicon, but was a triple mutant associated with fluoroquinolone resistance (D87N and S83F in gyrA and S80I in parC). This cluster of 10 isolates was supported by a 100% bootstrap value and a close genetic relationship (0–7 pairwise single nucleotide polymorphism [SNP] differences) in comparison to the other 4.3.1.2 sequences (Figure 1B). An alignment of the assembly contigs from each of the eleven genomes carrying blaSHV-12, qnrB7 and IncX3 to the complete sequence of the previously described plasmid pLHST2018 (accession CP052768, [9]) using the CGView Server [20] showed a complete match to the 42.8 Kbp plasmid sequence (Figure 1C). This was confirmed by mapping the sequence reads of each of the 89 genomes to the pLHST2018 plasmid and computing the sequence length coverage, which shows 100% coverage of the plasmid sequence only for the 11 genomes with blaSHV-12 (Figure 1A).
These data are consistent with 2 independent acquisitions of plasmid pLHST2018 within genotype 4.3.1.2, of which only 1 showed evidence of maintenance. The 10 isolates in the cluster were recovered between April 2017 and December 2018 from patients residing in 4 different neighborhoods within a 30 km radius of the hospital. We compared the 67 4.3.1.2 genomes from this study to global public genomes from the same genotype using Pathogenwatch. Four previously described ceftriaxone-resistant isolates from Mumbai carrying blaSHV-12 in the IncX3 plasmid pLHST2018 [9] formed a monophyletic cluster and were interspersed in the tree with the isolates from this study (Supplementary Figure 2), signifying that this lineage has been circulating since at least 2016. Our data suggest that the persistence of the IncX3 plasmid conferring ceftriaxone resistance is not as short-term as previously thought [9], coinciding with the acquisition of the ceftriaxone resistance within the genetic context of the fluoroquinolone-resistant triple mutant lineage [3, 21].
The extended temporal and geographical distribution of the isolates suggests broad environmental transmission. Transmission from contaminated food or water at a public gathering, with subsequent person-to-person transmission within neighborhoods is also plausible. The higher proportion of male patients observed amongst ceftriaxone-resistant isolates (9/12, 75.0%) compared to ceftriaxone-susceptible isolates (34/80, 42.5%) is notable in this context but not significant (Fisher exact test 0.06, P>.05, Supplementary Table 1). The absence of an epidemiological investigation based on patient exposures limits our understanding of the circulation of this high-risk clone. Although the isolates analyzed were collected by only 1 hospital in Mumbai, the patients were distributed broadly across the city, and the 4.3.1.2 S. Typhi genomes in this study were interspersed with those from previous studies in the tree, suggesting adequate representation.
The World Health Organization classified third-, fourth-, and fifth-generation cephalosporins as critically important for human medicine. We characterized an emergent high-risk lineage of S. Typhi with plasmid-borne ceftriaxone (blaSHV-12) and ciprofloxacin (qnrB7) resistance in Mumbai, nested successively within a high-risk clone of chromosomally encoded ciprofloxacin resistance found in several countries in South Asia and associated with fluoroquinolone treatment failure [21], the 4.3.1.2 genotype that has been expanding clonally in India [3], and the epidemic drug-resistant clone 4.3.1 clade or haplotype H58 [19]. The evidence of persistent transmission of this high-risk lineage in a location with high burden of typhoid fever calls for active surveillance and effective prevention measures to avert a large outbreak and international dissemination, as seen with XDR S. Typhi carrying the ESBL gene blaCTX-M-15 in an IncY plasmid in neighboring Pakistan [5]. Through technology transfer and capacity building, the Central Research Laboratory at KIMS, Bangalore, is now equipped to carry out whole genome sequencing [22] and bioinformatics analysis [23] locally to support genomic surveillance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to the DNA Pipelines and Pathogen Informatics teams at the Wellcome Sanger Institute for their support.
Financial support. This work was supported by Official Development Assistance (ODA) funding from the National Institute of Health Research (NIHR grant number 16_136_111). The study was approved by the Kalinga Institute of Medical Sciences (KIMS) Institutional Ethics Committee (KIMS/IEC/S12-2017, dated 15 February 2018), and the Breach Candy Hospital Ethics Committee (BCMRC/P3/2018, dated 7 February 2018).
Potential conflicts of interest. A. K. V., V. S., D. S., K. L. R., G. N., and A. P. report grant funding and travel support from NIHR UK. S. A. and D. M. A. report grants from NIHR UK (grant number 16_136_111) awarded to David M. Aanensen, during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Silvia Argimón, Centre for Genomic Pathogen Surveillance, Wellcome Genome Campus, Hinxton, Cambridge, United Kingdom.
Geetha Nagaraj, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
Varun Shamanna, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
Dharmavaram Sravani, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
Ashwini Kodlipet Vasanth, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
Akshatha Prasanna, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
Aruna Poojary, Department of Pathology and Microbiology, Breach Candy Hospital Trust, Mumbai, India.
Anurag Kumar Bari, Department of Pathology and Microbiology, Breach Candy Hospital Trust, Mumbai, India.
Anthony Underwood, Centre for Genomic Pathogen Surveillance, Wellcome Genome Campus, Hinxton, Cambridge, United Kingdom.
Mihir Kekre, Centre for Genomic Pathogen Surveillance, Wellcome Genome Campus, Hinxton, Cambridge, United Kingdom.
Stephen Baker, Cambridge Institute of Therapeutic Immunology & Infectious Disease, Department of Medicine, University of Cambridge, Cambridge, United Kingdom.
David M Aanensen, Centre for Genomic Pathogen Surveillance, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Old Road Campus, Oxford, United Kingdom.
Ravikumar Kadahalli Lingegowda, Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India.
REFERENCES
- 1. John J, Van Aart CJ, Grassly NC.. The burden of typhoid and paratyphoid in India: systematic review and meta-analysis. PLoS Negl Trop Dis 2016; 10:e0004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Divyashree S, Nabarro LE, Veeraraghavan B, Rupali P.. Enteric fever in India: current scenario and future directions. Trop Med Int Health 2016; 21:1255–62. [DOI] [PubMed] [Google Scholar]
- 3. Britto CD, Dyson ZA, Mathias S, et al. Persistent circulation of a fluoroquinolone-resistant Salmonella enterica Typhi clone in the Indian subcontinent. J Antimicrob Chemother 2020; 75:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar P, Kumar R.. Enteric fever. Indian J Pediatr 2017; 84:227–30. [DOI] [PubMed] [Google Scholar]
- 5. Klemm EJ, Shakoor S, Page AJ, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumarasamy K, Krishnan P.. Report of a Salmonella enterica serovar Typhi isolate from India producing CMY-2 AmpC β-lactamase. J Antimicrob Chemother 2012; 67:775–6. [DOI] [PubMed] [Google Scholar]
- 7. Gokul BN, Menezes GA, Harish BN.. ACC-1 beta-lactamase-producing Salmonella enterica serovar Typhi, India. Emerg Infect Dis 2010; 16:1170–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Shankar BA, Munusamy E, Anandan S, Veeraraghavan B.. Draft genome sequence of blaTEM-1-mediated cephalosporin-resistant Salmonella enterica serovar Typhi from bloodstream infection. J Glob Antimicrob Resist 2016; 7:11–2. [DOI] [PubMed] [Google Scholar]
- 9. Jacob JJ, Pragasam AK, Vasudevan K, et al. Salmonella Typhi acquires diverse plasmids from other Enterobacteriaceae to develop cephalosporin resistance. Genomics 2021; 113:2171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balaji V, Kapil A, Shastri J, et al. Longitudinal typhoid fever trends in India from 2000 to 2015. Am J Trop Med Hyg 2018; 99(3_Suppl): 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kokare RS, Bari AK, Pereira JV, Patel K, Poojary A.. Minimum inhibitory concentration (MIC) of ceftriaxone and azithromycin for blood culture isolates of Salmonella enterica spp. J Infect Dev Ctries 2021; 15:538–43. [DOI] [PubMed] [Google Scholar]
- 12. Underwood A. GHRU (Genomic Surveillance of Antimicrobial Resistance) Retrospective 1 Bioinformatics Methods V.4. Available at: https://www.protocols.io/view/ghru-genomic-surveillance-of-antimicrobial-resista-bpn6mmhe.
- 13. Hunt M, Mather AE, Sánchez-Busó L, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom 2017; 3:e000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Center for Biotechnology Information (NCBI). Bacterial Antimicrobial Resistance Reference Gene. Available at: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047. Accessed 30 October 2019.
- 15. Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM.. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother 2017; 72:2764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyson ZA, Holt KE.. Five years of GenoTyphi: updates to the global Salmonella Typhi genotyping framework. bioRxiv 441766 [Preprint]. April 28, 2021. Available from: 10.1101/2021.04.28.441766. Accessed 19 August 2019. [DOI] [PMC free article] [PubMed]
- 17. Carattoli A, Hasman H.. PlasmidFinder and In Silico pMLST: identification and typing of plasmid replicons in whole-genome sequencing (WGS). Methods Mol Biol 2020; 2075:285–94. [DOI] [PubMed] [Google Scholar]
- 18. Argimón S, Yeats CA, Goater RJ, et al. A global resource for genomic predictions of antimicrobial resistance and surveillance of Salmonella Typhi at pathogenwatch. Nat Commun 2021; 12:2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong VK, Baker S, Pickard DJ, et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 2015; 47:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grant JR, Stothard P.. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 2008; 36:W181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham Thanh D, Karkey A, Dongol S, et al. A novel ciprofloxacin-resistant subclade of H58 Salmonella Typhi is associated with fluoroquinolone treatment failure. Elife 2016; 5:e14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kekre M, Arevalo SA, Valencia MF, et al. Integrating scalable genome sequencing into microbiology laboratories for routine AMR surveillance. Research Square [Preprint]. June 28, 2021. Available from: 10.21203/rs.3.rs-659029/v1. Accessed 19 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afolayan AO, Bernal JF, Gayeta JM, et al. Overcoming data bottlenecks in genomic pathogen surveillance. OSF Preprints [Preprint]. June 26, 2021. Available from: 10.31219/osf.io/uxrg5. Accessed 19 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.