Abstract
Background
Vulvovaginal candidiasis affects approximately 75% of women in their lifetime. Approved treatment options are limited to oral or topical azoles. Ibrexafungerp, a novel, first-in-class oral triterpenoid glucan synthase inhibitor, has demonstrated broad fungicidal Candida activity and a favorable tolerability profile. The primary objective of this dose-finding study was to identify the optimal dose of oral ibrexafungerp in patients with acute vulvovaginal candidiasis.
Methods
Patients with vulvovaginal signs and symptoms score ≥7 were randomized equally to 6 treatments groups: 5 treatment doses of oral ibrexafungerp or oral fluconazole 150 mg. The primary endpoint was the percentage of patients with a clinical cure (complete resolution of vulvovaginal signs and symptoms) at the test-of-cure visit (day 10).
Results
Overall, 186 patients were randomized into the 6 treatment groups. Results, using the modified intent-to-treat population (baseline positive culture), are reported for ibrexafungerp 300 mg twice daily (BID) for 1 day (n = 27), which was the dose selected for phase 3 studies, and fluconazole 150 mg for 1 day (n = 24). At day 10, the clinical cure rates for ibrexafungerp and fluconazole were 51.9% and 58.3%, respectively; at day 25, patients with no signs or symptoms were 70.4% and 50.0%, respectively. During the study ibrexafungerp patients required less antifungal rescue medications compared with fluconazole (3.7% vs 29.2%, respectively). Ibrexafungerp was well tolerated, with the most common treatment-related adverse events being mild gastrointestinal events.
Conclusions
Ibrexafungerp is a well-tolerated novel antifungal with comparable efficacy to fluconazole in the treatment of acute vulvovaginal candidiasis.
Clinical Trials Registration
Keywords: Candida albicans, fluconazole, ibrexafungerp, SCY-078, vulvovaginal candidiasis
Single-day ibrexafungerp treatment, the first in a new class of fungicidal drugs, is well tolerated and provides similar efficacy to single-dose fluconazole 150 mg for acute vulvovaginal candidiasis.
Vulvovaginal candidiasis (VVC), more commonly known as vaginal yeast infections, is one of the most common causes of vaginitis. In 80%–95% of women, VVC is caused by Candida albicans with fewer remaining infections caused by non-albicans Candida species including Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida krusei, or other fungi [1]. Approximately 75% of women will have ≥1 episode of VVC in their lifetime, and 40%–45% of women will experience ≥2 episodes [1, 2]. By the age of 25 years, an estimated 50% of all women will have experienced ≥1 episode of VVC after the onset of sexual activity [3]. Current treatment options for VVC are predominantly limited to the azole class of fungistatic agents and include short courses of topical formulations of various agents or oral fluconazole given as a 150-mg single or multiple dose regimen [2, 4, 5]. Limitations of available treatments include concerns of intolerance, adverse events (AEs), and with fluconazole, a potential risk of miscarriage and fetal harm [6–9]. More recent VVC treatment limitations include an increasing prevalence of fluconazole resistance and intrinsic resistance or low susceptibility of non-albicans Candida species to azole antifungals [10, 11].
Currently, there are no Food and Drug Administration (FDA)-approved oral non-azole treatment options available for patients with VVC. This absence negatively impacts VVC treatment options for patients with azole-resistant organisms, those not responding to or not tolerating fluconazole or for whom its use is contraindicated. New agents should ideally have broader fungal coverage, minimal AEs, no risk of fetal harm, and limited drug to drug interactions.
Ibrexafungerp (formerly SCY-078) is a first-in-class triterpenoid antifungal [12]. Similar to the echinocandins, its mechanism of action targets the glucan synthase enzyme, resulting in decreased (1,3)-β-D-glucan polymers, which weakens the fungal cell wall leading to fungal cell lysis and death. Due to its unique structure, ibrexafungerp binds to a site on glucan synthase that only partially overlaps with the echinocandin binding site [13]. As a non-azole antifungal, ibrexafungerp exerts broad spectrum activity against an extensive range of Candida isolates, including many with fks1 and fks2 point mutations that cause echinocandin resistance among C. glabrata and Candida auris [14]. Because glucan synthase is uniquely found in fungal cell walls and not human cells, there is less chance of off-target effects (eg, cytochrome P450 interactions) as observed with azole treatments [15].
Ibrexafungerp has demonstrated activity against several Candida species, including C. albicans and non-albicans Candida species such as C. glabrata, C. krusei, and C. auris [14, 16]. Preclinically, ibrexafungerp demonstrated good vaginal penetration, with tissue levels 2- to 9- fold higher than plasma levels [16, 17], and unlike fluconazole, the activity of ibrexafungerp is not negatively affected by low vaginal pH (<4.5) typical of vaginal milieu in VVC patients [16].
Given the high combined clinical cure rate (35/50 [70%] at day 24; ibrexafungerp 1250 mg loading dose followed by either 750 mg for 2 or 4 days) and favorable tolerability profile demonstrated in a phase 2 proof-of-concept study evaluating ibrexafungerp in women with moderate-to-severe acute VVC, we furthered the investigation of ibrexafungerp [18]. In this study, we selected a range of doses that were well-tolerated in previous phase 1 studies [19, 20]. Here we report results of DOVE (Double Blind Oral SCY-078 Acute VVC Evaluation; ClinicalTrials.gov NCT03253094), a phase 2, double-blind, randomized, active-control, dose-finding study of ibrexafungerp compared with fluconazole 150 mg with a focus on patients receiving ibrexafungerp 300 mg twice daily (BID) for 1 day, the dose selected for phase 3 study evaluation based on patient convenience, safety, and efficacy.
METHODS
Study Design and Patients
Enrolled patients were ≥18 years of age with a diagnosis of symptomatic moderate-to-severe acute VVC determined by a vulvovaginal signs and symptoms (VSS) score of ≥7. Other eligibility criteria included a positive microscopic examination with 10% potassium hydroxide (KOH) in a vaginal sample revealing yeast forms (hyphae/pseudohyphae) or budding yeasts and a vaginal pH of ≤4.5. Exclusion criteria included patients with any vaginal condition that would interfere with the diagnosis and evaluation of VVC (suspected or concurrent causes of vulvovaginitis and/or cervicitis including bacterial vaginosis, Trichomonas, active herpes virus or human papillomavirus infection, positive tests for Neisseria gonorrheae or Chlamydia trachomatis, or other mixed infections), the use of antifungal treatments (topical or systemic) within 28 days of baseline visit, vaginal contraceptives, use of CYP3A4/3A5 inducers and strong time-dependent CYP3A4/3A5 inhibitors 14 days before enrollment and during treatment, strong or moderate reversible CYP3A4/3A5 inhibitors including azoles or grapefruit juice 48 hours before enrollment and during treatment, select CYP2C8 substrates (ie, amiodarone, amodiaquine, paclitaxel, repaglinide, montelukast, pioglitazone, and rosiglitazone) within 48 hours before enrollment and during treatment, or select P-gp substrates (ie, digoxin, colchicine) 48 hours before enrollment and during treatment, patients menstruating at the baseline visit, patients with uncontrolled diabetes mellitus, HIV infection, active cervical or vaginal cancer, and patients who were pregnant. This study was conducted in accordance with the general principles of the Declaration of Helsinki. Each study site obtained institution review board approval for the protocol, informed consent form, recruitment flyers, and other written information before study initiation.
Randomization and Masking
Patients were randomized in equal allocations (at a 1:1:1:1:1:1 ratio) to 1 of 6 treatment groups: oral ibrexafungerp at doses of 750 mg day 1; 300 mg BID for 1 day; 450 mg BID for 1 day; 150 mg BID days 1–3; 300 mg BID days 1–3; or fluconazole 150 mg day 1. Randomization was completed electronically through an interactive web response system. Fluconazole capsules were encapsulated to maintain treatment blinding. All randomized patients received matching ibrexafungerp placebo tablets and/or matching fluconazole placebo capsules based on treatment assignment, in a double-blind, double-dummy fashion. Both active and placebo ibrexafungerp tablets were manufactured by Corealis Pharma; fluconazole active tablets were manufactured by Teva Pharmaceuticals and encapsulated by Corealis Pharma for blinding purposes. All site and sponsor personnel were blinded to treatment assignment except for a sponsor representative who was involved in safety assessments.
Study Assessments
Vulvovaginal samples for 10% KOH microscopic assessment (assessed locally) and fungal culture (assessed by a central laboratory) were collected at baseline, test-of-cure (TOC, day 10), and follow-up (day 25) visits. Susceptibility testing was performed per Clinical Laboratory Standards Institute M27-A3 guidelines for all cultures positive for Candida species. A vulvovaginal sample was also assessed at baseline for pH and other pathogens (eg, bacterial vaginosis, trichomoniasis, N. gonorrheae, C. trachomatis). VSS were assessed using a standardized, predefined scale for which each sign and symptom was given a numerical rating based on severity (absent = 0, mild = 1, moderate = 2, and severe = 3) to calculate a total composite score (range, 0–18). Vulvovaginal signs (edema, erythema, excoriation, or fissures) were rated by the investigator (scale of 0–3; maximum score of 9) and vulvovaginal symptoms (itching, burning, irritation) were rated by the patient (scale of 0–3; maximum score of 9) at baseline, days 1–10 (TOC), and day 25. Safety was assessed by physical exams, hematology and blood chemistry laboratory tests, and vitals at baseline and day 10, and continuous AE monitoring throughout the study.
Outcomes
The primary objective was to identify an optimal dose of oral ibrexafungerp in patients with moderate-to-severe acute VVC. The primary efficacy endpoint was the percentage of patients with a clinical cure (complete resolution of signs and symptoms; VSS = 0) at TOC; clinical failure was defined as no response to therapy or incomplete resolution of VSS or need for additional vulvovaginal or systemic antifungal therapy before the TOC visit. Secondary objectives were to evaluate the efficacy of oral ibrexafungerp in patients with VVC based on mycological and clinical outcomes and to evaluate the safety and tolerability of ibrexafungerp. Secondary efficacy endpoints were the percentage of patients with mycological eradication (negative fungal culture) at TOC and day 25, percentage of patients with both clinical cure and mycological eradication at TOC, percentage of patients with both absence of VSS and mycological eradication at day 25, and percentage of patients with continued clinical response (ongoing absence of symptoms in patients achieving clinical cure at TOC) at day 25. Exploratory endpoints included percentage of patients at day 25 who were symptom-free (absence of symptoms regardless of clinical outcome at TOC; patients that received additional antifungal therapy were considered not being free of VSS at day 25). Post hoc analyses included VSS score ≤1 (clinical improvement) at TOC and day 25 and the use of antifungal rescue medications.
Statistical Analysis
As a dose-finding study with no formal sample size calculation performed, this study was not statistically powered. All analyses are descriptive in nature. Approximately 180 patients were planned to be enrolled and equally randomized to the 6 study treatment groups. Thirty patients per group was estimated to be adequate for an initial assessment of safety and potential efficacy. The intent-to-treat (ITT) population included all randomized patients. The modified ITT (mITT) population included all randomized patients who had a positive KOH test and a confirmed positive mycological culture for yeast at baseline; all efficacy results will be reported using the mITT population. The safety population included all randomized patients who received ≥1 dose of study drug and had ≥1 postbaseline evaluation.
Role of the Sponsor
The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors’ personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
RESULTS
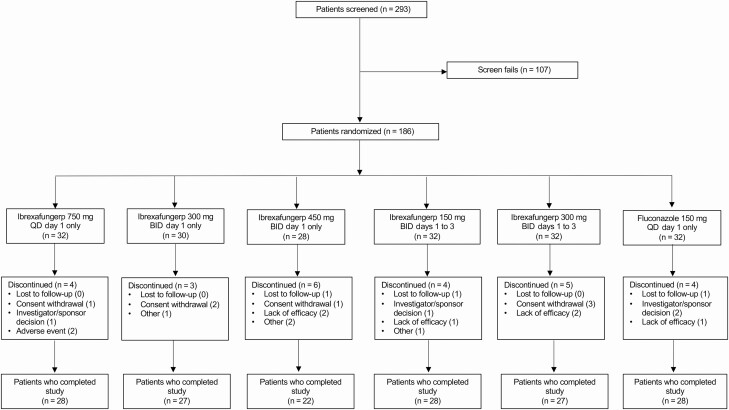
Between August 2017 and May 2018, 293 patients were screened for eligibility, and 186 were enrolled and randomized into 1 of 6 treatment groups (Figure 1, Supplementary Table 1); 153 patients had a confirmed culture for yeast at baseline and were included in the mITT population. All patients included in the mITT population had moderate to severe VVC with VSS scores ranging from 7.0 to 16.0. Based on patient convenience with 1 day dosing and our results demonstrating an increase in gastrointestinal treatment-related treatment-emergent adverse events (TEAEs) with larger doses without a corresponding increase in efficacy, ibrexafungerp 300 mg BID for 1 day was selected as the dose in phase 3 studies (Supplementary Tables 2 and 4). Therefore, results reported here will be limited to ibrexafungerp 300 mg BID for 1 day and fluconazole. A total of 62 patients were enrolled in these 2 treatment groups (ibrexafungerp 300 mg BID for 1 day, n = 30; fluconazole, n = 32); 51 patients were included in the mITT population (ibrexafungerp 300 mg BID for 1 day, n = 27; fluconazole, n = 24). Results for the other treatment groups are provided in the supplement (Supplementary Tables 1, 2, and 4). Overall, there were no differences between treatment groups for baseline characteristics (Table 1).
Figure 1.
Patient disposition. Abbreviations: BID, twice daily; QD, once daily.
Table 1.
Baseline Characteristics (ITT Population)
| Ibrexafungerp 300 mg BID for 1 Day (n = 30) | Fluconazole 150 mg Day 1 (n = 32) | |
|---|---|---|
| Age, years | ||
| Mean ± SD | 34.4 ± 11.3 | 33.8 ± 10.1 |
| Median (min, max) | 32 (18, 55) | 32 (18, 59) |
| Race, no. (%) | ||
| White | 20 (66.7) | 19 (59.4) |
| Black or African American | 10 (33.3) | 13 (40.6) |
| Asian | 0 | 0 |
| Other | 0 | 0 |
| Ethnicity, no. (%) | ||
| Hispanic or Latino | 9 (30.0) | 11 (34.4) |
| Non-Hispanic or Latino | 21 (70.0) | 21 (65.6) |
| BMI (kg/m2)a | ||
| Mean ± SD | 28.1 ± 7.3 | 27.4 ± 7.0 |
| Median (min, max) | 26 (16, 42) | 26 (18, 46) |
Abbreviations: BID, twice daily; BMI, body mass index; ITT, intent-to-treat; max, maximum; min, minimum; SD, standard deviation.
aBaseline BMI is calculated as baseline weight/(baseline height).
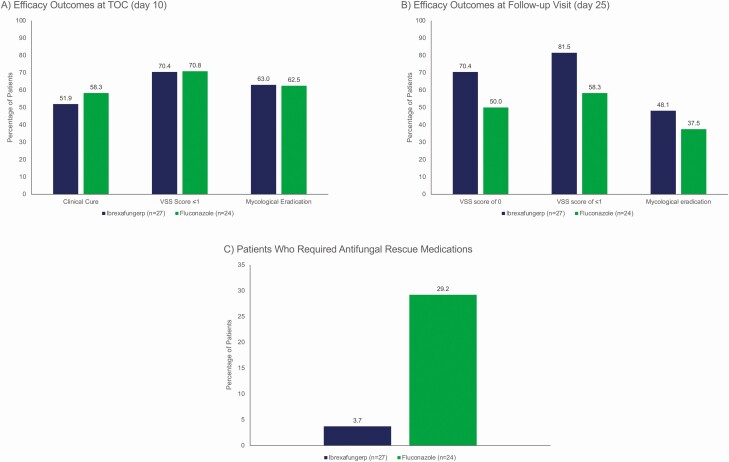
Clinical cure (51.9% vs 58.3%, respectively) and mycological eradication (63.0% vs 62.5%, respectively) rates at TOC were similar between ibrexafungerp and fluconazole (Figure 2A). The composite clinical cure rate with mycological eradication at TOC was also similar between ibrexafungerp and fluconazole (37% vs 41.7%, respectively) (Supplementary Table 2). In patients that achieved a clinical cure at TOC, the continued absence of VSS at day 25 (continued clinical response) was reported in 11/27 (40.7%) and 10/24 (41.7%) of patients receiving ibrexafungerp and fluconazole, respectively. At day 25, mycological eradication (48.1% vs 37.5%, respectively) (Figure 2B) and composite absence of VSS and mycological eradication rates (40.7% vs 33.3%, respectively) (Supplementary Table 2) were reported with ibrexafungerp versus fluconazole. Similar efficacy results were seen across the other ibrexafungerp treatment groups (Supplementary Table 2). In a post hoc analysis, similar rates of clinical improvement (VSS scores ≤1) were reported with ibrexafungerp and fluconazole at TOC (70.4% vs 70.8%, respectively) (Figure 2A); at day 25, clinical improvement rates with ibrexafungerp versus fluconazole were 81.5% vs 58.3%, respectively; a VSS of 0 was reported in 70.4% of patients receiving ibrexafungerp versus 50.0% in the fluconazole group (Figure 2B).
Figure 2.
Efficacy outcomes for ibrexafungerp 300 mg BID for 1 day from baseline to TOC (day 10), and follow-up (day 25) visits. A, Clinical cure, VSS scores ≤1, and rate of mycological eradication at TOC visit (day 10). B, VSS score of 0, VSS scores ≤1, and rate of mycological eradication at follow-up (day 25). C, Percentage of patients who required antifungal rescue medication while participating in the study. Clinical cure was defined as a complete resolution of VSS of acute vulvovaginal candidiasis at the TOC visit (day 10); the VSS scale is a standardized, predefined assessment for which each sign and symptom was given a numerical rating based on severity (absent = 0, mild = 1, moderate = 2, and severe = 3) to calculate a total composite score (range, 0–18); mycological eradication was defined as a negative fungal culture. Rescue medications used in these 2 treatment arms included fluconazole, Lotrisone (clotrimazole-betamethasone dipropionate), and terconazole. Abbreviations: BID, twice daily; TOC, test-of-cure; VSS, vulvovaginal signs and symptoms.
In a post hoc analysis, antifungal rescue medication use was reported in 3.7% of patients receiving ibrexafungerp 300 mg BID for 1 day versus 29.2% of patients receiving fluconazole (Figure 2C). Details of rescue medication use are provided in Supplementary Table 3.
Treatment-related TEAEs were reported in all treatment groups, with higher proportions seen with ibrexafungerp compared with fluconazole (Supplementary Table 4). Increased rates of treatment-related TEAEs (Supplementary Table 4), most of which were gastrointestinal related and mild to moderate in severity, were reported with the administration of ibrexafungerp doses exceeding 300 mg BID per day (750 mg day 1, 62.5% [20/32] of patients and 450 mg BID for 1 day, 57.1% [16/28] of patients) compared with administration of the lowest daily dose of ibrexafungerp (150 mg BID days 1–3, 38.7% [12/31] of patients). TEAEs were reported in 46.7% (14/30) of patients receiving ibrexafungerp 300 mg BID for 1 day and 25% (8/32) of patients receiving fluconazole (Table 2). No serious AEs or deaths were reported for any treatment group. Two patients in the 750 mg day 1 group discontinued treatment due to gastrointestinal-related TEAEs that resolved within a day. A normal pregnancy and delivery with no birth defects was reported in 1 patient in the ibrexafungerp 150 mg BID days 1–3 group.
Table 2.
Summary of Treatment-Related TEAEs Reported in ≥2 Patients (Safety Population). Data Presented are no. (%)
| Ibrexafungerp 300 mg BID for 1 Day (n = 30) | Fluconazole 150 mg Day 1 (n = 32) | |
|---|---|---|
| Any Treatment-related TEAE | 14 (46.7) | 8 (25.0) |
| Diarrhea | 5 (16.7) | 1 (3.1) |
| Nausea | 3 (10.0) | 2 (6.3) |
| Headache | 2 (6.7) | 1 (3.1) |
| Abdominal pain | 1 (3.3) | 2 (6.3) |
| Somnolence | 0 | 2 (6.3) |
Abbreviations: BID, twice daily; TEAE, treatment emergent adverse event.
DISCUSSION
Based on patient convenience and efficacy and safety data in this study, ibrexafungerp 300 mg BID for 1 day was the dosage selected for further evaluation in phase 3 studies. Our study suggested comparable clinical cure rates between ibrexafungerp 300 mg BID for 1 day and fluconazole at TOC. In this phase 2 study, we found it encouraging that certain parameters, such as improved and sustained VSS scores, mycological eradication at day 25, and the need for rescue medication appeared to be improved with ibrexafungerp compared with fluconazole. Because our study was not statistically powered, these initial findings will need further study to see if the observed differences are meaningful.
In 2019, the FDA provided pharmaceutical industry guidance for drug development in the treatment of VVC and recommended clinical cure, defined as the complete absence of all VSS, as the primary efficacy endpoint [21]. In our study, a clinical cure was defined as the complete resolution of VSS (VSS score = 0) by the TOC visit without the need for further antifungal treatment. Mycological eradication was not a primary endpoint, as Candida is normally found in the vagina [2]. Given variations in efficacy outcome definitions in previous studies of VVC, historical comparisons are difficult [22–25]. However, some previous studies have reported a decreased sustained response with azole treatment from day 14 to day 35 [24, 25]. In comparison, the percentage of patients receiving ibrexafungerp with no VSS increased from 51.9% at TOC to 70.4% at day 25 in our study suggesting continued improvement and sustained efficacy.
Although more patients receiving ibrexafungerp reported a treatment-related TEAE (46.7% [14/30 patients]) compared with fluconazole (25.0% [8/32 patients]), ibrexafungerp was generally well tolerated, with self-limited (generally 1-day duration), mild to moderate gastrointestinal TEAEs. The incidence and nature of treatment-related TEAEs for fluconazole were similar to those reported for single-dose use in VVC [7].
The results of our study have clinical implications. Since the approval of fluconazole for the treatment of VVC, no other medications have been approved for this indication. This exploratory phase 2 study, which included a single-dose fluconazole treatment group, suggests that ibrexafungerp may have a potential role in managing a very common infection. With its novel fungicidal mechanism of action [15] and ongoing in vitro activity at lower vaginal pH values where fluconazole activity is decreased [16], ibrexafungerp has theoretical advantages over existing azole therapies. Furthermore, because preclinical penetration of ibrexafungerp into vaginal tissue is 2- to 9-fold higher than plasma levels, as opposed to ratios of 0.4–0.7 reported clinically with fluconazole [6, 15, 16], the drug seems to be delivered very effectively to the area that requires treatment. However, it is uncertain how the benefit of these preclinical characteristics of ibrexafungerp will translate in clinical use.
This dose-finding study is limited as no formal sample size calculation was performed and therefore, the study was not statistically powered. All analyses were descriptive in nature only. Also, sample sizes for the treatment groups were small. One strength of the study was use of an active comparator to ibrexafungerp, fluconazole, instead of a placebo. Although patients in this study received only 1 dose, we recognize that current practice guidelines recommend patients receive 2–3 doses of fluconazole for patients with severe Candida vulvovaginitis, which would have most likely affected the outcomes of the fluconazole arm in our study [2, 5]. Single dose fluconazole was used to provide an active FDA-approved comparator to ibrexafungerp.
In conclusion, ibrexafungerp provides a safe, new, and novel treatment for moderate-to-severe acute VVC. Establishing the clinical role for single day ibrexafungerp treatment in women with acute VVC will be forthcoming following analysis of the 2 large phase 3 studies, VANISH-303 (NCT03734991) and VANISH-306 (NCT03987620), in which the potential advantages of ibrexafungerp against azole resistant isolates and different Candida species can be evaluated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The DOVE study was funded by SCYNEXIS, Inc. We thank the patients, the investigators, and the investigational staff of the DOVE study. The authors acknowledge the medical writing assistance of Laura Jung, PharmD, and Joe Scobey, PhD, of PRECISIONscientia in Yardley, Pennsylvania, USA, which was supported financially by SCYNEXIS, Inc, in compliance with international Good Publication Practice guidelines.
Data sharing statement. Qualified scientific and medical researchers may make requests for individual participant data that underlie the results (text, tables, figures, and supplement) reported in this article, after de-identification, at medicalaffairs@SCYNEXIS.com. Methodologically sound proposals for such data will be evaluated and approved by SCYNEXIS, Inc, in its sole discretion. All approved researchers must sign a data access agreement prior to accessing the data. Data will be available as soon as possible but no later than within 1 year of the acceptance of the article for publication, and for 3 years following article publication. SCYNEXIS, Inc, will not share identified participant data or a data dictionary.
Financial support. This work was supported by SCYNEXIS, Inc.
Potential conflicts of interest. P. N. has received consulting fees from Mycovia Pharmaceuticals, Hologic Inc, and SCYNEXIS, Inc. has received support or grants (to institution) from Hologic, Inc, Mycovia Pharmaceuticals, and SCYNEXIS, Inc, consulting fees from Talis, Hologic, Inc, and SCYNEXIS, Inc; has received educational fees from Hologic and holds stock options in Talis. D. A. A. and N. E. A. are employed by and hold stocks in SCYNEXIS, Inc. I. A. H. is a consultant with SCYNEXIS, Inc. J. D. S. has received research fund from SCYNEXIS, Inc, and Mycovia Pharmaceuticals.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Paul Nyirjesy, Jefferson Vulvovaginal Health Center, Department of Obstetrics and Gynecology, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Jane R Schwebke, University of Alabama at Birmingham, Birmingham, Alabama, USA.
David A Angulo, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
Itzel A Harriott, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
Nkechi E Azie, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
Jack D Sobel, Wayne State University, Detroit, Michigan, USA.
References
- 1. Mendling W, Brasch J, Cornely OA, et al. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015; 58:1–15. [DOI] [PubMed] [Google Scholar]
- 2. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 3. Makanjuola O, Bongomin F, Fayemiwo SA. An update on the roles of non-albicans Candida species in vulvovaginitis. J Fungi 2018; 4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeanmonod R, Jeanmonod D.. Vaginal candidiasis. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459317/. Updated 21 November 2020. Accessed 9 December 2020. [Google Scholar]
- 5. Committee on Practice Bulletins—Gynecology. Vaginitis in nonpregnant patients: ACOG practice bulletin, Number 215. Obstet Gynecol 2020; 135:e1–17. [DOI] [PubMed] [Google Scholar]
- 6. Diflucan [package insert]. New York: Pfizer Inc, 2020. [Google Scholar]
- 7. Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ 2019; 191:E179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Schalkwyk J, Yudin MH; INFECTIOUS DISEASE COMMITTEE. . Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can 2015; 37:266–74. [DOI] [PubMed] [Google Scholar]
- 9. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med 2013; 369:830–9. [DOI] [PubMed] [Google Scholar]
- 10. Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 2012; 120:1407–14. [DOI] [PubMed] [Google Scholar]
- 11. Mintz JD, Martens MG. Prevalence of non-albicans Candida infections in women with recurrent vulvovaginal symptomatology. Adv Infect Dis 2013; 3:238–42. [Google Scholar]
- 12. Hasim S, Coleman JJ. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med Chem 2019; 11:869–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiménez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. De novo acquisition of resistance to SCY-078 in Candida glabrata involves FKS mutations that both overlap and are distinct from those conferring Echinocandin resistance. Antimicrob Agents Chemother 2017; 61:e00833–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azie N, Angulo D, Dehn B, Sobel JD. Oral Ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin Investig Drugs 2020; 29:893–900. [DOI] [PubMed] [Google Scholar]
- 15. Gintjee TJ, Donnelley MA, Thompson GR III. Aspiring antifungals: review of current antifungal pipeline developments. J Fungi (Basel) 2020; 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larkin EL, Long L, Isham N, et al. A novel 1,3-beta-D-glucan inhibitor, ibrexafungerp (formerly SCY-078), shows potent activity in the lower pH environment of vulvovaginitis. Antimicrob Agents Chemother 2019; 63:e02611–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wring S, Borroto-Esoda K, Solon E, Angulo D. SCY-078, a novel fungicidal agent, demonstrates distribution to tissues associated with fungal infections during mass balance studies with intravenous and oral [14 C]SCY-078 in albino and pigmented rats. Antimicrob Agents Chemother 2019; 63:e02119–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roman M, Hernandez C, Blanco D, Obrycki G, Helou S, Angulo D. SCY-078 phase 2 study in moderate and severe vulvovaginal candidiasis (VVC) [abstract P1748]. In: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017.
- 19. Angulo D, Murphy G, Atiee G, Corr C, Willett M, Wring S. Effect of SCY-078 on the pharmacokinetics of tacrolimus: results from a phase 1 clinical drug-drug interaction trial [abstract P1738]. In: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017.
- 20. Angulo D, Murphy G, Atiee G, Corr C, Willett M, Wring S. Effect of SCY-078 on the pharmacokinetics of CYP2C8 substrate (rosiglitazone): results from a phase 1 clinical trial [abstract P1713]. In: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017.
- 21. US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Vulvovaginal candidiasis: developing drugs for treatment. Guidance for industry. 2019. Available at: https://www.fda.gov/media/129537/download. Accessed 9 December 2020.
- 22. Fan S, Liu X, Liang Y. Miconazole nitrate vaginal suppository 1,200 mg versus oral fluconazole 150 mg in treating severe vulvovaginal candidiasis. Gynecol Obstet Invest 2015; 80:113–8. [DOI] [PubMed] [Google Scholar]
- 23. Sekhavat L, Tabatabaii A, Tezerjani FZ. Oral fluconazole 150 mg single dose versus intra-vaginal clotrimazole treatment of acute vulvovaginal candidiasis. J Infect Public Health 2011; 4:195–9. [DOI] [PubMed] [Google Scholar]
- 24. Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–9. [DOI] [PubMed] [Google Scholar]
- 25. Zhou X, Li T, Fan S, et al. The efficacy and safety of clotrimazole vaginal tablet vs. oral fluconazole in treating severe vulvovaginal candidiasis. Mycoses 2016; 59:419–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.