Abstract
A plethora of treatment options exist for cancer therapeutics, but many are limited by side effects and either intrinsic or acquired resistance. The need for more effective targeted cancer treatment has led to the focus on forkhead box (FOX) transcription factors as possible drug targets. Forkhead factors such as FOXA1 and FOXM1 are involved in hormone regulation, immune system modulation, and disease progression through their regulation of the epithelial–mesenchymal transition. Forkhead factors can influence cancer development, progression, metastasis, and drug resistance. In this review, we discuss the various roles of forkhead factors in biological processes that support cancer as well as their function as pioneering factors and their potential as targetable transcription factors in the fight against cancer.
Introduction
Current options for cancer treatment include surgery, radiotherapy, chemotherapy, and more recently developed targeted immunotherapies and checkpoint blockade therapies (1, 2). Both radiation and chemotherapy have a plethora of limitations, including side effects that require that clinical benefits be weighed against toxicity and the emergence of resistance that may result from intratumor heterogeneity (1–3). Targeted treatments also often cause side effects, including but not limited to gastrointestinal symptoms, and cancer frequently recurs or progresses despite treatment (3). Cancer metastasis and recurrence account for almost all cancer-associated deaths (4). Patients with advanced-stage cancer have a poor prognosis and high rates of resistance to available treatments (4).
There has been growing interest in the forkhead box (FOX) family of transcription factors as targets for anticancer drug development. Members of this family have an evolutionarily conserved DNA-binding domain and are involved in the regulation of cell growth, differentiation, and embryogenesis (5). In various cancers, including ovarian, breast, and prostate cancer, FOX factors are implicated in cancer initiation, progression, and chemoresistance (6–9). In this review, we discuss the roles of FOX factors in the epithelial–mesenchymal transition (EMT), hormone signaling, drug resistance, metabolism, and immune system regulation and their functions as pioneering factors. Based on our current understanding of the intertwined and complex roles of FOX proteins in cancer development and progression, these factors are important emerging therapeutic targets.
EMT, EM Plasticity, and Cancer Stem Cell Properties
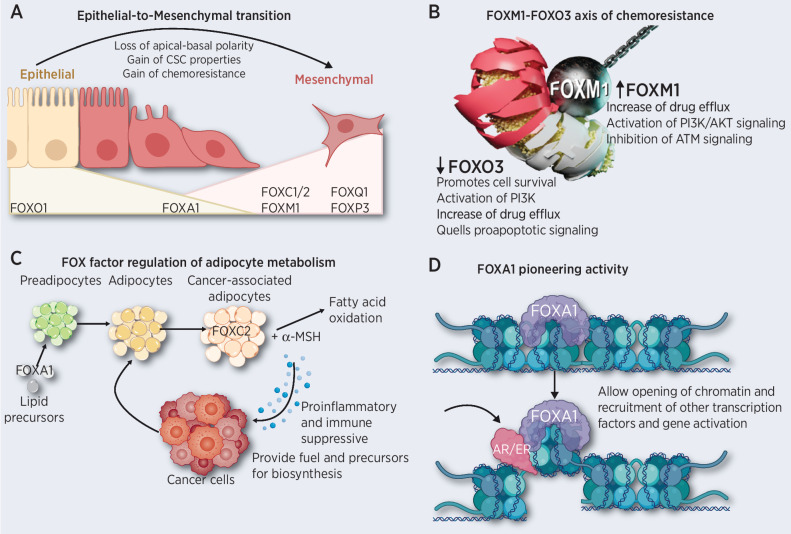
EMT is at the epicenter of cancer progression (10). The transition from the epithelial phenotype to the mesenchymal-like phenotype is a dynamic process necessary during normal development that is also involved in tumor progression and the acquisition of resistance to chemotherapy (10). EMT involves the reorganization of the epithelial cell cytoskeleton, loss of cell–cell junctions and apical–basal polarity, and a change in signaling programs that alter the expression of proteins defining cell shape (Fig. 1A) (11). In addition, EMT endows cancer cells with stem cell–like properties (12). Cancer stem cells (CSC) are a rare subtype of cancer cells that are intrinsically resistant to chemotherapy that are implicated in disease recurrence. Therapeutic agents that target transcription factors, such as the FOX proteins that regulate EMT, may enable the inhibition of metastasis, eradication of CSCs, and reversal of drug resistance.
Figure 1.
Role of FOX factors in cancer. A, Depiction of the two extreme phenotypes of EMT with the associated physiologic changes and the FOX factors that elicit each phenotype. FOXA1 can induce and inhibit EMT, depending on the cancer subtype. B, Summary of the functions of the FOXO3–FOXM1 axis in drug resistance. A drug-resistant state occurs upon inhibition of FOXO3 or upregulation of FOXM1, often in combination, leading to an increase in prosurvival mechanisms and drug efflux transporter activation. C, Schematic of how FOX factors regulate adipocyte metabolism, creating an immunosuppressive environment. FOXA1 mediates the acquisition of lipid precursors to fuel tumor proliferation. These precursors can become cancer-associated adipocytes expressing FOXC2. The cooperation between FOXC2 and α-MSH to promote fatty-acid oxidation creates an energy source for cancer cells. This creates a feedback loop in which cancer cells signal adipocytes to become cancer-associated adipocytes, and the cycle continues. D, Pioneering activity of FOXA1, a potential mechanism of action of other transcription factors. FOXA1 is capable of binding condensed chromatin to create an opening for easier accessibility of other transcription factors. In this case, FOXA1 allows for the binding of ER or AR to condensed chromatin, thereby activating an altered hormone response in cancer cells.
FOXC2 is the central mediator of EMT through the activation of various signaling pathways, including MAPK and PI3K/AKT, or by modulation of other transcription factors such as FOXO3 (13–15). We have also shown that FOXC2 results in the enrichment of CSC populations, thereby linking EMT to the induction of CSCs (12, 16). This leads to a chemoresistant state in various cancer types (6, 17, 18). The interconnectedness of CSCs, EMT, and drug resistance through FOXC2 places this transcription factor at the center of cancer progression and recurrence. Thus, the specific inhibition of FOXC2 is an attractive treatment option. Several methods of FOXC2 downregulation have been studied. For example, p38 inhibition, which leads to a decrease in FOXC2 levels, blocks the migratory and invasive capabilities of cancer cells (16).
Like FOXC2, FOXC1 has been linked to the induction of EMT (19, 20). FOXC1-mediated activation of the EMT program occurs through the transactivation of the transcription factor Snail and direct activation of ZEB2, both well-known EMT regulators (21, 22). FOXC1 is also able to activate the PI3K/AKT pathway (22), which, in a mechanism similar to that of FOXC2, leads to the downregulation of FOXO3 and the emergence of a chemoresistant state.
Several other FOX factors also influence EMT progression. The FOXA proteins are members of the class of transcription factors known as pioneering factors; these are transcription factors that associate with compacted chromatin to mediate binding of other transcription factors. The loss of FOXA family members affects the accessibility of enhancer regions of epithelial genes, triggering a switch from epithelial to mesenchymal gene expression (23, 24). The precise effect of FOXA factors on EMT induction is tissue-specific: In prostate cancer, the inhibition of FOXA leads to EMT inhibition, whereas loss of FOXA1 is necessary for EMT induction in pancreatic and breast cancer (25–27). The link between FOX factors and EMT is multifaceted. The varying induction of EMT through FOXA1 can be partially attributed to the reversibility element associated with EMT and to the intertwined programs such as EMT and metabolism and hormone regulation. FOXA1 is also involved in the regulation of androgen receptor (AR) and estrogen receptor (ER) signaling. This complex system of regulation by FOXA1 is potentially the cause of its dual roles in EMT induction.
Two FOX factors, FOXM1 and FOXO, are closely linked to drug resistance and are also mediators of EMT. Depletion of FOXM1 inhibits the proliferative and invasive capabilities of cancer cells, and its overexpression is linked to EMT induction (28–30). Several FOXM1 binding sites have been identified in the promoter of Slug, a well-characterized EMT-inducing transcription factor (31). Unlike FOXM1, which induces EMT and leads to a chemoresistant state, FOXO1 inhibits EMT. FOXO1 inhibits TGFβ–induced EMT, negatively regulates EMT transcription factors, and interacts with ZEB2 (32). In pancreatic ductal carcinoma, FOXO depletion induces EMT via activation of the β-catenin/transcription factor 4 (TCF4) pathway, a known CSC-associated signaling pathway (33).
Other FOX factors are also linked to EMT induction. FOXP3 induces EMT and promotes tumor growth by activating β-catenin/Wnt-mediated signaling by binding directly to β-catenin and by activating NF-κβ signaling, a regulatory pathway involved in inflammatory responses and cell survival (34–36). FOXQ1 induces signaling mediated by TGFβ to initiate EMT (37, 38) and represses the expression of E-cadherin and induces mesenchymal properties through induction of ZEB2 (37, 39). FOXF2 induces EMT induction and cancer progression with induction of bone metastasis seen in breast cancer (40, 41). However, FOXF2 induction is cancer-specific (42). Thus, various FOX factors are involved in EMT induction (Fig. 1A) and acquisition of CSC traits and the mesenchymal phenotype. These examples also demonstrate that the actions of FOX factors in cancer initiation and progression are complex. The prevalence of FOX factors in the induction of EMT sets the stage for their involvement in various cancer-associated pathways due to the complex role of EMT in cancer progression. It remains to be seen whether targeting these transcription factors alone will be enough to halt or reverse EMT or whether this process is so complex that a multifaceted approach is necessary.
Regulation of Hormone Signaling
In order to understand how FOX factors influence cancer progression, we must examine their roles in the regulation of hormone signaling pathways. Hormone-receptive cancers are often treated with therapies that target the relevant hormone signaling pathway. FOX factors are intricately involved in ER, HER2, and AR signaling. Hormone signaling is abnormal in over 60% of breast cancers (43). Targeting of hormone-mediated signaling pathways can be an effective treatment option; however, endocrine treatment (e.g., tamoxifen or aromatase inhibitors) often leads to treatment resistance and recurrence (44).
A key determinant of the response to estrogen is the level of FOXA1 expression (45). FOXA1 can activate ER-mediated signaling (46), which is associated with the activation of various protein kinase cascades and signaling pathways involved in cell proliferation and survival (47). In addition, this genome-wide reprogramming drives the activation of the hypoxia-inducible factor-2α (HIF-2α) transcription factor and its induction of a prometastatic program in breast cancer (23).
FOXM1 and FOXP1 also function in endocrine sensitivity and resistance in breast cancer. FOXM1 is correlated with ER and HER2 expression, and FOXM1 is able to activate ER expression by binding to forkhead-response elements located in the proximal region of the ER promoter (48–50). In addition, FOXP1 increases recruitment of ERα to ER binding sites to increase cellular proliferation (51, 52). FOXM1 can bypass the need for ER in downstream ER signaling pathways, resulting in endocrine resistance and tumor growth in breast cancer as well as an increase in CSC frequency (7, 53, 54). In addition, FOXM1 expression is dependent on HER2 expression, making it a likely downstream target of HER2 signaling (55). The functions of FOXM1 in both ER and HER2 signaling allow for a switch away from hormone-dependent signaling and make FOXM1 a prime target to combat acquired endocrine resistance in patients with cancer.
FOX factors can also regulate AR signaling. Prostate cancer cell proliferation is often dependent on AR-mediated signaling, and androgen deprivation therapy (ADT) is the first line of therapy for these tumors (56, 57). AR is required for normal male physiology and is also expressed in prostate cancer cells. ADT often fails in advanced stages of prostate cancer, which is termed castration-resistant prostate cancer (CRPC) (25, 58). Members of the FOX family of transcription factors are critical in AR-mediated signaling, particularly in CRPC (59).
In addition to regulating ER-mediated signaling, FOXA1 mediates AR expression (60). The forkhead domain of FOXA1 binds to sequences on the AR gene and physically interacts with AR, acting as a pioneering factor to recruit the AR protein. Thus, FOXA1 positively regulates prostate-related gene expression induced by androgens. It is expressed at high levels in patients with metastatic prostate cancer (60). Much like FOXA1, FOXA2 interacts with AR, resulting in activation of the promoter of the gene encoding the prostate-specific antigen (PSA). PSA, in turn, regulates the growth of epididymal cells, which are required for prostate physiology (61). Both FOXA1 and FOXA2 can upregulate AR-targeted genes in the absence of AR (62, 63), increasing prostate cancer growth, even in the absence of androgens, leading to the failure of ADT.
Other FOX transcription factors can also regulate AR-mediated signaling. For example, FOXM1 influences the binding of AR to the CDC6 promoter, increases the AR protein levels and induces PSA gene transcription in the absence of androgen stimulation (64, 65). FOXO3 can directly bind to AR promoter and the function of FOXO3 is regulated by the PIK3/AKT pathway (66). In contrast to these positive regulators of AR-mediated gene expression, FOXP1 is able to repress AR target genes (67). FOXP1 functions as a homodimer or heterodimer with FOXP2 and FOXP4 (67). When AR enters the nucleus, it is ligand-dependently recruited to bind FOXP1, leading to the negative regulation of AR target genes (67). In summary, FOX factors are essential players in hormone signaling in various cancer types with roles as both tumor promoters and suppressors. The involvement of FOX factors in hormone signaling links hormone regulation to drug response in cancer. Most importantly, FOX factors create hormone-independent states in which cancer cells can bypass the typical hormone regulatory pathways needed for normal physiologic growth, such as those seen in breast and prostate cancer. This scenario allows for unsuppressed growth and cancer progression.
Resistance to Therapeutics
Drug resistance remains a major hurdle in cancer treatment (2, 3). Drug resistance can develop through acquired resistance to drug regimens, tumor heterogeneity, or altered immune system intervention (68). In hormone-receptive cancers, single-use agents to target hormone signaling have been shown to be ineffective long term because of acquired resistance, as these agents cause a selective pressure that increases the frequency of CSCs (68). The drug resistance mechanisms include the development of resistance to apoptosis, increased DNA damage repair efficiency, elimination of the chemotherapeutic drugs by increased efflux, and drug inactivation.
Several of the FOX factors involved in the regulation of hormone signaling and EMT, including FOXO3 and FOXM1, are also implicated in drug resistance. FOXO3 depletion induces the expression of proteins important for drug efflux (such as MDR1) and proliferation in cancer, mostly through the activation of the PI3K signaling pathway, a well-known cancer-driving signaling pathway (69, 70). The inactivation of FOXO3 is due to the hyper-activation of the PI3K/AKT pathway, which results in the cytoplasmic translocation and degradation of FOXO protein (71). The degradation of FOXO proteins leads to a reversal of the proapoptotic properties of FOXO family members (13, 72, 73).
Although inactivation of FOXO3 alone can induce drug resistance, the FOXO3–FOXM1 axis is a better marker of drug resistance modulation (Fig. 1B; refs. 74, 75). FOXM1 and FOXO3 compete for the same binding sites in gene promoters, and FOXO3 can negatively regulate FOXM1 expression (76). When FOXM1 is upregulated, or FOXO3 is downregulated, the expression of genes involved in drug efflux, DNA repair, and cell survival are impacted (75). FOXM1 transcription is activated by an increase in reactive oxygen species (ROS), creating a feedback loop between ROS and FOXM1 expression (77). This leads to regulation of cell-cycle transition points by FOXM1 and increased resistance via overexpression of DNA damage response genes and the development of resistance to DNA damaging agents (78, 79). This acquired resistance may be due to the expansion of CSCs induced by FOXM1 (80–82). By acting synergistically with FOXO3 inactivation, FOXM1 blocks the action of trastuzumab by increasing the proteolytic degradation of p27, an inhibitor of cell-cycle progression (7, 78). FOXM1 creates a perfect axis for drug resistance by increasing the number of resistant CSCs, effectively pumping out drugs from the cell, and downregulating the expression of genes that serve as tumor suppressors.
Recently, another member of the FOX family, FOXC2, has been shown to be important in drug resistance and metastasis in multiple cancer types, including those of the prostate, breast, and ovary (83–85). High levels of FOXC2 expression are observed in advanced metastatic cancers, and its expression is correlated with poor prognosis (86). Inhibition of FOXC2 expression by treating cells with an inhibitor of p38 kinase, an upstream regulator of FOXC2, reduces resistance to chemotherapy agents (85). In accordance with these data, in ovarian cancer, the inhibition of FOXC2 expression increases chemosensitivity by decreasing the concentrations of phosphorylated forms of AKT and ERK protein kinases, which are involved in multiple cellular processes tied to cell proliferation and migration (6). In summary, FOXC2 is able to upregulate not only cell proliferation and migration but also chemoresistance through its modulation of EMT.
FOXQ1 is also involved in drug resistance. Elevated FOXQ1 expression is linked to poor prognosis in patients with cancer patients (87). FOXQ1 upregulation leads to the activation of PDGFR-α and PDGFR-β, upregulation of Twist and ZEB2, and initiation of EMT (88, 89). All of these proteins are involved in cancer progression and the generation of a drug-resistant state. FOXQ1 has been shown to control the expression of MDM2, which is a negative regulator of the expression of the tumor suppressor p53 (87). Thus, FOXQ1 upregulates EMT-involved transcription factors and downregulates tumor suppressor expression, leading to drug resistance. In summary, a large body of evidence points to multiple mechanisms by which various FOX transcription factors can confer chemoresistant traits to cancer cells, with factors involved in hormone signaling and EMT induction being closely tied to the induction of chemoresistance.
Cancer Metabolism
In addition to chemoresistance, FOX factors influence cancer growth through metabolic regulation. Cancer cells depend on aerobic glycolysis, a process known as the Warburg effect (90, 91). Normal cells utilize oxidative phosphorylation; however, in tumors, a hypoxic environment creates an environment in which oxidative phosphorylation is inefficient. Thus, cancer cells resort to glycolysis, in which two ATP molecules and intermediate building blocks are produced for every glucose consumed (92). Even though the production of ATP from glycolysis is lower on a molar basis than that of oxidative phosphorylation under normal conditions, the amount of ATP produced by a cancer cell can equal or even surpass that produced by a cell through oxidative phosphorylation (91, 93). Through glycolysis, cancer cells can generate an adequate amount of energy while also creating intermediates needed to promote cell growth. Two FOX factors have been implicated in the regulation of the Warburg effect: FOXM1 and FOXO.
FOXM1 has been shown to promote the Warburg effect and regulate glycolysis by upregulation of the expression of the lactate dehydrogenase (LDHA; refs. 94, 95). The resulting elevation of lactate production and glucose consumption causes an increase in glycolytic flux, fueling cancer cell growth. In addition, FOXM1 has been shown to upregulate the expression of the glucose transporter GLUT1 and the kinase HK2, which catalyzes the first step in glucose metabolism, further stimulating the Warburg effect (95). In contrast, FOXO has been shown to inhibit glycolysis through its antagonization of MYC (96). MYC is an oncogene that promotes a shift in metabolism primarily to glycolysis. Elevated FOXO levels lead to a decrease in ROS and a shift away from glycolysis (96). Thus, the FOXM1–FOXO axis, which plays a role in drug resistance, also plays a critical role in the metabolic shift in cancer cells.
Aerobic glycolysis leads to the production of the precursors needed for the biosynthesis of lipids and adipocytes; both have been shown to promote cancer metastasis (97, 98). Adipocytes store lipids and also secrete proinflammatory and immune-suppressing molecules. When adipocytes are close to cancer cells, they are reprogrammed to secrete proinvasive and prometastatic signals (98). The cancer-associated adipocytes can also provide cancer cells with energy sources through lipolysis (98). Thus, lipids and adipocytes create an environment that induces survival and metastasis.
FOX factors mediate adipocyte function (Fig. 1C). FOXA is expressed in preadipocytes and regulates the expression of LIPG, an enzyme responsible for generating lipid precursors (99, 100). Thus, FOXA mediates the acquisition of extracellular lipids that fuel tumor proliferation (99). FOXC2 is involved in lipid and adipocyte metabolism (101) and cooperates with α-MSH to promote fatty acid oxidation, creating an energy source for cancer cells (102). FOXC2 suppresses apoptosis in preadipocytes and promotes adipogenesis, another mechanism of synthesis of precursors that cancer cells utilize for energy and biosynthesis (103). In addition, FOXC2 promotes glycolysis by regulating the YAP/TAZ pathway, leading to an increase in tumor progression (104). Thus, FOX factors regulate cancer cell metabolism and adipogenesis, providing cancer cells with energy sources and creating a proinflammatory, prosurvival, and prometastatic environment. The interplay between FOX factors that regulate EMT, chemoresistance, and cancer metabolism demonstrates the complex and interconnected nature of signaling pathways involved in cancer progression. Based on this, a valid concern is that inhibiting the function of one of these FOX transcription factors, such as targeting FOXC2-regulated metabolism, will not be sufficient to halt cancer progression.
Evasion and Regulation of the Immune System
In addition to a prosurvival and metastatic environment, FOX factors can regulate the immune system to allow cancer cells to evade immune detection. Some FOX factors inhibit the immune response and others activate senescence. Senescence, the arrest of the proliferation of cells, leads to a change in the microenvironment that can allow tumor development (105). Senescent cells secrete numerous proinflammatory cytokines, chemokines, growth factors, and proteases that allow cancer cells to evade the immune system (105). Recovery from a senescent state after exposure to therapy is called proliferative recovery, and it has been shown to contribute to cancer recurrence (106). Senescent cells are not targetable with existing therapies because they do not actively proliferate (106).
Several FOX factors contribute to the induction of senescence. FOXM1 is a key regulator of senescence induction through regulation of the G2–M transition (107). Depletion of FOXM1 leads to the induction of a senescent state in multiple cancer cell types (107–109). FOXM1 downregulation also causes an increase in the abundance of ROS and the downregulation of c-MYC in cancer cells (107, 110). This, in turn, limits mitosis and increases the release of prosenescence markers that ultimately halt proliferation (108).
FOXO4 also has a demonstrated function in senescence. The activation of FOXO4 is correlated with an increase in levels of p21 protein and an increase in the population of senescent cancer cells (111, 112). FOXO4 binds to p53 at regions of DNA damage, enabling p53 to bind and transactivate the p21 promoter (111, 113). p21 executes the senescence program by causing cell cycle arrest (114). The arrest in the cell cycle leads to a survival advantage in the presence of chemotherapeutic drugs. A better understanding of how FOX factors initiate senescence through the upregulation of an inflammatory environment and inhibition of cell cycle regulators will guide the development of targeting strategies to overcome this phenomenon.
Although FOXQ1 is not as well characterized as some other FOX factors, it is known to induce EMT and has recently been shown to play a role in the repression of cancer senescence (115). FOXQ1 is responsible for the induction of proliferation through its suppression of IL6 and IL8 through the modulation of sirtulin 1 (115). IL6 and IL8 are proinflammatory chemokines responsible for senescence induction, and the inflammatory environment created by IL8 and IL6 further enhances survival. Downregulation of FOXQ1 induces premature senescence in fibroblasts, and its overexpression thwarts senescence induction (115). Thus, additional studies should be performed on FOXQ1 to determine its relationship to immune system modulation, as FOXQ1 may inhibit cancer growth by reducing inflammation.
Immunomodulatory cancer therapies have been developed that augment patients’ immune responses to cancer cells. Several FOX factors modulate key immune system signaling pathways, leading to an interest in their use as immunotherapy agents. Regulatory T (Treg) cells are an immunosuppressive subset of CD4+ T cells that account for about 5% of the CD4+ T-cell population (116). Treg cells are prevalent in tumor samples, and they are early responders during tumor development that are associated with poor patient prognosis (116, 117). Th cells are the primary line of adaptive immunity, activating B cells and cytotoxic T cells (118). Thus, helper T cells have functions opposite those of Treg cells in cancer progression.
FOXO3 can inhibit inflammatory transcriptional activity by blocking NF-κβ activation, leading to inhibition of Th-cell function and immune tolerance (119). FOXO3 expression is increased after T-cell receptor engagement and is involved in Th-cell differentiation (120). FOXO3 positively affects immune system activation, whereas FOXO3 inhibition has been linked to drug resistance acquisition. FOXO1 induces the production of Treg cells by binding to the FOXP3 promoter and triggering robust FOXP3 expression (121). FOXP3 is considered to be the master regulator of Treg cells (122). Sustained FOXP3 expression is required for the proper function of Treg cells (123). In addition, FOXP3 induces an immunosuppressive environment by repressing cytokines such as IL4 that promote a proper immune response (124, 125). Thus, FOXO1 and FOXP3 work together to elicit an immunosuppressive, prosurvival microenvironment, leading to the activation of Treg cells. The emergence of these immunosuppressive environments leads to the inhibition or resistance to immune checkpoint blockade therapy. Targeting the FOX family of transcription factors in combination with standard-of-care chemotherapeutics can aid in combating chemoresistance, one of the biggest hurdles of cancer treatment. Thus, a double targeted approach of chemotherapeutics plus FOX factor-targeting compounds could inhibit the bulk of the tumor, target the CSC population, and inhibit the emergence of resistance.
Pioneering Factors
A fundamental reason for targeting FOX proteins is that many FOX proteins function as pioneering factors. Pioneering factors are the first to engage at target sites on chromatin and therefore serve to initiate transcriptional programs (126). Under normal conditions, most transcription factors do not directly access their target sites on compacted chromatin but rather bind after the binding of a pioneering factor (126, 127). Pioneering factors are crucial for cellular reprogramming and are also implicated in gene regulatory networks that occur during cancer progression (128). Under some conditions, pioneering factors can activate regulatory programs that result in cancer cell propagation without normal checks and balances that restrict proliferation. The FOX proteins have a highly conserved winged-helix DNA-binding domain that resembles a linker histone and allows binding to nucleosomes (129).
FOXA1 and FOXA2 are capable of displacing histone H1, and they maintain enhancer regions in an open conformation, allowing other transcription factors to bind and activate gene expression (128, 130). The FOXA factors can activate redundant pathways despite the loss of any individual transcription factors (131). Loss of hormone production in hormone-dependent cancers, which should halt cancer progression, is thwarted as FOXA factors turn on otherwise hormone-dependent pathways in the absence of hormone secretion. For example, FOXA1 has been shown to open compact chromatin to facilitate AR recruitment to maintain the prostate epithelial phenotype and regulate the expression of genes encoding AR and ER (Fig. 1D; refs. 62, 132). In addition, FOXA proteins remain bound to chromatin during mitosis and help to activate signaling pathways that regulate cell fate (Fig. 1D; ref. 133). More studies are needed to explore the roles of FOXA transcription factors in cell fate determination during cancer progression.
Another FOX factor with pioneering capabilities is FOXO1, which opens and remodels chromatin via its conserved winged-helix domain (134). FOXO1 and FOXA1/2 cooperate to open condensed chromatin around insulin-regulated genes (135). FOXO1 decondenses chromatin arrays to regulate metabolism, cell survival, apoptosis, cell-cycle progression, and DNA repair (136). FOXI1 associates with mitotic chromatin and remains bound throughout mitosis (137, 138) but little is known about the role of this factor in cancer progression. FOXM1 expression has been shown to be required for proper chromosome segregation and mitotic progression (139, 140).
FOXC2 regulates mitotic entry via PLK1, a kinase responsible for G2–M regulation, and levels of FOXC2 fluctuate during cell-cycle progression, a known characteristic of mitotic bookmarkers (141). Mitotic bookmarkers are a subset of pioneering factors that possess the ability to bind to chromosomes during mitosis to rapidly activate a subset of genes following mitotic exit. Additional studies are needed to determine whether other FOX factors are both pioneering factors and mitotic bookmarkers. The ability of FOX factors to impact a plethora of critical pathways and regulatory mechanisms involved in cancer progression is likely due to their roles as pioneering factors that regulate cell-fate determination and acquisition of CSC properties. FOX factors are not only involved in the regulation of cancer cell signaling but also in the activation of these pathways through the regulation of chromosome accessibility. Thus, FOX factors may control more than just the cancer signaling pathways; they may also be involved in the epigenetics and cell fate control during cancer progression.
Future Directions
Our ability to successfully treat cancer has improved over the last decade; yet, there is no cure for highly invasive or metastatic cancers. Studies of FOX factors have provided unique perspectives into the mechanisms that drive cancer progression. Often, FOX factors are involved in more than one aspect of cancer progression (Table 1). Clarification of the functions of FOX factors in cancer modulation and the identification of druggable targets that alter the activities of these transcription factors could lead to additional options for the treatment of cancer. Inhibition of FOX factors, or the pathways they mediate, can potentially reverse drug resistance, inhibit immune evasion, and block metastatic progression. Inhibitors of FOX factors, the kinases involved in FOX-mediated pathways, and the agents that mediate RNAi to silence the expression of FOX factors are promising tools to treat patients with various types of cancer.
Table 1.
Functions of FOX factors in cancer.
| FOX factors | Roles in cancer |
|---|---|
| FOXA1 and | Bind to sequences on AR gene (58, 61) |
| FOXA2 | Open compact chromatin for AR and ER recruitment (45, 58, 132) |
| Duality in EMT activation, cancer (26, 58) | |
| Promote lipid precursor accumulation (99) | |
| Pioneering factors (130) | |
| FOXM1 | Increases AR levels via recruitment of AR to CDC6 in the absence of androgen stimulation (65) |
| Correlates with ER and HER2 expression (48, 65, 80) | |
| Binds to forkhead response elements located in the proximal region of ER promoter (49) | |
| Increases drug resistance via overexpression of DNA damage response genes (70, 75, 76) | |
| Initiates EMT through binding of Slug promoter (31) | |
| Induces senescence via regulation of cell division at G2–M (107, 109) | |
| Promotes Warburg effects (94) | |
| FOXO3 | Binds to AR promoter (66) |
| Regulates cell fate via apoptosis induction (13, 73) | |
| Upregulates drug efflux and activation of the PI3K pathway (13, 14, 72, 75) | |
| FOXC2 | Upregulates EMT, CSC population, and AKT signaling, leading to drug resistance (16, 80, 97) |
| Enriches the CSC population in tumors (16) | |
| Mediates G2–M transition (141) | |
| Promotes fatty-acid oxidation (102) | |
| FOXP1 | Represses AR-targeting genes via the formation of dimers with FOXP2 and FOXP4 (67) |
| FOXO4 | Increases p21, a senescence marker (113) |
| Binds to p53 at the area of DNA damage and activates the p21 promoter (113) | |
| FOXO1 | Stimulates dendritic cells and T and B lymphocytes (121) |
| Binds to FOXP3 promoter and regulates the production of Treg cells (135) | |
| Elicits an immunosuppressive environment (121) | |
| Pioneering factor (136) | |
| FOXP3 | Binds directly to β-catenin and upregulates GSK3β (34) |
| Maintains proper function of Treg cells (122, 123, 125) | |
| FOXQ1 | Activates PDGFR-α and β, Twist, and ZEB2, leading to EMT induction (37, 38, 88) |
| Controls expression of MDM2, a regulator of p53 (87, 89) | |
| Induces proliferation through suppression of IL6 and IL8 (115) | |
| FOXF2 | Promotes bone metastasis in breast cancer (40) |
| Induces EMT and cancer progression, cancer-specific (41, 42) |
However, additional studies are required to shed light on the complexity of FOX factors. The dual natures of certain FOX factors during EMT raise the concern that inhibition of these transcription factors during different stages of cancer progression could lead to undesirable outcomes (42, 142). In addition, certain FOX factors have contradictory outcomes depending on the tissue. For example, FOXA has inhibitory effects in pancreatic cancer but stimulatory effects in breast and prostate cancers (25, 26). This duality in EMT and metastatic progression may be due to the intricate nature of FOXA's involvement with hormone signaling.
Of particular interest will be the study of the abilities of FOX factors to function as pioneering factors. Their highly conserved winged-helix DNA-binding domain mimics a histone linker, and therefore, it is well-matched to the binding of condensed chromatin structures (129). All FOX factors may have the pioneering ability, although this remains to be experimentally confirmed. Their abilities to serve as pioneering factors allow FOX factors to activate compensatory mechanisms needed for cancer growth as well as to determine cancer cell fate. FOX transcription factors are linked to EMT, hormone signaling, drug resistance, metabolic reprogramming, and immune system modulation, and their study may be key to understanding cancer.
Acknowledgments
This article was supported by grants from the NIH (grant nos. 1R01CA155243; 2R01CA155243), NSF (grant no. PHY-1935762), the Bowes Foundation to S.A. Mani, supported in part by the MD Anderson Cancer Center (Houston, TX) Support Grant (grant no. CA016672; MRP to S.A. Mani), and an NCI-T32 fellowship (5T32CA217789-04) to M. Castaneda. Editing services were provided through Editing Services, Research Medical Library at MD Anderson Cancer Research Center. Figures were created by the creative commons team at MD Anderson Cancer Research Center.
Authors' Disclosures
No disclosures were reported.
References
- 1. Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med 12:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol 43:723–37. [DOI] [PubMed] [Google Scholar]
- 3. Schnipper LE. American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 33:2563–77,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu S-G, Li H, Tang L-Y, Sun J-Y, Zhang W-W, Li F-Y, et al. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: A SEER database analysis. Tumour Biol 2017;39:1010428317705082. [DOI] [PubMed] [Google Scholar]
- 5. Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (Fox) gene families. Hum Genomics 2010;4:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li C, Ding H, Tian J, Wu L, Wang Y, Xing Y, et al. Forkhead Box Protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol Biochem 39:242–52. [DOI] [PubMed] [Google Scholar]
- 7. Carr JR, Park HJ, Wang Z, Kiefer MM, Raychaudhuri P. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res 70:5054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan B, Liu Y, Yu X, Yin L, Peng Y, Gao Y, et al. FOXM1 contributes to taxane resistance by regulating UHRF1-controlled cancer cell stemness. Cell Death Dis. Springer US; 2018;9:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu N, Zhang X, Wang X, Ge H, Wang X, Garfield D, et al. FoxM1 mediated resistance to gefitinib in non-smallcell lung cancer cells. Acta Pharmacol Sin 2012;33:675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–90. [DOI] [PubMed] [Google Scholar]
- 11. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol cell Biol 15:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arun RP, Sivanesan D, Vidyasekar P, Verma RS. PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of colorectal cancer cells under simulated microgravity. Sci Rep 2017;7:5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Das TP, Suman S, Alatassi H, Ankem MK, Damodaran C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis 2016;7:2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mani SA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci 2007;104:10069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, et al. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res 2013 Mar 15;73:1981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett 2015;363:137–45. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Deng G, Fu Y, Han Y, Guo C, Yin L, et al. FOXC2 promotes oxaliplatin resistance by inducing epithelial-mesenchymal transition via MAPK/ERK signaling in colorectal cancer. Onco Targets Ther 2020;13:1625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X, Wei L, Bai Y, Wu S, Han S. FoxC1 promotes epithelial-mesenchymal transition through PBX1 dependent transactivation of ZEB2 in esophageal cancer. Am J Cancer Res 2017;7:1642. [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Z-Y, Ding S-M, Zhou L, Xie H-Y, Chen K-J, Zhang W, et al. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int J Biol Sci 2012;8:1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song X, Chang H, Liang Q, Guo Z, Wu J. ZEB1 promotes prostate cancer proliferation and invasion through ERK1/2 signaling pathway. Eur Rev Med Pharmacol Sci 21:4032–8. [PubMed] [Google Scholar]
- 22. Huang L. FOXC1 promotes proliferation and epithelial-mesenchymal transition in cervical carcinoma through the PI3K-AKT signal pathway. Am J Transl Res 2017;9:1297. [PMC free article] [PubMed] [Google Scholar]
- 23. Trivedi MV, Fu X, Pereira R, Osbornea CK, Schiff R, Shea M, et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc Natl Acad Sci 2016;113:6600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jägle S, Busch H, Freihen V, Beyes S, Schrempp M, Boerries M, et al. SNAIL1-mediated downregulation of FOXA proteins facilitates the inactivation of transcriptional enhancer elements at key epithelial genes in colorectal cancer cells. PLoS Genet 2017;13:e1007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerhardt J. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol 2012;180:848–61. [DOI] [PubMed] [Google Scholar]
- 26. Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancreatic cancer. Cancer Res 2010;70:2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. BenAyed-Guerfali D, Dabbèche-Bouricha E, Ayadi W, Trifa F, Charfi S, Khabir A, et al. Association of FOXA1 and EMT markers (Twist1 and E-cadherin) in breast cancer. Mol Biol Rep 2019;46:3247–55. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Yao B, Wang Y, Zhang M, Fu S, Gao H, et al. Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int J Mol Med 33:1514–22. [DOI] [PubMed] [Google Scholar]
- 29. Kong F-F, Qu Z-Q, Yuan H-H, Wang J-Y, Zhao M, Guo Y-H, et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep 2014;31:2660–8. [DOI] [PubMed] [Google Scholar]
- 30. Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim K, et al. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med 2011;3:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang C, Chen H, Tan G, Gao W, Cheng L, Jiang X, et al. FOXM1 promotes the epithelial to mesenchymal transition by stimulating the transcription of Slug in human breast cancer. Cancer Lett 2013;340:104–12. [DOI] [PubMed] [Google Scholar]
- 32. Dong T, Zhang Y, Chen Y, Liu P, An T, Zhang J, et al. FOXO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget 2017;8:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Yang R, Dong Y, Chen M, Wang Y, Wang G. Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes metastasis of pancreatic ductal adenocarcinoma by activation of the β-catenin/TCF4 pathway through SPRY2. J Exp Clin Cancer Res 2019;38:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Yang S, Liu Y, Li M-Y, Ng CSH, Yang S, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer 2017;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Liu Y, Dai L, Liu Q, Jia L, Wang H, et al. Foxp3 downregulation in NSCLC mediates epithelial-mesenchymal transition via NF-κB signaling. Oncol Rep 2016;36:2282–8. [DOI] [PubMed] [Google Scholar]
- 36. Pan D, Zeng X, Ma G, Gao J, Li N, Miao Q, et al. Label-free quantitative proteomic analysis identifies CTNNB1 as a direct target of FOXP3 in gastric cancer cells. Oncol Lett 2018;15:7655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu F, et al. Forkhead transcription factor Foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res 2011;71:1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiao Y, Jiang X, Lee S, Karuturi R, Hooi S, Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res 2011;71:3076. [DOI] [PubMed] [Google Scholar]
- 39. Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, et al. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology 2014;59:958–73. [DOI] [PubMed] [Google Scholar]
- 40. Wang S, Li G-X, Tan C-C, He R, Kang L-J, Lu J-T, et al. FOXF2 reprograms breast cancer cells into bone metastasis seeds. Nat Commun 2019;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lo P-K, Lee JS, Liang X, Sukumar S. The dual role of FOXF2 in regulation of DNA replication and the epithelial-mesenchymal transition in breast cancer progression. Cell Signal 2016;28:1502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer-Schaller N, Heck C, Tiede S, Yilmaz M, Christofori G. Foxf2 plays a dual role during transforming growth factor beta-induced epithelial to mesenchymal transition by promoting apoptosis yet enabling cell junction dissolution and migration. Breast Cancer Res 2018;20:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hilton HN, Doan TB, Graham JD, Oakes SR, Silvestri A, Santucci N, et al. Acquired convergence of hormone signaling in breast cancer: ER and PR transition from functionally distinct in normal breast to predictors of metastatic disease. Oncotarget 2014;5:8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robinson J, Carroll J. FoxA1 is a key mediator of hormonal response in breast and prostate cancer. Front Endocrinol (Lausanne) 2012;3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 2005;122:33–43. [DOI] [PubMed] [Google Scholar]
- 47. Frasor J, Danes JM, Komm B, Chang KCN, Richard Lyttle C, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 2003;144:4562–74. [DOI] [PubMed] [Google Scholar]
- 48. Francis RE. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. Int J Oncol 2009;35: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Millour J, Lam EW. FOXM1 is a transcriptional target of ERα and has a critical role in breast cancer endocrine sensitivity and resistance. Breast Cancer Res 2010; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bektas N. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer 2008;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fox SB, Brown P, Han C, Ashe S, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor α and improved survival in primary human breast carcinomas. Clin Cancer Res 2004;10:3521–7. [DOI] [PubMed] [Google Scholar]
- 52. Shigekawa T, Ijichi N, Ikeda K, Horie-Inoue K, Shimizu C, Saji S, et al. FOXP1, an estrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer. Horm Cancer 2011;2:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Madureira PA. The Forkhead box M1 protein regulates the transcription of the estrogen receptor α in breast cancer cells. J Biol Chem 2006;281:25167–76. [DOI] [PubMed] [Google Scholar]
- 54. Ziegler Y, Laws MJ, Sanabria Guillen V, Kim SH, Dey P, Smith BP, et al. Suppression of FOXM1 activities and breast cancer growth in vitro and in vivo by a new class of compounds. npj Breast Cancer 2019;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roßwag S, Cotarelo CL, Pantel K, Riethdorf S, Sleeman JP, Schmidt M, et al. Functional characterization of circulating tumor cells (CTCs) from metastatic ER+/HER2− breast cancer reveals dependence on HER2 and FOXM1 for endocrine therapy resistance and tumor cell survival: implications for treatment of ER+/HER2− breast cancer. Cancers 2021;13:1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li P, Yang R, Gao W-Q. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Mol Cancer 2014;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med 2017;7:a030452. [DOI] [PMC free article] [PubMed]
- 58. Sahu B. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J 30:3962–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Der Heul-Nieuwenhuijsen L, Dits NF, Jenster G, Van Der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int 103:1574–80. [DOI] [PubMed] [Google Scholar]
- 60. Vasaikar SV, Deshmukh AP, den Hollander P, Addanki S, Kuburich NA, Kudaravalli S. EMTome: a resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br J Cancer 2021;124:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu X. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann New York Acad Sci 2005;1061:77–93. [DOI] [PubMed] [Google Scholar]
- 62. Jin H-J, Zhao JC, Ogden I, Bergan RC, Yu J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res 2013;73:3725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Connelly ZM, Yang S, Chen F, Yeh Y, Khater N, Jin R, et al. Foxa2 activates the transcription of androgen receptor target genes in castrate resistant prostatic tumors. Am J Clin Exp Urol 2018;6:172–81. [PMC free article] [PubMed] [Google Scholar]
- 64. Liu Y, Liu Y, Yuan B, Yin L, Peng Y, Yu X, et al. FOXM1 promotes the progression of prostate cancer by regulating PSA gene transcription. Oncotarget 2017;8:17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Gong Z, Sun L, Li X. FOXM1 and androgen receptor co-regulate CDC6 gene transcription and DNA replication in prostate cancer cells. Biochim Biophys Acta (BBA)-Gene Regul Mech 2014;1839:297–305. [DOI] [PubMed] [Google Scholar]
- 66. Yang L. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem 2005;280:33558–65. [DOI] [PubMed] [Google Scholar]
- 67. Takayama K. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Biophys Res Commun 2008;374:388–93. [DOI] [PubMed] [Google Scholar]
- 68. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 2019;575:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gao Z, Li Z, Liu Y, Liu Z. Forkhead box O3 promotes colon cancer proliferation and drug resistance by activating MDR1 expression. Mol Genet genomic Med 2019;7:e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wilson MSC, Brosens JJ, Schwenen HDC, Lam EW-F. FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets 2011;12:1256–66. [DOI] [PubMed] [Google Scholar]
- 71. Dubrovska A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci 2009;106:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu Y, Xiang A, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer 2018;17:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mattos SF, Villalonga P, Clardy J, Lam EW. FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol Cancer Ther 2008;7:3237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim MY. High FOXM1 expression is a prognostic marker for poor clinical outcomes in prostate cancer. J Cancer 2019;10:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yao S, Fan LY-N, Lam EW-F. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin Cancer Biol 2018;50:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Moraes G, Bella L, Zona S, Burton J, LW-F M., E. Insights into a critical role of the FOXO3a-FOXM1 axis in DNA damage response and genotoxic drug resistance. Curr Drug Targets 2016;17:164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J 2009;28:2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saba R, Alsayed A, Zacny JP, Dudek AZ. The role of forkhead box protein m1 in breast cancer progression and resistance to therapy. Int J Breast Cancer 2016;2016:9768183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hwang C. Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol 2012;4:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bergamaschi A. The forkhead transcription factor FOXM1 promotes endocrine resistance and invasiveness in estrogen receptor-positive breast cancer by expansion of stem-like cancer cells. Breast Cancer Res 2014;16:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kopanja D, Pandey A, Kiefer M, Wang Z, Chandan N, Carr JR, et al. Essential roles of FoxM1 in Ras-induced liver cancer progression and in cancer cells with stem cell features. J Hepatol 2015;63:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang Z, Park HJ, Carr JR, Chen Y, Zheng Y, Tyner AL, et al. FoxM1 in tumorigenicity of the neuroblastoma cells and renewal of the neural progenitors. Cancer Res. AACR; 2011;71:4292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A 2007;104:10069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Børretzen A. FOXC2 expression and epithelial–mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. J Pathol Clin Res 2019;5:272–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Paranjape A. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene 2016;35:5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Y-W. High expression of forkhead box protein C2 is related to poor prognosis in human gliomas. Asian Pac J Cancer Prev 2014;15:10621–5. [DOI] [PubMed] [Google Scholar]
- 87. Zhang X, Wang L, Wang Y, Shi S, Zhu H, Xiao F, et al. Inhibition of FOXQ1 induces apoptosis and suppresses proliferation in prostate cancer cells by controlling BCL11A/MDM2 expression. Oncol Rep 2016;36:2349–56. [DOI] [PubMed] [Google Scholar]
- 88. Meng F, Speyer C, Zhang B, Zhao Y, Chen W, Gorski D, et al. PDGFRα and β play critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res 2015;75:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, et al. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res 2010;70:2053–63. [DOI] [PubMed] [Google Scholar]
- 90. Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Warburg O. On the origin of cancer cells. Science (80-) JSTOR; 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- 92. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 93. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001;292:504–7. [DOI] [PubMed] [Google Scholar]
- 94. Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, et al. FOXM1 promotes the Warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res 2014;20:2595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang Y, Yun Y, Wu B, Wen L, Wen M, Yang H, et al. FOXM1 promotes reprogramming of glucose metabolism in epithelial ovarian cancer cells via activation of GLUT1 and HK2 transcription. Oncotarget 2016;7:47985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Peck B, Ferber EC, Schulze A. Antagonism between FOXO and MYC regulates cellular powerhouse. Front Oncol 2013;3:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17:1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013;1831:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Slebe F, Rojo F, Vinaixa M, García-Rocha M, Testoni G, Guiu M, et al. , Gomis RR. FoxA and LIPG endothelial lipase control the uptake of extracellular lipids for breast cancer growth. Nat Commun 2016;7:11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wolfrum C, Shih DQ, Kuwajima S, Norris AW, Kahn CR, Stoffel M. Role of Foxa-2 in adipocyte metabolism and differentiation. J Clin Invest 2003;112:345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang T, Zheng L, Wang Q, Hu Y-W. Emerging roles and mechanisms of FOXC2 in cancer. Clin Chim Acta 2018;479:84–93. [DOI] [PubMed] [Google Scholar]
- 102. Gan L, Liu Z, Chen Y, Luo D, Feng F, Liu G, et al. α-MSH and Foxc2 promote fatty acid oxidation through C/EBPβ negative transcription in mice adipose tissue. Sci Rep 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gan L, Liu Z, Jin W, Zhou Z, Sun C. Foxc2 enhances proliferation and inhibits apoptosis through activating Akt/mTORC1 signaling pathway in mouse preadipocytes. J Lipid Res 2015;56:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Song L, Tang H, Liao W, Luo X, Li Y, Chen T, et al. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp Cell Res 2017;357:17–24. [DOI] [PubMed] [Google Scholar]
- 105. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saleh T, Tyutyunyk-Massey L, Gewirtz DA. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res 2019;79:1044–6. [DOI] [PubMed] [Google Scholar]
- 107. Smirnov A. FOXM1 regulates proliferation, senescence and oxidative stress in keratinocytes and cancer cells. Aging 2016;8:1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Macedo JC. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat Commun 2018;9:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, et al. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27kip1. J Pathol A J Pathol Soc Gt Britain Irel 2009;218:419–27. [DOI] [PubMed] [Google Scholar]
- 110. Pan H, Zhu Y, Wei W, Shao S, Rui X. Transcription factor FoxM1 is the downstream target of c-Myc and contributes to the development of prostate cancer. World J Surg Oncol 2018;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bourgeois B, Madl T. Regulation of cellular senescence via the FOXO 4-p53 axis. FEBS Lett 2018;592:2083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jung-Hynes B, Ahmad N. Role of p53 in the anti-proliferative effects of Sirt1 inhibition in prostate cancer cells. Cell Cycle 2009;8:1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yuedi D, Houbao L, Pinxiang L, Hui W, Min T, Dexiang Z. KLF2 induces the senescence of pancreatic cancer cells by cooperating with FOXO4 to upregulate p21. Exp Cell Res 2020;388:111784. [DOI] [PubMed] [Google Scholar]
- 114. Tonnessen-Murray CA, Lozano G, Jackson JG. The regulation of cellular functions by the p53 protein: cellular senescence. Cold Spring Harb Perspect Med 2017;7:a026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang P, Lv C, Zhang T, Liu J, Yang J, Guan F, et al. FOXQ1 regulates senescence-associated inflammation via activation of SIRT1 expression. Cell Death Dis 2017;8:e2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Najafi M, Farhood B, Mortezaee K. Contribution of regulatory T cells to cancer: A review. J Cell Physiol 2019;234:7983–93. [DOI] [PubMed] [Google Scholar]
- 117. Shen Y-C, Ghasemzadeh A, Kochel CM, Nirschl TR, Francica BJ, Lopez-Bujanda ZA, et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis 2018;21:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mosmann TR, Cherwinski H, Bond MW. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–57. [PubMed] [Google Scholar]
- 119. Lin L, Hron JD, Peng SL. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004;21:203–13. [DOI] [PubMed] [Google Scholar]
- 120. Stienne C, Michieletto MF, Benamar M, Carrié N, Bernard I, Nguyen X-H, et al. Foxo3 transcription factor drives pathogenic T helper 1 differentiation by inducing the expression of eomes. Immunity 2016;45:774–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cabrera-Ortega AA, Feinberg D, Graves DT. The role of forkhead box 1 (FOXO1) in the immune system: dendritic cells, T cells, B cells, and hematopoietic stem cells. Crit Rev Immunol 2017;37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 123. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 124. Fleskens V, Boxtel R. Forkhead Box P family members at the crossroad between tolerance and immunity: a balancing act. Int Rev Immunol 2014;33:94–109. [DOI] [PubMed] [Google Scholar]
- 125. Kiniwa Y. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 2007;13:6947–58. [DOI] [PubMed] [Google Scholar]
- 126. Zaret KS, Carroll JS. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev 2011;25:2227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015;161:555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Iwafuchi-Doi M, Zaret KS. Cell fate control by pioneer transcription factors. Dev 2016;143:1833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev 2009;23:804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, et al. The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol Cell 2016;62:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Reizel Y, Morgan A, Gao L, Lan Y, Manduchi E, Waite EL, et al. Collapse of the hepatic gene regulatory network in the absence of FoxA FACTORS. Genes Dev 2020;34:1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Augello MA, Hickey TE, Knudsen KE. FOXA1: master of steroid receptor function in cancer. EMBO J 2011;30:3885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: Programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol 2008;73:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yalley A, Schill D, Hatta M, Johnson N, Cirillo LA. Loss of interdependent binding by the FoxO1 and FoxA1/A2 forkhead transcription factors culminates in perturbation of active chromatin marks and binding of transcriptional regulators at insulin-sensitive genes. J Biol Chem 2016;291:8848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schill D, Nord J, Cirillo LA. FoxO1 and FoxA1/2 form a complex on DNA and cooperate to open chromatin at insulin-regulated genes. Biochem Cell Biol 2019;97:118–29. [DOI] [PubMed] [Google Scholar]
- 136. Hatta M, Cirillo LA. Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J Biol Chem 2007;282:35583–93. [DOI] [PubMed] [Google Scholar]
- 137. Lalmansingh AS, Karmakar S, Jin Y, Nagaich AK. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta 2012;1819:707–15. [DOI] [PubMed] [Google Scholar]
- 138. Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol 2006;26:155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Laoukili J, Kooistra MRH, Brás A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 2005;7:126–36. [DOI] [PubMed] [Google Scholar]
- 140. Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res 2005;65:5181–9. [DOI] [PubMed] [Google Scholar]
- 141. Pietila M, Vijay GV, Soundararajan R, Yu X, Symmans WF, Sphyris N, et al. FOXC2 regulates the G2-M transition of stem cell-rich breast cancer cells and sensitizes them to PLK1 inhibition. Sci Rep 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cai J, Tian A-X, Wang Q-S, Kong P-Z, Du X, Li X-Q, et al. FOXF2 suppresses the FOXC2-mediated epithelial-mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett 2015;367:129–37. [DOI] [PubMed] [Google Scholar]