The omicron variant of SARS-CoV-2 (B.1.1.529) was first detected in South Africa in November, 2021.1, 2, 3 Declared a variant of concern on Nov 26, 2021,4 omicron spread exponentially, replacing the delta variant (B.1.617.2) and driving rapid increases in COVID-19 cases.5, 6 In-vitro experiments show that the omicron variant escapes antibody neutralisation in people who have previously been infected with or vaccinated against SARS-CoV-2.7, 8 Epidemiological data suggest that vaccine effectiveness is reduced,9 and reinfection rates are higher for omicron than for the beta (B.1.351) and delta variants.10
Here we describe breakthrough infections—positive SARS-CoV-2 PCR or antigen tests 28 days or more after vaccination—in the South African Sisonke phase 3B Ad26.COV2.S vaccine trial (NCT04838795) during periods of time when delta and omicron variants were in circulation.11 The trial enrolled health-care workers in 360 vaccination centres across South Africa's nine provinces. Study procedures included an electronic consent process, an on-site check for vaccination eligibility, and post-vaccination safety monitoring. 477 234 health-care workers received a single dose of Ad26.COV2.S vaccine between Feb 17, 2021, and May 17, 2021; 230 488 health-care workers voluntarily received a second dose of Ad26.COV2.S between Nov 9, 2021, and Dec 16, 2021. We used proxy dates to define the contiguous time periods during which each of the three SARS-CoV-2 variants dominated in circulation: from Feb 17 (the start of the Sisonke study) to May 17, 2021, for the beta period; from May 18 to Nov 14, 2021, for the delta period; and from Nov 15, 2021, to Jan 31, 2022, for the omicron period. Since Feb 17–May 17, 2021, was the tail end of the beta period, we do not present the detailed analysis of this period here.
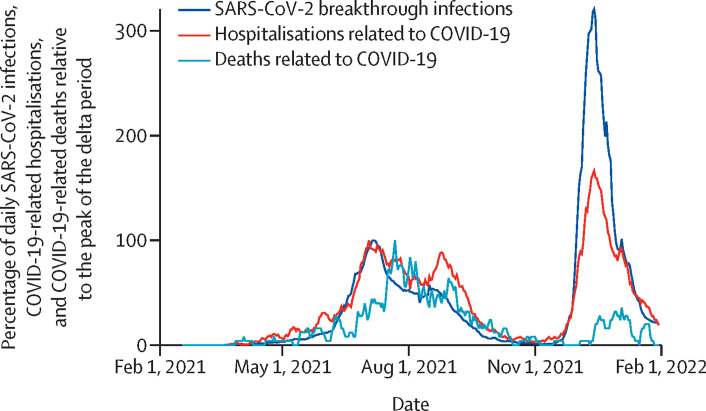
We analysed breakthrough infection patterns, COVID-19-related hospitalisations (ie, hospitalisations of participants who tested positive for COVID-19), and COVID-19-related deaths overall between Feb 17, 2021, and Jan 31, 2022—inclusive of when participants received their first and second doses of Ad26.COV2.S (figure ). We also evaluated the frequency and severity of breakthrough infections during the first 78 days during which participants were predominantly exposed to the delta and omicron variants after a single dose of the Ad26.COV2.S vaccine.
Figure.
Daily SARS-CoV2 infections, COVID-19-related hospitalisations, and COVID-19-related deaths expressed as a percentage of those at the peak of the delta period
Demographic characteristics and comorbidities were self-reported, and vaccinators recorded vaccination details in the Electronic Vaccination Data System. The definition of a person under investigation for COVID-19 remained constant throughout the study period. SARS-CoV-2 PCR remained the gold standard for diagnosis; however, nationally accredited antigen testing was introduced as an alternative to PCR in October, 2021. Criteria for SARS-CoV-2 testing also remained constant during the study. Breakthrough infections were monitored using active and passive surveillance. Linkage of the Sisonke trial data with the COVID-19 Notifiable Medical Conditions Sentinel Surveillance master list, the DATCOV list (COVID-19-related hospitalisations), and the National Population Register (held by the South African Medical Research Council) identified participants with SARS-CoV-2 infections or reinfections, COVID-19-related hospitalisations, and COVID-19-related deaths. Additionally, participants received text messages after vaccination encouraging them to report breakthrough infections by telephone or online. The protocol safety team contacted all hospitalised participants to confirm the severity of disease and ascertain outcome. All deaths associated with breakthrough infections were investigated. Health-care workers with reinfections were included in all relevant study periods. We considered two positive tests for SARS-CoV-2 within 90 days of each other as the same infection. Age was categorised into tertiles using hospitalisation data. Adjusted odds ratios, which assessed the effect of age and gender on infection by omicron versus delta variants, were computed using logistic regression including province as a variable and adjusting for clustering within patients for repeated events. A similar analysis was conducted for hospitalisation.
At the peak of the omicron period (Dec 14, 2021), daily infections were more than three times higher than daily infections at the peak of the delta period (July 6, 2021; figure). However, unlike the delta period, the omicron period showed a clear and early decoupling of hospitalisations from cases (figure).
Relative to the first 78 days of the delta period, the odds of breakthrough infections within the first 78 days of the omicron period was significantly reduced among men (11% lower odds) and older age groups (28% lower odds in the 31–54 year age group; 53% lower odds in the ≥55 year age group; appendix). The median age of participants with breakthrough infections was 43 years (IQR 35–51) during the delta period and 40 years (32–49) during the omicron period. The median number of days after initial vaccination at which breakthrough infections were reported was 98 days (IQR 67–120) during the delta period and 259 days (224–280) during the omicron period. The odds of hospitalisation were significantly lower (by 56%) during the omicron period than during the delta period among men and among older age groups (56% lower odds in the 31–54 year age group; 76% lower odds in the ≥55 year age group; appendix). Patients hospitalised with breakthrough infections during the omicron period were 1·54 times more likely to have HIV than patients were during the delta period; by contrast, the omicron period saw significantly lower odds of hypertension (21% for omicron period vs 35% for delta period) and diabetes (10% for omicron period vs 23% for delta period; appendix). The omicron period also saw lower proportions of patients hospitalised with breakthrough infections and needing intensive care (7% for delta period vs 3% for omicron period; p<0·001), ventilation (7% vs 2%), and oxygen (42% vs 16%; appendix). No significant differences between the omicron and delta periods were seen in the prevalences of cancer (0·8–1·5%), tuberculosis (0·1%), or chronic lung disease (0·6–1·2%) among patients hospitalised with breakthrough infections. Adjusting for comorbidities, the level of care needed (as measured by the need for oxygenation and whether the patient was ever ventilated) was still significantly lower in the omicron period than in the delta period (appendix). Additionally, a shift from intensive care towards high care was observed during the omicron period (appendix).
Among health-care workers hospitalised during the first 78 days of each period and for whom hospital discharge data were available (n=1926), the median length of hospital stay was 5 days (IQR 2–11; n=47) during the beta period, 5 days during the delta period (3–10; n=1416), and 3 days (2–6; n=882) during the omicron period (p<0·0001).
Of 17 650 health-care workers infected during the omicron period, 28 (0·16%) and 786 (4·5%) had been previously infected during the beta period and delta period, respectively, suggesting possible cross-protection from previous infections with the beta variant or reactivation of infection from the delta variant, due to adjacent time periods.12 No significant differences were found when comparing baseline characteristics (age, sex, and comorbidities) of reinfected health-care workers who had initially been infected during beta and delta periods.
Our analysis has several limitations. We report on the first 78 days of the omicron period, which might be too early to measure the full effect of the omicron variant. However, 78 days into the omicron period, the number of breakthrough infections had by far surpassed that of the delta peak. We also compare periods of time in which a specific variant dominated circulation rather than the specific variants themselves, as not every breakthrough infection was sequenced. We included all health-care workers who were hospitalised with breakthrough infections across all study periods, including those who received incidental diagnoses while hospitalised for other reasons such as surgery. The number and proportion of patients with breakthrough infections who required hospitalisation because of severe illness caused by SARS-CoV-2 is therefore probably overestimated. Breakthrough infection data were obtained from the national line list and include symptomatic and asymptomatic infections. Given that the study population consisted of health-care workers, SARS-CoV-2 testing occurred for several reasons, including occupational testing requirements, close contact with a family member or friend with COVID-19, travel screening, and symptoms of COVID-19. Separation of cases by reason for testing was not possible. COVID-19 was an incidental finding in some hospitalisations that were classed as COVID-19-related; however, all deaths were investigated, and those reported here all followed positive COVID-19 tests and were therefore deemed related to COVID-19. The significantly increased prevalence of HIV infections among health-care workers who were hospitalised during the omicron period and who had received a single dose of Ad26.COV2.S could be explained by waning immunity in this group, pointing to the need for booster doses.
The course of COVID-19 caused by omicron in South Africa could have been tempered by high population seroprevalence of SARS-CoV-2, which reached 68% in some populations by April, 2021; our findings therefore might not be generalisable to all settings or to all populations globally.13 Despite these limitations, our large dataset provides a snapshot of the effect omicron has had in a low-to-middle-income setting with a high SARS-CoV-2 seroprevalence. We show that there were more breakthrough infections during the omicron period than during the beta and delta periods, but reassuringly, COVID-19 disease associated with omicron infection was less severe. The decreased severity of disease is probably driven by high population seroprevalence of SARS-CoV-2. The steep increase in breakthrough infections during the omicron period, including both reinfections and primary infections, was probably driven by waning vaccine effectiveness over time, increased infectivity of the omicron variant, immune evasion by the omicron variant, or a combination of these factors. Further investigation is required.
We declare no competing interests.
Supplementary Material
References
- 1.Chotiner I. How South African researchers identified the omicron variant of COVID. The New Yorker. Nov 30, 2021. https://www.newyorker.com/news/q-and-a/how-south-african-researchers-identified-the-omicron-variant-of-covid
- 2.Callaway E. Heavily mutated omicron variant puts scientists on alert. Scientific American. Nov 29, 2021. https://www.scientificamerican.com/article/heavily-mutated-omicron-variant-puts-scientists-on-alert/ [DOI] [PubMed]
- 3.National Institute for Communicable Diseases New COVID-19 variant detected in South Africa. Nov 25, 2021. https://www.nicd.ac.za/new-covid-19-variant-detected-in-south-africa/
- 4.World Health Organization Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Nov 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 5.Grabowski F, Kochańczyk M, Lipniacki T. Omicron strain spreads with the doubling time of 3.2–3.6 days in South Africa province of Gauteng that achieved herd immunity to delta variant. MedRxiv. 2021 doi: 10.1101/2021.12.08.21267494. published online Dec 9. (preprint). [DOI] [Google Scholar]
- 6.de Oliveira T, Venter M, Bhiman J, Scheepers C, Preiser W. Here's what omicron can tell us about how COVID-19 variants are discovered. World Economic Forum. Nov 29, 2021. https://www.weforum.org/agenda/2021/11/coronavirus-variant-discovery-omicron-health/
- 7.Callaway E. Omicron likely to weaken COVID vaccine protection. Nov 29, 2021. https://www.scientificamerican.com/article/heavily-mutated-omicron-variant-puts-scientists-on-alert/ [DOI] [PubMed]
- 8.Wilhelm A, Widera M, Grikscheit K, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. MedRxiv. 2021 doi: 10.1101/2021.12.07.21267432. published online Dec 13. (preprint). [DOI] [Google Scholar]
- 9.Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. New Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulliam J, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the omicron variant in South Africa. medRxiv. 2021 doi: 10.1101/2021.11.11.21266068. published online Dec 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekker L, Garrett N, Goga AE, et al. Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet. 2022;399:1141–1153. doi: 10.1016/S0140-6736(22)00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Xie W, Ge Z, et al. Reactivation of SARS-CoV-2 infection following recovery from COVID-19. J Infect Public Health. 2021;14:620–627. doi: 10.1016/j.jiph.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 seroprevalence in a rural and urban household cohort during first and second waves of infections, South Africa, July 2020–March 2021. Emerg Infect Dis. 2021;27:3020–3029. doi: 10.3201/eid2712.211465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.