Abstract
Objective:
To investigate gut microbiota and intestinal barrier function changes after orthopedic surgery in elderly patients with either normal cognition (NC) or a prodromal Alzheimer disease phenotype (pAD) comprising either subjective cognitive decline (SCD) or amnestic mild cognitive impairment (aMCI).
Background:
Homeostatic disturbances induced by surgical trauma and/or stress can potentially alter the gut microbiota and intestinal barrier function in elderly patients before and after orthopedic surgery.
Methods:
In this prospective cohort study, 135 patients were subject to preoperative neuropsychological assessment and then classified into: NC (n=40), SCD (n=58), or aMCI (n=37). Their gut microbiota, bacterial endotoxin (lipopolysaccharide), tight junction (TJ) protein, and inflammatory cytokines in blood were measured before surgery and on postsurgical day 1, 3, and 7 (or before discharge).
Results:
The short-chain fatty acid (SCFA)-producing bacteria were lower while the gram-negative bacteria, lipopolysaccharide and TJ were higher preoperatively in both the SCD and aMCI (pAD) groups compared with the NC group. After surgery, a decrease in SCFA-producing bacteria, and an increase in both gram-negative bacteria and plasma claudin were significant in the pAD groups relative to the NC group. SCFA-producing bacteria were negatively correlated with TJ and cytokines in pAD patients on postsurgical day 7. Furthermore, surgery-induced perioperative metabolic stress and inflammatory responses were associated with gut microbiota alterations.
Conclusions:
Surgery exacerbates both preexisting microbiota dysbiosis and intestinal barrier dysfunction in pAD patients, all of which may be associated with systemic inflammation and, in turn, may lead to further cognitive deterioration.
Keywords: gut microbiota dysbiosis, intestinal barrier, prodromal Alzheimer disease, orthopedic surgery, cognitive impairment
A large proportion of elderly individuals undergo surgery each year.1 Within this demographic, surgical trauma and stress may increase the risk of cognitive decline, particularly in those with prodromal Alzheimer disease (pAD) who either have subjective cognitive decline (SCD) or have amnestic mild cognitive impairment (aMCI).2 Postoperative systemic inflammation, together with increased plasma bacterial endotoxin,3 has been demonstrated to synergistically cause neuroinflammation that may trigger the development of cognitive dysfunction.4,5 Furthermore, higher postoperative levels of plasma bacterial endotoxin were reported to be associated with gastrointestinal microbiota dysbiosis including a reduced diversity and altered composition of the gut microbiota6 and intestinal barrier damage.7
Gut microbiota dysbiosis and intestinal barrier dysfunction may be related to the progression of Alzheimer disease (AD).8 During the prodromal stages of AD, such as mild cognitive impairment, increased levels of gram-negative bacteria (GNB)9 and decreased levels of short-chain fatty acid (SCFA)-producing bacteria have been reported.10 This gut microbiota dysbiosis may lead to a systemic inflammatory state that consequently activates microglia, neuroinflammation, and increases blood–brain barrier disruption.11 Furthermore, this inflammation contributes to neuroinflammation and the resultant production of injurious products including tau protein.12
Surgery-related disturbances, such as oral intake reduction and gut physiological compromise which included poor microcirculatory oxygen supply and intestinal perfusion, alter the mucosal barrier function and the gut environment.13–15 Surgical trauma and stress-induced systemic inflammation5 can also damage the intestinal barrier.16 The present study was, therefore, designed to investigate postoperative alterations of the gut microbiota and intestinal barrier function together with perioperative inflammatory responses in elderly patients with normal cognition (NC) or pAD.
METHODS
Study Design
The study protocol of this prospective observational cohort study was approved by the Ethics Committee, Xuanwu Hospital, Beijing, China, and then registered in the Chinese clinical trial registry (ChiCTR1800018420). A total of 176 patients who underwent orthopedic surgery from September 2018 to December 2019 and provided written informed contents were screened for study recruitment. The inclusion criteria were elderly patients (65–79 years old), receiving knee joint arthroplasty or lumbar interbody fusion surgery, having an American Society of Anesthesiologists (ASA) status of I–III, and having received at least 9 years of education. The exclusion criteria were lower gastrointestinal tract diseases, severe deafness or vision problems, illiteracy and/or communication difficulties related to pronunciation or dialect, acute or chronic infectious diseases, taking anti-inflammatory drugs or immunosuppressants, other central nervous system diseases (including diagnosis of AD prior and after admission), postoperative delirium, and an unexpectedly long hospital stay (>8 days). After exclusion, the remaining eligible participants were assessed by the same senior neurologist for their cognition before surgery and on postsurgical day 7 or before discharge for the cases whose hospital stay was <7 days, as reported previously.17 The standard neurological evaluation protocol (see Supplement, Supplemental Digital Content 1, http://links.lww.com/SLA/D918: Neuropsychological Test) was used to classify patients as NC (n=40), SCD (n=58) or aMCI (n=37). Hence, 135 participants in total were recruited (Fig. 1). The study procedures and time frame are listed in Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
FIGURE 1.
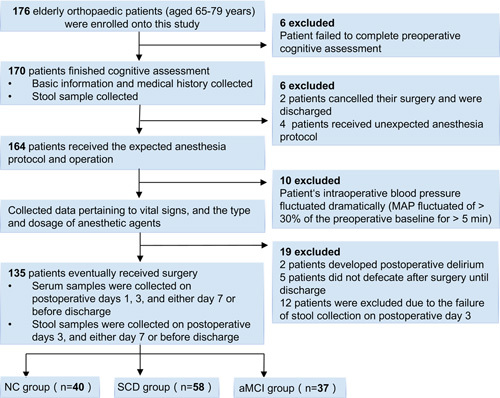
Flow chart of patient recruitment. MAP indicate mean arterial pressure.
Data Collection
All patients were routinely monitored. Arterial blood gases were measured before and after induction of anesthesia and at the end of surgery. Mean blood pressure was maintained with <20% variation from the basal mean blood pressure throughout the surgery. Intravenous infusion loading volume, urine output, bleeding, and blood transfusion were recorded during the operation. Anesthesia, surgical interventions, and postoperative care were aligned with routine clinical practice and therefore similar in all patients. Postoperative pain was controlled with multimodal analgesia.
Fresh stool samples were collected after enrollment, on postsurgery day 3, and either postsurgery day 7 or before discharge for gut microbiota analysis use. Blood samples were collected upon entering the operating room, on postoperative day 1 and 3, and either on postoperative day 7 or before discharge, and were represented by Pre, Pos1, Pos3, and Pos7 hereafter. Blood samples were then used for plasma endotoxin, inflammatory cytokines and tight junction (TJ) protein measurements with ELISA kits (Supplementary Materials for details, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
Outcomes Measures
The complexity of gut microbiota diversity can be assessed by Alpha-diversity through several indices such as the Sobs and Shannon index. The Sobs index reflects the community richness whilst the Shannon index accounts for the number of species (richness) and their relative abundance (evenness).18 The primary outcome was the dysbiosis of gut microbiota as assessed by changes of the gut microbiota (the Sobs index, the Shannon index and the composition of SCFA-producing bacteria as well as GNB) and the intestinal barrier function (plasma endotoxin, occluding, and claudin-1) after surgery. The secondary outcome was the postoperative inflammation as assessed by changes in inflammatory biomarkers including C-reactive protein (CRP), interleukin (IL)-6, and IL-10 in peripheral blood. Blood loss, surgery time and other laboratory measurements were also collected.
Data Analysis
Data were presented as mean (SD) or median (interquartile range) together with dot plots, or frequency (percentage) where appropriate. Continuous variables such as the plasma concentration of lipopolysaccharide (LPS), TJ proteins and inflammatory cytokines were log or square root transformed and analyzed with the analysis of variance (ANOVA) followed by post hoc Fisher least significant difference (LSD) tests for comparison. Kruskal-Wallis sum-rank test was used when data were not normally distributed. Pearson analysis was used to calculate correlations if data were normally distributed; otherwise, Spearman rank was used. Multiple comparisons were adjusted through the Benjamini-Hochberg false discovery rate (FDR). For microbiota analyses, the FDR adjusted P value of 0.25 was chosen as the threshold to determine statistical significance.19 For other analyses, a P value <0.05 was considered to be statistically significant. All data analysis was carried out using R 2.15.3 packages (R Foundation for Statistical Computing, Vienna, Austria) and SPSS 21.0 statistical software (SPSS, IBM Corp., Armonk, NY).
RESULTS
The General Demographics and Postoperative Cognitive Function Changes
Patients’ demographics, laboratory measurements, and surgery time and blood loss during surgery were comparable among the 3 groups (Table 1 and Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/SLA/D918). All patients cooperated well throughout the study and there were no major complications and in-hospital death after surgery. Their neurocognitive performance scores assessed with the Montreal Cognitive Assessment-Basic (MoCA-B) [median (interquartile range)] after surgery were slightly lower than before surgery [25 (24, 27) vs 24 (23, 26) in NC, P=0.006; 24 (22, 26) vs 23 (21, 25) in SCD, P=0.084; 19 (17, 21) vs 18 (16, 21) in aMCI, P=0.126]. Further analysis with Z score revealed that the incidence of postoperative cognitive impairment was 17.5%, 24.1%, and 36.4% in the NC, SCD, aMCI group, respectively, with no significant difference between NC and the other 2 groups (Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
TABLE 1.
The Baseline Characteristics of the Study Population
| NC (n=40) | SCD (n=58) | aMCI (n=37) | P | |
|---|---|---|---|---|
| Characteristics | ||||
| Gender, N, female/male | 13/27 | 14/44 | 6/31 | 0.251 |
| Age, mean (SD), year | 69.75 (4.20) | 70.28 (3.73) | 71.95 (4.62) | 0.054 |
| BMI, mean (SD), kg/m2 | 27.56 (3.19) | 26.63 (3.39) | 26.86 (3.56) | 0.398 |
| Food, high/low fat | 5/35 | 10/48 | 5/32 | 0.783 |
| NYHA, N, II/III | 38/2 | 55/3 | 33/4 | 0.563 |
| ASA, N, I /II/III | 0/37/3 | 1/48/9 | 0/24/13 | 0.010* |
| Comorbidity, n (%) | ||||
| Hypertension | 29 (72.5) | 35 (60.3) | 27 (73.0) | 0.315 |
| CAD (prior MI) | 7 (17.5) | 12 (20.7) | 8 (21.6) | 0.889 |
| Diabetes | 14 (35.0) | 16 (33.3) | 14 (37.8) | 0.540 |
| Antibiotic | 4 (10.0) | 11 (19.0) | 6 (16.2) | 0.518 |
| Smoking | 2 (5.0) | 7 (12.1) | 2 (5.4) | 0.444 |
| Surgery, N, lumbar interbody fusion/knee joint arthroplasty | 16/24 | 27/31 | 13/24 | 0.531 |
P<0.05.
ASA indicates American Standard Association; BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; NYHA, New York Heart Association.
The Reduction of α-Diversity of Gut Microbiota in the aMCI Group After Surgery
Before surgery, the pAD groups displayed significantly lower Sobs index and Shannon index when compared to the NC group (Sobs indexes, NC vs SCD, P=0.014, NC vs aMCI, P=0.024; Shannon index, NC vs SCD, P=0.048) (Figs. 2A, B). On postsurgery day 3, the Sobs index was lower in the SCD group when compared with that in the NC group (P=0.001) (Fig. 2C). The Shannon index in the SCD was the lowest among the 3 groups (SCD vs NC, P=0.011; SCD vs aMCI, P=0.015) (Fig. 2D). The Sobs index and Shannon index were lower in the aMCI group than in the NC group on postoperative day 7 (Sobs indexes, P=0.012; Shannon index, P=0.014) (Figs. 2E, F). There was only a significant reduction of the Shannon index in the aMCI group on postoperative day 7 compared with the baseline presurgery among the 3 groups (P=0.011) (Figs. 2G, H). β-Diversity (Bray-Curtis and Weighted-UniFrac distance) metrics also showed difference on postoperative day 3 compared with presurgery among the 3 groups (Figs. 2I–N).
FIGURE 2.
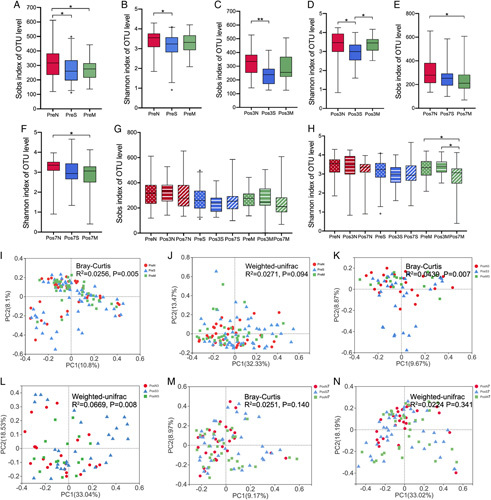
Changes of fecal microbial diversities among the 3 groups. A, The Sobs index was lower in the pAD group. B, The NC group displayed significantly higher Shannon index compared with the SCD group before surgery. C, The NC group had a higher Sobs index than the SCD group on postoperative day 3. D, On postsurgery day 3, the Shannon index in the SCD was the lowest among the 3 groups. E and F, The Sobs index and Shannon index was lower in the aMCI group than in the NC group on postoperative day 7. G, The perioperative alteration of Sobs index among the 3 groups. H, There was a significant reduction in the Shannon index in the aMCI group on the postoperative day 7. I–N, Principal coordinate analysis (PCoA) of Bray-Curtis and weighted UniFrac analysis computed among the 3 groups during preoperative period. The Significance between the 3 groups at each time point was determined using Kruskal-Wallis test.
Preoperative pAD-associated Bacterial Composition
Before surgery, the NC group had the highest relative abundance of the phylum Firmicutes (median=53.63%) in contrast to that in the SCD (median=47.66%) and aMCI (median=50.60%) group. The level of the phylum Bacteroidetes in the SCD group was higher than that in the aMCI and NC group; whereas, the SCD group had the lowest F:B ratio (median=1.276) among the 3 groups (Online supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
The total preoperative proportion of GNB was the highest in the SCD group (Fig. 3A). The relative abundance of family Bacteroidaceae, Enterobacteriaceae, Acidaminococcaceae before operation was higher in the pAD groups than that in the NC group (Fig. 3B). Preoperative abundance of the family Butyricicoccaceae, Christensenellaceae, and genera Butyricicoccus, Christensenellaceae_R-7_group was higher in the NC group than in the pAD groups (Supplementary Figs. 2 and 3C, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
FIGURE 3.
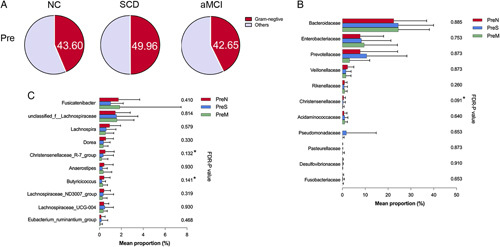
Preoperative pAD-associated bacterial composition. A, Increased levels of preoperative GNB proportion at the family level in the SCD group compared with others. B, The difference in GNB at the family level among the 3 groups before surgery. C, The difference in SCFA-producing bacteria at the genus level among the 3 groups before surgery. ANOVA test was used to compare the GNB proportion among the 3 groups. Kruskal-Wallis sum-rank test was used for comparisons of the relative abundances of bacteria among the 3 groups. *FDR-P<0.25.
Postoperative Changes of Bacteria Within Each Group
The phylum Firmicutes remained stable in the NC group across all time points but gradually decreased over time in the aMCI group and decreased to the lowest level (median=37.04%) in the SCD group on postoperative day 3. Within the aMCI group, the Firmicutes: Bacteroidetes ratio declined dramatically from 1.74 to 1.00 (P=0.022) during hospitalization, whereas the other 2 groups decreased by <25% (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
There was a significant difference in the proportion of GNB between the 3 groups on postoperative day 3 (P=0.039) (Fig. 4A). Levels of bacterial families Bacteroidaceae, Prevotellaceae, and Desulfovibrionaceae were highest on day 3 postoperatively in the SCD group (Fig. 4B). The relative abundance of family Enterobacteriaceae and Rikenellaceae was the highest in the aMCI group on postoperative day 3 and 7 (Figs. 4B, C). Postoperative levels of GNB in the aMCI group increased compared with the baseline and this increase was attributed to increases in Bacteroidaceae, Enterobacteriaceae, and Veillonellaceae families although without significant difference (Fig. 4D). After surgery, the Lachnospiraceae family decreased consistently in both the SCD and aMCI groups (FDR-P SCD=0.001; FDR-P aMCI=0.002), whereas the abundance of this family did not decrease further in the NC group on postsurgery day 7 (median=12.82%, FDR-PNC <0.0001) (Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
FIGURE 4.
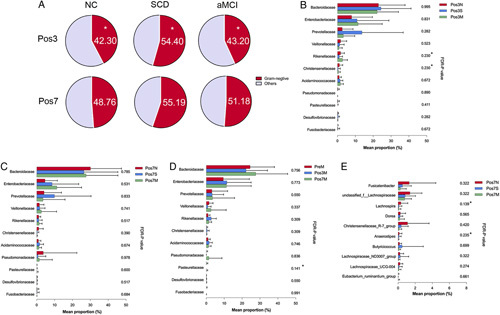
Postoperative changes of bacteria within each group. A, The ANOVA test was used to compare the GNB proportion at the family level in the NC, SCD, and aMCI groups during the postoperative period. B and C, Kruskal-Wallis sum-rank test was used to compare the relative abundance of GNB among the 3 groups on postoperative day 3 and 7. D, Change in the relative abundance of GNB in the aMCI group during the perioperative period. E, The difference in SCFA-producing bacteria between the 3 groups after surgery. *P<0.05, *FDR-P<0.25.
At the genus level, the relative abundance of Fusicatenibacter, unclassified_f__Lachnospiraceae, Dorea, Christensenellaceae_R-7_group, Lachnospiraceae_UCG-004 was higher in the NC group than in the other 2 groups on day 7 postoperation. Meanwhile, the relative abundance of Lachnospira was remarkably higher in the NC group on day 7 postoperation (FDR-P=0.139) (Fig. 4E). Postoperative levels of 20 genera of SCFA-producing bacteria were significantly altered in the aMCI group. Sixteen of them kept decreasing over time and only 1 genus (Characteristics_f__Lachnospiraceae) stopped declining after postoperative day 3 (Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/SLA/D918). Furthermore, 13 genera of SCFA-producing bacteria were significantly altered in the SCD group and 11 of them significantly decreased and remained low up to postsurgery day 7 (Supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/SLA/D918). By contrast, in the NC group, 3 out of 11 genera of SCFA-producing bacteria did not decrease after postoperative day 3 (Supplementary Fig. 5, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
A significant difference in bacteria alteration due to surgery was only detected in genus Lachnospiraceae_UCG-010 among the 3 groups on day 3 postoperation (medianNC=0.022% vs medianSCD=−0.002% vs medianaMCI=−0.015%, P=0.008) and genus Subdoligranulum on 7 postoperation (medianNC=0.299% vs medianSCD=−0.427% vs medianaMCI=−0.007%, P=0.002) (data unshown).
Orthopedic Surgery Resulted in Increased Plasma Endotoxin and TJ Protein Concentrations
Preoperative concentrations of plasma endotoxin in the pAD groups were significantly higher than that in the NC group (P=0.003; post hoc LSD test: SCD vs NC, P=0.001; aMCI vs NC, P=0.026) (Supplementary Fig. 6, Supplemental Digital Content 1, http://links.lww.com/SLA/D918). Levels of plasma endotoxin increased postoperatively in both NC and aMCI group and peaked on postoperative day 3 (Figs. 5A–C). Plasma endotoxin was higher postsurgery than presurgery in the NC group (P Pos3=0.007 and P Pos7=0.019), but no significant change was found in the pAD groups.
FIGURE 5.
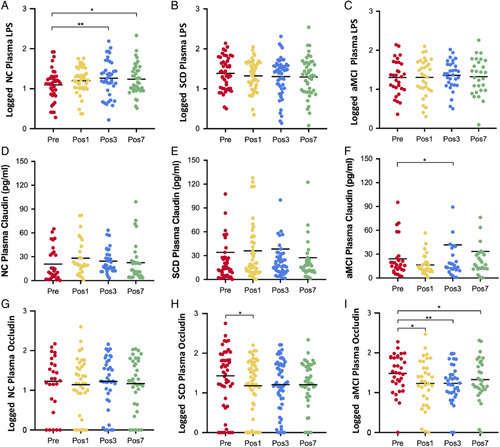
Perioperative alteration of plasma LPS, TJ protein in each group. A, The level of LPS was significantly increased after surgery than that before surgery in the NC group (LPS was log transformed). B and C, The changes of plasma LPS in the SCD and the aMCI groups. D–F, The alteration of plasma claudin1 during the perioperative period in the 3 groups. G, The change of plasma occludin in the NC group during perioperative period (occludin was log transformed). H, The level of occludin on postoperative day 1 was significantly lower than that before surgery in the SCD group. I, Post-operation plasma occludin was significantly lower than that before surgery in the aMCI group. The paired-sample t test was used to compare the difference between before surgery and after surgery. *P<0.05, **P<0.01.
Preoperative baseline levels of plasma occludin and claudin-1 were elevated in the pAD groups than those in the NC group, but the differences did not reach statistical significance (Supplementary Fig. 6, Supplemental Digital Content 1, http://links.lww.com/SLA/D918). Postoperative plasma claudin-1 concentration peaked on day 1 in the NC group and on day 3 in the pAD groups (Figs. 5D–F). In the aMCI group, plasma claudin1 increased significantly on day 3 postsurgery (P=0.036). Postoperatively, there was no significant difference in plasma occludin in the NC group (Fig. 5G); however, in the SCD group, the occludin level was significantly lower on post-surgical day 1 (P=0.045) (Fig. 5H). The postoperative concentration of occludin was significantly lower in the aMCI group (P Pos1=0.044, P Pos3=0.004, and P Pos7=0.025, respectively) (Fig. 5I).
SCFA-producing Bacteria Were Negatively Correlated With Postoperative Levels of TJ Proteins
The genus Lachnospira was negatively correlated with plasma claudin-1 in the aMCI group on postsurgical day 7 (r=−0.577, P=0.002). The genus Ruminococcus showed a negative correlation with plasma claudin-1 in the SCD group on postsurgical day 7 (r=−0.358, P=0.040) (Supplementary Fig. 7, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
Changes in Postoperative Plasma CRP and Inflammatory Cytokines
Postoperative plasma CRP increased, peaked on day 3 (P<0.0001) and remained high until day 7 in all patients (P<0.0001). This was supported by LSD tests that indicated significant differences in perioperative CRP between the aMCI group and the NC group (P=0.015). Plasma IL-6 concentration peaked on day 1 (P<0.01) and remained at a high level in all 3 groups until day 3 postoperatively (P<0.05) (Supplementary Fig. 8, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
The postoperative level of plasma IL-10 was elevated in all groups with significant differences found between the 3 group; IL-10 remained elevated in the SCD group—perioperatively, this had been higher than the other 2 groups (P Pre=0.006 and P Pos7 <0.001, respectively). Plasma IL-10 concentration peaked on postoperative day 1 in the aMCI and the NC groups, and, before discharge, returned to its baseline in the NC group only. The LSD post-hoc test indicated a significantly higher IL-10 in the pAD groups compared to the NC group after surgery. Plasma LPS level was positively correlated with the plasma IL-10 level before surgery (r=0.428, P<0.001) (Supplementary Fig. 8, Supplemental Digital Content 1, http://links.lww.com/SLA/D918) and on postoperative day 7 (r=0.308, P=0.001) but not with IL-6 (data unshown).
SCFA-producing Bacteria Were Negatively Correlated With Plasma Inflammatory Cytokines
Preoperatively, plasma CRP was negatively correlated with the genera Faecalibacterium and Lachnospira (r=−0.365 and −0.409, respectively, both P<0.05) in the NC group as well as with the genus Roseburia (r=−0.406, P=0.016) in the aMCI group. Before surgery, plasma IL-6 was negatively correlated with the genus Fusicatenibacter (r=−0.350, P=0.040) in the NC group and the genera Roseburia, Lachnospiraceae_UCG-004 (r=−0.397 and r=−0.486, respectively, both P<0.05) in the aMCI group.
After surgery, plasma CRP levels were negatively correlated with the genus norank_f__Ruminococcaceae (r=−0.389, P=0.045) in the aMCI group on day 7. In the NC group, the postoperative levels of plasma IL-6 were negatively correlated with the genus unclassified_f__Lachnospiraceae (r=−0.488, P=0.021) on day 7 (Supplementary Fig. 9, Supplemental Digital Content 1, http://links.lww.com/SLA/D918).
Associations Between Surgery-related Factors and the Gut Microbiota
The correlation between preoperative intrinsic factors, extrinsic factors, and gut microbiota was visualized using a heatmap. Plasma lactate and glucose levels were negatively correlated with the families Lachnospiraceae and Butycicoccaceae (P<0.01 and <0.05) (Fig. 6A). Plasma CRP had a significant negative correlation with levels of the following families: Butycicoccaceae, Lachnospiraceae, Rikenellaceae, Streptococcaceae (all P<0.05) and positively correlated with the genus Enterobacter (r=0.281, P=0.002) (Figs. 6A, B). Plasma glucose, CRP, and IL-6 levels were negatively correlated with the genus Roseburia (all P<0.05).
FIGURE 6.
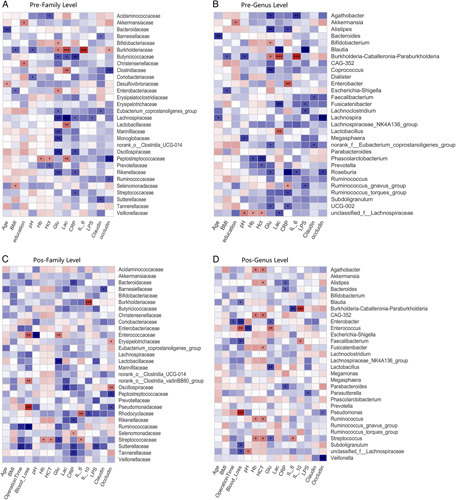
Correlation analysis of clinical parameters and the gut microbiota among the 3 groups before surgery and after surgery. A and B, Associations between 13 clinical parameters and relative abundance of 30 families and 30 genera presurgery in all patients. C and D, Association between 15 clinical parameters and the relative abundance of 30 families and 30 genera on day 7 in all patients. The correlation was estimated by applying Spearman correlation analysis and visualized using Heatmap. Color intensity represents the magnitude of correlation, with red indicating positive correlations and blue indicating negative correlations. *P value <0.05, **P value <0.01, ***P value <0.001.
The correlation between postoperative intrinsic factors, extrinsic factors, and gut microbiota was explored. On day 7 postsurgery, there was a negative correlation between the level of plasma CRP and the abundance of Bacteroidaceae and Rikenellaceae (Fig. 6C). Arterial blood pH values (Supplementary Table. 2, Supplemental Digital Content 1, http://links.lww.com/SLA/D918) at the end of the operation were positively correlated with the genus unclassified_f__Lachnospiraceae (Fig. 6D). Blood loss was negatively correlated with 5 SCFA-producing bacteria, including Blautia, Enterobacter, Faecalibacterium, Subdoligranulum, and unclassified_f__Lachnospiraceae. Hemoglobin and hematocrit levels were positively correlated with 2 SCFA-producing bacteria: Ruminococcus and Streptococcus.
DISCUSSION
The current study found surgery induced microbiota disorder and intestinal barrier dysfunction in patients with NC, and aggravated preexisting microbiota disorders and intestinal barrier dysfunction in pAD patients. Before surgery, the α-diversity of gut microbiota and the relative abundance of SCFA-producing bacteria were higher in NC patients than those in the pAD patients. Also, the proportion of GNB, and the plasma endotoxin and TJ protein significantly increased in the pAD group, especially in patients with SCD. In NC patients, postoperative alteration of gut microbiota was characterized by a reduction of butyrate-produced bacteria whilst in the pAD patients, postoperative microbiota dysbiosis included a reduced α-diversity, an increased level of GNB, and a decreased level of SCFA-producing bacteria. Furthermore, intestinal barrier dysfunction alongside an increase in systemic inflammatory cytokines was associated with postoperative microbiota dysbiosis in pAD patients.
Compared with NC patients, pAD patients displayed decreased species richness presurgery, which is in line with the findings of previous studies in aMCI and pAD patients.8,20 A lower α-diversity in pAD patients was found after surgery in our study, which was also reported in an animal study on a postoperative delirium-like phenotype.21 These findings implicated that low α-diversity resulted in the gut being susceptible to invasion and a concomitant decrease in resilience.22,23 A previous study reported that a decrease in bacterial diversity was associated with frailty and cognitive decline in the elderly.24
In our study, the alterations in the composition of the microbiota entailed an increase in GNB and a decrease in SCFA-producing bacteria. The increased GNB was correlated with surgery and the family Veillonellaceae increased postoperatively as reported in a previous study.25 The increase was associated with cognitive decline, endotoxemia, and inflammation.26 It has been documented that Enterobacteriaceae displays a progressive and enriched prevalence in aMCI and pAD patients compared with the NC healthy controls.8 We found that GNB levels were more stable in the NC patients perioperatively. Overrepresentation of GNB was associated with the development of AD.9,27 The GNB molecules and GNB-derived endotoxins were colocalized with Aβ in amyloid plaques and also with Aβ located around blood vessels in AD brains.28 GNB-derived endotoxins also directly down-regulated critical neuron-specific cytoskeletal and synaptic signaling proteins.29 In addition, animal experiments in a rat AD model showed that intraperitoneal injection of LPS caused hippocampal Aβ accumulation, resulting in cognitive impairment.30 Together, these findings suggested that increased levels of GNB following surgery may have a further impact on postoperative cognitive decline in pAD patients.
In this study, pAD patients demonstrated reduced pre-surgical butyrate-producing bacteria compared with the controls; similar finding has also been reported in patients with prodromal MCI toward AD.8,10 We observed that the relative abundance of SCFA-producing bacteria continuously decreased in pAD patients, especially in those with aMCI, suggesting that pAD patients have weaker resilience against surgery compared with the controls. A reduction in SCFA-producing bacteria was associated with intestinal barrier disruption because SCFAs play key roles in gut motility, immune response, and gut barrier function.30–33 It was found that SCFA-producing bacteria may reinforce the intestinal barrier by regulating the expression of TJ protein and mucin to prevent the translocation of bacterial products (LPS, amyloid) and reduce systemic inflammation.34 The anti-inflammatory mechanism entails direct interaction with immune cells to block histone deacetylases, resulting in more transcriptionally active chromatin and down-regulated inflammatory cytokines, such as TNF-a and IL-12.35 In addition, SCFAs were reported to be directly associated with memory function recovery and an increase in gene expression involved associative learning.31
By applying elevations of plasma TJ protein and endotoxin as markers of gut barrier dysfunction, researchers observed increased intestinal permeability after surgery.36 In our study, plasma LPS, claudin-1, and occludin were higher in pAD patients prior to surgery, particularly in SCD patients, which may suggest preexisting high intestinal permeability in pAD patients. Wang et al37 also found that the level of plasma zonulin significantly increased in AD and aMCI patients, and that this increase was related to lower MMSE scores. Aside from perioperative intestinal hypoperfusion,38 a previous study also found that surgery-related trauma and stress response triggered the production of inflammatory mediators and oxygen-free radicals, which may increase intestinal epithelial cell apoptosis and therefore undermine intestinal barrier integrity.39 A reduction in the abundance of SCFA-producing bacteria was associated with a reduction in SCFA, intestinal epithelial barrier impairment, high levels of serum endotoxin, and hippocampal neuroinflammation in fructose-fed mice.40 In contrast, a fiber-enriched diet increased the abundance of SCFA-producing bacteria in the gut which may promote a balance in the gut microbiota and restore intestinal barrier function.41 We found that postoperative abundance of Lachnospira and Ruminococcus was negatively correlated with plasma claudin-1 in pAD patients. Taken together, postsurgical reduction in SCFA-producing bacteria, as shown in our study, was possibly associated with intestinal barrier dysfunction as reflected by an increase in plasma claudin and endotoxin.
Interestingly, endotoxin was found to be significantly increased in NC patients but not in pAD patients in our study, which may further indicate that pAD patients already had intestinal barrier dysfunction before surgery. In addition, plasma endotoxin was positively correlated with plasma IL-10 either before or after surgery in our study. A previous study suggested that plasma endotoxin may activate regulatory T cells (Tregs) which then stimulate neutrophils releasing IL-10.42 IL-10 promoted TJ protein expression and hence protected intestinal epithelial barrier function.43,44 Therefore, the increase in IL-10 found in the present study was likely due to patients’ negative feedback mechanism, but this protection is limited in both aMCI and SCD patients. In line with this, it has been well documented that both proinflammatory interleukin-1β and anti-inflammatory IL-1 receptor antagonist were increased in the blood and cerebrospinal fluid of elderly patients after acute hip fracture and surgery.45 In such cases, peripheral inflammation may likely exaggerate neuroinflammation and trigger neuronal and synaptic destruction, with a decline of cognitive function postoperatively.46
Further, postsurgical alterations in the gut microbiota were associated with perioperative metabolic stress responses and surgery-related disturbances such as changes in the levels of lactate, glucose, and pH values. In this study, postoperative lower blood pH values likely reflect reduced intestinal perfusion, accompanied by a decrease in a SCFA-producing bacteria: unclassified_f__Lachnospiraceae. Parameters such as plasma lactate, glucose, and blood pH indicate the extent of the stress response and intestinal perfusion and, indeed, poor intestinal perfusion led to lactic acidosis and an unstable arterial pH.47,48 Lower microcirculatory oxygen supply ultimately damaged mucosal barrier function, resulting in translocation of bacterial toxins into circulation.48 In pAD patients, microbiota disturbance and barrier function impairment synergistically aggravated postoperative inflammation per se.
We may preserve neurocognition of the pAD patients from the systemic inflammation or other metabolites originated from gut microbiota by promoting microbiome diversity and intestinal barrier function. Preventing hypoperfusion of the gut and the usage of anti-inflammatory medicine during perioperative period may protect intestinal barrier function in pAD patients.49 A high level of physical exercise, vegetarian diets, and weight control can improve microbiota conditions before and after surgery alongside other treatments such as probiotics50,51 and fecal microbiota transplantation18 which have been reported to benefit gut bacteriome diversity. Given the individualization of microbiota and the numerous influencing factors on colonization, more studies on microbiota therapy are needed.
This study had some limitations. First, an investigation of fecal SCFAs was not included, and it remained unknown whether altered SCFA-producing microbiota in the gut led to a reduction in fecal SCFAs and damaged the gut barrier. Further, how the preoperative microbiota changes were closely associated with the postoperative changes including IL-6 or IL-10 is also unknown. Second, our study had a limited sample size and only included patients who underwent orthopedic surgery. As a result, our findings may not generalize to other types of surgeries. Lastly, other clinical outcomes including delirium and long-term impact of gut microbiota and barrier function on cognition are unknown.
In summary, our data suggested that pAD patients exhibited gut microbiota dysbiosis and barrier dysfunction prior to surgery. Furthermore, surgery was an additional detriment to these parameters in the pAD patients while it induced mild or moderate changes in the patients whose cognition was normal preoperatively. The long-term impact of gut microbiota and barrier changes induced by surgery and the subsequent trauma requires further study.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Tong Liang Zhou, China-Japan Friendship Hospital, Beijing, China, for providing assistance of ELASA tests, the Microeco Tech Co. Ltd. Shenzhen China, for the feces microbiota analysis through 16S ribosomal RNA (rRNA) gene amplification and sequencing. The authors also also thank Dr Chun Xiu Wang, Evidence-Based Medical Center, Xuanwu Hospital, Capital Medical University, for the statistics consultation and Dr Jia Li, Reader, Imperial College London, for her critical comments during manuscript preparation.
Footnotes
Fangyan Liu and Mei Duan contributed equally and share first authorship.
Funding: This study was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201818). Prof. Ma’s current research is supported by grants from National Institute for Health Research and British Journal of Anaesthesia, London, UK.
T.W., H.F., F.Y.L., and M.D.: designed the experiment. F.Y.L., H.F., and M.D.: made substantial contributions to acquisition of data, and analysis and interpretation of data. F.L., G.Z., G.C., Z.L., Y.H.: coordinated and were involved in data acquisition, interpretation of the data. F.Y.L., H.F., Z.A., T.W., and D.M.: analyzed and interpreted data and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Fangyan Liu, Email: xiami0104@126.com.
Mei Duan, Email: dm18473425440@163.com.
Huiqun Fu, Email: fhq1972@hotmail.com.
Guoguang Zhao, Email: ggzhao@vip.sina.com.
Ying Han, Email: hanying@xwh.ccmu.edu.cn.
Fei Lan, Email: Lanfei206@hotmail.com.
Zara Ahmed, Email: zara.ahmed@kcl.ac.uk.
Guanglei Cao, Email: gregary111@163.com.
Zheng Li, Email: xwgklz@163.com.
Daqing Ma, Email: d.ma@imperial.ac.uk.
Tianlong Wang, Email: w_tl5595@hotmail.com.
REFERENCES
- 1. Evered L, Scott DA, Silbert B. Cognitive decline associated with anesthesia and surgery in the elderly: does this contribute to dementia prevalence? Curr Opin Psychiatry. 2017;30:220–226. [DOI] [PubMed] [Google Scholar]
- 2. Gold S, Forryan S. Postoperative cognitive decline: a current problem with a difficult future. Trends Anaesth Crit Care. 2019;24:49–58. [Google Scholar]
- 3. Sandiego CM, Gallezot JD, Pittman B, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA. 2015;112:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alam A, Ma D. Is it time to assess neurological status before surgery to improve postoperative outcomes? Ann Surg. 2022;275:644–645. [DOI] [PubMed] [Google Scholar]
- 5. Alam A, Hana Z, Jin Z, et al. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu H, Liu Y, Li S, et al. Altered gut microbiota after traumatic splenectomy is associated with endotoxemia. Emerg Microbes Infect. 2018;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Satokari R. Contentious host-microbiota relationship in inflammatory bowel disease—can foes become friends again? Scand J Gastroenterol. 2015;50:34–42. [DOI] [PubMed] [Google Scholar]
- 8. Liu P, Wu L, Peng G, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–643. [DOI] [PubMed] [Google Scholar]
- 9. Li B, He Y, Ma J. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019;15:1357–1366. [DOI] [PubMed] [Google Scholar]
- 10. Hu L, Zhu S, Peng X, et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis. 2020;77:629–640. [DOI] [PubMed] [Google Scholar]
- 11. Kamer AR, Craig RG, Dasanayake AP, et al. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4:242–250. [DOI] [PubMed] [Google Scholar]
- 12. Fulop T, Witkowski JM, Bourgade K, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s Disease? Front Aging Neurosci. 2018;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reese AT, Dunn RR. Drivers of microbiome biodiversity: a review of general rules, feces, and ignorance. MBio. 2018;9:e01294–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. [DOI] [PubMed] [Google Scholar]
- 15. Tewari N, Awad S, Lobo DN. Regulation of food intake after surgery and the gut brain axis. Curr Opin Clin Nutr Metab Care. 2013;16:569–575. [DOI] [PubMed] [Google Scholar]
- 16. He W, Wang Y, Wang P, et al. Intestinal barrier dysfunction in severe burn injury. Burns Trauma. 2019;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan T, Wang W, Yang L, et al. Rich club disturbances of the human connectome from subjective cognitive decline to Alzheimer’s disease. Theranostics. 2018;8:3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gotoh K, Sakaguchi Y, Kato H, et al. Fecal microbiota transplantation as therapy for recurrent Clostridioides difficile infection is associated with amelioration of delirium and accompanied by changes in fecal microbiota and the metabolome. Anaerobe. 2021;73:102502. [DOI] [PubMed] [Google Scholar]
- 19. Vanegas SM, Meydani M, Barnett JB, et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 2017;105:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vogt NM, Kerby RL, Dill-Mcfarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Bi JJ, Guo GJ, et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci Ther. 2019;25:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bodelier P. Toward understanding, managing, and protecting microbial ecosystems. Front Microbiol. 2011;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark RI, Walker DW. Role of gut microbiota in aging-related health decline: insights from invertebrate models. Cell Mol Life Sci. 2018;75:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paganelli FL, Luyer M, Hazelbag CM. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci Rep. 2019;9:10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ticinesi A, Tana C, Nouvenne A, et al. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhan X, Stamova B, Jin LW, et al. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Y, Sharfman NM, Jaber VR, et al. Down-regulation of essential synaptic components by GI-tract microbiome-derived lipopolysaccharide (LPS) in LPS-treated human neuronal-glial (HNG) cells in primary culture: relevance to Alzheimer’s disease (AD). Front Cell Neurosci. 2019;13:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15. [DOI] [PubMed] [Google Scholar]
- 31. Maekawa M, Yoshitani K, Yahagi M, et al. Association between postoperative changes in the gut microbiota and pseudopsia after cardiac surgery: prospective observational study. BMC Surg. 2020;20:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Branca JJV, Gulisano M, Nicoletti C, et al. Intestinal epithelial barrier functions in ageing. Nutrients. 2019;11:pii: E1853. [DOI] [PubMed] [Google Scholar]
- 33. Yang Y, Zhang Y, Xu Y, et al. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct. 2019;10:5952–5968. [DOI] [PubMed] [Google Scholar]
- 34. Koh A, Vadder FD, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 35. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. [DOI] [PubMed] [Google Scholar]
- 36. Typpo KV, Larmonier CB, Deschenes J, et al. Clinical characteristics associated with postoperative intestinal epithelial barrier dysfunction in children with congenital heart disease. Pediatr Crit Care Med. 2015;16:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Liu GJ, Gao Q, et al. C-type lectin-like receptor 2 and zonulin are associated with mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2020;141:250–255. [DOI] [PubMed] [Google Scholar]
- 38. Koning NJ, de Lange F, van Meurs M, et al. Reduction of vascular leakage by imatinib is associated with preserved microcirculatory perfusion and reduced renal injury markers in a rat model of cardiopulmonary bypass. Br J Anaesth. 2018;120:1165–1175. [DOI] [PubMed] [Google Scholar]
- 39. Xu Q, Xu P, Cen Y, et al. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncol Lett. 2019;18:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li JM, Yu R, Zhang LP, et al. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. 2019;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. [DOI] [PubMed] [Google Scholar]
- 42. Lewkowicz N, Mycko MP, Przygodzka P, et al. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016;9:364–378. [DOI] [PubMed] [Google Scholar]
- 43. Zheng L, Kelly CJ, Battista KD, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor–dependent repression of Claudin-2. J Immunol. 2017;15;199:2976–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Wang X, Chen Q, et al. Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol Nutr Food Res. 2020;64:e1900943. [DOI] [PubMed] [Google Scholar]
- 45. Cape E, Hall RJ, van Munster BC, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014;77:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eckenhoff RG, Laudansky KF. Anesthesia, surgery, illness and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gillespie Í, Rosenstein PG, Hughes D. Update: clinical use of plasma lactate. Vet Clin North Am Small Anim Pract. 2017;47:325–342. [DOI] [PubMed] [Google Scholar]
- 48. Kalder J, Ajah D, Keschenau P, et al. Microcirculatory perfusion shift in the gut wall layers induced by extracorporeal circulation. J Vasc Surg. 2015;61:497–503. [DOI] [PubMed] [Google Scholar]
- 49. Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liufu N, Liu L, Shen S, et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging (Albany NY). 2020;12:1965–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Juan Z, Chen J, Ding B, et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur J Cancer. 2022;161:10–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


