Summary
Background
Vaccines against COVID-19 are needed to overcome challenges associated with mitigating the global pandemic. We report the safety and immunogenicity of V590, a live recombinant vesicular stomatitis virus-based COVID-19 vaccine candidate.
Methods
In this placebo-controlled, double-blind, three-part phase 1 study, healthy adults were randomised to receive a single intramuscular dose of vaccine or placebo. In Part 1, younger (18–54 years) and, in Part 2, older (≥55 years) adults seronegative for SARS-CoV-2 nucleocapsid received one of four V590 dose levels (5.00 × 105; 2.40 × 106; 1.15 × 107; or 5.55 × 107 plaque-forming units [pfu]) or placebo. In Part 3, a single V590 dose level (5.55 × 10⁷ pfu) or placebo was administered to younger SARS-CoV-2 seropositive adults. Primary endpoints included adverse events (AEs) and for Parts 1 and 2 anti-SARS-CoV-2 serum neutralising antibody responses measured by 50% plaque reduction neutralisation (PRNT50) assay at Day 28. Registration NCT04569786 [P001-02].
Findings
232 participants were randomised and 219 completed the study. In seronegative participants, anti-SARS-CoV-2 spike-specific antibody responses to V590 were low and comparable to placebo across the lower dose levels. At the highest dose level (5.55 × 107 pfu), anti-SARS-CoV-2 spike-specific PRNT50 was 2.3-fold higher than placebo. The most frequently reported AEs were injection-site pain (38.4%), headache (15.1%) and fatigue (13.4%).
Interpretation
V590 was generally well-tolerated. However, Day 28 anti-SARS-Cov-2 spike-specific antibody responses in seronegative participants following a single intramuscular administration of V590 were not sufficient to warrant continued development.
Funding
The study was funded by Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Keywords: SARS-CoV-2, COVID-19, Vaccine, V590, Vesicular stomatitis virus (VSV)
Research in context.
Evidence before this study
Experience with the recombinant vesicular stomatitis virus (VSV) vaccine platform in the highly efficacious Zaire ebolavirus vaccine rVSVΔG-ZEBOV-GP (ERVEBO™) was the basis for the development of V590, a viral vector COVID-19 vaccine candidate. Pre-clinical studies showed that a single intramuscular injection with rVSV∆G-SARS-CoV-2 protected hamsters against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) challenge.
Added value of this study
We present the first-in-human results of the COVID-19 vaccine candidate V590, which, while generally well tolerated, demonstrated low immunogenicity following single intramuscular administration in SARS-CoV-2 seronegative participants. Given the strong immunogenicity observed with the Zaire ebolavirus vaccine (ERVEBO™) using the same dosing regimen and route of administration, the low immunogenicity observed with V590 is unexpected.
Implications of all the available evidence
Based on these findings, development of single-dose intramuscularly-administered V590 was discontinued. The reasons for the low immunogenicity observed in this study warrant further investigation to inform the development of future VSV-based vaccination strategies targeting SARS-CoV-2.
Alt-text: Unlabelled box
Introduction
The coronavirus disease 2019 (COVID-19) global pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a rapidly evolving health crisis associated with significant mortality and morbidity.1 There is an urgent need for effective options that are accessible and readily implementable in order to reduce the impact of COVID-19.2 While several vaccines against COVID-19 have received either emergency use authorization or full marketing approval, there remains a need for increased access to vaccines to overcome the many challenges associated with mitigating the pandemic, including immunising the global population.3
V590 is a live, recombinant viral vector COVID-19 vaccine candidate based on the vesicular stomatitis virus (VSV) (Figure 1). V590 is based on the recombinant VSV platform used for the highly efficacious vaccine, rVSVΔG-ZEBOV-GP (ERVEBO™)4 licensed to prevent disease caused by Zaire ebolavirus. rVSVΔG-ZEBOV-GP produces a rapid immune response against the ebolavirus surface glycoprotein (GP) following a single dose – as demonstrated in a cluster-randomised ring vaccination trial in 3,537 participants, in which vaccine efficacy of 100% (95% confidence interval [CI]: 79.3%–100.0%) was reported4,5 – and has also been used effectively in response to additional ebolavirus outbreaks in Africa.6 In the V590 vaccine the VSV envelope glycoprotein (G) gene is replaced with the coding sequence for the SARS-CoV-2 spike glycoprotein (S) and the cellular tropism of the V590 recombinant is thus determined by the S glycoprotein. During development of V590 it acquired several stable mutations that improved growth in cell culture, including a point mutation that reduced furin cleavage at the Spike S1/S2 subunit boundary as well as a C-terminal truncation. V590, as well as a VSV-based construct designed similarly to V590, have been reported to provide protection against SARS-CoV-2 challenge in a Syrian hamster in vivo model.7
Figure 1.
Schematic of the VSV SARS-CoV-2 construct (a) and a cryo transmission electron microscopy image of V590 GMP bulk drug substance (image courtesy of Irene Yin-Ting Chang and Douglas D. Richardson) (b).
This randomised, double-blind, placebo-controlled, dose-ranging phase 1 trial was designed to evaluate the safety and immunogenicity of V590 in healthy adults.
Methods
Study design and participants
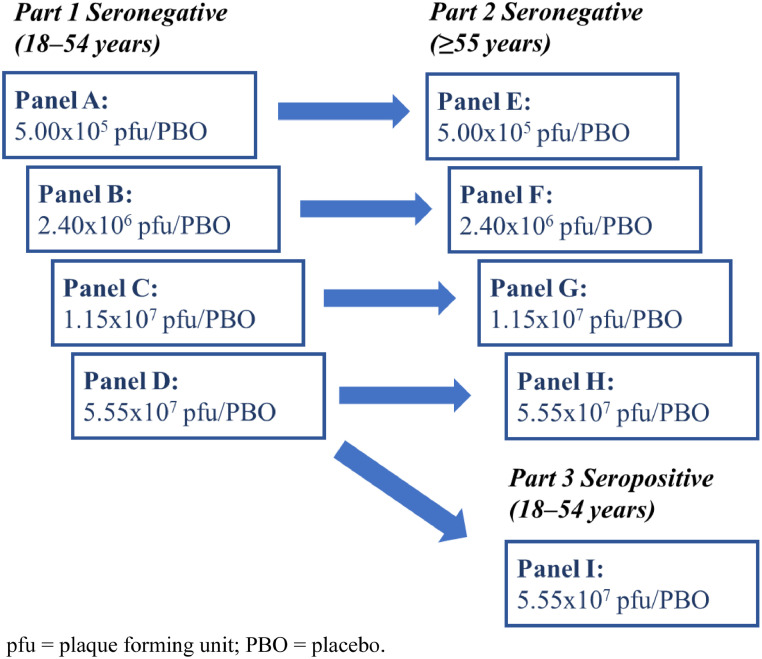
This multicentre, phase 1, randomised, double-blind, placebo-controlled, dose-ranging study (NCT04569786) was initiated on November 2, 2020, at seven phase 1 clinical sites in the United States (Supplementary Table S1).8 The trial consisted of three parts (Figure 2). In Parts 1 and 2, younger (aged 18–54 years) and older (≥55 years) SARS-CoV-2 nucleocapsid seronegative adults were randomly assigned to receive one of four V590 dose levels (5.00 × 105 plaque-forming units [pfu]; 2.40 × 106 pfu; 1.15 × 107 pfu; or 5.55 × 107 pfu) or matched placebo. In Part 3, a single V590 dose level (5.55 × 10⁷ pfu) or placebo was administered to younger SARS-CoV-2 seropositive adults. Safety data were reviewed until Day 7 for at least six participants from Part 1 (younger participants 18–54 years), as well as all other available safety data, before dosing either the next higher dose level in Part 1 or the same dose level in older participants ≥55 years (Part 2). In all parts of the study, participants were domiciled for 7 days after vaccination to facilitate close monitoring and specimen acquisition.
Figure 2.
Study design.
Eligible adults were 18 years of age or older with a body mass index ≤30 kg/m2 and in overall good health based on medical history, physical examination, electrocardiogram, and vital sign measurements performed prior to randomisation, as well as on laboratory tests obtained at screening. Participants included in Parts 1 and 2 of the study tested negative for SARS-CoV-2 based on reverse transcription polymerase chain reaction (RT-PCR) and anti-SARS-CoV-2 nucleocapsid antibody testing available under emergency use authorisation at screening and upon start of domiciling. Participants in Part 3 of the study were required to have positive serology (antibody to nucleocapsid) testing for SARS-CoV-2, also with negative SARS CoV-2 RT-PCR testing at screening and upon start of domiciling and be without symptoms of respiratory infection for at minimum 3 weeks preceding screening.
All participants must have been practicing social distancing for at least 2 weeks prior to planned Day 1 vaccination and have had no close contacts with known active SARS-CoV-2 infection during this period. Key exclusion criteria included severe reaction to vaccine administration, treatment with immunosuppressive therapy, diagnosis with an immunocompromising condition, or history of a current clinically significant condition that puts or may put a participant at increased risk for severe illness from SARS-CoV-2 infection.
Ethics
The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the central Institutional Review Board Advarra (ref: Pro00046862) and was conducted under an FDA-reviewed Investigational New Drug Application. Participants signed informed consent prior to any procedures being conducted.
Randomisation and masking
For each part of the study, a randomisation allocation schedule was generated by an unblinded statistician using the clinical schedule generation system (CSGS) – a randomisation application for the generation and storage of allocation and component schedules. The randomization numbers were first assigned to Panels A to I in a sequential fashion (for example, 0001 to 0084 for Panel A, 0101 to 0184 for Panel B). Within each panel, allocation of V590 or matching placebo was randomly assigned in a 3:1 ratio (active:placebo) with allocation block factor equal to 4. Therefore, for each 4 allocation numbers, 3 were assigned to V590 and 1 was assigned to placebo, with order randomly permutated. Upon the completion of the allocation schedule generation, site specific allocation schedules were distributed to unblinded pharmacists of the 7 sites via Aspera®, a secured data transfer platform. In instances when individual sites could not enroll participants as planned, replacement allocation numbers were assigned to other sites with available participants in a blinded fashion by the Merck & Co., Inc., Rahway, NJ, USA unblinded statistician. On site, each participant that met the inclusion/exclusion criteria and was considered to have passed screening was assigned a randomisation number by sequence.
Treatment assignment (V590 vs placebo) was blinded to participants, Investigators, all site staff (except the pre-designated unblinded pharmacist), and the Merck & Co., Inc., Rahway, NJ, USA study team (except the unblinded statistician and programmer, and unblinded clinical research associate, as per standard Merck & Co., Inc., Rahway, NJ, USA clinical process). Panel assignments, and consequently dose levels, were not blinded.
Vaccine was diluted by an unblinded pharmacist to the assigned dose level prior to administration, with an injection volume of 0.5 mL for all dose levels except for 5.55 × 107, which was 1.0 mL (1.0 mL of placebo was administered at this vaccine dose level to maintain blinding). The vaccine was administered by intramuscular injection, with the deltoid area of the upper non-dominant arm preferred.
Study intervention
The V590 vaccine and diluent/placebo were manufactured by Merck & Co., Inc. (West Point, USA) according to Good Manufacturing Practices. The placebo, and the diluent used for the preparation of the lower three V590 dose levels, was a TRIS-buffer based solution. Vials containing V590 drug product were stored frozen (-60 to -90°C) and protected from light and vials containing the diluent/placebo were stored refrigerated (2–8°C) and also protected from light.
Objectives
The primary safety objective of all parts of the study was to assess the safety and tolerability of single-dose intramuscular V590 compared with placebo. Safety endpoints included solicited injection-site adverse events (AEs) (swelling, erythema, pain/tenderness) from Day 1 to Day 5 following study intervention, solicited systemic AEs (myalgia, arthralgia, headache, fatigue, rash, nausea, joint swelling, oral lesions, hyperhidrosis) from Day 1 to Day 28, unsolicited AEs from Day 1 to Day 28, serious AEs (SAEs) from Day 1 to Day 365, and medically-attended AEs (MAAEs, events in which medical attention is received outside of a routine visit) collected from Day 1 through Day 180. The protocol was amended based on Day 28 immunogenicity data to follow MAAEs through Day 28 and SAEs until database lock (and through at least Day 28), as part of terminating the study.
Participants were provided with a vaccine report card (VRC) to fill out daily from Day 1 to Day 28. The VRC was used to collect injection site and systemic AEs, using both specific questions for solicited AEs and a space to record unsolicited AEs as “other complaints”. Any AEs recorded in the VRC were reviewed and fully assessed by the investigator. AEs were graded by the study investigators based on the guidance document ‘Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials of the Food and Drug Administration’ (September 2007).9
The primary immunogenicity objective of Parts 1 and 2 of the study was to assess if V590 increased anti-SARS-CoV-2 spike-specific neutralising antibodies measured by the 50% plaque reduction neutralisation test (PRNT50) in serum samples at Day 28 compared with placebo. Secondary immunogenicity analysis endpoints included anti-SARS-CoV-2 spike-specific neutralising antibody responses at Day 1 predose, Day 7, and Day 14, and anti-SARS-CoV-2 spike-specific immunoglobulin G (IgG) responses measured by the enzyme linked immunosorbent assay (ELISA) at Day 1 predose, Day 7, Day 14, and Day 28. Timepoints beyond Day 28 were removed from the protocol through an amendment following evaluation of Day 28 immunogenicity.
Immunogenicity assays to assess SARS-CoV-2 antibody responses following vaccination with V590 were developed and qualified to demonstrate acceptable sensitivity, specificity and precision and subsequent sample testing was conducted in a regulated laboratory. A semi-quantitative plaque reduction neutralisation test, based on neutralization of rVSV∆G-SARS-CoV-2 (V590), PRNT50, was developed and qualified to measure SARS-CoV-2 neutralising antibodies in human serum with a limit of detection of 10. Serial dilutions of heat-inactivated serum samples are incubated with a fixed concentration of rVSV∆G-V590 virus (VSV expressing SARS-CoV-2 spike protein on the surface) to initiate spike-specific antibody neutralisation of the virus. The serum-virus mixture was added to Vero CCL-81 cells seeded in a 96-well plate and incubated for approximately 4 hours before addition of methylcellulose overlay to localise infection and allow for the growth of viral plaques. After an incubation of 20–24 hours, the cells were fixed, permeabilised and immunostained with a monoclonal rabbit anti SARS-CoV-2 spike neutralising antibody, followed by Alexa Fluor-488-conjugated goat anti-rabbit IgG for the detection of SARS-CoV-2-S proteins expressed in infected cells. The stained plaques were scanned for immunofluorescence and counted on the BioTek Cytation 5 cell imaging multimode reader. Responses were reported as a 50% neutralisation titre (PRNT50) which represents the inverse dilution of the highest serum sample that neutralises the virus control by 50%. A subset of serum samples from clinical study participants and from COVID-19 convalescent donors was tested in a validated live SARS-CoV-2 microneutralization assay (Battelle Memorial Institute, West Jefferson, OH, USA) to demonstrate concordance across assays and thus acceptability of utilizing the V590 PRNT assay to detect antibodies capable of neutralizing live SARS-CoV-2 (see Supplementary Figure S1).
A semi-quantitative ELISA was developed and qualified for endpoint titre determination of anti-SARS-CoV-2 spike protein IgG antibodies in human serum. Microtitre plates were coated with SARS-CoV-2 spike (prefusion stabilised trimer; Lake Pharma #46328). Serially diluted serum samples were incubated in the S-coated wells, allowing SARS-CoV-2 spike–specific antibodies to bind. Positive and negative controls were also included on each plate. Bound S-specific IgG was detected with goat anti-human IgG horseradish peroxidase conjugate (Fcγ fragment-specific; Jackson ImmunoResearch Laboratories) followed by development with tetramethylbenzidine substrate. Optical density at 450nm was measured on an ELISA plate reader. Endpoint titres were defined as the reciprocal of the highest sample dilution that gives a reading above the plate cut-off of two-fold over background. The assay was qualified to demonstrate acceptable sensitivity, specificity and precision for the detection of anti-SARS CoV-2 spike-specific protein IgG antibodies in human serum, with a limit of detection of 100. Additional secondary endpoints included plasma V590 vector viraemia and viral shedding in saliva and urine. Samples were collected on Day 1 post-dose, and Days 2, 3, 4, 5, 6, 7, 14, and 28 for all parts of the study. Viraemia samples were analysed for all participants from samples collected on Days 2, 3, and 4, as well as on Days 7, 14 and 28 if Day 4 was positive. For shedding, Day 7 samples were analysed for all participants, and if Day 7 was positive Days 14 and 28 were additionally analysed. Plasma, urine, and saliva samples were assessed for the presence of vaccine virus RNA in a qualified RT-qPCR assay specific for the V590 VSV nucleoprotein gene sequence. The assay consisted of three principal steps: 1) RNA extraction from clinical specimens using the MagMax Viral Pathogen Kit on the KingFisher, 2) reverse transcription and parallel amplification/detection of the vector-specific nucleoprotein gene sequence using TaqMan chemistry and, 3) quantification of results using a standard curve generated from spiking quantified standards into Basematrix at multiple concentrations. An internal control (MS2 phage) was used to verify RNA extraction from each specimen and to confirm the absence of nonspecific RT-qPCR inhibition. The limit of detection for the plasma, urine and saliva samples was defined at 182.9 copies/mL, 188.9 copies/mL, and 173.3 copies/mL, respectively. The limit of quantification for all three matrices was defined as 297 copies/mL with inter-assay precision less than 80% CV and relative accuracy between 0.8 and 1.25 throughout range.
Exploratory immunogenicity endpoints included anti-SARS-CoV-2 spike-specific neutralising antibody responses and anti-SARS-CoV-2 spike-specific IgG responses in Part 3 of the study (using ELISA and PRNT50 assays as described), as well as SARS-CoV-2 spike-specific T cell responses (measured by intracellular cytokine staining [ICS] of peripheral blood mononuclear cells [PBMC]) and serum cytokines/chemokines from participants in Parts 1, 2, and 3. A multiparametric ICS assay was developed and qualified in PBMC by CellCarta (Montreal, Canada) to characterise CD4 and CD8 T-cell response multifunctionality and the Th1 and Th2 response in CD4 T cells, upon stimulation using a mix of resuspended peptide pools specific to the S-protein of SARS-CoV-2 (JPT Innovate Peptides Solutions; PM-WCPV-S2 >70% purity). Day 1 cryopreserved PBMCs were thawed and rested overnight in an incubator set at 37°C, 5% CO2. On Day 2 the cells were plated (1 × 106 cells/well) and treated for 6 hours with either matched DMSO (non-stimulated), SARS-CoV-2 specific peptide pools mix, or polyclonal stimulator SEB (positive control). After 1 hour of treatment, GolgiPlug™ and GolgiStop™ were added and incubated for the remaining 5 hours. The plates were then sealed and kept at 2–8°C overnight. On Day 3, the cells were treated with Fc Block for 10 min at room temperature to block non-specific antibody binding sites, followed by staining with surface antibody cocktail for 30 min at 2–8°C. Cells were then permeabilised with Cytofix/Cytoperm for 20 min followed by staining with the intracellular antibody cocktail for 30 min at 2–8°C. The stained samples were acquired on a BD LSR Fortessa flow cytometer. Flow cytometry data analysis was performed offline using FlowJo™ software using a pre-defined analysis template. Layouts of each analysed sample were captured and data tables were produced.
The gating strategy used for data analytics is as follows; 1) exclusion of debris and fluidics instabilities, 2) exclusion of sample aggregates, 3) exclusion of doublets using FSC-A versus FSC-H and SSC-A versus SSC-H, 4) exclusion of dead cells, 5) gating for lymphocytes using FSC-A/SSC-A, 6) gating on CD3+ T-cells using CD3 by Dump channel (containing antibodies against CD14, CD56, CD19) dot plot and gating on CD3+Dump- cells, 7) identification of CD4+ and CD8+T-cells from CD3+ T-cells, 8) for CD4+ T-cells, IFN-γ+, IL-2+, TNF-α+, IL-4+, and IL-13+ cells were gated, 9) for CD8+ T-cells, IFN-γ+, IL-2+, and TNF-α+ cells were gated, 10) Boolean gate rules were performed to analyze polyfunctional populations.
A representative gating strategy from a seropositive antigen experienced donor prior to vaccination is provided in Supplementary Figure S2.
For the serum cytokine assay, an electrochemiluminescence (ECL) method, the V-PLEX human Proinflammatory Panel 1, for the quantitation of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL12p70, IL-13 and TNF-α was developed by Meso Scale Discovery (MSD) (Rockville, MD, USA; Catalog number: K15049D) and qualified in human serum by PPD Laboratories (Richmond, VA). The MSD kit included a 96-well plate pre-coated with capture antibodies on independent and well-defined spots. After a plate was washed, serum samples (1:2 dilution) and calibrators were added into the plate, allowing cytokine analytes in samples to bind capture antibodies immobilised on the working electrode surface. Three quality control samples (human serum samples with different levels of cytokines) and a blank sample were also included on each plate. After the plate was washed, detection antibodies conjugated with electrochemiluminescent labels were added into each well and recruited by the bound analytes to complete the sandwich. After the plate was washed, an MSD read buffer was added into each well and the plate loaded into an MSD instrument where a voltage applied to the plate electrodes causes the captured labels to emit light. The instrument measures the intensity of emitted light which was proportional to the amount of analyte present in the sample and provides a quantitative measure of each analyte in the sample.
Statistical analysis
Using SAS 9.4 statistical software, safety, viraemia and viral shedding analyses were conducted in the ‘all participants as treated’ population, which consisted of all randomly assigned participants who received study intervention. Participants were included in the treatment group corresponding to the study intervention they received for the analysis.
Safety data including AEs, post-vaccination temperatures, and laboratory measurements were assessed by point estimates. Risk differences with 95% CIs for between-group differences were evaluated for solicited AEs, maximum temperatures, MAAEs, SAEs, and AEs with an incidence of at least four participants in any one vaccination group.
Numbers and proportions of participants with positive V590 plasma viraemia and positive viral shedding in urine and saliva were calculated by dose and time point. Geometric means (GMs) and 95% CIs were estimated, and descriptive statistics including median, inter quartile range, minimum, and maximum were provided. Positive viraemia and viral shedding were detectable by RT-PCR results greater than or equal to the limit of detection. Half of the lower limit of quantification (LLOQ) value was used for analysis of positive sample titres below LLOQ.
The immunogenicity analyses were conducted in the per-protocol immunogenicity (PPI) population, which consists of all randomly assigned participants without deviations from the protocol that may substantially affect the results of the immunogenicity endpoints. For Parts 1 and 2, participants should also be seronegative to anti-SARS-CoV-2 nucleocapsid antibody through Day 28 to be included in the PPI. Potential deviations that may have resulted in the exclusion of a participant from the per protocol population for immunogenicity analyses include failure to receive the correct dose of study vaccine at Day 1, receipt of prohibited medication and prohibited vaccine, or the collection of blood sample outside of the pre-specified window. Participants were included in the vaccination group to which they were randomly assigned for the analysis of immunogenicity data using the per protocol population.
For immunogenicity endpoints, GMs with within-group 95% CIs were calculated by dose and time point. Treatment differences were evaluated for PRNT and ELISA at Day 28 by geometric mean titre (GMT) ratio (V590/placebo) with 90% CI (evaluated at a one-sided 5% alpha level, thus equivalent to a one-sided 95% CI), which were calculated using a longitudinal data analysis method.10 For anti-SARS-CoV-2 spike-specific PRNT and ELISA below LLOQ and serum cytokine titres below the minimal reportable value, half of the LLOQ or half of the minimal reportable value was used for analysis.
The study sample size was calculated to achieve 90% power at a 1-sided 5% alpha-level to demonstrate V590 increases anti-SARS-CoV-2 SNA GMTs, compared to placebo, based on a true GMT ratio of V590 versus placebo ≥2.17, assuming: 1) an 85% evaluability rate at Day 28 in Parts 1 and 2 (i.e., 36 evaluable participants out of 42 V590 recipients in each dose level, and 48 evaluable participants out of 56 placebo recipients); and 2) a comparable variability as observed in rVSVΔG-ZEBOV-GP. The sample size was also calculated to ensure adequate power for the safety evaluation.
Role of the funding source
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and the International AIDS Vaccine Initiative (IAVI), participated in study design, data collection, data analysis, data interpretation, and writing of the report. The funders reviewed the draft of the manuscript. All authors had access to the study results and approved the decision to submit for publication.
Results
Participants
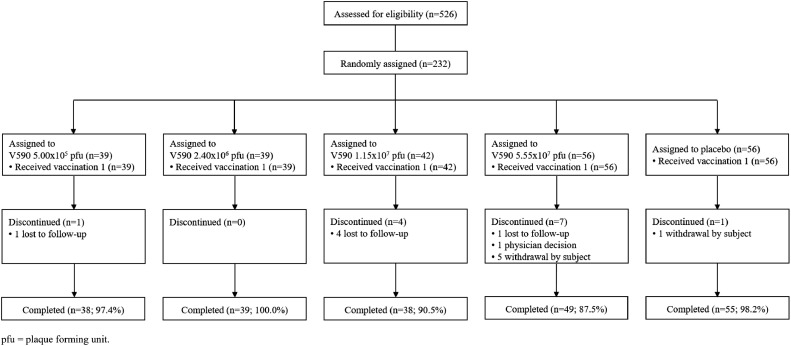
From November 2, 2020 to January 7, 2021, a total of 526 participants underwent screening for enrolment (Figure 2). Of these, 232 participants were enrolled into the study and randomly assigned, 219 of whom completed the study (Figure 3). Baseline characteristics for participants randomly assigned into the study are given in Table 1.
Figure 3.
Participant disposition.
Table 1.
Characteristics of the participants at baseline (all randomised participants – Parts 1, 2 and 3).
| V590 5.00 × 105 pfu |
V590 2.40 × 106 pfu |
V590 1.15 × 107 pfu |
V590 5.55 × 107 pfu |
Placebo |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Participants in population | 39 | 39 | 42 | 56 | 56 | 232 | ||||||
| Sex | ||||||||||||
| Male | 27 | (69.2) | 17 | (43.6) | 19 | (45.2) | 24 | (42.9) | 27 | (48.2) | 114 | (49.1) |
| Female | 12 | (30.8) | 22 | (56.4) | 23 | (54.8) | 32 | (57.1) | 29 | (51.8) | 118 | (50.9) |
| Age (years) | ||||||||||||
| 18 to 54 | 21 | (53.8) | 20 | (51.3) | 21 | (50.0) | 35 | (62.5) | 32 | (57.1) | 129 | (55.6) |
| ≥55 | 18 | (46.2) | 19 | (48.7) | 21 | (50.0) | 21 | (37.5) | 24 | (42.9) | 103 | (44.4) |
| Mean | 48.9 | 50.9 | 51.0 | 48.0 | 47.3 | 49.0 | ||||||
| SD | 18.8 | 16.0 | 13.7 | 16.1 | 16.9 | 16.3 | ||||||
| Median | 49.0 | 54.0 | 54.5 | 49.0 | 50.0 | 50.0 | ||||||
| Range | 19 to 79 | 19 to 84 | 22 to 71 | 18 to 77 | 19 to 85 | 18 to 85 | ||||||
| Race | ||||||||||||
| American Indian or Alaska Native | 1 | (2.6) | 0 | (0.0) | 0 | (0.0) | 1 | (1.8) | 0 | (0.0) | 2 | (0.9) |
| Asian | 0 | (0.0) | 1 | (2.6) | 1 | (2.4) | 0 | (0.0) | 2 | (3.6) | 4 | (1.7) |
| Black or African American | 3 | (7.7) | 5 | (12.8) | 4 | (9.5) | 7 | (12.5) | 8 | (14.3) | 27 | (11.6) |
| Multiple | 0 | (0.0) | 0 | (0.0) | 2 | (4.8) | 0 | (0.0) | 0 | (0.0) | 2 | (0.9) |
| White | 35 | (89.7) | 33 | (84.6) | 35 | (83.3) | 48 | (85.7) | 46 | (82.1) | 197 | (84.9) |
| Ethnicity | ||||||||||||
| Hispanic or Latino | 13 | (33.3) | 20 | (51.3) | 13 | (31.0) | 31 | (55.4) | 19 | (33.9) | 96 | (41.4) |
| Not Hispanic or Latino | 26 | (66.7) | 19 | (48.7) | 29 | (69.0) | 25 | (44.6) | 37 | (66.1) | 136 | (58.6) |
Part 1 and 2 participants are seronegative for anti-SARS-CoV-2 nucleocapsid antibody at baseline prior to vaccination. Part 3 participants are seropositive for anti-SARS-CoV-2 nucleocapsid antibody at baseline prior to vaccination.
pfu = plaque forming unit; SD = standard deviation.
Vaccine safety
Of the 232 participants included in the safety analysis, 140 (60.3%) experienced one or more AEs during the study, 90 (38.8%) experienced an injection-site AE, and 99 (42.7%) experienced a non-injection-site AE (Table 2). The proportions of participants with these AEs were generally comparable across V590 and placebo groups.
Table 2.
Adverse event summary (all participants as treated population) following V590 or placebo (Parts 1, 2 and 3).
| V590 5.00 × 105 pfu |
V590 2.40 × 106 pfu |
V590 1.15 × 107 pfu |
V590 5.55 × 107 pfu |
Placebo |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Participants in population | 39 | 39 | 42 | 56 | 56 | 232 | ||||||
| with one or more adverse events | 19 | (48.7) | 23 | (59.0) | 23 | (54.8) | 39 | (69.6) | 36 | (64.3) | 140 | (60.3) |
| injection-site | 11 | (28.2) | 14 | (35.9) | 14 | (33.3) | 24 | (42.9) | 27 | (48.2) | 90 | (38.8) |
| non-injection-site | 14 | (35.9) | 15 | (38.5) | 16 | (38.1) | 30 | (53.6) | 24 | (42.9) | 99 | (42.7) |
| with no adverse event | 20 | (51.3) | 16 | (41.0) | 19 | (45.2) | 17 | (30.4) | 20 | (35.7) | 92 | (39.7) |
| with vaccine-relateda adverse events | 17 | (43.6) | 22 | (56.4) | 18 | (42.9) | 36 | (64.3) | 33 | (58.9) | 126 | (54.3) |
| injection-site | 11 | (28.2) | 14 | (35.9) | 13 | (31.0) | 24 | (42.9) | 27 | (48.2) | 89 | (38.4) |
| non-injection-site | 12 | (30.8) | 10 | (25.6) | 8 | (19.0) | 23 | (41.1) | 14 | (25.0) | 67 | (28.9) |
| with toxicity grade 3-4 adverse events | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (1.8) | 0 | (0.0) | 1 | (0.4) |
| with toxicity grade 3-4 vaccine-relateda adverse events | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (1.8) | 0 | (0.0) | 1 | (0.4) |
| with non-serious adverse events | 19 | (48.7) | 23 | (59.0) | 23 | (54.8) | 39 | (69.6) | 36 | (64.3) | 140 | (60.3) |
| with serious adverse events | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (1.8) | 0 | (0.0) | 1 | (0.4) |
| with serious vaccine-relateda adverse events | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| with adverse events of clinical interest | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| with vaccine-relateda adverse events of clinical interest | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
Determined by the investigator to be related to the vaccine.
Reported adverse events include nonserious adverse events within 28 days of vaccination and serious adverse events occurring from Day 1 through the duration of the study until final database lock.
Part 1 and 2 participants are seronegative for anti-SARS-CoV-2 nucleocapsid antibody at baseline prior to vaccination. Part 3 participants are seropositive for anti-SARS-CoV-2 nucleocapsid antibody at baseline prior to vaccination.
pfu = plaque forming unit.
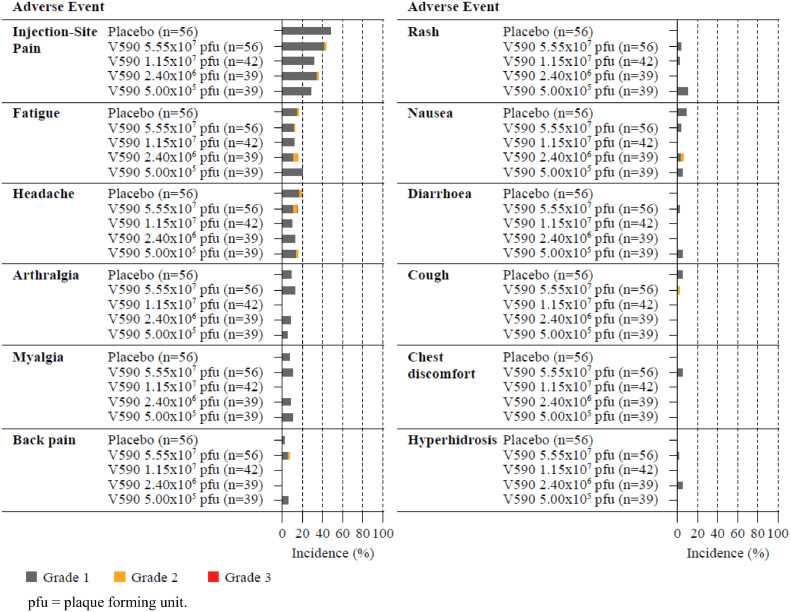
For all intervention groups in Parts 1, 2, and 3 combined, the most frequently reported (≥5%) AEs were the solicited injection-site AE of injection-site pain (89 [38.4%]) and the solicited systemic AEs of headache (35 [15.1%]), fatigue (31 [13.4%]), arthralgia (17 [7.3%]), and myalgia (17 [7.3%]) (Figure 4). There was no clear dose-dependence of these events and overall incidences were generally comparable to placebo (most frequently reported [≥5%] AEs with placebo were injection-site pain [27 {48.2%}], headache [11 {19.6%}], fatigue [9 {16.1%}], nausea [5 {8.9%}], arthralgia [5 {8.9%}], myalgia [4 {7.1%}] and cough [4 {5.4%}]).
Figure 4.
Incidence and severity of adverse events after injection of V590 or placebo.
Adverse events with an incidence of 5% or higher reported up to 28 days after injection. Each panel presents a specific adverse event. Data are grouped by treatment group.
A total of 126 participants (54.3%) reported any AE that was determined by the investigator to be vaccine related, 89 (38.4%) experienced a vaccine-related injection-site AE, and 67 (28.9%) experienced a vaccine-related non-injection-site AE. Across V590 dose groups the proportion of participants who reported injection-site pain was not higher than in the placebo group (V590: 11 [28.2%] to 24 [42.9%]; placebo: 27 [48.2%]). A lower proportion of participants with vaccine-related AEs was observed for older seronegative participants than for younger seronegative participants (49 [47.6%] vs 67 [60.4%], respectively), primarily due to the proportion of participants who experienced injection-site pain. Within each age group, the proportions of V590 and placebo recipients who reported specific vaccine-related AEs (including injection-site pain) were comparable. All vaccine-related AEs were considered not serious; all AEs were mild to moderate in intensity except for one event (a severe [Grade 3] headache reported on Day 23 experienced by a Part 1, V590 5.55 × 107 pfu dose group participant considered to be related to study vaccination by the investigator).
One SAE was reported during this study, a Part 2 participant who received V590 5.55 × 107 pfu was diagnosed with amaurosis fugax, which was also classified as a MAAE. The participant reported blurred/reduced vision in the left eye 10 days after vaccination, lasting 7 minutes with spontaneous resolution, and was subsequently admitted to hospital and diagnosed with amaurosis fugax. Laboratory testing and imaging (brain magnetic resonance imaging, computed tomography angiography of the head, and vascular ultrasound of the lower extremities) showed no evidence of thromboembolic disease, and an echocardiogram revealed an interatrial shunt. Platelet count and coagulation tests were unremarkable. This event was assessed by the investigator as unrelated to the study intervention. There were two additional MAAEs reported during the study: 1) oral ulcers, classified as moderate intensity, were reported by a participant in Part 1 of the study, 10 days after receiving V590 5.55 × 107 pfu, lasting 3 weeks and considered to be related to study vaccination; and 2) a case of mild urethritis reported in a Part 3 participant, 3 days after receiving V590 5.55 × 107 pfu, resolved (following treatment) approximately 1.5 weeks after onset, which was not considered related to study vaccination.
No clinically meaningful patterns were observed following study vaccination for laboratory safety tests or vital signs. At Day 3 postvaccination, a transient decrease in neutrophils was observed in participants who received V590 (mean change from baseline [lowest to highest V590 dose group] was -0.26 × 109/L [standard deviation {SD}: 0.70] to -0.97 × 109/L [SD: 0.96]), and neutrophil counts improved by Day 7 postvaccination. Two participants who received V590 5.55 × 107 pfu had laboratory-identified AEs of neutropenia, which resolved by Day 7, compared with zero participants vaccinated with placebo.
Immunogenicity
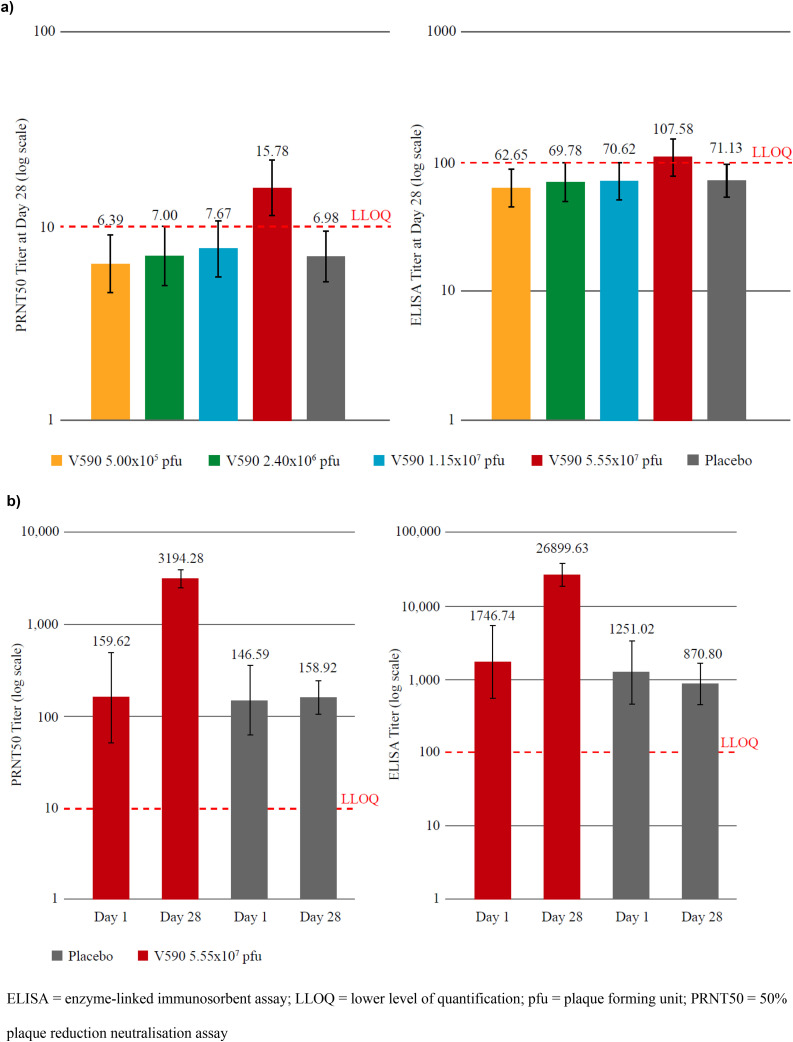
In the 214 seronegative participants (Parts 1 and 2), the overall proportions of participants with anti-SARS-CoV-2 spike-specific antibody responses to V590 were low and comparable to participants administered placebo (Supplementary Table S2). PRNT50 and ELISA titres at Day 28 were comparable across V590 and placebo groups except in the V590 5.55 × 107 pfu dose group, in which titres were approximately 2.3-fold (90% CI, 1.56-3.28, p<0.001) and 1.5-fold (90% CI, 1.04-2.19) higher than placebo, respectively (Figure 5 and Supplementary Table S3).
Figure 5.
Analysis of anti-SARS-CoV-2 spike-specific antibody responses at Day 28 (per-protocol immunogenicity population) ([a] Parts 1 and 2: seronegative participants) and compared with Day 1 for seropositive participants in Part 3 ([b] 18–54 years).
Following vaccination with V590 5.55 × 107 pfu, increases in ELISA and PRNT50 titres were observed in both younger and older seronegative participants by Day 14 which then plateaued until Day 28 (Supplementary Table S4). The fold increase in GMTs following vaccination with V590 5.55 × 107 pfu from baseline to Day 28 compared with placebo was comparable in younger and older participants, as measured by ELISA, and modestly greater in younger participants than in older participants, as measured by PRNT50.
The overall magnitude of anti-SARS-CoV-2 spike-specific antibody response was low across V590 dose levels, with ELISA titres at Day 28 below the LLOQ for the first three dose levels, and modestly above the LLOQ for the highest dose level. Similarly, PRNT50 GMTs at Day 28 were below the LLOQ for the first three dose levels, and approximately 1.5-fold above the LLOQ at the highest dose level. The percentage of participants with a Day 28 ELISA or PRNT50 titre value above the LLOQ in the highest dose level was higher in the younger participants as compared with the older participants (Supplementary Table S4). For ELISA, 62% of younger participants were above the LLOQ compared with 24% of older participants. Similarly, for PRNT50, 76% of younger participants had titres above the LLOQ at Day 28, compared with 29% of older participants.
In Part 3 of the study, in seropositive adults following vaccination with V590 5.55 × 107 pfu, anti-SARS-CoV-2 spike-specific IgG GMTs increased by approximately 15-fold (GMT: 1746.74 [95% CI, 554.08–5506.61] at baseline; 26899.63 [95% CI, 18106.23–39963.58] at Day 28) and PRNT50 GMTs increased by approximately 20-fold (GMT: 159.62 [95% CI, 51.21–497.53] at baseline; 3194.28 [95% CI, 2450.18–4164.37] at Day 28) at Day 28 relative to baseline (Figure 5).
Overall, serum cytokine responses (as measured by multiplex binding assay) and SARS-CoV-2-specific CD4+ and CD8+ T-cell responses (as measured by ICS) were generally similar across V590 and placebo groups in seronegative participants, in whom the induction of SARS-CoV-2 specific Th1 ( IFN-γ, TNF-α, and IL-2) and Th2 (IL-4, IL-13) cytokine responses in CD4+ T cells at Day 28 post vaccination was low to negligible (Supplementary Table S5).
In seropositive participants SARS-CoV-2 specific Th1 (IFN-γ, TNF-α, and IL-2) responses were increased in CD4+ T cells prior to vaccination, when compared with seronegative participants, and further increased by approximately 2-fold (GM: 0.081 [95% CI, 0.037–0.178] at baseline; 0.156 [95% CI, 0.096–0.252] at Day 28) on Day 28 following vaccination with V590 5.55 × 107 pfu (Supplementary Table S6). No change was observed in the placebo group. Th1-type responses in CD8+ T cells and Th2 responses in CD4+ T cells were low to negligible in the V590 5.55 × 107 pfu group and placebo groups at Day 1 and Day 28.
Serum cytokine responses, as measured by multiplex immunoassay, were also comparable across all treatment groups (V590 and placebo) in seronegative and seropositive participants (Supplementary Figures S3 and S4 and Supplementary Tables S7 and S8). Specifically, GMs of serum IFN-γ, IL-2, IL-12p70, TNF-α, IL-1 β, IL-8, IL-4, IL-10, and IL-13 were comparable across V590 and placebo groups at Day 1 predose, Day 1 postdose, and Day 4. The GMs of serum IL-6 were comparable at Day 1 predose and Day 4 but modestly elevated at Day 1 postdose across V590 and placebo groups. The same trends were observed in Part 1 (seronegative participants 18–54 years old), Part 2 (seronegative ≥55 years old), and in Part 3 (seropositive participants aged 18–54 years old). The modest elevation of serum IL-6 in both V590 and placebo groups suggests this is not induced by V590.
V590 viraemia and viral shedding
Overall, 65% of V590 recipients had detectable, low-level vaccine viraemia for at least one assayed time point within 7 days post vaccination based on detection of viral RNA using RT-qPCR. This low-level viraemia, which was detected in all four V590 dose groups, was undetectable by Day 7 in all participants. The proportion of participants with vaccine viraemia for at least one assayed time point increased in a dose-dependent manner, ranging from approximately 25% in the lowest dose group to approaching 100% in the highest dose group. In both younger and older participants, a similar proportion of participants with vaccine viraemia was observed and was not influenced by baseline serostatus (Supplementary Table S9).
Viral shedding in saliva and urine was assessed in all participants for samples collected on Day 7 and was detected in the saliva of two participants: one seronegative participant who received placebo and one seropositive participant who received V590 5.55 × 107 pfu (Supplementary Table S10). Shedding was not detected in either of these participants at Day 14. The positive saliva result in the placebo recipient was investigated, with no evidence of laboratory cross-contamination or sample-handling error, and no other evidence of infection with V590 in this participant; this result was considered most consistent with a false positive result. No viral shedding for any study participant was detected in urine (Supplementary Table S11).
Discussion
We report the findings of the first-in-human dose-ranging study assessing safety, tolerability, and immunogenicity of a single, intramuscular administration of V590 SARS-CoV-2 vaccine among healthy younger and older adults.
V590 was generally well-tolerated and the results provide evidence that a rVSV SARS-CoV-2 spike chimeric virus can elicit an immune response. However, the immune response following single dose intramuscular administration of V590 to SARS-CoV-2 seronegative participants was limited in magnitude. Day 28 ELISA and neutralising antibody GMTs at the highest dose tested were approximately 10-fold lower than those observed with natural SARS-CoV-2 infection, when comparing with the seropositive Part 3 participants at baseline (Figure 4), as well as COVID-19 convalescent donors previously tested using the same assays (Supplemental Figure S1). Day 28 ELISA and neutralising antibody GMTs were below the LLOQ for the lower three dose levels tested in this study. Since our objective was to develop a single-dose vaccine to simplify vaccination during an ongoing pandemic, immunity induced by a prime-boost regimen was not evaluated in this study.
V590 was developed leveraging rVSV platform experience from rVSVΔG-ZEBOV-GP, with the prediction that V590 as a similarly designed replication-competent chimeric virus would elicit rapid immunity and protection against SARS-CoV-2 following a single intramuscular dose. In the context of a global pandemic, building on the non-clinical, clinical, and manufacturing experience from rVSVΔG-ZEBOV-GP facilitated efficient clinical testing of intramuscularly administered V590 as a single dose vaccine. Based on the data from rVSVΔG-ZEBOV-GP, the low immunogenicity observed following V590 vaccination in seronegative participants was unexpected. Strategies that might be considered to enhance the immunogenicity of V590 based on replacing the native spike with a modified immunogen-like stabilised spike trimer, or an isolated receptor binding domain (RBD), cannot be readily adapted to the VSV∆G chimeric virus approach because the recombinant virus must express a functional glycoprotein that supports virus attachment to ACE2, membrane fusion needed for cell entry, and maturation of infectious progeny.
There are several possible explanations for the limited immunogenicity observed in SARS-CoV-2 seronegative individuals. Reduced viral fitness of V590 relative to rVSVΔG-ZEBOV-GP or wild type VSV is a possibility. One contributor to reduced replicative fitness might be that the spike predominantly localizes to intracellular membranes where SARS-CoV-2 matures while VSV assembles and buds from the cell surface membrane.11,12
The cellular-level tropism of V590 offers an additional, and perhaps leading, hypothesis for the limited immunogenicity observed in this study. Since V590 expresses SARS-COV-2 spike protein on the viral surface, similar to SARS-CoV-2 virus, V590 is expected to enter cells in an angiotensin-converting enzyme 2 (ACE2)-dependent fashion.13 While ACE2 is widely expressed in mucosal, endothelial and epithelial tissues, it is not expressed in skeletal muscle, which is the major tissue type present at the site of an intramuscular injection, and this may have limited V590 infection of host cells, replication, and immune response.14
The innate immune response to V590 as assessed by early circulating cytokines appeared limited compared with that reported for rVSVΔG-ZEBOV-GP.15 The reason for this is unclear but could be due to reduced vaccine replication of V590 compared with rVSVΔG-ZEBOV-GP, and/or a difference in the innate immune response induced by SARS-CoV-2 spike versus ebolavirus glycoprotein. Ebolavirus glycoprotein has been reported to elicit a strong innate immune response by itself,16 which may contribute to the difference in postvaccination circulating cytokine responses between V590 and rVSVΔG-ZEBOV-GP. A strong innate response is needed to enable development of an effective adaptive response, and therefore this reduced innate response could contribute to the limited humoral response observed with V590.
The cellular immune response to V590 was also limited, particularly in seronegative individuals. Importantly, no Th2 bias was noted in CD4+ T cell responses, which has been associated with the potential for disease enhancement for other pathogens.17 An increase in spike-specific CD4+ Th1 cells, but not Th2 cells, was observed following vaccination of seropositive individuals with the highest dose, supporting an overall Th1 bias for the platform. This Th1 bias is consistent with prior findings with rVSVΔG-ZEBOV-GP.18
Notably, in seropositive younger adults vaccinated with 5.55 × 107 pfu V590 approximately 15-fold increases in ELISA and 20-fold increases in PRNT GMTs were observed at Day 28 relative to baseline. Thus, in the setting of prior natural SARS-CoV-2 infection, V590 is able to elicit an immune response of greater magnitude relative to baseline than was observed in seronegative individuals. It is noted that only the highest dose in the study was tested in seropositive participants, in order to assess safety of V590 in the seropositive population, and therefore the immune response in seropositive participants to lower doses of V590 is unknown. Additionally, among seropositive participants, V590 was tested only in younger individuals; among seronegative participants who received the same highest dose level, a higher proportion of younger than older individuals had quantifiable postdose ELISA or PRNT titres. The magnitude of seropositive individuals’ immune response to lower dose-levels of V590 is not known, nor is that of older subjects at any dose level. It is not known if a stronger immune response would have been observed at a later timepoint, as the study was terminated based on review of the Day 28 immunogenicity and consequently no further samples were collected or processed beyond the time of this assessment. There were a small number of Day 90 samples collected (10 in total) and tested by ELISA prior to this data review, predominantly from younger participants at the lower V590 dose levels. All 10 had Day 28 ELISA values below the LLOQ, and from Day 28 to Day 90, 2 were infected with SARS-CoV-2 based on positive anti-nucleocapsid antibodies (with ELISA responses likely attributable to natural infection), 5 had ELISA titers around or below the LLOQ, and 3 participants had high titres at Day 90. The overall sample size is too limited to draw clear conclusions from, and the high proportion of non-responders at this later time point in this very limited dataset would not be anticipated to support further development.
Overall, single-dose administration of intramuscular V590 over the dose range of 5.00 × 105 pfu to 5.55 × 107 pfu was generally well-tolerated. Single-dose intramuscular V590 at 5.55 × 107 pfu, the highest dose tested, was superior to placebo in seronegative adults. However, the overall magnitude of immune response was low in seronegative participants, and likely not clinically meaningful.19,20 Based on these immunogenicity results in seronegative participants, this study was terminated.
Contributors
JR contributed to the trial conceptualisation and design, data collection, data analysis, data interpretation and the writing of this report. DT and DG contributed to the trial conceptualisation and design, data interpretation and the writing of this report. SD contributed to the assay methodology, data collection, data interpretation and writing of this report. JC and JL contributed to the trial investigation – project administration and supervision – and the writing of this report. QH contributed to the trial conceptualisation and design, trial administration, data collection, data analysis, data interpretation and the writing of this report. TC contributed to the trial conceptualisation, trial design (methodology), data interpretation and the writing of this report. JS, EL, SJ contributed to the trial design, data analysis, data interpretation and the writing of this report. PA contributed to the trial design and data interpretation. HP contributed to the trial conceptualisation and the writing of this report. SAS contributed to the trial design, conduct and data interpretation. KR contributed to the trial design (methodology) and the writing of this report. CH contributed to the safety data analysis and interpretation. XZ contributed to the data collection and interpretation and writing of this report. RW contributed to the technical conceptualisation of the trial, trial design (methodology), supervision of TH1/TH2 intracellular cytokine assays and sample analyses. RS, WJH, JCR, MH-I, TO and WS contributed to the trial conduct, data collection, data analysis and data interpretation. AC contributed to the provision of data, methodologies for trial sample analysis and data interpretation. The underlying data was verified by JR, QH, SD, TC, JC, JL and RW. The final version of this paper was reviewed and approved by all authors.
Data sharing statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php through the EngageZone site or via email to dataaccess@merck.com.
Declaration of interests
JAR, QH, SD, TC, JC, JL, JS, CH, XZ, RW, EL, PA, DG and SAS are employees of Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. JRS is an employee of Merck Sharpe & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA holding stocks alongside stocks in various pharmaceutical and biotechnology companies and service providers, has participated on various editorial boards and committees for Society for Industrial and Applied Mathematics and is a member and participant in committees for the International Society of Pharmacometrics. All other authors report no conflicts of interest.
Acknowledgements
The VSV vector was licensed from the Public Health Agency of Canada (“PHAC”) for use in the development of a COVID vaccine. The authors wish to thank the participants and the Merck & Co., Inc., Rahway, NJ, USA, IAVI, and clinical site staff involved in the conduct of the study. The authors additionally acknowledge Dr Robert Bass of Worldwide Clinical Trials for supporting clinical conduct, and Irene Yin-Ting Chang and Douglas D. Richardson of Merck & Co., Inc., Rahway, NJ, USA for supporting drug manufacture and providing the representative electron micrograph included in this publication. Professional medical writing and editorial assistance was provided by Megan Perkins of ApotheCom (London, UK) and was funded by Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104138.
Appendix. Supplementary materials
References
- 1.John Hopkins University & Medicine [Webpage]. COVID-19 dashboard by The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [Cited November 2021]. Available from: https://www.coronavirus.jhu.edu/map.html.
- 2.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richman DD. COVID-19 vaccines: implementation, limitations and opportunities. Global Health Med. 2021;3(1):1–5. doi: 10.35772/ghm.2021.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet (London, England) 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus ebola vaccine. N Engl J Med. 2017;376(4):330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization [Webpage]. 10th Ebola outbreak in the Democratic Republic of the Congo declared over; vigilance against flare-ups and support for survivors must continue. 2020 Jun 25 [cited November 2021]. Available from: https://www.who.int/news/item/25-06-2020-10th-ebola-outbreak-in-the-democratic-republic-of-the-congo-declared-over-vigilance-against-flare-ups-and-support-for-survivors-must-continue.
- 7.Yahalom-Ronen Y, Tamir H, Melamed S, et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11(1):6402. doi: 10.1038/s41467-020-20228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US National Library of Medicine [Webpage]. ClinicalTrials.gov: Dose Ranging Trial to Assess Safety and Immunogenicity of V590 (COVID-19 Vaccine) in Healthy Adults (V590-001) [Cited November 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04569786.
- 9.US Department of Health and Human Services FaDA. Toxicity Grading Scale for Healthy Adults and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007. [DOI] [PubMed]
- 10.Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Indian J Statis. 2000;Series B:134–148. [Google Scholar]
- 11.Philip V'kovski AK, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himangi R, Jayakar EJ, Whitt MA. Rhabdovirus assembly and budding. Virus Res. 2004;106(2):117–132. doi: 10.1016/j.virusres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waradon Sungnak NH, Bécavin C, Berg M, et al. Barnes & HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angela Huttner CC, Grillet S, Haks MC., et al. Ottenhoff and Claire-Anne Siegrist. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Science Transl Med. 2019;12(385):1701. doi: 10.1126/scitranslmed.aaj1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai C-Y, Strange DP, Wong TAS, Lehrer AT, Verma S. Ebola virus glycoprotein induces an innate immune response in vivo via TLR4. Front Microbiol. 2017;8:1571. doi: 10.3389/fmicb.2017.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruckwardt TJ, Morabito KM, Graham BS. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity. 2019;51(3):429–442. doi: 10.1016/j.immuni.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Dahlke C, Kasonta R, Lunemann S, et al. Dose-dependent t-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine. 2017;19:107–118. doi: 10.1016/j.ebiom.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A Largajolli, N Plok, B Kandala, et al. Cross-species translation of correlates of protection for COVID-19 vaccine candidates using quantitative tools. Poster (#1010) presented at ID week [virtual]; 2021 Sep 29–Oct 3.
- 20.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.