Abstract
The number of awake craniotomies is increasing because of its beneficial features. However, not enough information is available regarding the current status of awake craniotomy in Japan. To evaluate the current status of awake craniotomy in institutes, a nationwide questionnaire survey was conducted. From June to August 2019, we conducted a questionnaire survey on awake craniotomy in the neurosurgery department of 45 institutes that perform awake craniotomies in Japan. Responses were obtained from 39 institutes (response rate, 86.7%). The main methods of awake craniotomy were almost the same in all institutes. Twenty-six institutes (66.7%) had fewer than 10 awake craniotomies (low-volume institutes) per year, and 13 high-volume institutes (33.3%) performed more than 10 awake craniotomies annually. Some institutes experienced a relatively high frequency of adverse events. In 11 institutes (28.2%), the frequency of intraoperative seizures was more than 10%. An intraoperative seizure frequency of 1%-9%, 10%-29%, and over 30% was identified in 12 (92%), 0 (0%), and 1 (8%) of the high-volume institutes, which was significantly less than in 16 (62%), 10 (38%), and 0 (0%) of the low-volume institutes (p = 0.0059). The routine usage of preoperative antiepileptic drugs was not different between them, but the old type was used more often in the low-volume institutes (p = 0.0022). Taken together, the annual number of awake craniotomies was less than 10 in over two-thirds of the institutes. Fewer intraoperative seizures were reported in the high-volume institutes, which tend not to preoperatively use the old type of antiepileptic drugs.
Keywords: awake craniotomy, Japan, questionnaire investigation, intraoperative seizures, brain tumor
Introduction
Awake craniotomy has been introduced in many institutes because of its beneficial features that minimize the complication risk and enable proper resection of lesions while evaluating the symptoms of the patient. Typical indications include epilepsy, glioma, and cavernous hemangioma.1) In 2012, the Japanese Society of Awake Surgery published “Guidelines for Awake Craniotomy.”2) The guidelines consist of three parts: 1) surgical maneuvers for awake craniotomy, 2) anesthetic management for awake craniotomy, and 3) language assessment during awake craniotomy. These guidelines ensure the safety and precision of an awake craniotomy and describe the method.
In 2014, the Awake Craniotomy Institute Certification System was launched in Japan. The management of brain mapping/monitoring in awake patients with brain tumors is covered by health insurance. Awake craniotomy can contribute to improve the clinical outcome by maximizing safe resection of gliomas.3,4) Although awake craniotomy has been performed in many institutes, the current status of awake craniotomy remains unclear, especially regarding whether or not the same methods are used in all institutes. Understanding the overall situation for improving the safety of awake craniotomy is therefore beneficial. In the present study, we conducted a nationwide questionnaire survey to clarify the actual state of awake craniotomy in Japan.
Materials and Methods
From June to August 2019, we conducted a questionnaire survey on awake craniotomy in the neurosurgery department of 45 institutes that perform awake craniotomy in Japan. A request for cooperation as well as the URL of the questionnaire website, login ID, and password was mailed to each institute.
The questions included (1) the frequency of awake craniotomy, (2) the medical professional who confirms the intraoperative neurological symptoms, (3) the use and method of intraoperative electrophysiological monitoring in awake patients, (4) the type and frequency of adverse events experienced during awake craniotomy, and (5) perioperative seizure management. This questionnaire study, which was carried out as a project of the 17th meeting of the Japan Awake Surgery Society, was approved by the Steering Committee of the Japan Awake Surgery Society and was carried out in accordance with the principles of the Declaration of Helsinki. This study did not require patient consent because no individual information regarding patients was collected.
Statistical analyses were performed using the R project (version 3.3.0, www.r-project.org) software. Categorical variables were compared with the Fisher's exact test. A p-value of <0.05 was considered statistically significant.
Results
Responses were obtained from 39 of the 45 institutes to which we sent the cooperation request (response rate, 86.7%).
Current status of awake craniotomy in Japanese institutes
Table 1 shows the frequency of awake craniotomies, the type of medical professional who confirms the intraoperative neurological symptoms, the use of intraoperative electrophysiological monitoring in awake patients, and the survey results of the methods. Twenty-six institutes (66.7%) performed fewer than 10 awake craniotomies annually (low-volume institutes), whereas eight institutes (20.5%) performed 10-30 awake craniotomies, and five institutes (12.8%) performed more than 30 (high-volume institutes) annually.
Table 1.
The current status of awake craniotomy in Japanese institutes
| 1) Annual number of awake craniotomies (39 institutes responded to the survey) | ||
| Under 10 | 26 (66.7%) | |
| 10-30 | 8 (20.5%) | |
| Over 30 | 5 (12.8%) | |
| 2) Medical professionals that observe neurological findings (39 institutes responded to the survey; multiple answers possible) | ||
| Surgeons | 31 (79.5%) | |
| Speech therapists | 24 (61.5%) | |
| Physical therapists | 8 (20.5%) | |
| Occupational therapists | 2 (5.2%) | |
| Clinical engineers | 1 (2.6%) | |
| Nurses | 1 (2.6%) | |
| 3) Electrophysiological monitoring during awake craniotomy (39 institutes responded to the survey; multiple answers possible) | ||
| High-frequency (50-60 Hz) electrical stimulation mapping (cortex) | 37 (94.9%) | |
| High-frequency (50-60 Hz) electrical stimulation mapping (white matter) | 32 (82.1%) | |
| Electroencephalography | 32 (82.1%) | |
| Motor-evoked potentials | 29 (74.4%) | |
| Somatosensory-evoked potentials | 20 (51.3%) | |
| Cortico-cortical-evoked potentials | 2 (5.2%) | |
Physicians were involved in the confirmation of neurological symptoms in awake patients at 31 institutes (79.5%). Of these, medical doctors confirmed neurological symptoms at 12 institutes (30.8%), and a speech therapist confirmed the symptoms in eight institutes (20.5%).
Intraoperative electrophysiological monitoring in awake patients was also performed at all responding institutes. Functional mapping (cortex) of high-frequency (50-60 Hz) stimulation was performed at 37 institutes (94.9%, multiple answers allowed). In addition, functional mapping (white matter) of high-frequency (50-60 Hz) stimulation was performed at 32 institutes (82.1%, multiple answers allowed). Intraoperative electrophysiological monitoring with electroencephalography (EEG) was performed at 32 institutes (82.1%, multiple answers allowed). Furthermore, motor-evoked potentials were measured at 29 institutes (74.4%, multiple answers allowed), and somatosensory-evoked potentials were measured at 20 institutes (51.3%, multiple answers allowed). No apparent differences were seen between the low-volume and high-volume institutes regarding the method of local anesthesia, electrical stimulation, and EEG and electrocorticography monitoring during surgery.
Types and frequencies of adverse events experienced during awake craniotomy
Figure 1 shows the frequency of adverse events of seizures, pain, nausea, vomiting, respiratory obstruction, no awakening, and restlessness experienced during awake craniotomy. In 11 institutes (28.2%), the frequency of seizures was more than 10%. Patients experienced “pain” with a frequency of more than 10% in 24 institutes (61.5%), “nausea” of more than 10% in 12 institutes (30.8%), and “vomiting” of more than 10% in 5 institutes (12.8%). “Respiratory obstruction” was experienced at a frequency of 5%-9% in one institute (2.6%) and 1%-4% in eight institutes (20.5%). In no institutes did patients experience “Respiratory obstruction” at a frequency of more than 10%. Fourteen institutes (35.9%) experienced a frequency of more than 10% of “no awakening” in patients who could not perform task tests. Patients experienced “restlessness,” which requires re-sedation before completion of the task, at a frequency more than 10% at three institutes (7.7%). At all 39 institutes, if a “seizure” occurred during awake craniotomy, cold water was applied to the brain surface.
Fig. 1.
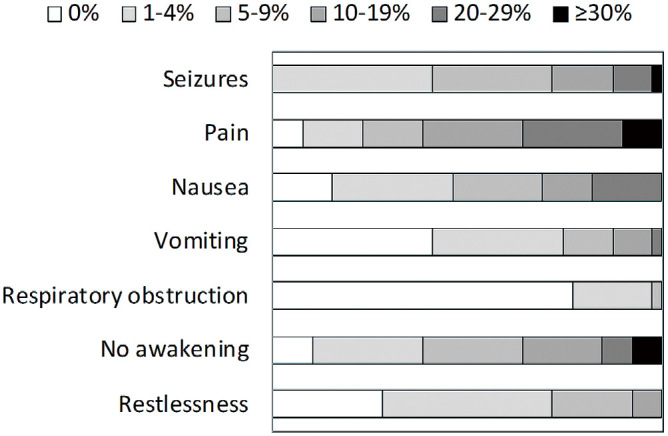
Frequency of adverse events (39 institutes responded to the survey).
Pain: painkillers were required; Nausea: antiemetics were required; Respiratory obstruction: tracheal intubation and laryngeal mask airway were required; No awakening: Patients could not perform the task; Restlessness: sedation before completion of the task was required.
Perioperative usage of antiepileptic drugs in patients with brain tumors
In patients with brain tumors, all patients were administered prophylactic antiepileptic drugs before awake craniotomy at 26 institutes (66.7%) (Table 2). Only patients with a history of epileptic seizures were administered antiepileptic drugs at 12 institutes (30.8%). Furthermore, levetiracetam, which is the most frequently used antiepileptic drug, was administered at 38 institutes (97.4%, multiple answers allowed) in patients with a history of epileptic seizures. At 24 institutes (61.5%, multiple answers allowed), levetiracetam was also used in patients with no history of epileptic seizures.
Table 2.
Perioperative usage of antiepileptic drugs in patients with brain tumors (39 institutes responded to the survey)
| Preoperative usage | Intraoperative usage | Postoperative usage | ||||
|---|---|---|---|---|---|---|
| No epileptic history | Epileptic history | No epileptic history | Epileptic history | No epileptic history | Epileptic history | |
| No usage of antiepileptic drugs | 13 (33.3%) | 1 (2.6%) | 17 (43.6%) | 13 (33.3%) | 19 (48.7%) | 0 (0%) |
| Usage of antiepileptic drugs | 26 (66.7%) | 38 (97.4%) | 22 (56.4%) | 26 (66.7%) | 20 (51.3%) | 39 (100%) |
| Breakdown list (multiple answers possible) | ||||||
| LEV | 24 | 38 | 7 (div) | 11 (div) | 19 | 37 |
| LCM | 3 | 14 | 0 | 1 (div) | 7 | 20 |
| PHT (fosPHT) | 3 | 5 | 16 (div) | 14 (div) | 3 | 3 |
| VPA | 3 | 4 | 0 | 0 | 2 | 4 |
| CBZ | 1 | 10 | 0 | 0 | 3 | 5 |
| LTG | 1 | 3 | 0 | 0 | 2 | 2 |
| PER | 1 | 6 | 0 | 0 | 3 | 11 |
LEV, Levetiracetam; LCM, Lacosamide; PHT, Phenytoin; fosPHT, Fosphenytoin; VPA, Sodium valproate; CBZ, Carbamazepine; LTG, Lamotrigine; PER, Perampanel hydrate; div, drip infusion in vein
Antiepileptic drugs were administered intravenously to all patients during awake craniotomy at 22 institutes (56.4%), whereas they were not used during awake craniotomy at 13 institutes (33.3%). At four institutes (10.2%), antiepileptic drugs were only used in patients with a history of epileptic seizures. Specifically, in patients with a history of epileptic seizures, fosphenytoin was used intraoperatively at 14 institutes (35.9%), whereas levetiracetam was used at 11 institutes (28.2%). Moreover, for patients without a history of epileptic seizures, fosphenytoin was used intraoperatively at 16 institutes (41.0%), whereas levetiracetam was used at 7 institutes (18.0%).
All patients were administered antiepileptic drugs after surgery at 20 institutes (51.3%). Antiepileptic drugs were administered only to patients with a history of epileptic seizures at 19 institutes (48.7%). As a postoperative prophylactic antiepileptic drug, levetiracetam was used at 37 institutes (94.9%) in patients with an epileptic history. Levetiracetam was also used at 19 institutes (48.7%) in patients without an epileptic history.
Intraoperative seizures and antiepileptic drugs in patients with brain tumors
Importantly, a frequency of intraoperative seizures, 1%-9%, 10%-29%, and over 30%, was identified in 12 (92%), 0 (0%), and 1 (8%) of the high-volume institutes, which was significantly less than that in 16 (62%), 10 (38%), and 0 (0%) of the low-volume institutes (p = 0.017) (Table 3). Although the routine usage of preoperative antiepileptic drugs was not different between the low-volume institutes (73%) and high-volume institutes (54%) (p = 0.29), the routine usage of intraoperative antiepileptic drugs was significantly more frequent in the low-volume institutes (69%) than in the high-volume institutes (31%) (p = 0.039) (Table 4).
Table 3.
Frequencies of intraoperative seizures based on the annual number of awake craniotomies
| Frequency of
seizures |
Awake craniotomies
<10 (26) |
Awake craniotomies
≥10 (13) |
|---|---|---|
| 1%-4% | 9 (35%) | 7 (54%) |
| 5%-9% | 7 (27%) | 5 (38%) |
| 10%-19% | 6 (23%) | 0 (0%) |
| 20%-29% | 4 (15%) | 0 (0%) |
| Over 30% | 0 (0%) | 1 (8%) |
Table 4.
Usage of antiepileptic drugs based on the annual number of awake craniotomies
| Preoperative AED | Awake craniotomies <10 (26) | Awake craniotomies ≥10 (13) | ||
|---|---|---|---|---|
| All patients | 19 (73%) | 7 (54%) | ||
| Selective | 6 (23%) | 6 (46%) | ||
| None | 1 (4%) | 0 (0%) | ||
| No epileptic history | Epileptic history | No epileptic history | Epileptic history | |
| LEV | 17 | 25 | 7 | 13 |
| LCM | 2 | 10 | 1 | 4 |
| LTG | 1 | 2 | 0 | 1 |
| PER | 0 | 4 | 1 | 2 |
| New type AEDs | 20 (74%) | 41 (69%) | 9 (100%) | 20 (95%) |
| PHT (fosPHT) | 3 | 5 | 0 | 0 |
| VPA | 3 | 4 | 0 | 0 |
| CBZ | 1 | 9 | 0 | 1 |
| Old type AEDs | 7 (26%) | 18 (31%) | 0 (0%) | 1 (5%) |
| Intraoperative AED | Awake craniotomies <10 (26) | Awake craniotomies ≥10 (13) | ||
| All patients | 18 (69%) | 4 (31%) | ||
| Selective | 2 (8%) | 2 (15%) | ||
| None | 6 (23%) | 7 (54%) | ||
| No epileptic history | Epileptic history | No epileptic history | Epileptic history | |
| LEV | 6 | 9 | 1 | 2 |
| LCM | 0 | 1 | 0 | 0 |
| LTG | 0 | 0 | 0 | 0 |
| PER | 0 | 0 | 0 | 0 |
| New type AEDs | 6 (32%) | 10 (50%) | 1 (25%) | 2 (33%) |
| PHT (fosPHT) | 13 | 10 | 3 | 4 |
| VPA | 0 | 0 | 0 | 0 |
| CBZ | 0 | 0 | 0 | 0 |
| Old type AEDs | 13 (68%) | 10 (50%) | 3 (75%) | 4 (67%) |
| Postoperative AED | Awake craniotomies <10 (26) | Awake craniotomies ≥10 (13) | ||
| All patients | 13 (50%) | 7 (54%) | ||
| Selective | 13 (50%) | 6 (46%) | ||
| None | 0 (0%) | 0 (0%) | ||
| No epileptic history | Epileptic history | No epileptic history | Epileptic history | |
| LEV | 13 | 25 | 6 | 12 |
| LCM | 4 | 15 | 3 | 5 |
| LTG | 2 | 2 | 0 | 0 |
| PER | 1 | 6 | 2 | 5 |
| New type AEDs | 20 (77%) | 48 (83%) | 11 (85%) | 22 (92%) |
| PHT (fosPHT) | 2 | 2 | 1 | 1 |
| VPA | 2 | 4 | 0 | 0 |
| CBZ | 2 | 4 | 1 | 1 |
| Old type AEDs | 6 (23%) | 10 (17%) | 2 (15%) | 2 (8%) |
AED: antiepileptic drug
In comparison between the new type of antiepileptic drugs (levetiracetam, lacosamide, lamotrigine, and perampanel hydrate) and old type (phenytoin, sodium valproate, and carbamazepine), the preoperative usage of the old type was more often in the low-volume institutes than in the high-volume institutes (26% vs. 0% for patients without a history of epilepsy, p = 0.16; 18% vs. 5% for patients with a history of epilepsy, p = 0.018; total, p = 0.0022) (Table 4). However, the intraoperative antiepileptic drugs were not different between them (50% vs. 67% for patients without a history of epilepsy, p > 0.999; 68% vs. 75% for patients with a history of epilepsy, p = 0.65; total; p = 0.72) (Table 4).
The routine usage of postoperative antiepileptic drugs was not different between the low-volume institutes (50%) and high-volume institutes (54%). As for preoperative antiepileptic drugs, the postoperative usage of the old type tends to be more often in the low-volume institutes (Table 4).
Discussion
A nationwide questionnaire survey was conducted to reveal the actual conditions of awake craniotomy in Japan. Twenty-six institutes (66%) performed fewer than 10 awake craniotomies annually, and 13 institutes (33%) performed more than 10 awake craniotomies annually. In Europe, the survey of awake diffuse low-grade gliomas (DLGG) surgery within the European Low-Grade Glioma Network centers showed that a median of 15 (range, 2-165) DLGG patients were annually operated on in each center.5) In the United Kingdom, approximately 33.5 awake craniotomies per year were performed in a single neurosurgical center.6) Awake craniotomy is carried out at a relatively large number of institutes in Japan, but the annual number of awake craniotomies in over two-thirds of the institutes was less than 10.
Although the basic methods of anesthesia, surgery, and intraoperative brain function examinations are unified, clearly some differences are present in the details. Most institutes involved surgeons to confirm the neurological symptoms during awake craniotomy. In addition, more than 25 of 39 institutes involved speech therapists or physical therapists for this. Some institutes involved only speech therapists or physical therapists for assessing neurological symptoms without involving surgeons. Building a good relationship of trust between the patient and the surgical staff when performing an awake craniotomy is most important7) and shows that the participation of medical professionals is reliable when performing an awake craniotomy. Moreover, electrophysiological monitoring was performed at all institutes, with the majority performing functional mapping (cortex or white matter) with high-frequency (50-60 Hz) stimulation. For this feature, no apparent difference among the institutes was observed. Electric cortical stimulation during awake craniotomy has been the gold standard for reversible cortical perturbation, which is valuable for functional cortical mapping and safe surgical resections.8-10) Modern functional mapping can support a more patient-specific approach.11)
Awake craniotomy is a well-defined procedure with a very low rate of complications.11) The guidelines, which were published in 2012, describe complications and their countermeasures.2) In the present study, we investigated the frequency of adverse events that required drug administration or that led to the inability to perform tasks, and each institution clearly experienced a relatively high frequency of adverse events. Some institutes reported that the frequency of seizures, pain, and no awakening was more than 20%, which is also an issue for proper awake craniotomy. In a cohort study of 609 awake craniotomies by Takami et al., intraoperative adverse events with impossible awake condition were identified in 21 patients, including emotional intolerance in 3 (0.5%), air embolism in 3 (0.5%), generalized seizures in 4 (0.7%), and unexpected subarachnoid hemorrhage in 1 (0.2%).12) Preoperative cognitive decline, dysphasia, and low performance status (poor Karnofsky Performance Status score) were risk factors for emotional intolerance. Intraoperative adverse events tended to cause inpatient admission, longer hospital stay, and difficult discharge to home.12) Kuribara et al. reported that the inappropriately awake conditions were identified in 26 of 136 patients with awake craniotomy (19%) because of insufficient wakefulness in 15 patients, restless state in 6, and intraoperative seizures in 5. The lack of preoperative seizures and left-sided lesions were identified as risk factors for inappropriately awake condition.13) On the basis of these results, preoperative conditions are important to select patients for awake craniotomy.
In particular, the frequency of intraoperative seizures during awake craniotomy has been reported to be approximately 0%-24%, although it depends on the target disease and its definition.12-17) Afterdischarges, which are defined as repetitive epileptiform discharges provoked by a stimulus,18) are also identified on electrocorticography.19) In recommendations for intraoperative seizures in the guidelines, surgical operations, especially electrical stimulation, were discontinued, and cold water was applied to the brain surface at the site of the seizure. Boetto et al. reported that all intraoperative seizures identified in 13 (3.4%) of 374 patients were partial seizures, which quickly resolved by irrigation with cold Ringer lactate.20)
In the analysis of 477 patients with awake craniotomy by Nossek et al., intraoperative seizures were associated with younger patients, frontal lobe involvement, and a history of seizures.21) In the other analysis of the same institute, history of seizures and treatment with multiple antiepileptic drugs were related to intraoperative seizures.22) Abecassis et al. identified intraoperative seizures in 35 patients (15%) and afterdischarges in 40 patients (18%) in 229 patients undergoing awake craniotomy, which were commonly observed during intraoperative stimulation for brain mapping.19) They found that patients (23%) with intraoperative seizures had afterdischarges prior to their seizure, although intraoperative seizures and afterdischarges were not statistically associated. Stimulation-induced seizures happen on lower stimulation intensities than afterdischarge thresholds detected by concurrent electrocorticography.8,18) Zanello et al. reported that intraoperative seizures occurred in 3.5% of patients during cortical stimulation and no predictor of intraoperative seizures was identified in 202 patients with diffuse glioma.16) Failures of awake craniotomy were associated with a lower incidence of gross-total resection and increased postoperative morbidity.22) The review of literature in 2020 indicated that stimulation-related intraoperative seizures do not always cause permanent and severe postoperative deficits, but they can affect the patient's perioperative status and the duration of hospitalization.17)
Regarding antiepileptic drugs, the guideline states,2) “in cases where awake craniotomy is planned, it is desirable to start administration of anticonvulsants in advance and maintain the effective blood concentration if there is time to surgery.” However, a previous report suggested that even if the blood concentration of antiepileptic drugs is within the effective range, no difference is present in the preventive effect of intraoperative convulsive seizures, and intraoperative seizures depend on the conditions of electrical stimulation.23) In this study, the low-volume institutes experienced more frequent intraoperative seizures than the high-volume institutes. The methods of local anesthesia, electrical stimulation, cold water, and EEG and electrocorticography monitoring during surgery were almost the same between the low-volume and high-volume institutes. The routine usage of intraoperative antiepileptic drugs was significantly more frequent in the low-volume institutes. However, as the preoperative antiepileptic drugs, the old type tends not to be used in the high-volume institutes. Recently, some molecular aberrations associated with drug-resistant epilepsy in gliomas have been reported.24) Therefore, these findings are needed to evaluate in a future large cohort study.
This study has limitations. It was an analysis of a questionnaire survey with limited numbers of questions. Therefore, accurate investigation of the relationship among the patient's characteristics, methods, medications, and adverse events was difficult.
This questionnaire survey revealed that the frequency of adverse events such as seizures, pain, and no awakening is different from 0% to over 30% among the institutes. This fact suggests that it is necessary to identify the technical and operational causes of adverse events, which should be reflected in training courses and guidelines in professional societies to realize the equalization of the best management in awake craniotomy.
Conclusions
Considering the results of the present questionnaire study, the main methods used during awake craniotomy are the same, but a few differences were noted among the institutes, including functional evaluation methods, antiepileptic drugs, local anesthesia, and postwake management. The annual number of awake craniotomies was less than 10 in over two-thirds of the institutes. Some institutes experienced a relatively high frequency of adverse events. Fewer intraoperative seizures were reported in the high-volume institutes. Although its reason was not clear in the present survey, the high-volume institutes tend not to preoperatively use the old type of antiepileptic drugs. These clinical questions are needed to evaluate in a future large cohort study.
List of Abbreviations
EEG: Electroencephalography
MEP: Motor-evoked potential
SEP: Somatosensory-evoked potential
Ethics Approval and Consent to Participate
This questionnaire plan, which was carried out as a project of the 17th meeting of the Japan Awake Surgery Society, was approved by the Steering Committee of the Japan Awake Surgery Society.
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest Disclosure
All authors have no conflict of interest for this study.
Acknowledgments
We would like to express our deep gratitude to the following neurosurgery institutes for their cooperation in the questionnaire survey.
Asahikawa Medical University Hospital, Iwate Medical University Hospital, Ube-kohsan Central Hospital, Osaka Medical College Hospital, Osaka City General Hospital, Osaka City University Hospital, Osaka University Hospital, Ohnishi Neurological Center, Kagawa University Hospital, Kanazawa University Hospital, Kitazato University Hospital, Gifu University Hospital, Kyushu University Hospital, Kyorin University Hospital, Kindai University Hospital, Kobe University Hospital, National Cancer Center Hospital, Saga University Hospital, Saitama Medical University International Medical Center, Sapporo Medical University Hospital, Shiga University of Medical Science Hospital, Juntendo University Hospital, Shinshu University Hospital, Kitano Hospital, The Tazuke Kofukai Medical Research Institute, University of Tsukuba Hospital, Tokyo Women's Medical University Hospital, The University of Tokyo Hospital, Tohoku University Hospital, Nagoya University Hospital, Nara Medical University Hospital, Niigata University Medical & Dental Hospital, Nippon Medical School Hospital, Nihon University Itabashi Hospital, Hiroshima University Hospital, Fukushima Medical University Hospital, University of Miyazaki Hospital, Yamagata University Hospital, Yokohama City University Hospital.
References
- 1). Muragaki Y, Maruyama T, Iseki H, Takakura K, Hori T: Functional brain mapping and electrophysiological monitoring during awake craniotomy for intra axial brain lesions. Jpn J Neurosurg (Tokyo) 17: 38-47, 2008 [Google Scholar]
- 2). Kayama T, conference GcotJas: The guidelines for awake craniotomy guidelines committee of the Japan awake surgery conference. Neurol Med Chir (Tokyo) 52: 119-141, 2012 [DOI] [PubMed] [Google Scholar]
- 3). Kumabe T, Sato K, Iwasaki M, et al. : Summary of 15 years experience of awake surgeries for neuroepithelial tumors in Tohoku university. Neurol Med Chir (Tokyo) 53: 455-466, 2013 [DOI] [PubMed] [Google Scholar]
- 4). Otani N, Bjeljac M, Muroi C, et al. : Awake surgery for glioma resection in eloquent areas--Zurich's experience and review. Neurol Med Chir (Tokyo) 45: 501-510; discussion 510-501, 2005 [DOI] [PubMed] [Google Scholar]
- 5). Arzoine J, Leve C, Perez-Hick A, et al. : Anesthesia management for low-grade glioma awake surgery: a European Low-Grade Glioma Network survey. Acta Neurochir 162: 1701-1707, 2020 [DOI] [PubMed] [Google Scholar]
- 6). Halla S, Kabwamaa S, Sadeka AR, et al. : Awake craniotomy for tumour resection: The safety and feasibility of a simple technique. Interdiscip Neurosurg 24: 101070, 2021 [Google Scholar]
- 7). Whittle IR, Midgley S, Georges H, Pringle AM, Taylor R: Patient perceptions of “awake” brain tumour surgery. Acta Neurochir 147: 275-277, 2005 [DOI] [PubMed] [Google Scholar]
- 8). Ibayashi K, Cardenas AR, Oya H, et al. : Focal cortical surface cooling is a novel and safe method for intraoperative functional brain mapping. World Neurosurg 147: e118-e129, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Kinoshita M, Miyashita K, Tsutsui T, Furuta T, Nakada M: Critical neural networks in awake surgery for gliomas. Neurol Med Chir (Tokyo) 56: 674-686, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Nakajima R, Nakada M, Miyashita K, et al. : Intraoperative motor symptoms during brain tumor resection in the supplementary motor area (SMA) without positive mapping during awake surgery. Neurol Med Chir (Tokyo) 55: 442-450, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Spena G, Panciani PP, Fontanella MM: Resection of supratentorial gliomas: the need to merge microsurgical technical cornerstones with modern functional mapping concepts. An overview. Neurosurg Rev 38: 59-70; discussion 70, 2015 [DOI] [PubMed] [Google Scholar]
- 12). Takami H, Khoshnood N, Bernstein M: Preoperative factors associated with adverse events during awake craniotomy: analysis of 609 consecutive cases. J Neurosurg 134: 1631-1639, 2020 [DOI] [PubMed] [Google Scholar]
- 13). Kuribara T, Akiyama Y, Mikami T, et al. : Preoperative prediction of communication difficulties during awake craniotomy in glioma patients: a retrospective evaluation of 136 cases at a single institution. Neurol Med Chir (Tokyo) 61: 21-32, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Skucas AP, Artru AA: Anesthetic complications of awake craniotomies for epilepsy surgery. Anesth Analg 102: 882-887, 2006 [DOI] [PubMed] [Google Scholar]
- 15). Conte V, Baratta P, Tomaselli P, Songa V, Magni L, Stocchetti N: Awake neurosurgery: an update. Minerva Anestesiol 74: 289-292, 2008 [PubMed] [Google Scholar]
- 16). Zanello M, Roux A, Zah-Bi G, et al. : Predictors of early postoperative epileptic seizures after awake surgery in supratentorial diffuse gliomas. J Neurosurg 134: 683-692, 2020 [DOI] [PubMed] [Google Scholar]
- 17). Roca E, Pallud J, Guerrini F, Panciani PP, Fontanella M, Spena G: Stimulation-related intraoperative seizures during awake surgery: a review of available evidences. Neurosurg Rev 43: 87-93, 2020 [DOI] [PubMed] [Google Scholar]
- 18). Blume WT, Jones DC, Pathak P: Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol 115: 982-989, 2004 [DOI] [PubMed] [Google Scholar]
- 19). Abecassis ZA, Ayer AB, Templer JW, Yerneni K, Murthy NK, Tate MC: Analysis of risk factors and clinical sequelae of direct electrical cortical stimulation-induced seizures and afterdischarges in patients undergoing awake mapping. J Neurosurg 134: 1610-1617, 2021 [DOI] [PubMed] [Google Scholar]
- 20). Boetto J, Bertram L, Moulinie G, Herbet G, Moritz-Gasser S, Duffau H: Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: electrocorticography is not mandatory. World Neurosurg 84: 1838-1844, 2015 [DOI] [PubMed] [Google Scholar]
- 21). Nossek E, Matot I, Shahar T, et al. : Intraoperative seizures during awake craniotomy: incidence and consequences: analysis of 477 patients. Neurosurgery 73: 135-140; discussion 140, 2013 [DOI] [PubMed] [Google Scholar]
- 22). Nossek E, Matot I, Shahar T, et al. : Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J Neurosurg 118: 243-249, 2013 [DOI] [PubMed] [Google Scholar]
- 23). Hervey-Jumper SL, Li J, Lau D, et al. : Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg 123: 325-339, 2015 [DOI] [PubMed] [Google Scholar]
- 24). Suzuki H, Mikuni N, Sugita S, et al. : Molecular aberrations associated with seizure control in diffuse astrocytic and oligodendroglial tumors. Neurol Med Chir (Tokyo) 60: 147-155, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.


