Abstract
Background and Objectives
People with Parkinson disease (PD) commonly experience cognitive decline, which may relate to increased α-synuclein, tau, and β-amyloid accumulation. This study examines whether the different proteins predict longitudinal cognitive decline in PD.
Methods
All participants (PD n = 152, controls n = 52) were part of a longitudinal study and completed a lumbar puncture for CSF protein analysis (α-synuclein, total tau [tau], and β-amyloid42 [β-amyloid]), a β-amyloid PET scan, and/or provided a blood sample for APOE genotype (ε4+, ε4−), which is a risk factor for β-amyloid accumulation. Participants also had comprehensive, longitudinal clinical assessments of overall cognitive function and dementia status, as well as cognitive testing of attention, language, memory, and visuospatial and executive function. We used hierarchical linear growth models to examine whether the different protein metrics predict cognitive change and multivariate Cox proportional hazard models to predict time to dementia conversion. Akaike information criterion was used to compare models for best fit.
Results
Baseline measures of CSF β-amyloid predicted decline for memory (p = 0.04) and overall cognitive function (p = 0.01). APOE genotypes showed a significant group (ε4+, ε4−) effect such that ε4+ individuals declined faster than ε4− individuals in visuospatial function (p = 0.03). Baseline β-amyloid PET significantly predicted decline in all cognitive measures (all p ≤ 0.004). Neither baseline CSF α-synuclein nor tau predicted cognitive decline. All 3 β-amyloid-–related metrics (CSF, PET, APOE) also predicted time to dementia. Models with β-amyloid PET as a predictor fit the data the best.
Discussion
Presence or risk of β-amyloid accumulation consistently predicted cognitive decline and time to dementia in PD. This suggests that β-amyloid has high potential as a prognostic indicator and biomarker for cognitive changes in PD.
Parkinson disease (PD) is a neurodegenerative disease characterized by the accumulation of α-synuclein Lewy bodies throughout the brain, affecting cognitive function.1,2 In addition, some research suggests that tau protein, a component of tangles within neurons related to the onset of dementia in Alzheimer disease (AD),3 may play a role in cognitive decline in PD.4 However, most people with PD do not have significant increases in tau accumulation in the brain.5-7 Instead, research more consistently indicates that β-amyloid, a protein that contributes to plaque formation in AD,3 relates to cognitive decline in PD.8,9 Altogether, 1 or more of these 3 proteins may be useful prognostic biomarkers for understanding and predicting cognitive decline in PD.
Prior studies mainly investigated these 3 proteins separately. People with PD have lower levels of total α-synuclein in CSF levels than controls5,10; however, CSF total a-synuclein levels may not relate to disease progression.2 CSF tau in PD may4,11,12 or may not5-7 play an important role in PD. Last, β-amyloid measures in CSF1,13 or with PET14,15 relate to cognitive function in PD. Not surprisingly, the presence of an APOE ε4 allele, a risk factor for β-amyloid accumulation, also predicts cognitive performance in PD.16
Only a few studies investigated 1 or more of these proteins or used different approaches to explore them.17-19 Akhtar et al.17 reported that higher β-amyloid accumulation, along with the presence of APOE ε4 allele, correlates with verbal memory performance. Using PET, Buongiorno et al.18 showed that higher β-amyloid binding relates to cognitive decline, dementia, and reduced levels of β-amyloid in CSF. Shahid et al.19 found that individuals with PD with low β-amyloid in CSF and the presence of an APOE ε4 allele have a higher rate of cognitive decline. To the best of our knowledge, no study applied all 3 methods (i.e., CSF, PET, and APOE genotype) to investigate the role of β-amyloid.
The relationship of any of the 3 proteins to longitudinal cognitive decline remains unclear,16,20 as is how the different methods compare to one another for predicting cognitive change. Therefore, this study, using multiple measurement approaches, evaluates the relationships of α-synuclein, tau, and β-amyloid to longitudinal cognitive decline in people with PD.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Washington University in St. Louis Human Research Protection Office approved this study, and all participants provided written informed consent.
Participants
All participants were part of 2 larger longitudinal studies21,22 examining PD progression (total PD n = 337; total controls n = 85). For inclusion in the parent studies, all participants needed to be at least 50 years old, to have a minimum of 12 years of education, and to agree to brain donation. Participants with PD had a clinical diagnosis of PD based on the modified United Kingdom PD Society Brain Bank clinical diagnostic criteria23 with clear motor response to levodopa. In addition, participants in the parent studies could not have (1) other neurologic diagnoses, (2) head injury with loss of consciousness >5 minutes or neurologic sequelae, or (3) schizophrenia or bipolar disorder. In addition, controls needed to have normal cognition, defined as receiving a Clinical Dementia Rating (CDR)24 global score of 0, and no first-degree family history of PD.
For inclusion in the present study, participants needed (1) protein biomarker data (CSF, β-amyloid PET, and/or APOE genotyping), (2) to be without dementia at the baseline visit (CDR global score <1), and (3) at least 1 subsequent clinical evaluation with cognitive testing after their baseline protein biomarker collection. In addition, controls needed a mean cortical binding potential (MCBP) ≤0.18 to reduce preclinical AD risk.25 eFigure 1 (links.lww.com/WNL/B939) gives for more details on participant inclusion and exclusion for these analyses.
Data Collection and Processing
Clinical and Cognitive Assessments
All participants completed longitudinal study visits, which included completion of the CDR clinical assessment and comprehensive cognitive testing. Participants with PD completed cognitive testing in the “off” medication state, defined as overnight withdrawal (>8 hours) from PD medications, to reduce possible medication confounds26; therefore, tests were chosen to minimize motor demands. For cognitive evaluations, participants completed multiple tests for each cognitive domain: attention (Digit Span,27 Digit Symbol27), memory (California Verbal Learning Test–II, short-form28; Logical Memory29), language (Boston Naming Test30), visuospatial function (Judgement of Line Orientation,31 Spatial Relations Test32), and executive function (Trail Making Test,31 Verbal Fluency- Switching,33 Color-Word Interference33). The CDR sum of boxes (CDR-SB) score was also collected to measure overall cognitive function.
For participants who developed severe cognitive impairment and could not complete the entire cognitive battery (e.g., had a CDR score ≥1, failed the practice portion, or were unable to complete the task), missing test scores were imputed as the lowest (worst) score possible. All other missing scores remained blank (i.e., missing). For the longitudinal analyses, number of cognitive testing sessions (exposures) was used to control for potential practice effects. If participants completed testing on medication (“on”), took medication during the testing session, or were unable to complete the testing session for any reason, the cognitive test data from that session were omitted from the longitudinal analyses but included in the number of testing exposures. Thus, some study visit evaluations included only the CDR clinical assessment without formal cognitive testing (Table 1).
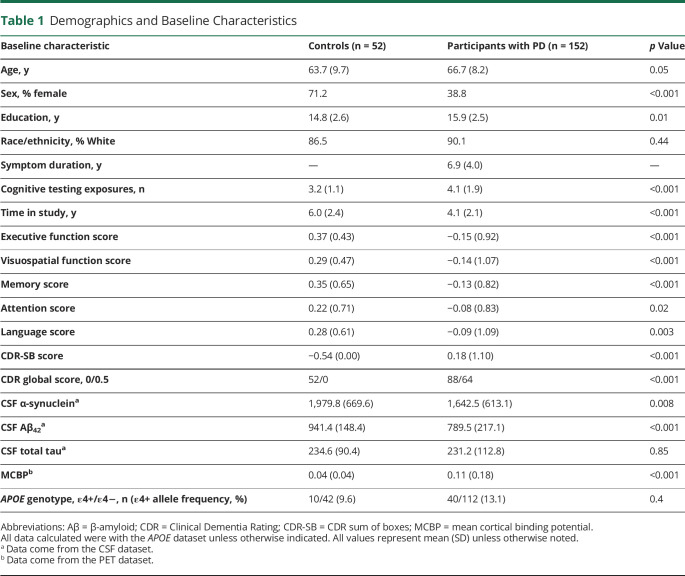
Table 1.
Demographics and Baseline Characteristics
CSF Collection and Processing
Procedures for CSF collection and processing are described in detail by Buddhala et al.5 In brief, participants underwent a lumbar puncture to collect CSF. Samples were collected between 1 and 2 pm at the study visit. A 22-gauge atraumatic Sprotte needle was used to collect ≈25 mL fluid. CSF samples were pulse vortexed and then centrifuged at 2,000g for 15 minutes at 4°C. After removal of all but the last 500 μL supernatant, 0.5- and 1-mL CSF aliquots were prepared and frozen at −80°C. CSF collection and freezing took ≈30 minutes.
To quantify levels of CSF α-synuclein, β-amyloid42 (β-amyloid), and total tau (tau), sandwich ELISAs were used.6 The Covance α-syn ELISA kit (Covance, Inc, Indianapolis, IN) measured α-synuclein. A CSF dilution of 1:4 provided the optimal CSF signal, such that all values fell within the range of the standard curve. The Innotest ELISA kit (Fujirebio US, Inc, Seguin, TX) quantified β-amyloid and tau. Hemoglobin levels in CSF were measured with the Human Hemoglobin ELISA Quantitation Set (Bethyl Laboratories, Inc, Montgomery, TX) to assess the potential contribution of red blood cell a-synuclein to CSF measures. Because no correlation was observed between hemoglobin and a-synuclein for the a-synuclein assay, no samples were excluded on the basis of hemoglobin levels. Each ELISA plate contained at least 8 CSF samples run on previous ELISA plates to assess interassay variance. The interassay coefficient of variation was <15% for all CSF assay data included in this analysis.
APOE Genotyping
Participants provided blood samples for APOE genotyping. Specifically, TaqMan assays (Applied Biosystems, Waltham, MA) for rs429358 (ABI#C_3084793_20) and rs7412 (ABI#C_904973_10) were used, as we previously published.34 ABI Sequence Detection software was used to detect the 6 combinations of APOE ε2, ε3, and ε4.
PET Scan and Image Processing
Dynamic [11C] Pittsburgh compound B (PiB) PET images were acquired with a Siemens (Munich, Germany) 962 HR+ ECAT scanner to measure fibrillar β-amyloid. Scans were processed as described previously.35 Sixty-minute dynamic scans were reconstructed with 3-dimensional filtered-back projection with a ramp filter to a voxel size of 2.1 × 2.1 × 2.4 mm with an approximate full-width half-maximum of 5 mm. Images were aligned to T1-weighted structural MRI scans with vector-gradient registration.36 Segmentation was conducted with FreeSurfer5.337 for region-based analyses. Reference-region–based Logan binding potentials were calculated from a model window of 30 to 60 minutes of PiB injection with the cerebellar gray matter used as the reference region. To account for partial volume effects, region-spread function partial volume correction was applied.35 Mean cortical binding potentials used regions defined by Su et al.35
Datasets
We created 3 datasets for each method (CSF, APOE, and PET) in which each participant's baseline (i.e., first visit) included data collection for the respective protein metric. We also compiled a fourth dataset (all protein), which included participants with all 3 protein metrics, specifically with the PET and CSF data collected at the same visit. Thus, for the CSF, PET, and all-protein datasets, the baseline visit was defined as the visit at which the lumbar puncture, PET scan, or both were performed. For the APOE dataset, the baseline visit was defined as the participant's first study visit with complete cognitive data. For each dataset, symptom duration equaled time from first motor symptom to baseline visit. Age in each dataset equaled age at baseline visit. To account for practice effects in cognitive testing, we calculated the number of times a participant was exposed to cognitive testing; thus, the number of cognitive testing exposures remained constant across all protein biomarker datasets. Last, for the APOE dataset, participants carrying at least 1 ε4 allele were categorized as ε4+, and participants without an ε4 allele were categorized as ε4−.
Data Standardizing
For each dataset, age, education, symptom duration, number of cognitive testing exposures, and raw values for each cognitive test were standardized to the mean and SD of the baseline visit. Next, cognitive domain scores (attention, language, memory, visuospatial function, and executive function) and CDR-SB score were computed for each participant for each visit by averaging the standardized test scores for each domain. Thus, scores represent how individuals change over time relative to baseline performance.
Statistical Analyses
Longitudinal Cognitive Change
We used hierarchical linear growth models (HLMs) to examine longitudinal cognitive changes in our participants. These statistical models account for individual and group variance within the models and predict an individual's cognitive performance over time. For our analyses, a participant's intercept and time-slope were specified as random effects for each model, meaning that each participant's intercept and time-slope were specific to their data and thus varied across individuals. HLMs do not require participants to have the same number of data points (visits) or the same length of time between visits. This allows greater flexibility with participant inclusion and provides a more complete picture of between-group differences. All HLMs were run with the lmer function in the lme4 R package (R Foundation for Statistical Computing, Vienna, Austria).38 The time between visits was calculated as the time from a participant's baseline visit within a dataset with the lubridate package in R. Covariates for all models included sex, symptom duration, education, number of cognitive testing session exposures, and age at baseline visit. After running of the growth models, slopes were extracted with ggeffects to assess the magnitude of change over time for each cognitive domain and CDR-SB score. We retested each significant model using the all-protein dataset to assess which growth model best predicts cognitive decline (i.e., all participants completed β-amyloid PET and lumbar punctures at the same visit). We ran growth models with only covariates to reduce the number of predictors to identify significant covariates for each cognitive measure. Baseline age, education, and sex were significant (α < 0.05) for executive function, memory, and attention. Only age and education were significant for visuospatial function and CDR-SB score. We then compared the Akaike information criterion (AIC) for each significant model with its specific, significant covariates. An AIC difference of ≥239 was used as a threshold criterion for indicating the best-fitting model, and a χ2 test compared models for statistically significant differences(α < 0.05).
Conversion to Dementia
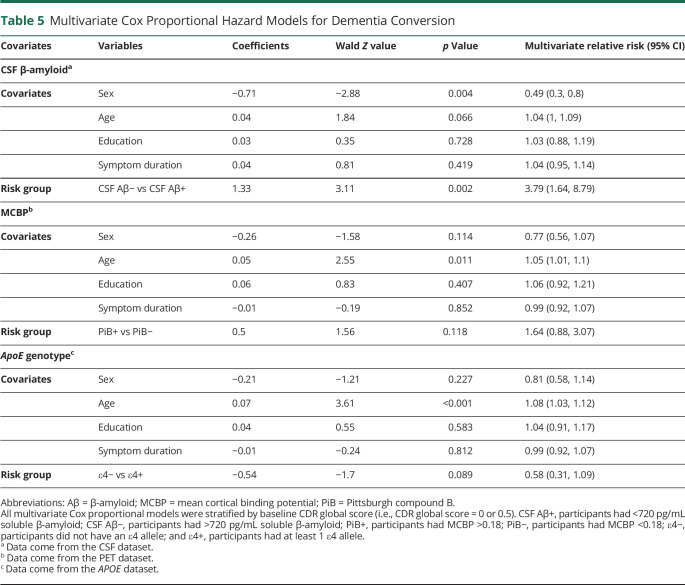
We used multivariate Cox proportional hazards regression models to determine whether different protein metrics predict the rate of dementia conversion in the PD group. All regressions were run with the SURVIVAL40 and SURVMINER41 packages in R. Censoring was based on the last date of contact. Events were defined as the date when a participant first received a CDR global score ≥1. Survival time was calculated as time since the baseline clinical assessment to the most recent CDR. Covariates included age, sex, symptom duration, and education, and all models were stratified by baseline CDR global score. Participants with PD were divided into high- and low-risk groups for converting to dementia according to protein levels or genetic risk (APOE) (see eTables 1–3, links.lww.com/WNL/B940 for demographic information based on risk group). For CSF β-amyloid, high-risk (CSF Aβ+) participants had CSF β-amyloid <720 pg/mL, the lowest tertile, and low-risk (CSF Aβ−) participants had CSF β-amyloid >720 pg/mL. For APOE, the ε4+ group was considered high risk, and the ε4− group was considered low risk.16 For MCBP, participants with MCBP >0.1825 were considered high risk (PiB+), and individuals with MCBP <0.18 were considered low risk (PiB−).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Baseline characteristics for PD and control groups can be found in Table 1. Controls and participants with PD differed in all baseline characteristics except CSF total tau and APOE allele ε4+ status.
Longitudinal Changes
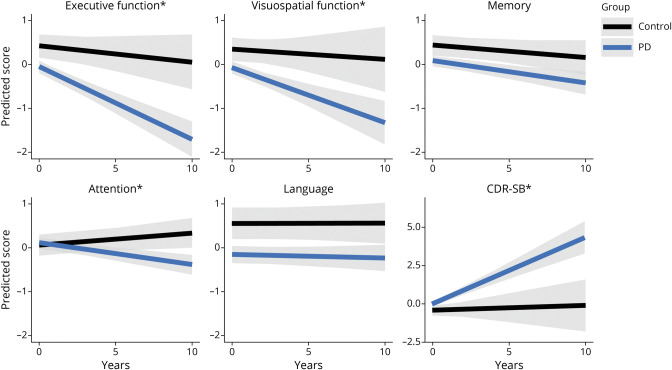
First, growth models for each cognitive domain and overall cognitive function (CDR-SB group) showed group (PD vs control) differences in change over time (Figure 1). Compared to controls, participants with PD demonstrated significant decline in executive function (p < 0.001), visuospatial function (p = 0.03), attention (p < 0.001), and CDR-SB score (p < 0.001), showing a well-differentiated sample of controls from participants with PD. To better understand cognitive dysfunction changes in PD, controls were removed from further analyses. In addition, the language domain was removed from further analyses because this domain did not change over time. The memory domain was retained because of its prominent role in dementia.
Figure 1. PD vs Controls.
Group (Parkinson disease [PD] n = 152, controls n = 52) changes in each cognitive domain and Clinical Dementia Rating Scale sum of boxes (CDR-SB) scores are depicted using the APOE genotype dataset to best depict change over time from the beginning of the study. *Significant difference in change over time between participants with PD and controls.
Individual Protein Measures
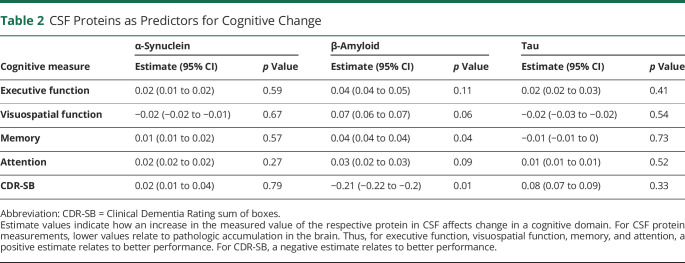
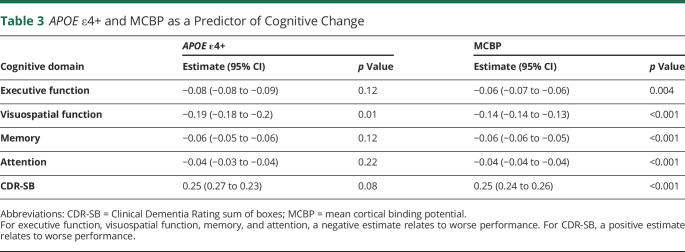
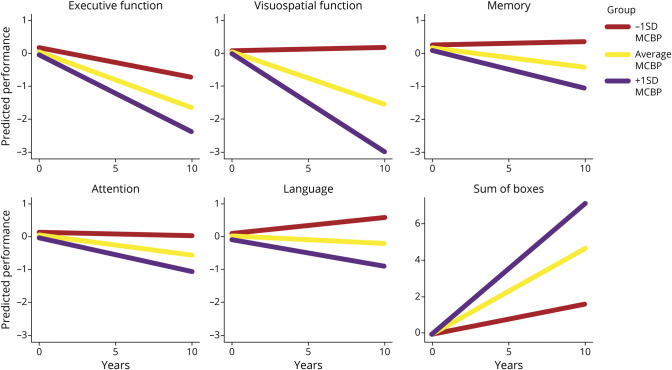
Overall, CSF β-amyloid measures consistently predicted cognitive decline, whereas CSF α-synuclein and tau did not (all p > 0.15). Specifically, baseline CSF β-amyloid related to the rate of decline for memory (p = 0.04) and CDR-SB score (p = 0.01) (Table 2). For APOE genotypes, growth models showed that the ε4+ group declined faster than the ε4− group in visuospatial function (p = 0.01) (Table 3). Last, growth models with β-amyloid PET (i.e., MCBP) showed that baseline MCBP relates to all cognitive measures (all p ≤ 0.004) (Table 3 and Figure 2) such that a higher MCBP predicted a faster rate of decline across all measures.
Table 2.
CSF Proteins as Predictors for Cognitive Change
Table 3.
APOE ε4+ and MCBP as a Predictor of Cognitive Change
Figure 2. MCBP Predicts Cognitive Decline.
Changes in cognitive performance as predicted by mean cortical binding potential (MCBP) are shown. For each graph, predicted change for the average MCBP is depicted, as well as change for ±1 SD from the average MCBP.
β-Amyloid Comparison
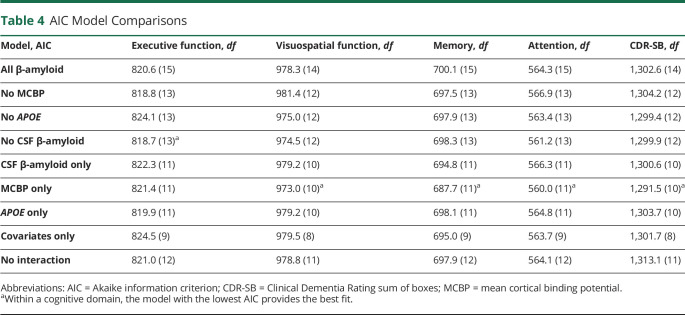
The individual models reveal that the 3 metrics of presence or risk for β-amyloid (CSF β-amyloid, APOE genotype, and MCBP) consistently predict cognitive decline. To determine the relative contribution of each metric to predicting cognitive decline, we built growth models with the significant covariates and interaction terms between time and CSF β-amyloid, APOE genotype, and MCBP individually for each cognitive domain (full model). Next, we ran 3 models, each model containing only 2 of the measurement interactions. Similarly, we ran models with a single interaction. All growth models for a cognitive domain were then compared to the full model (Table 4). This permits the identification of the best metric(s) for predicting cognitive decline in PD.
Table 4.
AIC Model Comparisons
For executive function, the model including APOE and MCBP had the lowest AIC compared to the full model. However, the AIC difference was <2, so it did not reach the threshold criterion to be considered the best model. The MCBP model had the greatest AIC difference from the full model (>4) for visuospatial function, memory, attention, and CDR-SB score (Table 4), meeting the criterion for selection as the best model. In addition, this AIC difference reached statistical significance for both memory and CDR-SB score (p < 0.05) according to the χ2 test.
Conversion to Dementia
Last, we examined whether the different protein metrics predict conversion to dementia. A greater proportion of the CSF Aβ+ risk group converted to dementia compared to the CSF Aβ− risk group (CSF Aβ+: 21 of 45 [46.7%]; CSF Aβ−: 15 of 89 [16.9%]; χ2 = 12.0, p < 0.001); a greater proportion of APOE ε4+ individuals converted to dementia compared to APOE ε4− individuals (ε4+: 18 of 36 [50.0%]; ε4−: 27 of 112 [24.1%]; χ2 = 7.5, p = 0.006); and a greater proportion of PiB+ individuals converted to dementia compared to PiB− individuals (PiB+: 23 of 43 [53.5%]; PiB−: 26 of 112 [23.2%]; χ2 = 11.8, p < 0.001).
Multivariate Cox proportional hazard regression revealed a faster dementia conversion rate (relative risk [RR] 3.8, p = 0.001) for the CSF Aβ+ group (Table 5), but multivariate Cox proportional hazard regression showed no significant difference in risk between the ε4+ and ε4− groups (RR 0.54, p = 0.09) or the PiB+ and PiB− groups (RR 0.50, p = 0.11). We also ran the multivariate Cox proportional hazard regression with CSF β-amyloid and MCBP as continuous variables. This revealed higher risk of dementia for CSF β-amyloid (RR −3.6, p < 0.001, indicating that higher values of CSF β-amyloid reduced the risk of dementia conversion) and for MCBP (RR 2.16, p = 0.03, indicating that higher values of MCBP increased the risk of dementia conversion).
Table 5.
Multivariate Cox Proportional Hazard Models for Dementia Conversion
Discussion
This study examines the relationships between different proteins and longitudinal cognitive decline in PD, including α-synuclein, β-amyloid, and tau. Through multiple measurement modalities, the presence and risk of β-amyloid consistently predicted longitudinal cognitive decline. In addition, models with the direct measure of β-amyloid aggregation in the brain (MCBP) were the most parsimonious. Last, the results indicate that both CSF and PET measures of β-amyloid predict conversion to dementia, highlighting the potential role of β-amyloid as a prognostic biomarker of PD dementia.
Our results illustrate that β-amyloid provides the greatest utility and potential for understanding and predicting cognitive change in PD. While prior research investigated β-amyloid in CSF,1,13 PET,14,15 and APOE genotypes16 separately, the present study examines each metric separately and compares across modalities, at least for β-amyloid. This comparison established that for visuospatial function, memory, attention, and overall cognitive function (CDR-SB score), the growth model with only β-amyloid PET (MCBP) as a predictor was the best fit for the data (i.e., had the lowest AIC), meaning that it maintained the most information from the data compared to the other models. In the case of memory and overall cognitive function, the model with only β-amyloid PET (MCBP) as a predictor reached our threshold criterion (i.e., AIC difference >2 points) and statistical significance. The best-fitting model had both APOE and β-amyloid PET (MCBP) as predictors for executive function. While this model did not surpass the full model, the importance of β-amyloid PET (MCBP) in the model remains evident. These data suggest that performing a baseline PET scan at minimum has significant clinical relevance for cognitive prognosis in patients with PD.
Our results expand on the predictive utility of β-amyloid for cognitive decline and dementia in PD. Our data clearly show that the presence or risk of β-amyloid (whether in CSF, PET imaging, or APOE genotypes) predicts cognitive decline. In addition, we show that β-amyloid, as measured in CSF or through PET imaging, predicts risk and time to dementia. Although the risk groups we created from the PET measures did not predict time to dementia, this likely reflects both the proportions of dementia conversions within the different risk groups and lower variability in time to dementia within each group, limiting the overall power of the Cox regression. Regardless, these results suggest clinical utility: knowing the β-amyloid burden would allow clinicians to better understand the prognosis of a patient with PD and to offer stronger guidance to the patient and family on disease progression.
It is important to note that, while prior research investigated relationships between different β-amyloid measures and cognitive change, these studies either had small sample sizes11,14 or short follow-up periods14,42 or used global measures of cognition without consideration of specific domains.16,19 In comparison, our research has an average follow-up of 4.1 years (range 1–12 years), >150 participants with PD, and comprehensive neuropsychological evaluations. It also includes APOE genotype, β-amyloid PET, and CSF measures of α-synuclein, β-amyloid, and tau. Importantly, this study combined 3 methods (CSF, PET, and genotype) and multiple proteins, compared to prior research which only used 2 of these methods.17-19
Further, our results agree with prior research on the limited prognostic role of CSF α-synuclein in PD,10,43 indicating its low predictive ability of cognitive change, despite the findings that insoluble α-synuclein fibrils (i.e., Lewy bodies) represent key markers in the pathophysiology of PD.44,45 Recent studies have shown that β-amyloid accumulation relates to higher levels of pathologic α-synuclein accumulation5,46 and that increased α-synuclein accumulation has an association with the presence of APOE ε4 allele.16 Together, these studies suggest a link between β-amyloid and α-synuclein accumulation. While our results could suggest that cognitive dysfunction in PD is not related to α-synuclein, it is more likely that total α-synuclein levels in CSF are not related to levels of aggregated α-synuclein in the brain or in specific regions of the brain that are critical for cognitive function. A direct measure of pathologic, aggregated α-synuclein accumulation (i.e., PET) may yield different results from the CSF measure of total α-synuclein. Indeed, the main challenge of using total CSF α-synuclein levels as a predictor for cognitive change in PD is that we do not know the strength of the correlation between total α-synuclein in CSF and the pathologic accumulation of α-synuclein in the brain and whether CSF concentrations reflect areas of brain closer to CSF bordering surfaces. Furthermore, pathologic β-amyloid accumulation is associated with higher pathologic α-synuclein accumulation at autopsy,46 raising the possibility that the prognostic role of β-amyloid measures may relate to an association with a higher α-synuclein burden. In other words, β-amyloid may only reflect greater α-synuclein burden and does not independently contribute to dementia in PD. PET tracers for α-synuclein will be critical to disentangle the temporal sequence of proteinopathy in PD to delineate the role of α-synuclein and to determine the unique, synergistic, or nonessential marker role of β-amyloid.
In contrast, tau accumulation in the brain may not contribute to dysfunction in most people with PD,5,6,16 even at autopsy.7 Although the importance of tau in AD is clear, tau PET may be useful in only a small subgroup of people with PD and dementia who also have coexisting AD.7
Alternatively, cognitive decline and dementia in PD may represent neurotransmitter and synaptic dysfunction47,48 associated with protein aggregation. For example, β-amyloid peptides disrupt neural transmission and synaptic function,49 and Lewy body accumulation in brainstem nuclei disrupts various neurotransmitter systems.47,50 Thus, protein levels may be an indirect assessment of the underlying neuropathogenesis of cognitive impairment in PD. Future research incorporating both protein and neurotransmitter measures will help delineate the relative contributions of these overlapping pathologies.
The robust sample size of individuals whose average symptom duration at baseline is ≈7 years and whose follow-up time is ≈4 additional years, provides a strong idea of how PD progresses over time. Our results consistently indicate that the presence or risk of β-amyloid accumulation, regardless of the measurement modality, is a strong predictor of cognitive decline in PD. This does not, however, mean that α-synuclein and tau are not also related to cognition. However, we do not have a PET radiotracer for α-synuclein, and PET measures of tau may apply to only a minority of those with PD and coexisting AD. In addition, while we demonstrate that baseline measures of β-amyloid predict cognitive change, the impact of changes in β-amyloid or the other 2 proteins on cognition remains unknown. Future research needs to incorporate multiple measures of β-amyloid over time (e.g., longitudinal PET scans) to better illuminate the role of β-amyloid in PD and cognitive decline. To test the underlying pathophysiology of cognitive deficits in PD, participants were tested “off” dopaminergic medications, in contrast to standard clinical conditions during which patients take medications, and should be interpreted accordingly. However, it is worth noting that a similar pattern of results was obtained with the CDR (assessed “on” medication), demonstrating that the presence or risk of β-amyloid also predicts cognitive decline and dementia on the basis of their functional abilities while medicated. Last, we acknowledge the low racial/ethnic diversity of our cohort and thus understand that our results may not generalize to the greater PD population. In addition, not all patients with PD are willing and able to complete a lumbar puncture or PET scan, and more severe motor symptoms (i.e., marked tremor) self-select participants out of our cohort. In these cases, APOE genotype may offer sufficient prognostic value until blood-based protein biomarkers become available for PD.
In this study, we investigated the relationship between α-synuclein, β-amyloid, and tau and longitudinal cognitive changes in people with PD. We found that CSF and PET measures of β-amyloid, as well as APOE genotype, predict cognitive decline. While these different modalities have been studied individually in PD, we compared the different modalities to better understand the relative predictive power of each modality. Although PET imaging for α-synuclein and tau warrants further research and development, our results emphasize the potential of β-amyloid as a prognostic biomarker for predicting cognitive changes in PD.
Acknowledgment
The authors thank the study participants for their time and effort to aid our understanding of PD. They also thank the following study coordinators and research nurse coordinators at Washington University School of Medicine: Phil Lintzenich, Thomas Belcher, Jenny Zhen-Duan, Anja Pogarcic, My Vu, Jenny Petros, Barb Merz, Katharine Cummings, Selma Avdagic, Kelly McVey, Andrea Slavik, Chris Waller, Jake Wolf, and especially Johanna Hartlein, for assistance with data collection. They also acknowledge that Washington University is located on the traditional and ancestral lands of the Wazhazhe Manzhan (Osage), Myaamia (Miami), and Očeti Šakówiŋ (Sioux) peoples. They express their gratitude for the ancestors and recognize them as the original stewards of the land where Washington University resides.
Glossary
- AD
Alzheimer disease
- AIC
Akaike information criterion
- CDR
Clinical Dementia Rating
- CDR-SB
CDR sum of boxes
- HLM
hierarchical linear growth model
- MCBP
mean cortical binding potential
- PD
Parkinson disease
- PiB
Pittsburgh compound B
- RR
relative risk
Appendix. Authors
Study Funding
Support for this work was provided by grants from National Institute of Neurological Disorders and Stroke (NINDS) (NS097437, NS075321, NS41509, NS058714, NS48924, NS118146, P30 NS048056, NS097799, F32NS105365), NIH National Center for Research Resources (UL1RR024992); American Parkinson Disease Association (APDA) Advanced Research Center for PD at Washington University in St. Louis; Greater St. Louis Chapter of the APDA; Oertli Fund; Paula and Rodger Riney Fund; Barnes Jewish Hospital Foundation (BJHF) (Elliot Stein Family Fund & PD Research Fund).
Disclosure
P.S. Myers received funding from NINDS NS097437. J.L. O'Donnell received funding support from NINDS NS075321 and F32NS105365. J.J. Jackson received funding support from NINDS NS097437. C.N. Lessov-Schlaggar received funding support from NINDS NS097437. R.L. Miller received funding from NINDS NS097437, NS075321, and NS097799. E.R. Foster received funding from Advanced Research Center of the Greater St. Louis Chapter of the APDA. C. Cruchaga has no disclosures relevant to the manuscript. B.A. Benitez received funding from NINDS NS118146. P.T. Kotzbauer received funding from NINDS NS097437, NS075321, and NS097799. J.S. Perlmutter received funding from NINDS NS097437, NS075321, and NS097799; Advanced Research Center of the Greater St. Louis Chapter of the APDA; Oertli Fund; and BJHF (Elliot Stein Family Fund & PD Research Fund). M.C. Campbell received funding from NINDS NS097437, NS075321, and NS097799. Go to Neurology.org/N for full disclosures.
References
- 1.Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart T, Liu C, Ginghina C, et al. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol. 2014;184(4):966-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25(24):5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Gao F, Wang D, et al. Tau pathology in Parkinson's disease. Front Neurol. 2018;9:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buddhala C, Campbell MC, Perlmutter JS, Kotzbauer PT. Correlation between decreased CSF α-synuclein and Aβ1-42 in Parkinson disease. Neurobiol Aging. 2015;36(1):476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han F, Perrin RJ, Wang Q, et al. Neuroinflammation and myelin status in Alzheimer's disease, Parkinson's disease, and normal aging brains: a small sample study. Park Dis. 2019;2019:7975407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotzbauer PT, Cairns NJ, Campbell MC, et al. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch Neurol. 2012;69(10):1326-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colom-Cadena M, Grau-Rivera O, Planellas L, et al. Regional overlap of pathologies in Lewy body disorders. J Neuropathol Exp Neurol. 2017;76(3):216-224. [DOI] [PubMed] [Google Scholar]
- 9.Ruffmann C, Calboli FC, Bravi I, et al. Cortical Lewy bodies and Aβ burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol. 2016;42(5):436-450. [DOI] [PubMed] [Google Scholar]
- 10.Mollenhauer B, Caspell-Garcia CJ, Coffey CS, et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson's disease. Mov Disord Off J Mov Disord Soc. 2019;34(9):1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compta Y, Ibarretxe-Bilbao N, Pereira JB, et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson's disease and related dementia. Parkinsonism Relat Disord. 2012;18(8):941-947. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Cholerton B, Shi M, et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson's disease. Parkinsonism Relat Disord. 2015;21(3):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman JG, Andrews H, Amara A, et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: relationships among biomarkers and Parkinson's disease features. Mov Disord Off J Mov Disord Soc. 2018;33(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palermo G, Tommasini L, Aghakhanyan G, et al. Clinical correlates of cerebral amyloid deposition in Parkinson's disease dementia: evidence from a PET study. J Alzheimers Dis 2019;70(2):597-609. [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Frey KA, Müller MLTM, et al. Striatal and cortical β-amyloidopathy and cognition in Parkinson's disease. Mov Disord. 2016;31(1):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis AA, Inman CE, Wargel ZM, et al. APOE genotype regulates pathology and disease progression in synucleinopathy. Sci Transl Med. 2020;12(529):eaay3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhtar RS, Xie SX, Chen YJ, et al. Regional brain amyloid-β accumulation associates with domain-specific cognitive performance in Parkinson disease without dementia. PLoS One. 2017;12(5):e0177924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buongiorno M, Antonelli F, Compta Y, et al. Cross-sectional and longitudinal cognitive correlates of FDDNP PET and CSF amyloid-β and tau in Parkinson's Disease1. J Alzheimers Dis. 2017;55(3):1261-1272. [DOI] [PubMed] [Google Scholar]
- 19.Shahid M, Kim J, Leaver K, et al. An increased rate of longitudinal cognitive decline is observed in Parkinson's disease patients with low CSF Aß42 and an APOE ε4 allele. Neurobiol Dis. 2019;127:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim EW, Aarsland D, Ffytche D, et al. Amyloid-β and Parkinson's disease. J Neurol. 2019;266(11):2605-2619. [DOI] [PubMed] [Google Scholar]
- 21.Campbell MC, Myers PS, Weigand AJ, et al. Parkinson disease clinical subtypes: key features & clinical milestones. Ann Clin Transl Neurol. 2020;7(8):1272-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25(15):2516-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. [DOI] [PubMed] [Google Scholar]
- 25.Mintun M, Vlassenko A, Head D, et al. Quantifying the rate of beta-amyloid accumulation in cognitively normal participants using longitudinal [11C] PIB PET imaging. J Nucl Med Soc Nucl Med. 2010;51(Suppl 2):382. [Google Scholar]
- 26.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30(1):1-23. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale–III. Psychological Corp; 1997. [Google Scholar]
- 28.Delis D, Kaplan E, Kramer J. Over B. California Verbal Learning Test-II. Psychological Corp; 2000. [Google Scholar]
- 29.Wechsler D. Wechsler Memory Scale III. Psychological Corp; 1997. [Google Scholar]
- 30.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test, 2nd ed. Pro-ED; 2001. [Google Scholar]
- 31.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment, 4th ed. Oxford University Press; 2004:1016. [Google Scholar]
- 32.Woodcock R, McGrew K, Mather N. Woodcock-Johnson Tests of Achievement. Riverside Publishing Co; 2001. [Google Scholar]
- 33.Delis DC. Delis-Kaplan Executive Function System (D-KEFS). Psychological Corp; 2001. [Google Scholar]
- 34.Ibanez L, Bahena JA, Yang C, et al. Functional genomic analyses uncover APOE-mediated regulation of brain and cerebrospinal fluid beta-amyloid levels in Parkinson disease. Acta Neuropathol Commun. 2020;8(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. NeuroImage. 2015;107:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowland DJ, Garbow JR, Laforest R, Snyder AZ. Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol. 2005;32(6):567-572. [DOI] [PubMed] [Google Scholar]
- 37.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. [Google Scholar]
- 39.Raftery AE. Bayesian model selection in social research. Soc Methodol. 1995;25:111-163. [Google Scholar]
- 40.Therneau TM, Lumley T, Elizabeth A, Cynthia C. SURVIVAL: survival analysis. 2020. Accessed March 2, 2021. CRAN.R-project.org/package=survival [Google Scholar]
- 41.Kassambara A, Kosinski M, Biecek P, Fabian S. SURVMINER: drawing survival curves using “ggplot2.” 2020. Accessed March 2, 2021. CRAN.R-project.org/package=survminer [Google Scholar]
- 42.Dolatshahi M, Pourmirbabaei S, Kamalian A, Ashraf-Ganjouei A, Yaseri M, Aarabi MH. Longitudinal alterations of alpha-synuclein, amyloid beta, total, and phosphorylated tau in cerebrospinal fluid and correlations between their changes in Parkinson's disease. Front Neurol. 2018;9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terrelonge M, Marder KS, Weintraub D, Alcalay RN. CSF β-amyloid 1-42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J Mol Neurosci. 2016;58(1):88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50(2):140-148. [DOI] [PubMed] [Google Scholar]
- 45.Jellinger K. Neuropathological substrates of Alzheimer's disease and Parkinson's disease. J Neural Transm Suppl. 1987;24:109-129. [PubMed] [Google Scholar]
- 46.Miller R, Dhavale D, O'Shea J, et al. Quantifying regional alpha-synuclein and amyloid beta accumulation in Lewy body dementia. Ann Clin Transl Neurol. 2021;9(2):106-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primer. 2021;7(1):1-21. [DOI] [PubMed] [Google Scholar]
- 48.van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Cholinergic denervation patterns across cognitive domains in Parkinson's disease. Mov Disord Off J Mov Disord Soc. 2021;36(3):642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martorana A, Di Lorenzo F, Belli L, et al. Cerebrospinal fluid Aβ42 levels: when physiological become pathological state. CNS Neurosci Ther. 2015;21(12):921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buddhala C, Loftin SK, Kuley BM, et al. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol. 2015;2(10):949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.