Abstract
Background:
Sepsis affects millions of patients annually, resulting in substantial health and economic burdens globally. The role of esmolol potentially plays in the treatment of sepsis and septic shock in adult patients remains controversial.
Methods:
We undertook a systematic search of PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases from their inception to May 12, 2022, for randomized controlled trials that evaluated the efficacy of esmolol for sepsis and septic shock. A random-effects meta-analysis was performed. Two investigators independently screened articles, extracted data, and assessed the quality of included studies.
Results:
Eight studies from 7 randomized controlled trials were included in our meta-analysis of 503 patients with sepsis and/or septic shock. Compared with standard treatment, esmolol significantly decreased 28-day mortality (risk ratio 0.68, 95% confidence interval [CI] 0.52–0.88; P = .004), heart rate (standardized mean difference [SMD] −1.83, 95% CI −2.95 to −0.70, P = .001), tumor necrosis factor-a (SMD −0.48, 95% CI −0.94 to −0.02, P = .04), and the troponin I level (SMD −0.59, 95% CI −1.02 to −0.16, P = .008) 24 hours after treatment. No significant effect was found in terms of length of intensive care unit stay; mean arterial pressure, lactic acid, central venous pressure, or central venous oxygen saturation, interleukin 6, or white blood cell levels; stroke volume index; or the PaO2/FiO2 ratio.
Conclusions:
Esmolol treatment may be safe and effective in decreasing 28-day mortality, controlling heart rate, and providing cardioprotective function, but has no effect on lung injury in patients with sepsis or septic shock after early fluid resuscitation. Improvement in cardiac function may be related to changes in serum inflammatory mediators. No significant adverse effects on tissue perfusion and oxygen utilization were observed.
Keywords: esmolol, meta-analysis, mortality, sepsis, septic shock
1. Introduction
Sepsis is defined as a host’s unbalanced response to infection, leading to a variety of deleterious effects, including septic shock, multiple organ failure, and ultimately death. Severe sepsis, septic shock, and their complications affect millions of people each year, and in-hospital mortality rates remain high at 25% to 30%, resulting in substantial health and economic burdens globally.[1–7]
Severe sepsis is a complex syndrome characterized as dysfunction of one or more organs, particularly heart dysfunction, which features as a hemodynamic disorder.[8] Blanco et al[6] reported that the mortality rate for patients with myocardial dysfunction was significantly higher (70%) than that for patients with sepsis without myocardial insufficiency (20%).[6] Some studies have reported that mortality rates are 2 to 3 times higher when septic cardiomyopathy is present.[5,9] However, severe sepsis or septic shock requires vasopressor therapy to maintain adequate tissue perfusion, which can then incline patients to tachycardia and cardiac arrhythmias and increase the risk of adverse cardiovascular events.[10,11] Considering the function of β-adrenergic in cardiovascular dysfunction in sepsis and the elevated risk of tachycardia and atrial fibrillation, beta-blockade therapy is a reasonable therapeutic modality for improving outcomes in patients with sepsis and septic shock.[12]
Several meta-analyses[12–18] have shown that selective β1-adrenergic blockade therapy may reduce the heart rate (HR) and improve the survival rate. However, the findings of a recent randomized controlled trial (RCT) suggest that the current evidence remains controversial.[19] In this study, we aimed to undertake an up-to-date meta-analysis to investigate the effect of esmolol on sepsis and/or septic shock treatment in adult patients.
2. Methods
2.1. Literature search
We undertook a systematic search for RCTs in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from their inception to May 12, 2022, using the following key words in all fields: “esmolol” and “septic shock” or “sepsis.” We also scanned reference lists of relevant studies and key review articles to locate relevant studies. All analyses were based on previously published studies. Ethical approval and patient consent were not required.
2.2. Study selection
Study inclusion criteria comprised the following: participants: patients with sepsis or septic shock aged ≥18 years, with an HR of ≥95 beats/min after early goal-directed therapy; intervention: continuous infusion of esmolol titrated to maintain a target HR range between 75 and 100/min during the first 96 hours; comparison: basic treatment for sepsis; and outcomes: the primary outcome was 28-day mortality. Secondary outcomes were HR; length of intensive care unit (ICU) stay; mean arterial pressure (MAP), central venous pressure (CVP), central venous oxygen saturation (ScvO2), lactic acid (Lac); stroke volume index (SVI), cardiac index; troponin I (TnI), tumor necrosis factor-a (TNF-a), interleukin 6 (IL-6) levels, and white blood cell (WBC) count; and design: RCTs. If data were duplicated or shared in more than 1 study, the first published study was included in the meta-analysis. The language restriction is English. Discrepancies regarding study inclusion between authors were resolved through discussion. Two of the authors (CC and JZ) independently evaluated the eligibility of all studies obtained from the databases according to the above selection criteria.
2.3. Data extraction and risk of bias assessment
Extracted data were entered into a standardized Excel file. Disagreements between authors were resolved through discussion. The following data were extracted: study name (together with publication year and the name of the first author), country and design, participants (sample size, sex, and age), intervention arms and controls (intervention drug), and outcomes (primary and secondary outcomes). The Cochrane Collaboration’s tool for assessing the risk of bias was used for each RCT, which includes the following criteria: adequacy of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases.[20] Disagreements were resolved through a further check of the original articles. We also used the GRADE system to rate the quality of evidence from our meta-analysis, using GRADE pro, which was supported by the Italian Ministry of Health and developed by the GRADE Working Group.
2.4. Statistical analysis
We calculated a relative risk (RR) with 95% confidence intervals (95% CIs) for 28-day mortality and HR. Concerning length of ICU stay, and MAP, CVP, ScvO2, Lac, SVI, TnI, TNF-a, and IL-6 levels, and the WBC count and cardiac index, standard mean differences (SMDs) between the experimental and control groups were combined. Heterogeneity in results across studies was examined using Cochran Q and I2 statistics.[21] The null hypothesis that the studies are homogeneous was rejected if the P-value for heterogeneity was <.10 or if I2 was >50%. A random-effects model[22] was used to pool the studies.
A sensitivity analysis was conducted to assess the influence of individual studies on the pooled result when the P-value was <.10 or when I2 was >50% through excluding each study one at a time and recalculating the combined results on the remaining studies. We used funnel plot asymmetry proposed by Egger et al[21] to test for publication bias. All data analyses were performed using Review Manager 5.3 (Cochrane Informatics and Knowledge Management Department, available from http://tech.cochrane.org/UnitedKingdom) software.
3. Results
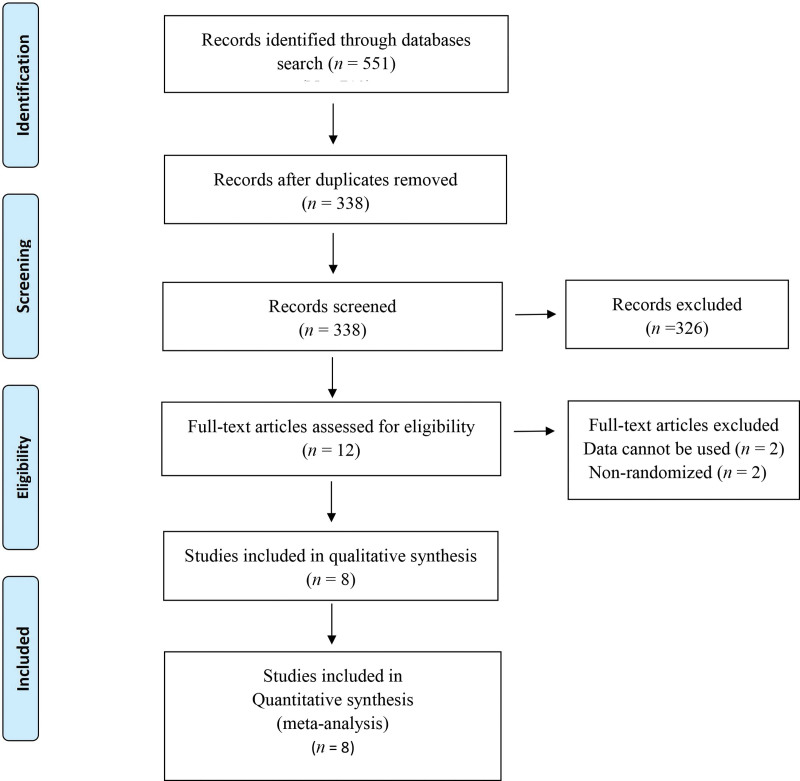
Figure 1 shows a flow diagram of the selection process. A total of 551 articles were initially identified from the databases. Of these, 213 articles were excluded as duplicates, and 326 articles were excluded after screening the titles and abstracts. The remaining 12 full-text articles were assessed for eligibility, of which 4 articles were further excluded. The remaining 8 RCTs[19,23–29] were included in the final meta-analysis.
Figure 1.
A flow diagram of the study selection process. All studies were randomized controlled trials.
3.1. Characteristics of the included studies
The characteristics of the studies included in our meta-analysis are summarized in Table 1. The 7 studies were published between 2013 and 2019, and sample sizes ranged from 40 to 154. Orbegozo Cortes et al[29] and Morelli et al[28] reported the same clinical trial but with different follow-up times. The 7 included 8 RCTs[19,23,24,26–29] involving septic shock. Wang et al[25] reported the effect of esmolol and milrinone on patients with severe sepsis, who were randomly divided into control, milrinone, and milrinone-esmolol groups. Wang et al[24] and Michael et al[19] used saline in control groups, while the remaining studies[23,26–28] used blank controls. Overall, 250 patients were included in esmolol groups, while 253 patients were included in control groups. All studies focused on adults, with a mean age of 34 to 67.2 and 38.0 to 69 years, respectively, in the intervention and control arms. Four studies[19,24,26,27] commenced at 0.05 mg/kg/h esmolol continuous intravenous titrate, while 4 trials[23,25,27,28] commenced at 25 mg/h esmolol continuous intravenous infusion, and adjusted the dosage according to HR until the predefined threshold rate had been reached.
Table 1.
Characteristics of included studies in meta-analysis.
| Authors | Country | Study design | Age (yrs) (mean ± SD) |
Comparisons | No. of patients (male) |
Target HR (beats/min) |
APACHE II score (I/C) |
Outcomes |
|---|---|---|---|---|---|---|---|---|
| Morelli et al[28] | Italian | RCT | 66 ± 17.03 | Esmolol* | 77 (54) | 80-94 | NA | 28-day mortality, Lac, WBC |
| 69 ± 14.82 | Control† | 77 (53) | NA | length of ICU stay, PO2/FiO2 | ||||
| Yang et al[27] | China | RCT | 51.0 ± 22.6 | Esmolol‡ | 21 (NA) | <100 | 20.1 ± 9.2 | HR, ScvO2, MAP, CVP, Lac, |
| 55.0 ± 25.4 | Control† | 20 (NA) | 21.3 ± 8.3 | TnI, cardiac index, SVI | ||||
| Orbegozo Cortes et al[29] | Italian | RCT | 66 ± 17.03 | Esmolol* | 77 (54) | 80-94 | NA | 28-day mortality |
| 69 ± 14.82 | Control† | 77 (53) | NA | length of ICU stay | ||||
| Wang et al[25] | China | RCT | 34 ± 28.89 | Esmolol§ | 30 (19) | 75-94 | 21.2 ± 5.7 | 28-day mortality, HR, MAP, CVP, Lac |
| 38 ± 27.41 | Control‖ | 30 (19) | 20.8 ± 5.6 | TnI, cardiac index, SVI, TNF-α, IL-6, | ||||
| Liu et al[26] | China | RCT | 61.4 ± 6.9 | Esmolol¶ | 24 (NA) | <100 | 20.75 ± 3.05 | 28-day mortality, length of ICU stay, HR |
| 61.2 ± 6.4 | Control† | 24 (NA) | 21.21 ± 2.67 | ScvO2, MAP, CVP, Lac, Cardiac index, SVI | ||||
| Wang et al[24] | China | RCT | 67.2 ± 12.5 | Esmolol# | 30 (18) | <95 | 18.4 ± 6.3 | 28-day mortality, HR, MAP, Lac |
| 62.5 ± 14.5 | Control** | 30 (21) | 15.7 ± 6.3 | Cardiac index, SVI, TNF-α, IL-6, WBC | ||||
| Liu et al[23] | China | RCT | 58 ± 15 | Eesmolol†† | 50 (29) | 80-100 | 18.8 ± 6.5 | 28-day mortality, length of ICU stay |
| 57 ± 18 | Control† | 50 (28) | 19.1 ± 7.5 | HR, Lac, WBC | ||||
| Michael et al[19] | Israel | RCT | 62 ± 10.37 | Esmolol‡‡ | 18 (10) | 80-94 | NA | HR, length of ICU stay, Lac, TNF-α |
| 64 ± 8.89 | Control§§ | 22 (13) | IL-6 |
CVP = central venous pressure, HR = heart rate, IL-6 = interleukin 6, MAP = mean arterial pressure, ScvO2 = central venous oxygen saturation, SVI = stroke volume index; TNF-a = tumor necrosis factor-a, TnI = troponin I, WBC = white blood cell.
Continuous esmolol infusion commenced at 25 mg/h and progressively increased the rate at 20-minute intervals in increments of 50 mg/h, or more slowly at the discretion of the investigators, to reach the target heart rate between 80/min and 94/min within 12 hours.
Basic treatment.
Micropump with dosage of esmolol 0.05 mg/kg/min to control HR below 100/min within 2 hours.
Continuous intravenous infusion of esmolol, milrinone that commenced with a loading dosage of 30 μg/kg and was maintained at 0.375 to 0.5 μg/kg/min.
Continuous intravenous infusion of milrinone that commenced with a loading dosage of 30 μg/kg and was maintained at 0.375 to 0.5 μg/kg/min.
Micropump with dosage of esmolol 0.05 mg/kg/min to control HR below 100/min within 24 hours.
#Continuous intravenous esmolol infusion for 24 hours, initial dose was 0.05 mg/kg/h, to control HR below 95/min within 4 hours.
Isotonic saline was given to control group through intravenous line at 3 mL/h for 24 hours.
Continuous esmolol micropump commenced at 25 mg/h to maintain HR 80 to 100/min within 12 hours.
Continuous esmolol micropump commenced at 0.05 mg/kg/min to maintain HR 80 to 94/min for 24 hours.
Saline was given at the beginning of study interventions.
3.2. Assessment of risk of bias and publication bias
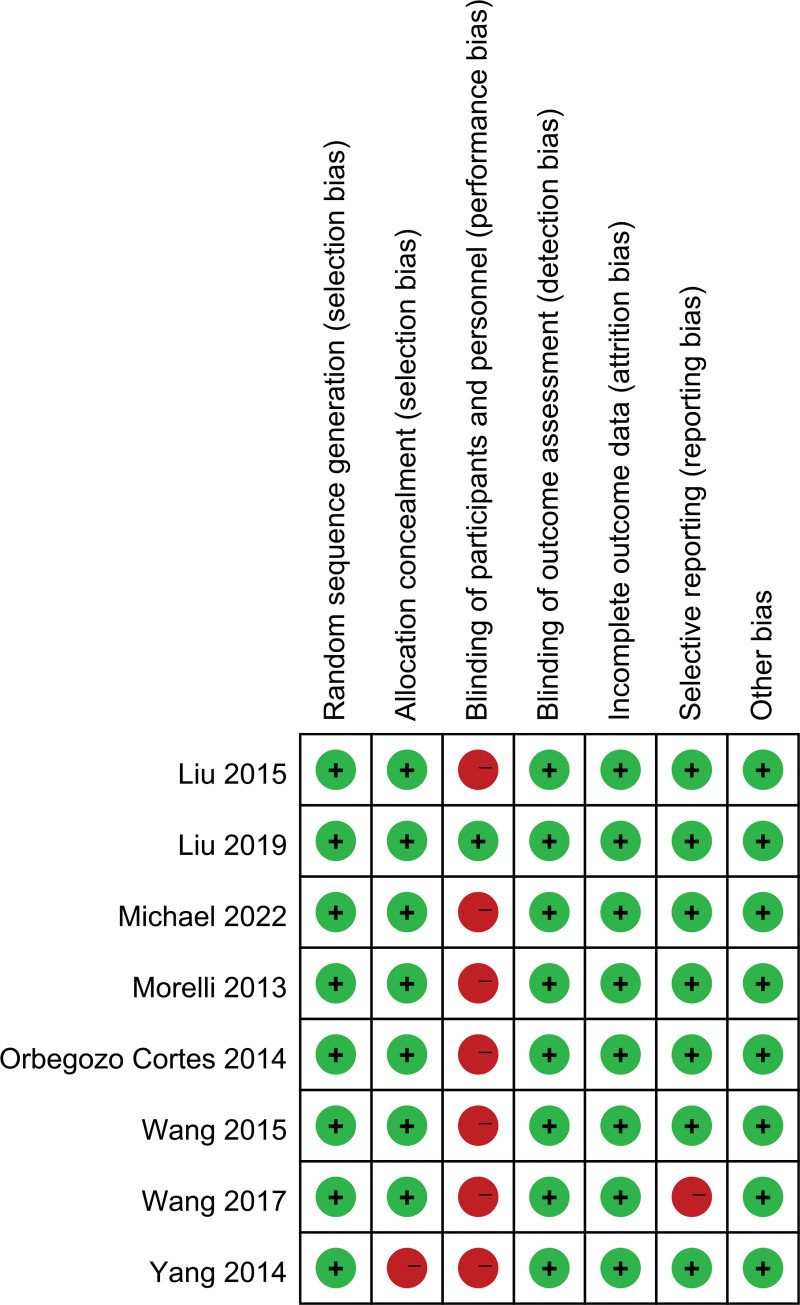
A risk of bias assessment for the included RCT is presented in Figure 2. The included RCTs had some methodological strengths and limitations. Seven[19,24–30] were adjudicated to have a high risk of bias in terms of blinding of participants and personnel. Yang et al[27] was adjudicated to have a high risk of bias in terms of allocation concealment as was Wang et al[25] in terms of selective reporting. Only Liu et al[23] was adjudicated to have a low risk of bias.
Figure 2.
Risk of bias summary for the included studies.
We were unable to assess publication bias using a funnel plot due to the small number of RCT (<10) included in this analysis. Therefore, publication bias could not be excluded.
3.3. Heterogeneity and sensitivity analysis
No heterogeneity was observed in MAP, CVP, Lac (12 hours), cardiac index (72 hours), WBC, PO2/FiO2 (24 and 48 hours), low heterogeneity in 28-day mortality, TnI (24 and 48 hours), SVI (24 hours), TNF-α, and PO2/FiO2 (72 and 96 hours). We found high heterogeneity in terms of the length of ICU stay, HR, ScvO2, Lac (24, 48, 72, and 96 hours), TnI (72 hours), cardiac index (12, 24, and 48 hours), SVI (48 and 72 hours), and IL-6. A sensitivity analysis was performed to evaluate the stability of the results. Each study was excluded one at a time and we recalculated the combined RR or SMD in the remaining studies. This analysis confirmed the stability of the results: the overall effects did not show statistically significant reversal, and recalculated pooled RR and SMD were consistent and without apparent fluctuation (data not shown).
3.4. Primary outcomes
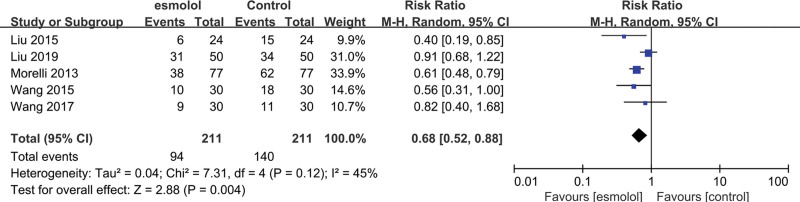
3.4.1. 28-day mortality.
Five trials[23–26,28] comprising 422 patients evaluated 28-day mortality. The esmolol groups had significantly decreased 28-day mortality compared with the control groups (RR 0.68, 95% CI 0.52–0.88, P = .004, I2 = 45%; Fig. 3).
Figure 3.
A forest plot of 28-day mortality between the esmolol and control groups.
3.5. Secondary outcomes
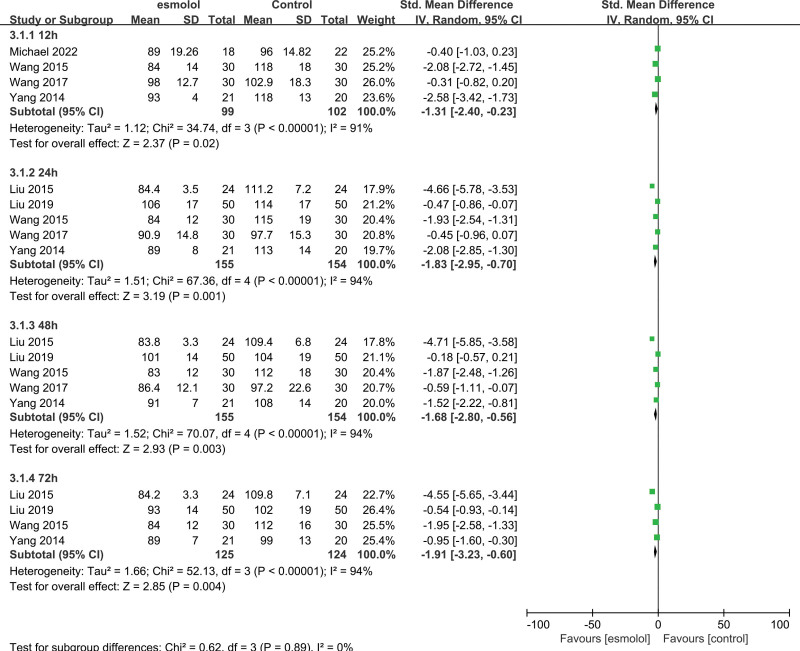
3.5.1. Heart rate.
Six trials[19,23–27] comprising 349 adults evaluated the effect of HR between esmolol and control groups. Pooled analysis results of these 349 adults indicated that esmolol significantly decreased the HR at 12, 24, 48, and 72 hours (SMD −1.31, 95% CI −2.4 to −0.23, P = .02, I2 = 91%; SMD −1.83, 95% CI −2.95 to −0.70, P = .001, I2 = 94%; SMD −1.68, 95% CI −2.8 to −0.56, P = .003, I2 = 94%; and SMD −1.91, 95% CI −3.23 to −0.60, P = .004, I2 = 94%, respectively); Figure 4.
Figure 4.
A forest plot of the heart rate between the esmolol and control groups.
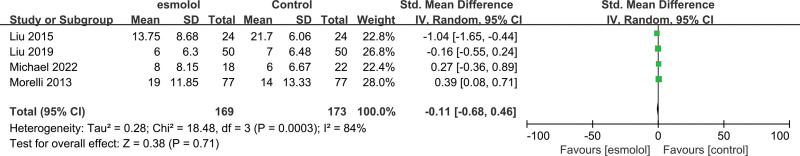
3.5.2. Length of ICU stay.
Four RCTs[19,23,26,28] comprising 342 adults evaluated the length of ICU stay between esmolol and control groups. Pooled analysis results showed no significant association between esmolol supplementation and septic shock treatment (SMD −0.11, 95% CI −0.68–0.46, P = .71, I2 = 84%; Fig. 5).
Figure 5.
A forest plot of the length of ICU stay between the esmolol and control groups. ICU = intensive care unit.
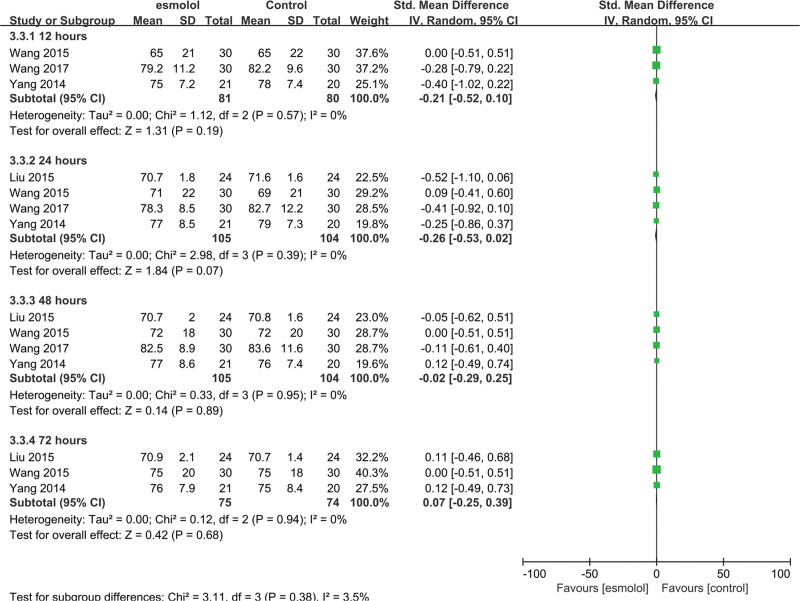
3.5.3. Mean arterial pressure.
Four trials[24–27] comprising 209 adults evaluated MAP between esmolol and control group. No significant differences were found at 12, 24, 48, and 72 hours (SMD −0.21, 95% CI −0.52 to 0.10, P = .19; SMD −0.26, 95% CI −0.53 to 0.02, P = .07; SMD −0.02, 95% CI −0.29 to 0.25, P = .89; and SMD 0.07, 95% CI −0.25 to 0.39, P = .68, respectively), and no heterogeneity was detected (Fig. 6).
Figure 6.
A forest plot of mean arterial pressure levels between the esmolol and control groups.
3.5.4. Lactic acid.
Seven RCTs[19,23–28] comprising 503 patients evaluated Lac levels and reported no significant differences between esmolol and control groups. Pooled SMDs at 12, 24, 48, and 72 hours were 0.03 (95% CI −0.24 to 0.31; P = .81, I2 = 0%), 0.12 (95% CI −0.43 to 0.67; P = .66, I2 = 88%), −0.5 (95% CI −1.01 to 0.01; P = .06, I2 = 83%), −0.60 (95% CI −1.24 to 0.03; P = .06, I2 = 88%), and −0.40 (95% CI −0.93 to 0.12; P = .13, I2 = 80%), respectively Figure 7.
Figure 7.
A forest plot of lactic acid levels between the esmolol and control groups.
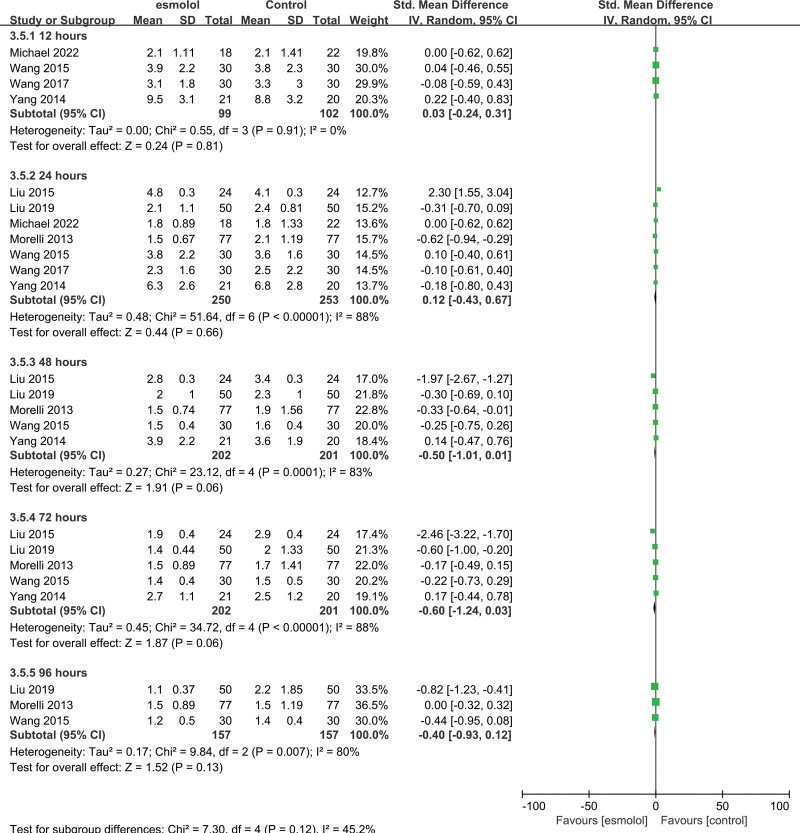
3.5.5. Stroke volume index.
Four studies[24–27] comprising 209 patients evaluated the SVI. No significant differences were found between esmolol and control groups. Pooled SMDs at 24, 48, and 72 hours were 0.16 (95% CI −0.12 to 0.44; P = .27, I2 = 7%), 0.44 (95% CI −0.27 to 1.15; P = .23, I2 = 84%), and 0.43 (95% CI −0.54 to 1.41; P = .39, I2 = 88%), respectively Figure 8.
Figure 8.
A forest plot of the stroke volume index between the esmolol and control groups.
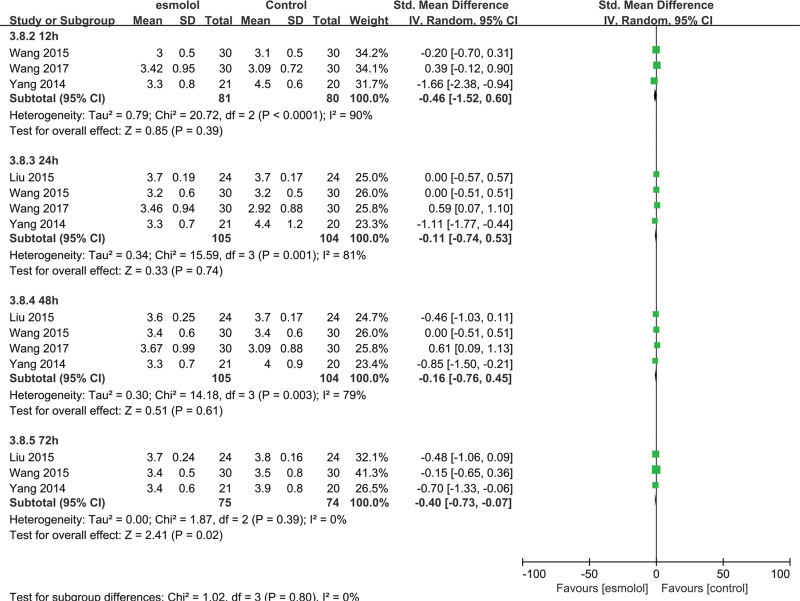
3.5.6. Cardiac index.
Four trials[24–27] comprising 209 adults evaluated the cardiac index. The esmolol group was found to have a significantly decreased cardiac index at 72 hours (SMD −0.4, 95% CI −0.73 to −0.07, P = .02, I2 = 0%) compared with the control group, but no significant differences were observed at 12, 24, and 48 hours (SMD −0.46, 95% CI −1.52 to 0.60, P = .39, I2 = 90%; SMD −0.11, 95% CI −0.74 to 0.53, P = .74, I2 = 81%; and SMD −0.16, 95% CI −0.76 to 0.45, P = .61, I2 = 79%, respectively Fig. 9).
Figure 9.
A forest plot of the cardiac index between the esmolol and control groups.
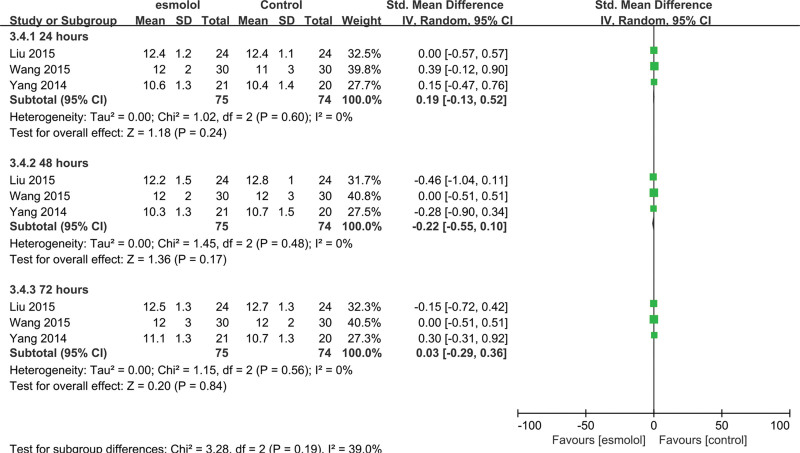
3.5.7. Central venous pressure.
Three studies[25–27] comprising 149 adults evaluated CVP levels. No significant differences were found at 24, 48, and 72 hours between esmolol and control groups (SMD 0.19, 95% CI −0.13 to 0.52, P = .24, I2 = 0%; SMD −0.22, 95% CI −0.55 to 0.1, P = .17, I2 = 0; and SMD 0.03, 95% CI −0.29 to 0.36, P = .84, I2 = 0%, respectively Fig. 10).
Figure 10.
A forest plot of central venous pressure levels between the esmolol and control groups.
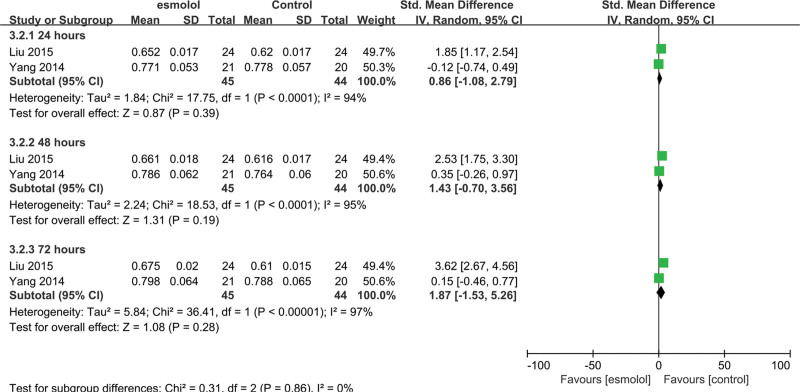
3.5.8. Central venous oxygen saturation.
Two trials[26,27] involving 89 patients evaluated ScvO2. No significant differences were found at 24, 48, and 72 hours between esmolol and control groups (SMD 0.86, 95% CI −1.08 to 2.79, P = .39, I2 = 94%; SMD 1.43, 95% CI −0.7 to 3.56, P = .19, I2 = 95%; and SMD 1.87, 95% CI −1.53 to 5.26, P = .28, I2 = 97%, respectively Fig. 11).
Figure 11.
A forest plot of central venous oxygen saturation levels between the esmolol and control groups.
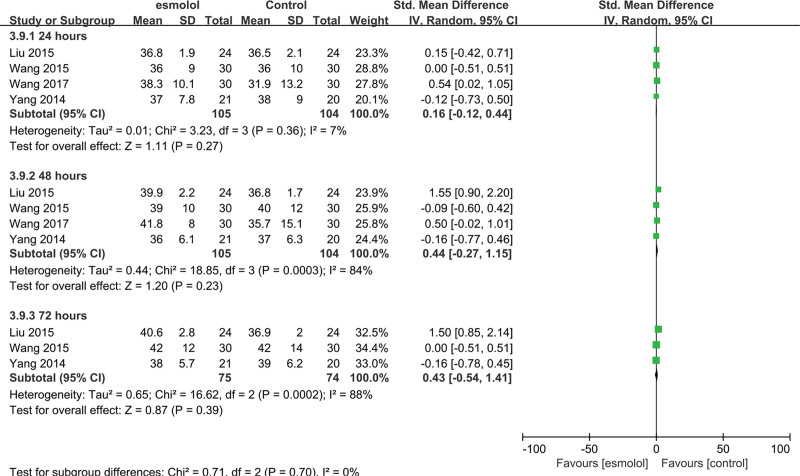
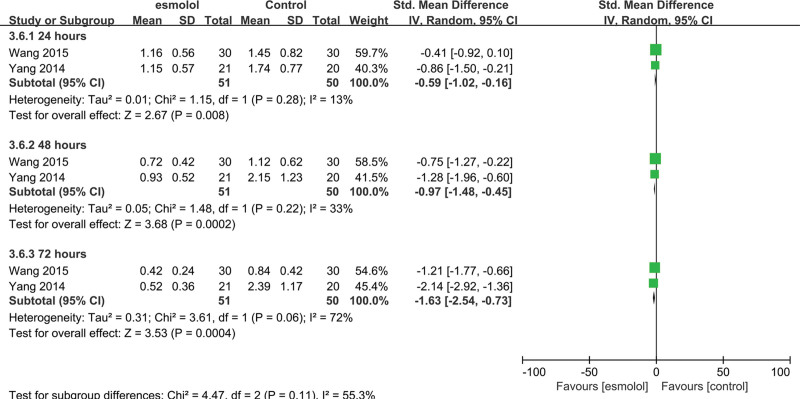
3.5.9. Tn1 levels.
Two trials[25,27] involving 101 adults evaluated Tn1 levels. The esmolol group was found to have significantly decreased TnI levels at 24, 48, and 72 hours (SMD −0.59, 95% CI −1.02 to −0.16, P = .008, I2 = 13%; SMD −0.97, 95% CI −1.48 to −0.45, P = .0002, I2 = 33%; and SMD −1.63, 95% CI −2.54 to −0.73, P = .0004, I2 = 72%, respectively); Figure 12.
Figure 12.
A forest plot of troponin I levels between the esmolol and control groups.
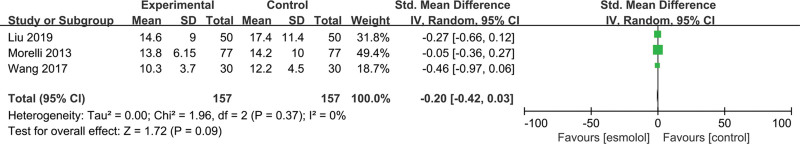
3.5.10. WBC counts.
Three studies[23,24,28] involving 314 adults evaluated WBC counts. No significant differences were found between esmolol and control groups (SMD −0.2, 95% CI −0.42 to 0.03, P = .09, I2 = 0%); Figure 13.
Figure 13.
A forest plot of white blood cell levels between the esmolol and control groups.
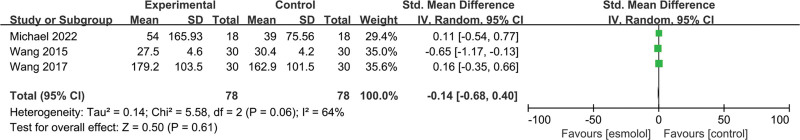
3.5.11. IL-6 levels.
Three trials[19,24,25] involving 156 patients evaluated IL-6 levels. Pooled analysis results reported that no significant differences were found between esmolol and control groups (SMD −0.14, 95% CI −0.68 to 0.4, P = .61, I2 = 64%); Figure 14.
Figure 14.
A forest plot of interleukin 6 levels between the esmolol and control groups.
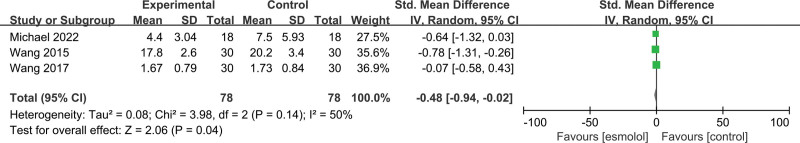
3.5.12. Tumor necrosis factor-a.
Three studies[19,25,26] involving 156 patients evaluated TNF-a levels. The esmolol group was found to have significantly decreased TNF-a level (SMD −0.48, 95% CI −0.94 to −0.02, P = .04, I2 = 50%); Figure 15.
Figure 15.
A forest plot of tumor necrosis factor-a levels between the esmolol and control groups.
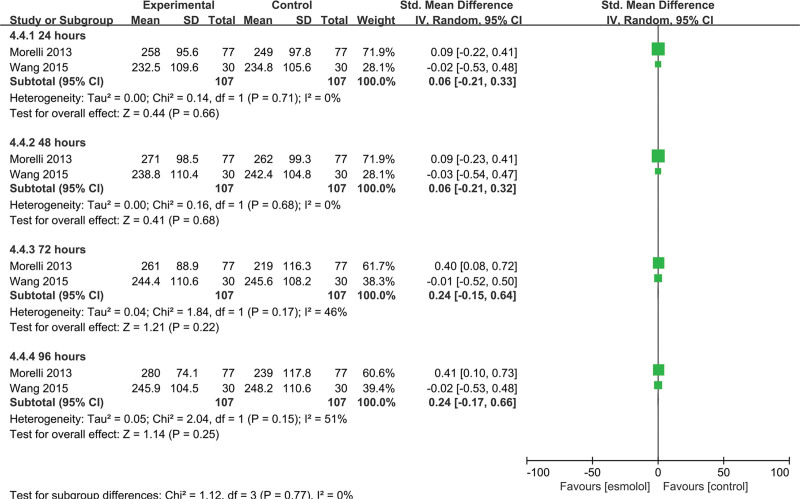
3.5.13. PO2/FiO2 ratio.
Two studies[22,25] involving 214 patients evaluated the PO2/FiO2 ratio. No significant differences were found between esmolol and control groups at 24, 48, 72, and 96 hours (SMD 0.06, 95% CI −0.21 to 0.33, P = .66, I2 = 0%; SMD 0.06, 95% CI −0.21 to 0.32, P = .68, I2 = 0%; SMD 0.24, 95% CI −0.15 to 0.64, P = .22, I2 = 46%; and SMD 0.24, 95% CI −0.17 to 0.66, P = .25, I2 = 51%, respectively); Figure 16.
Figure 16.
A forest plot of the PO2/FiO2 ratio between the esmolol and control groups. PO2/FiO2, the ratio of arterial oxygen partial pressure (PaO2 in mm Hg) to fractional inspired oxygen.
3.5.14. Quality of evidence.
We used the GRADE system to determine the quality of evidence in our meta-analysis. The PaO2/FiO2 ratio and 28-day mortality had “very-low”-quality evidence, with a serious risk of bias, inconsistency, and indirectness. The length of ICU stay and ScvO2 had “very-low”-quality evidence, with a risk of bias, inconsistency, and imprecision. HR, MAP, CVP, and TnI had “very-low”-quality evidence, with a risk of bias, indirectness, and imprecision. Lac, CI, SVI, TNF-a, and IL-6 had “very-low”-quality evidence, with a risk of bias, inconsistency, indirectness, and imprecision. WBC had “low”-quality evidence, with a risk of bias and imprecision.
4. Discussion
The included RCTs showed that esmolol could reduce 28-day mortality, control the HR, decrease the level of cardiac troponin I and TNF-a, and decrease the cardiac index at 72 hours. However, there was no significant difference in the length of ICU stay, or ScvO2, CVP, MAP, Lac, SVI, WBC, and IL-6 levels, or in the PO2/FiO2 ratio in patients with severe sepsis and septic shock after adequate fluid resuscitation in the early stages of standard treatment.
Sepsis-related cardiovascular failure is mainly associated with sustained systemic adrenergic activation, particularly via the β1-adrenergic pathway,[30] which augments cardiac contractility[31] and HR,[32] increasing energy demands. When energy demand outstrips supply, cardiac myocytes are at risk of cell death, with elevated troponin levels indicating such injury,[33,34] leading to detrimental cardiac effects including fibroblast hyperplasia, myocyte necrosis and apoptosis, and increased risk of arrhythmia.[35] Theoretically, adjusting the adrenergic system may be a new approach in the treatment of sepsis.[36,37] Berk et al[38] assessed the effects of propranolol, a nonselective β-blocker, on dogs with sepsis and found that propranolol significantly improved survival and arterial PO2 values, prevented the second phase of hypotension, and reduced fluid requirements. Patterson et al[39] reported a negative outcome in mice with sepsis treated with propranolol, which may be explained as nonselective β-blockers inhibiting the activation of β-2 receptors, which have cardioprotective properties. Aboab et al[40] reported that pigs with endotoxic shock treated with a continuous infusion of esmolol, a selective β-1 adrenergic blocker, tolerated this infusion well, and that it appeared to offset sepsis-induced cardiac dysfunction. Suzuki et al[41] reported that infusing esmolol into septic rats reduced HR and blood pressure, improved oxygen utilization of the myocardium, and preserved myocardial function, with lactate levels not increasing compared with controls. Ibrahim-Zada et al[42] also showed that esmolol significantly improved survival in a murine model of septic insult. One meta-analysis[43] of 67 RCTs involving 3766 patients showed that esmolol had the potential to protect against myocardial ischemia in patients undergoing noncardiac surgery.
Tachycardia is common in severe septic cardiomyopathy to compensate for low cardiac output.[15] An observation study[11] found that a prolonged elevated HR was associated with an increasing incidence of major cardiac events in patients who were critically ill. Beta-blockers reduce HR, have anti-inflammatory effects, improve myocardial oxygen supply and demand balance, and affect hemodynamics and metabolic and immune regulation in sepsis. Beta-blockers may be a new method for the treatment of sepsis, especially for patients with high catecholamine levels and tachycardia.[44] Esmolol is commonly used in the ICU because of its rapid effect and ease of titration.[45] We found that esmolol significantly decreased HR and the cardiac index at 72 hours compared with control groups, but that there were no differences in SVI, indicating that esmolol did not affect cardiac systolic function. The decreased cardiac index was mainly associated with a decreased HR. We also found that there was no significant difference in the level of MAP and CVP at various time points between esmolol and control groups. Gore and Wolfe[46] found that esmolol reduced the HR with a comparable decrease in cardiac output in patients with moderately severe sepsis, which may improve myocardial blood flow and have potential benefits in terms of reducing the incidence of cardiac demise without affecting oxygen utilization or hepatic, peripheral blood flow. Lac and ScvO2 levels usually reflect tissue infusion and oxygen metabolism at an early stage of sepsis. Gore and Wolfe[46] found that esmolol did not limit oxygen utilization, or affect systemic energy expenditure, or alter energy within muscles while reducing HR and cardiac output. Our meta-analysis found no significant difference between esmolol and control groups in terms of Lac and ScvO2, and these results were consistent with those reported by Li et al,[13] Liu et al,[15] and Huang et al,[18] suggesting that the dose control of esmolol did not have an adverse effect on tissue perfusion and circulatory function. Thus, no evidence was found to indicate that esmolol infusion adversely affects organ perfusion and oxygen and energy utilization.
Monocytes are activated in sepsis causing abundant release of proinflammatory factors such as TNF-a, IL-6, and high mobility group box-1,[47] which could cause significant myocardial depression and depress myocardial contractile function, even developing into septic cardiomyopathy.[48] Suzuki et al[41] showed that esmolol could significantly reduce TNF-a concentrations in sepsis rats and improve oxygen utilization of the myocardium and preserve myocardial function. Wang et al[25] showed that esmolol combined with milrinone could reduce TNF-a, IL-6, and high mobility group box-1 levels, improve patient cardiac function, and reduce mortality. Wang et al[24] found that the TNF-α levels in esmolol and control groups showed a downward trend over time. Our meta-analysis found that there was significant difference in TNF-α levels between esmolol and control groups, indicating that improvement in cardiac function may be related to changes in serum inflammatory mediators although published meta-analyses and our results showed no significant difference in WBC counts and IL-6 levels between esmolol and control groups. Considering the small sample sized involved and that the quality of evidence was “very low,” more robust RCTs with larger sample sizes are needed to validate these findings.
A study conducted by Mehta et al[49] found that TnI concentration levels in serum correlated with myocardial dysfunction in septic shock, and high serum TnI levels predicted increased sepsis severity and higher mortality. We found that esmolol could significantly reduce the level of TnI concentration in serum, which was consistent with published meta-analyses,[13–15,18] further confirming that esmolol has a cardioprotective role.
The present study showed that esmolol can significantly decrease 28-day mortality compared with control groups. An observation study with 9465 patients suggested that patients who received chronic β-blocker prescriptions may have a survival advantage if they subsequently develop sepsis.[50] Some studies[17,23,25] have shown a higher survival rate for septic shock in patients treated with esmolol after adequate and early fluid resuscitation, which is consistent with published meta-analyses.[13–15,18] Liu et al[26] showed that esmolol can significantly shorten the length of ICU stay and reduce 28-day mortality. Fuchs et al[51] reported an increased length of ICU stay despite showing substantial 90-day mortality benefits in patients with sepsis, whereas our study found that there was no difference in the length of ICU stay between esmolol and control groups. Considering that heterogeneity was high, our leave-one-out sensitivity analysis showed that the results were robust. However, as the quality of evidence was “very low,” there is insufficient evidence to indicate whether esmolol affects the length of ICU stay.
In an animal experiment by Berk et al,[38] propranolol was shown to reduce lung injury in dogs with sepsis. Morelli et al[28] found that esmolol significantly improved PaO2/FiO2 in patients with septic shock compared with a control group, whereas Higgins et al[22] found no difference between esmolol and control groups. Our meta-analysis also found no difference between esmolol and control groups. As patients numbers were few and the quality of evidence was “very low,” there is insufficient evidence to conclude whether esmolol affects the PaO2/FiO2 ratio; thus, more RCTs are needed to confirm this issue.
This study had the following strengths. First, Huang et al[18] included the largest number of published studies; however, they included several studies published in Chinese that could not be verified in English language PubMed, Embase, and Cochrane library databases, and these studies were of low quality. Our meta-analysis included the most recent RCTs and all of the studies could be verified in English language databases, which have the largest number of participants and studies for included RCTs in English. Second, we analyzed data at 12, 24, 48, 72, and 96 hours separately, when possible, which made our findings more robust, whereas previous meta-analyses have analyzed outcomes through pooling various time points. Third, we also examined the effects of esmolol on the length of ICU stay, SVI, cardiac index, IL-6, TNF-a levels, the WBC count, and the PaO2/FiO2 ratio at different time points when possible, which allowed for a more comprehensive understanding of the effectiveness of esmolol.
This study had several limitations. First, we were not able to assess for publication bias due to the small number of studies included in this analysis, and publication bias could be fully excluded. Second, each patient with sepsis has distinct individual differences in terms of myocardial inhibition, and the methods of esmolol treatment for sepsis differ, which may have affected the pooling results. Third, the best method and optimal dose of esmolol treatment remains to be elucidated. Finally, the quality of the evidence was “very low,” and further larger RCTs are required to validate our findings.
5. Conclusion
Esmolol treatment may be safe and effective in decreasing 28-day mortality, controlling HR, and have a cardioprotective role, while having no effect on lung injury, in patients with sepsis or septic shock after early fluid resuscitation. Improvements in cardiac function may be related to changes in inflammatory mediators in the serum. There were no significant adverse effects on tissue perfusion and oxygen utilization. However, patient numbers in the included studies were few and the quality of evidence was very low; thus, larger RCTs are needed.
Acknowledgments
We thank all the people who participated in the primary randomized controlled trials and the teams who did them.
Author contributions
J.Z. and C.C. initiated and coordinated this study. J.Z., C.C., and J.Y. were responsible for the literature research, data extraction, and statistical analysis. Y.L., Y.Y., and X.Y. participated in the data extraction and analysis. J.Z. wrote the first draft. These studies were reviewed by J.Y. All authors have read and approved the final manuscript.
Abbreviations:
- CI =
- confidence interval
- CENTRAL =
- the Cochrane Central Register of Controlled Trials
- CVP =
- central venous pressure
- HMGB-1 =
- high mobility group box-1
- HR =
- heart rate
- ICU =
- intensive care unit
- IL-6 =
- interleukin 6
- Lac =
- lactic acid
- MAP =
- mean arterial pressure
- RCT =
- randomized controlled trial
- RR =
- risk ratio
- ScvO2 =
- central venous oxygen saturation
- SMD =
- standardized mean difference
- SVI =
- stroke volume index
- TNF-a =
- tumor necrosis factor-a
- TnI =
- troponin I
- WBC =
- white blood cell
The authors have no funding and conflicts of interest to disclose.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
How to cite this article: Zhang J, Chen C, Liu Y, Yang Y, Yang X, Yang J. Benefits of esmolol in adults with sepsis and septic shock: an updated meta-analysis of randomized controlled trials. Medicine 2022;101:27(e29820).
Contributor Information
Jing Zhang, Email: 799138261@qq.com.
Chun Chen, Email: 387719141@qq.com.
Yi Liu, Email: 20200856@qq.com.
Yi Yang, Email: springofsilence@163.com.
Xiaolei Yang, Email: springofsilence@163.com.
References
- [1].Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: barriers and potential solutions. Crit Care. 2018;22:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- [3].Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. [DOI] [PubMed] [Google Scholar]
- [4].Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12:R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rabee HA, Tanbour R, Nazzal Z, et al. Epidemiology of sepsis syndrome among intensive care unit patients at a tertiary university hospital in Palestine in 2019. Indian J Crit Care Med. 2020;24:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ehrman RR, Sullivan AN, Favot MJ, et al. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care. 2018;22:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–7. [DOI] [PubMed] [Google Scholar]
- [10].Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–48. [DOI] [PubMed] [Google Scholar]
- [11].Sander O, Welters ID, Foëx P, et al. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33:81–8; discussion 241. [DOI] [PubMed] [Google Scholar]
- [12].Chacko CJ, Gopal S. Systematic review of use of β-blockers in sepsis. J Anaesthesiol Clin Pharmacol. 2015;31:460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Sun W, Guo Y, et al. Prognosis of β-adrenergic blockade therapy on septic shock and sepsis: a systematic review and meta-analysis of randomized controlled studies. Cytokine. 2020;126:154916. [DOI] [PubMed] [Google Scholar]
- [14].Shi K, Hu Y, Huang J, et al. Efficacy of esmolol for septic shock and sepsis: a meta-analysis of randomized controlled studies. Int J Clin Exp Med. 2018;11:11458–64. [Google Scholar]
- [15].Liu P, Wu Q, Tang Y, et al. The influence of esmolol on septic shock and sepsis: a meta-analysis of randomized controlled studies. Am J Emerg Med. 2018;36:470–4. [DOI] [PubMed] [Google Scholar]
- [16].Yu YW, Sun TW, Wan YD, et al. [Effects of β-blockers in patients with septic shock: a meta analysis]. Zhonghua Yi Xue Za Zhi. 2016;96:570–4. [DOI] [PubMed] [Google Scholar]
- [17].Hasegawa D, Sato R, Prasitlumkum N, et al. Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest. 2021;159:2289–300. [DOI] [PubMed] [Google Scholar]
- [18].Huang P, Zheng X, Liu Z, et al. The efficacy and safety of esmolol for septic shock: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:682232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cocchi MN, Dargin J, Chase M, et al. Esmolol to treat the hemodynamic effects of septic shock: a randomized controlled trial. Shock. 2022;57:508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Higgins J, Thompson S, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu H, Ding XF, Zhang SG, et al. Effect of esmolol in septic shock patients with tachycardia: a randomized clinical trial. Zhonghua yi xue za zhi. 2019;99:1317–22. [DOI] [PubMed] [Google Scholar]
- [24].Wang S, Li M, Duan J, et al. Effect of esmolol on hemodynamics and clinical outcomes in patients with septic shock. Zhonghua wei zhong bing ji jiu yi xue. 2017;29:390–5. [DOI] [PubMed] [Google Scholar]
- [25].Wang Z, Wu Q, Nie X, et al. Combination therapy with milrinone and esmolol for heart protection in patients with severe sepsis: a prospective, randomized trial. Clin Drug Investig. 2015;35:707–16. [DOI] [PubMed] [Google Scholar]
- [26].Liu X, Huang W, Wen M, et al. Esmolol improves clinical outcome and tissue oxygen metabolism in patients with septic shock through controlling heart rate. Zhonghua wei zhong bing ji jiu yi xue. 2015;27:759–63. [PubMed] [Google Scholar]
- [27].Yang S, Liu Z, Yang W, et al. Effects of the β-blockers on cardiac protection and hemodynamics in patients with septic shock: a prospective study. Zhonghua wei zhong bing ji jiu yi xue. 2014;26:714–7. [DOI] [PubMed] [Google Scholar]
- [28].Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–91. [DOI] [PubMed] [Google Scholar]
- [29].Orbegozo Cortes D, Njimi H, Dell’Anna AM, et al. Esmolol for septic shock: more than just heart rate control? Minerva Anestesiol. 2014;80:254–8. [PubMed] [Google Scholar]
- [30].Bristow MR, Feldman AM, Adams KF, Jr., et al. Selective versus nonselective beta-blockade for heart failure therapy: are there lessons to be learned from the COMET trial? J Card Fail. 2003;9:444–53. [DOI] [PubMed] [Google Scholar]
- [31].Jones AE, Craddock PA, Tayal VS, et al. Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with nontraumatic symptomatic undifferentiated hypotension. Shock. 2005;24:513–7. [DOI] [PubMed] [Google Scholar]
- [32].Azimi G, Vincent JL. Ultimate survival from septic shock. Resuscitation. 1986;14:245–53. [DOI] [PubMed] [Google Scholar]
- [33].Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–9. [DOI] [PubMed] [Google Scholar]
- [34].Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Crit Care Med. 1999;27:1775–80. [DOI] [PubMed] [Google Scholar]
- [35].Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–49. [DOI] [PubMed] [Google Scholar]
- [36].de Montmollin E, Aboab J, Mansart A, et al. Bench-to-bedside review: Beta-adrenergic modulation in sepsis. Crit Care. 2009;13:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Werdan K, Schmidt H, Ebelt H, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87:266–74. [DOI] [PubMed] [Google Scholar]
- [38].Berk JL, Hagen JF, Beyer WH, et al. The treatment of endotoxin shock by beta adrenergic blockade. Ann Surg. 1969;169:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Patterson AJ, Zhu W, Chow A, et al. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–8. [DOI] [PubMed] [Google Scholar]
- [40].Aboab J, Sebille V, Jourdain M, et al. Effects of esmolol on systemic and pulmonary hemodynamics and on oxygenation in pigs with hypodynamic endotoxin shock. Intensive Care Med. 2011;37:1344–51. [DOI] [PubMed] [Google Scholar]
- [41].Suzuki T, Morisaki H, Serita R, et al. Infusion of the β-adrenergic blocker esmolol attenuates myocardial dysfunction in septic rat. Crit Care Med. 2005;33:2294–301. [DOI] [PubMed] [Google Scholar]
- [42].Ibrahim-Zada I, Rhee P, Gomez CT, et al. Inhibition of sepsis-induced inflammatory response by β1-adrenergic antagonists. J Trauma Acute Care Surg. 2014;76:320–8. [DOI] [PubMed] [Google Scholar]
- [43].Yu SK, Tait G, Karkouti K, et al. The safety of perioperative esmolol: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2011;112:267–81. [DOI] [PubMed] [Google Scholar]
- [44].Hamzaoui O, Teboul JL. The role of beta-blockers in septic patients. Minerva Anestesiol. 2015;81:312–9. [PubMed] [Google Scholar]
- [45].Angaran DM, Schultz NJ, Tschida VH. Esmolol hydrochloride: an ultrashort-acting, beta-adrenergic blocking agent. Clin Pharm. 1986;5:288–303. [PubMed] [Google Scholar]
- [46].Gore DC, Wolfe RR. Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery. 2006;139:686–94. [DOI] [PubMed] [Google Scholar]
- [47].Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91. [DOI] [PubMed] [Google Scholar]
- [48].Pathan N, Hemingway C, Alizadeh A, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–9. [DOI] [PubMed] [Google Scholar]
- [49].Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95:13–7. [DOI] [PubMed] [Google Scholar]
- [50].Macchia A, Romero M, Comignani P, et al. Previous prescription of β-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med. 2012;40:2768–72. [DOI] [PubMed] [Google Scholar]
- [51].Fuchs C, Wauschkuhn S, Scheer C, et al. Continuing chronic beta-blockade in the acute phase of severe sepsis and septic shock is associated with decreased mortality rates up to 90 days. Br J Anaesth. 2017;119:616–25. [DOI] [PubMed] [Google Scholar]