Abstract
PURPOSE
To examine the overlap of homologous recombination deficiency (HRD) and microsatellite instability high (MSI-H) status, and to dissect driver versus bystander status of BRCA1/2 mutations (BRCAm) in this context.
METHODS
A pan-cancer comprehensive genomic profiling cohort (n = 213,199) was examined for overlap between BRCAm and MSI-H status. BRCA1/2 variant zygosity was examined and correlated with MSI-H status, tumor mutational burden, and genome-wide loss of heterozygosity (gLOH). Clinical histories of two patients with prostate cancer with co-occurring BRCAm and MSI-H are described.
RESULTS
HRD and MSI-H phenotypes were generally mutually exclusive events (P < .001). BRCAm that co-occurred together with high tumor mutational burden or MSI-H were predominantly monoallelic bystander alterations. In breast, ovarian, and pancreatic cancers, very few BRCAm occurred in the context of MSI-H; however, in prostate cancer, 12.8% of BRCA1 and 3.4% of BRCA2 alterations co-occurred with MSI-H. In these BRCA-associated cancers, co-occurring BRCAm were generally monoallelic and were not associated with elevated gLOH. Two patients with prostate cancer with co-occurring BRCAm and MSI-H showed resistance to poly (ADP-ribose) polymerase inhibition but sensitivity to subsequent anti–programmed cell death protein 1 therapy.
CONCLUSION
MSI-H status and HRD are generally mutually exclusive phenomena across cancer types, but may rarely co-occur, especially in prostate cancer. Although MSI-H samples had a higher BRCAm prevalence relative to microsatellite-stable tumors, these BRCA1/2 mutations were generally monoallelic and were not associated with elevated gLOH. Our findings suggest that most BRCAm coexisting with microsatellite instability are likely bystander events that may not result in sensitivity to poly (ADP-ribose) polymerase inhibitors.
INTRODUCTION
Personalized medicine relies on a patient's or a tumor's genomic profile to guide therapy selection and optimize outcomes. The number of targeted cancer therapies on the basis of the genomic profile has increased in recent years. When genomic profiling identifies multiple potential therapeutic options, this presents a diagnostic dilemma where the optimal initial choice of systemic therapy may be unclear.
CONTEXT
Key Objective
Do BRCAm occurring in the context of microsatellite instability high (MSI-H) status result in homologous recombination deficiency (HRD) and poly (ADP-ribose) polymerase inhibitor (PARPi) sensitivity?
Knowledge Generated
MSI-H and HRD were identified as mutually exclusive phenomena, with BRCAm in MSI-H samples being predominantly monoallelic alterations. Two patients with prostate cancer with co-occurring BRCAm and MSI-H were PARPi insensitive but responded to anti–programmed cell death protein 1 agents.
Relevance
BRCA1/2 mutations (BRCAm) and MSI-H represent actionable biomarkers that can guide precision medicine strategies in multiple cancers. Our findings demonstrate that BRCAm occurring in MSI-H cancers may not result in HRD or sensitivity to PARPi and imply that such patients should be treated preferentially with anti–programmed cell death protein 1 agents rather than PARPi.
Previous reports have identified a potential co-occurrence of BRCA1/BRCA2 mutations (BRCAm), a target for poly (ADP-ribose) polymerase inhibitors (PARPi), and microsatellite (MS) instability high (MSI-H), a biomarker of immune checkpoint blockade (ICB) response.1,2 However, these reports have not assessed whether such BRCA1 or BRCA2 mutations resulted in functional homologous recombination deficiency (HRD) or PARP inhibitor sensitivity.3,4 Since MSI-H tumors often harbor thousands of mutations across the exome, some of these BRCA mutations may represent monoallelic bystander events that leave the second copy of BRCA intact and do not result in PARPi sensitivity.
Here, we explored the overlap of microsatellite instability (MSI) with a scar-based measure of HRD, genome-wide loss of heterozygosity (gLOH), across multiple tumor types and examined the allelic status of BRCAm in these samples. gLOH signatures are used clinically in ovarian cancer for identifying patients who may respond to PARPi and have been previously reported to associate with biallelic BRCA1/2 alterations across tumor types.5,6 We report that BRCAm in MSI-H cancers are usually monoallelic and not associated with HRD signatures. We also present the clinical experience of two patients with prostate cancer with co-occurring BRCAm and MSI and their outcomes to consecutive PARPi and ICB agents.
METHODS
Comprehensive Genomic Profiling
Comprehensive genomic profiling for all classes of alterations in at least 324 genes was performed on all-comers during routine clinical care using hybrid capture-based next-generation sequencing in a Clinical Laboratory Improvement Amendments–certified laboratory as previously described (Foundation Medicine Inc, Cambridge, MA).7 Tumor mutational burden (TMB), gLOH, and MS status were called as previously described.5,8,9 Gene alteration status was categorized as biallelic (deleterious mutation with loss of heterozygosity [LOH] of the wild-type allele, homozygous deletion, or two or more deleterious variants), monoallelic (heterozygous deleterious mutation with wild-type second allele), or wild-type (no BRCA variants in either allele).10 Zygosity was determined as previously described.10 For each sample, we created a genome-wide copy number profile with circular binary segmentation and a Gibbs sampling Markov Chain Monte Carlo algorithm, on the basis of log-ratios to a process-matched control and allele frequencies at over 3,500 single nucleotide polymorphisms.7,10 To determine zygosity, we model every possible variant allele count and somatic/germline status using the modeled purity and variant allele fraction. For the modeled variant allele count and germline status, the goodness of fit is measured using a two-tailed binomial test; only samples with a > 99% fit are given a status, otherwise they are modeled as unknown status. Modeling was limited to samples with > 30% tumor purity and to samples that pass signal:noise copy number metrics; most unknown calls were a result of purity requirements. Homologous recombination repair (HRR) genes include ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, and RAD54L. Approval for this study, including a waiver of informed consent and Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (protocol No. 20152817). The two patients with prostate cancer treated with olaparib and pembrolizumab were approved by Johns Hopkins University institutional review board (with a waiver of informed consent for a retrospective study).
Statistical Analyses
Proportions were compared using a univariate Fisher's exact test with 95% binomial CIs. Continuous distributions were compared using a Mann-Whitney U test. Multiple hypothesis correction used the false-discovery rate method.
RESULTS
Overlap of BRCAm and MSI-H
We examined a cohort of 213,199 real-world cancer patients profiled with comprehensive genomic profiling. Consistent with previous reports, BRCAm were observed at a higher frequency in MSI-H cases relative to MS-stable cases (19.7% [678/3,446] in MSI-H and 5.3% [11,056/208,971] in MS-stable; odds ratio [OR] = 4.4; P < .001). When examining a scar-based measure of HRD (gLOH > 16%), however, MSI-H and gLOH-high cancers were found to be mutually exclusive (Fig 1A; OR = 0.18; P < .001), with only 0.07% [148 of 213,199] of samples showing co-occurrence of both events across the data set. This mutual exclusivity was observed consistently across most malignancies (Fig 1B), with strong mutual exclusivity observed in endometrial, ovarian, stomach, colorectal, and prostate cancers (all OR < 0.10, all P < .001).
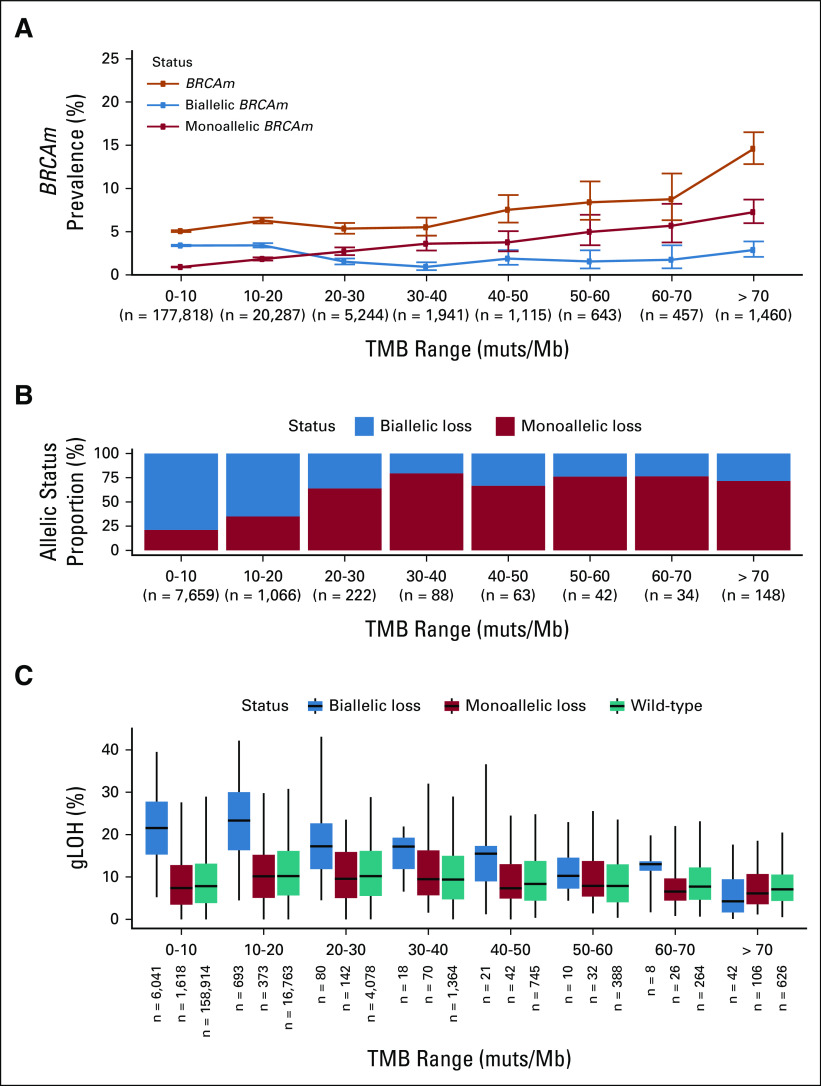
FIG 1.
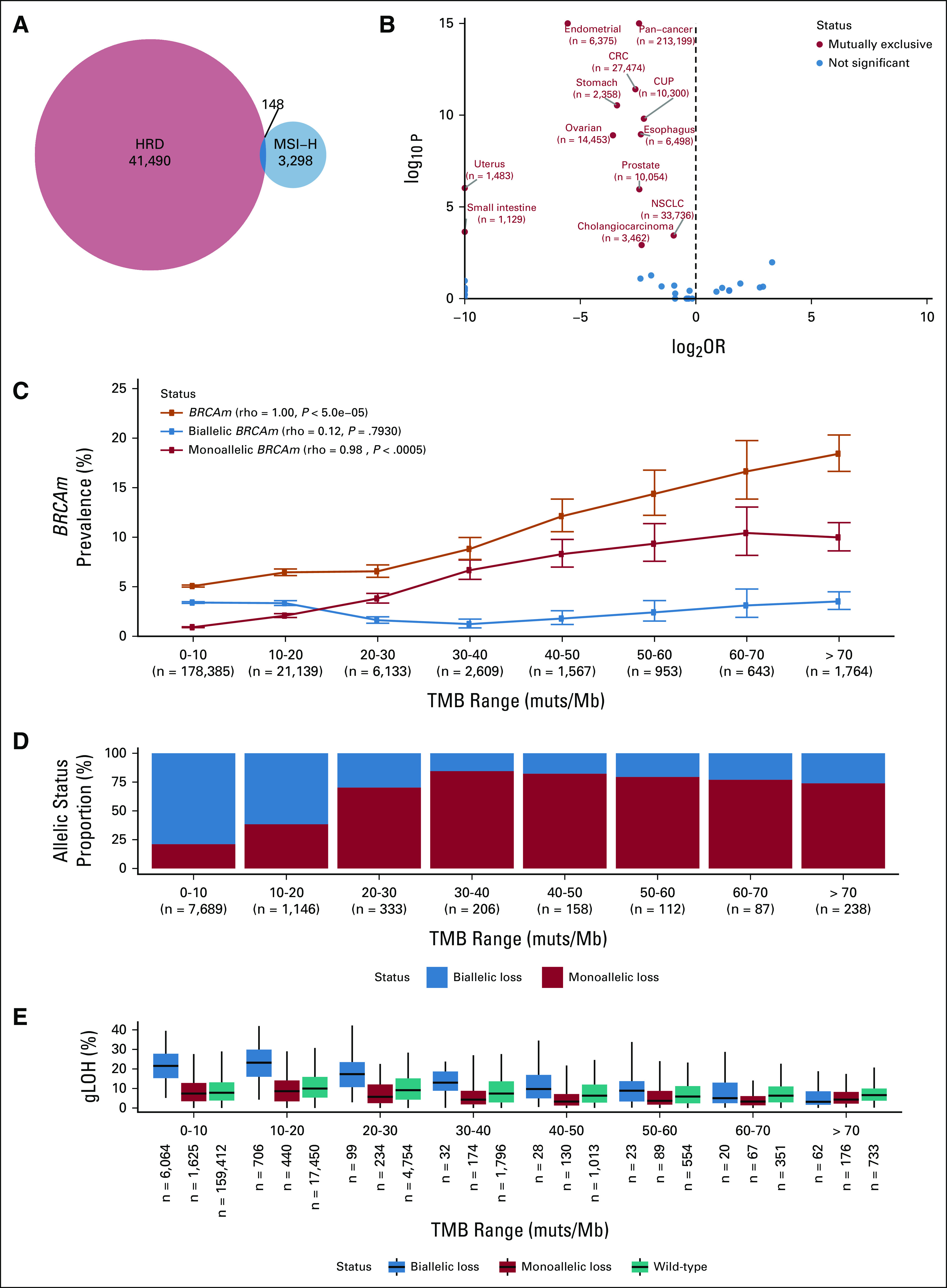
MSI-H and HRD are mutually exclusive phenomena. (A) HRD (gLOH ≥ 16%) and MSI-H were mutually exclusive in a pan-cancer analysis. (B) Volcano plot examining mutual exclusivity patterns across disease groups; log2 ORs were capped at ±10, and P values were capped at 1E-15. P values were multiple hypothesis corrected. (C) BRCA1/BRCA2 mutation prevalence binned on the basis of sample TMB (orange line). Predicted monoallelic (red) and biallelic (blue) BRCAm prevalence was plotted on the same axes. (D) Fraction of BRCAm predicted as monoallelic (red) and biallelic (blue) in each TMB bin; analyses were limited to samples where allelic status could be determined. (E) gLOH distribution for biallelic BRCAm, monoallelic BRCAm, and BRCA1/2 wild-type samples in each TMB bin. CRC, colorectal cancer; CUP, carcinoma of unknown primary; gLOH, genome-wide loss of heterozygosity; HRD, homologous recombination deficiency; MSI-H, microsatellite instability high; NSCLC, non–small-cell lung cancer; OR, odds ratio; TMB, tumor mutational burden.
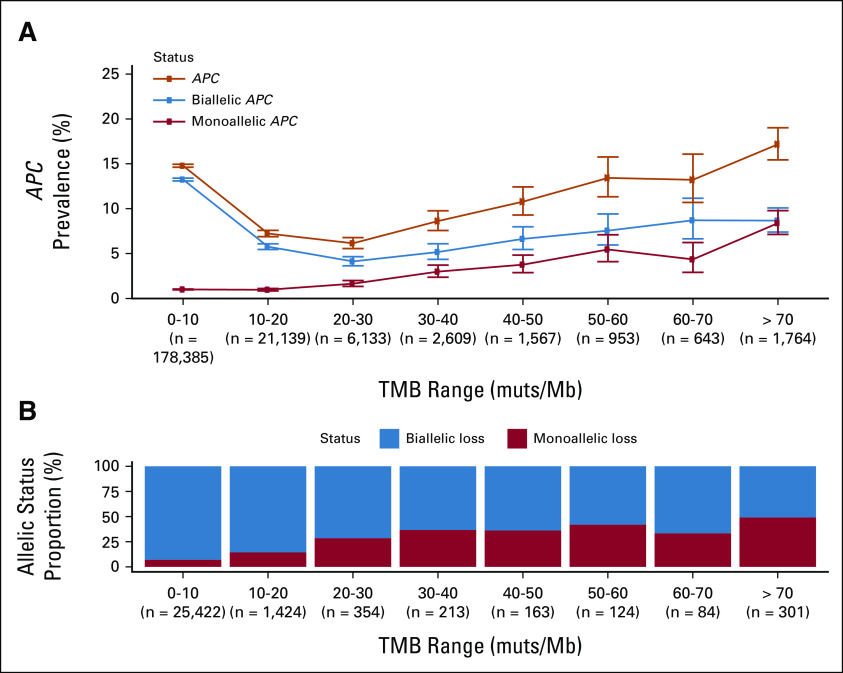
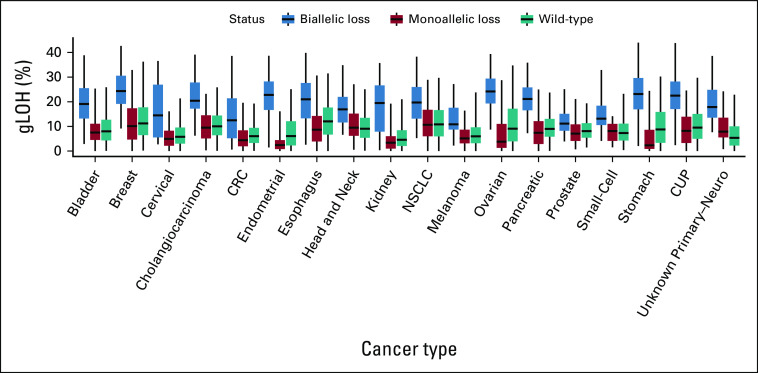
MSI leads to the accumulation of numerous genome-wide mutations, particularly insertion/deletion events and to a high TMB (median 31.3 muts/Mb in MSI-H) compared with 3.8 muts/Mb for MS-stable samples. We hypothesized that the higher prevalence of BRCAm was a result of accumulated bystander mutations in the context of high TMB. Consistent with this hypothesis, BRCAm frequency increased in a stepwise fashion with increasing TMB strata (Fig 1C), with BRCAm occurring in 8.8% of cases with TMB 30-40 muts/Mb and in 18.4% of cases with TMB > 70 muts/Mb. Similar results were observed in MS-stable samples, with elevated rates of BRCAm in higher TMB strata (Appendix Fig A1) and APC, another tumor suppressor gene (Appendix Fig A2). In these higher TMB cases, a larger relative proportion of BRCA alterations were monoallelic, implying they are likely bystander mutations (Figs 1C and 1D). Monoallelic alterations were linked to TMB (Spearman rho = 0.98) while biallelic mutation frequency remained stable (Spearman rho = 0.12). Across TMB strata and diseases, monoallelic BRCAm were associated with low gLOH scores (Fig 1E; Appendix Fig A3), suggesting that they do not result in HRD.
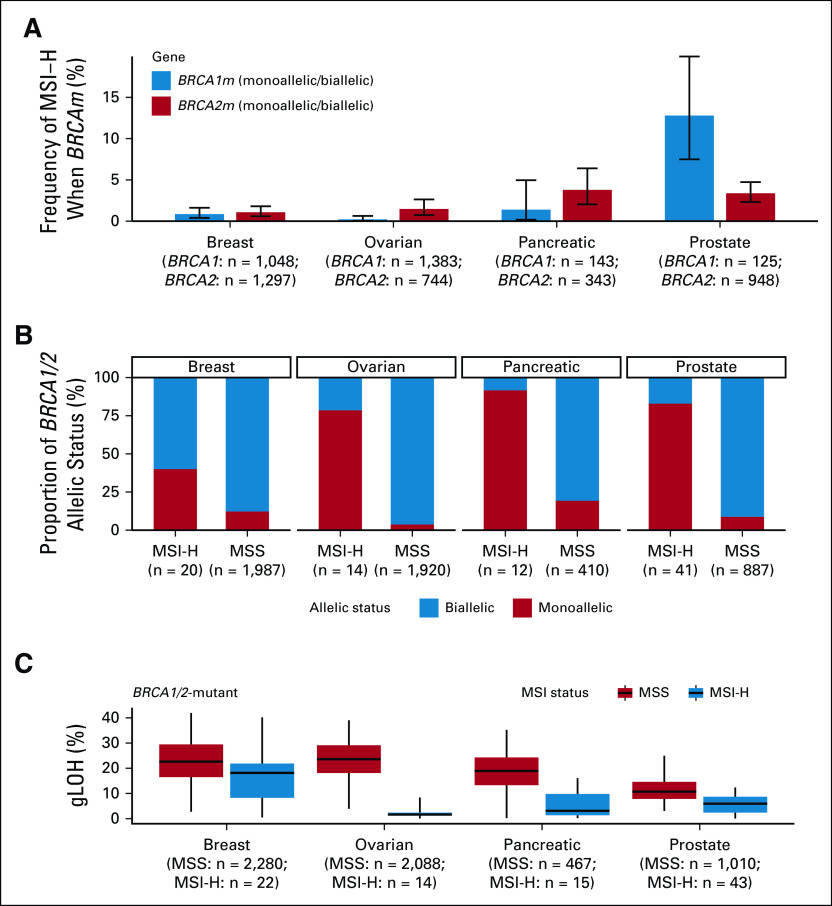
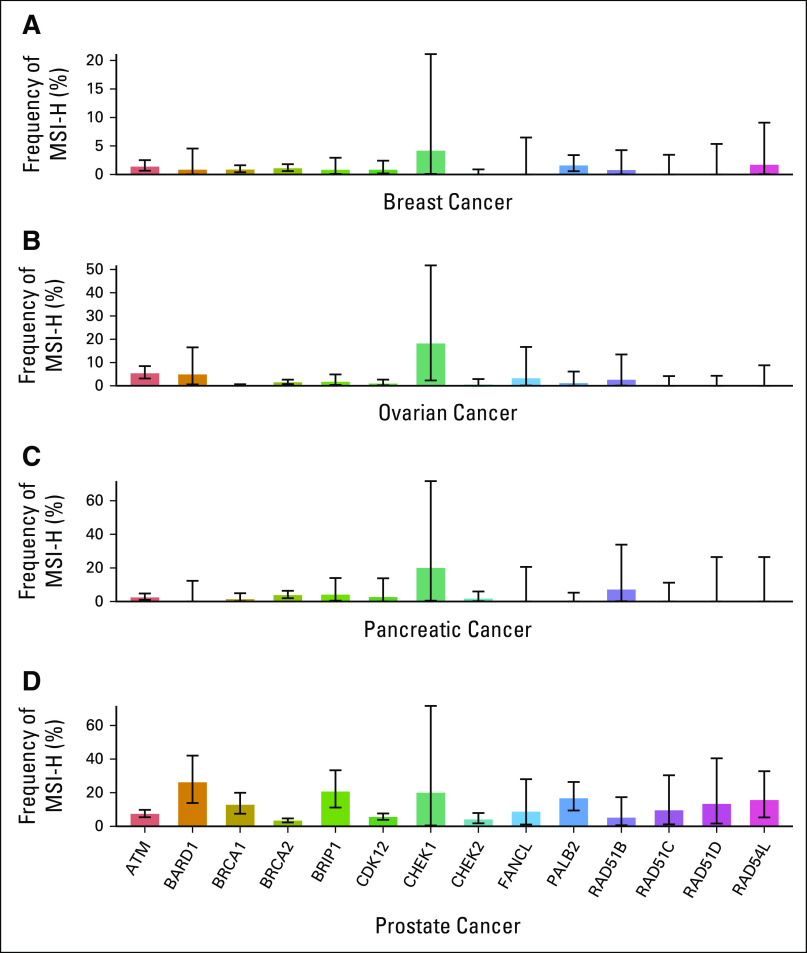
PARP inhibitors are currently approved for the treatment of advanced ovarian, breast, pancreatic, and prostate cancers in the setting of BRCA1/2 mutations.11-14 To understand how often MSI co-occurs with BRCAm, we examined the overlap of the two biomarkers in these diseases (Fig 2A). Although very few BRCAm cases were MSI-H in breast and ovarian cancers (< 2%) and only modest rates of overlap were seen in pancreatic cancer (1.4% BRCA1, 3.8% BRCA2), 12.8% of BRCA1 and 3.4% of BRCA2 alterations co-occurred with MSI-H in prostate cancer. When expanded to include the full set of 14 HRR genes approved as a companion diagnostic for olaparib use in prostate cancer,12 the overlap was even more dramatic with 46.3% of MSI-H prostate cancer samples harboring at least one HRR gene mutation (Appendix Fig A4). In cases of overlap of the two biomarkers, BRCAm were predominantly monoallelic (Fig 2B) and were associated with lower gLOH (Fig 2C) compared with biallelic BRCAm, suggesting that these alterations occurring in the context of MSI did not result in HRD.
FIG 2.
BRCAm frequently co-occur with MSI-H in prostate cancer but not in other BRCA-associated cancers. (A) Frequency of MSI-H status among patients with mutations in BRCA1 or BRCA2 for breast, ovarian, pancreatic, and prostate cancer. (B) Fraction of alterations in each cancer type that are predicted to be monoallelic/biallelic according to MSI status. (C) Distribution of gLOH in BRCAm samples, stratified by MSI status and cancer type. gLOH, genome-wide loss of heterozygosity; MSI, microsatellite instability; MSI-H, microsatellite instability high; MSS, microsatellite stable.
Clinical Outcomes in Two Patients With Prostate Cancer With BRCAm/MSI-H Overlap
We also report the outcomes of two patients with metastatic castration-resistant prostate cancer (CRPC) with concurrent MSI-H status and BRCA1/2 mutations. Neither patient had a prostate-specific antigen (PSA) response upon PARP inhibitor treatment, whereas both showed PSA responses to subsequent programmed cell death protein 1 (PD-1) inhibition of variable durations (Fig 3).
FIG 3.
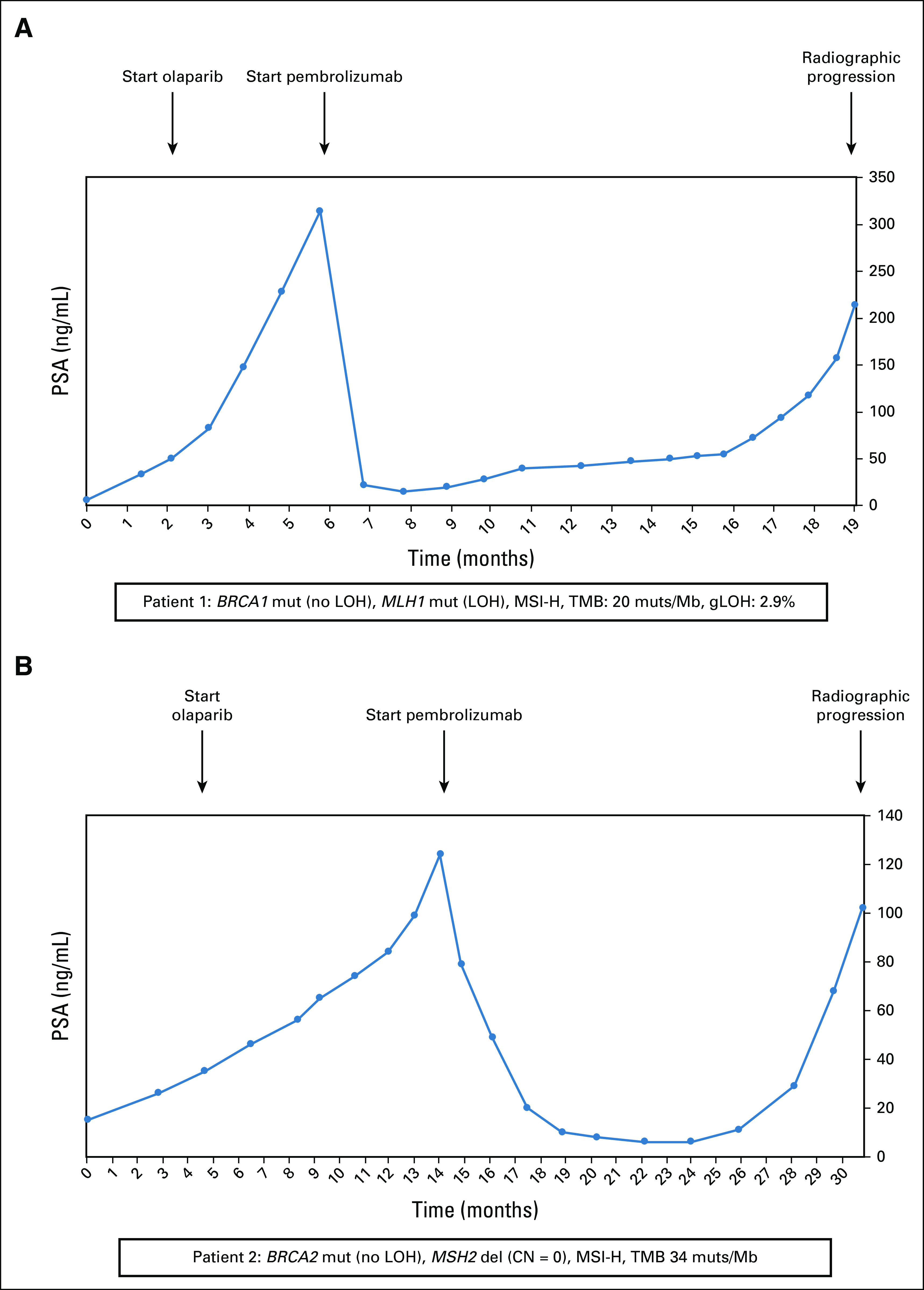
Treatment history of two patients with metastatic castration-resistant prostate cancer with co-occurring MSI-H and BRCAm. (A) Patient 1 was MSI-H with a BRCA1 mutation, and (B) patient 2 was MSI-H with a BRCA2 mutation. CN, copy number; gLOH, genome-wide loss of heterozygosity; LOH, loss of heterozygosity; MSI, microsatellite instability; MSI-H, microsatellite instability high; PSA, prostate-specific antigen; TMB, tumor mutational burden.
Patient 1 (Fig 3A) was a 72-year-old African American man who presented with a PSA level of 43.4 ng/mL and metastatic pelvic and retroperitoneal lymphadenopathy. His prostatic biopsy revealed Gleason 4 + 5 = 9 adenocarcinoma with cribriform morphology, and immunohistochemistry analysis demonstrated loss of MLH1 and PMS2 proteins, with intact MSH2 and MSH6 proteins. Germline DNA analysis was unremarkable. Genomic analysis from the prostate biopsy showed a somatic BRCA1 mutation (p.Q1111fs*5, without LOH) and a MLH1 mutation (p.T206fs*23, with LOH). He also had somatic mutations in PTEN (p.T319fs*1), CTNNB1 (p.T41A), NF1 (p.K1386fs*20), RNF43 (p.G659fs*41), CIC (p.P1116fs*45), JAK1 (p.P430fs*2), CSF1R (p.E317fs*55), and the classic TMPRSS2-ERG fusion. His tumor was characterized as MSI-H, with a TMB of 20 mutations/Mb. The gLOH score was low (2.9%), suggesting a lack of homologous recombination repair deficiency. The patient was treated with a combination of androgen deprivation therapy plus abiraterone but eventually developed metastatic CRPC. He then received enzalutamide, with a transient response to this agent. By month 2, he had developed disease progression with a PSA level rising to 50 ng/mL. Because of the pathogenic BRCA1 mutation, he was started on olaparib 300 mg orally twice daily, resulting in stabilization of his bone disease despite a continued rise in his PSA. His PSA level continued to rise to 314 ng/mL by month 5. Imaging showed progressive bone metastases and new liver metastases, and his olaparib therapy was stopped. At that time, his treatment was switched to pembrolizumab 200 mg intravenously once daily every 3 weeks, which resulted in a rapid PSA reduction accompanied by a partial radiographic remission of his liver lesions and an improvement in his bone pain. His response to PD-1 inhibition lasted approximately 13 months and was followed by a subsequent progression of his bone and liver metastases. Chemotherapy with docetaxel was initiated.
Patient 2 (Fig 3B) was a 54-year-old White man who presented with high-risk localized prostate cancer and a PSA level of 3.7 ng/mL. He underwent prostatectomy, which revealed Gleason 5 + 4 = 9 adenocarcinoma with ductal features. Immunohistochemistry analysis demonstrated loss of MSH2 and MSH6 proteins, with intact expression of MLH1 and PMS2 proteins. Germline genetic testing was unremarkable. Genomic analysis from the prostatectomy specimen showed a somatic BRCA2 mutation (p.N1784Kfs*3, without LOH) and a MSH2 homozygous deletion (ie, biallelic loss). He also had somatic mutations in MSH6 (p.F1088fs*5), JAK1 (p.K860fs*16), KMT2D (p.P2354fs*1), NOTCH1 (p.D1815fs*1), SPEN (p.R807fs*3), AXL (p.H292fs*1), LRP1B (p.C1859fs*1), RECQL4 (p.V155fs*1), and TP53 (p.R273C). His tumor was MSI-H, with a TMB of 34 mutations/Mb. After surgical resection, the patient developed a postoperative biochemical recurrence and was treated with salvage pelvic radiotherapy plus concurrent androgen deprivation therapy. Unfortunately, his disease rapidly progressed to CRPC with bone involvement. He received abiraterone, followed by enzalutamide, with transient control of his disease. By month 4, his PSA level had reached 35 ng/mL despite enzalutamide treatment. Because of the pathogenic BRCA2 mutation, he was started on olaparib 300 mg twice daily, but his disease continued to progress. Ten months after initiation of olaparib, his PSA level had reached 124 ng/mL despite PARP inhibitor treatment, and imaging tests showed bone scan progression. Olaparib exposure was stopped, and the patient was placed on pembrolizumab 200 mg intravenously once daily every 3 weeks, which resulted in a PSA reduction accompanied by a stabilization of his bone metastases. His clinical benefit from PD-1 inhibition lasted about 16 months, followed by another PSA elevation and eventual progression of his bone metastases. He was subsequently referred for clinical trial participation.
DISCUSSION
Our study identified MSI-H status and HRD as mutually exclusive phenomena across cancer types. Although MSI-H samples had a higher BRCA1/2 mutation rate relative to MS-stable samples across cancers, the resulting BRCA mutations were generally monoallelic and were not associated with elevated gLOH. These findings suggest that many BRCAm occurring in the context of MSI are likely bystander events that may not result in sensitivity to PARP inhibitors. Accordingly, two patients with BRCAm/MSI-H prostate cancer derived no benefit from PARP inhibitor treatment but subsequently responded favorably to pembrolizumab. Interestingly, the probability of a BRCA mutation being attributable to MSI is highest in prostate cancer relative to other BRCA-associated malignancies. Thus, this diagnostic and therapeutic dilemma may occur most commonly in the context of prostate cancer.
A recent Memorial Sloan Kettering Cancer Center report suggests that patients with BRCA2 mutations were more susceptible to ICB.15 Interestingly, the benefit was only observed in patients who were not typically rich in HRD (ie, melanoma, small-cell lung cancer), whereas patients with HR-associated tumors (breast, prostate, pancreatic, or ovarian) did not derive benefit from ICB. Our results indicate that most of the BRCA1 and BRCA2 deleterious alterations in HR indications are biallelic and sensitive to PARP inhibitors, but the small subset that is present in highly mutated tumors or tumors carrying MSI-H are monoallelic and insensitive to PARPi but responsive to ICB.
Currently, most clinical-grade genomic assays do not report the status of both BRCA alleles nor do they report gLOH scores (or other measures of HRR deficiency) except in the setting of ovarian cancer.5 Thus, if a clinician encounters a genomic report that shows both MSI-H status and BRCA mutation, it is difficult to decipher if that cancer is driven primarily by HRR deficiency or mismatch repair deficiency. Our data suggest that such patients should be treated preferentially with PD-1 inhibitors rather than a PARP inhibitor (in cases where both therapies have US Food and Drug Administration approval).
This study was limited by the small number of patients with combined BRCAm/MSI-H status who received PARP inhibitor treatment, and we do not know if our anecdotal findings in prostate cancer apply to other malignancies. Therefore, our clinical recommendations should be interpreted with caution. We encourage the international community to collectively study the outcomes of PARP and PD-1 inhibitors among patients with the combined BRCAm/MSI-H phenotype.
APPENDIX
FIG A1.
Association of TMB with BRCAm frequency in MS-stable tumors (A) BRCA1/BRCA2 mutation prevalence binned on the basis of sample TMB (orange line) in MS-stable samples. Predicted monoallelic (red) and biallelic (blue) BRCAm prevalence was plotted on the same axes. (B) Fraction of BRCAm predicted as monoallelic (red) and biallelic (blue) in each TMB bin; analyses were limited to samples where allelic status could be determined. (C) gLOH distribution for biallelic BRCAm, monoallelic BRCAm, and BRCA1/2 wild-type samples in each TMB bin. gLOH, genome-wide loss of heterozygosity; MS, microsatellite; TMB, tumor mutational burden.
FIG A2.
Association of TMB with APC mutations (A) APC mutation prevalence binned on the basis of sample TMB (orange line). Predicted monoallelic (red) and biallelic (blue) mutation prevalence was plotted on the same axes. (B) Fraction of APC mutations predicted as monoallelic (red) and biallelic (blue) in each TMB bin; analyses were limited to samples where allelic status could be determined. TMB, tumor mutational burden.
FIG A3.
Monoallelic BRCA alterations are not associated with elevated gLOH across cancer types. Distribution of gLOH scores is shown across cancer types for patients with biallelic BRCAm (blue), monoallelic BRCAm (red), and BRCA1/2 wild-type status (teal). CRC, colorectal cancer; CUP, carcinoma of unknown primary; gLOH, genome-wide loss of heterozygosity; NSCLC, non–small-cell lung cancer.
FIG A4.
Homologous recombination deficiency alterations are frequently associated with MSI-H status in prostate cancer. Frequency of MSI-H status among patients with mutations in a broad basket of 14 HRR-associated genes in patients with (A) breast, (B) ovarian, (C) pancreatic, and (D) prostate cancer. These 14 genes represent the biomarker panel for the recent US Food and Drug Administration approval of olaparib in advanced prostate cancer. MSI-H, microsatellite instability high.
Ethan S. Sokol
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Submitted patent for HRD calling methodology (Inst)
Dexter X. Jin
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Alexander Fine
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Sally E. Trabucco
Employment: Foundation Medicine
Stock and Other Ownership Interests: Neon Therapeutics, Roche, BioNTech, Bristol Myers Squibb
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent pending with Foundation Medicine and Genentech co-inventors
Sophia Maund
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Garrett Frampton
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Luciana Molinero
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: P35974-US Patent Application Inventorship
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
SUPPORT
Supported by Foundation Medicine Inc. E.S.A. is partially supported by Department of Defense grant W81XWH‐17‐2‐0027 and National Institutes of Health grant R01CA238384-A1.
E.S.S. and D.X.J. contributed equally to this work. L.M. and E.S.A. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Ethan S. Sokol, Dexter X. Jin, Sophia Maund, Garrett Frampton, Luciana Molinero, Emmanuel S. Antonarakis
Provision of study materials or patients: Emmanuel S. Antonarakis
Collection and assembly of data: Ethan S. Sokol, Sophia Maund, Garrett Frampton, Emmanuel S. Antonarakis
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ethan S. Sokol
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Submitted patent for HRD calling methodology (Inst)
Dexter X. Jin
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Alexander Fine
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Sally E. Trabucco
Employment: Foundation Medicine
Stock and Other Ownership Interests: Neon Therapeutics, Roche, BioNTech, Bristol Myers Squibb
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent pending with Foundation Medicine and Genentech co-inventors
Sophia Maund
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Garrett Frampton
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Luciana Molinero
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: P35974-US Patent Application Inventorship
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
No other potential conflicts of interest were reported.
REFERENCES
- 1.Patel PS, Algouneh A, Hakem R.Exploiting synthetic lethality to target BRCA1/2-deficient tumors: Where we stand Oncogene 403001–30142021 [DOI] [PubMed] [Google Scholar]
- 2.Adashek JJ, Subbiah V, Kurzrock R.From tissue-agnostic to N-of-One therapies:(R)Evolution of the precision paradigm Trends Cancer 715–282021 [DOI] [PubMed] [Google Scholar]
- 3.Segev Y, Pal T, Rosen B, et al. Risk factors for ovarian cancers with and without microsatellite instability Int J Gynecol Cancer 231010–10152013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Harpaz N, Gatt YE, Granit RZ, et al. Mucinous histology, BRCA1/2 mutations, and elevated tumor mutational burden in colorectal cancer (vol 2020, 6421205, 2020) J Oncol. 2020;2020:6421205. doi: 10.1155/2020/6421205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial Lancet 3901949–19612017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol ES, Pavlick D, Khiabanian H, et al. Pan-cancer analysis of BRCA1 and BRCA2 genomic alterations and their association with genomic instability as measured by genome-wide loss of heterozygosity JCO Precis Oncol 4442–4652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing Nat Biotechnol 311023–10312013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trabucco SE, Gowen K, Maund SL, et al. A novel next-generation sequencing approach to detecting microsatellite instability and pan-tumor characterization of 1000 microsatellite instability-high cases in 67,000 patient samples J Mol Diagn 211053–10662019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun JX, He Y, Sanford E, et al. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput Biol. 2018;14:e1005965. doi: 10.1371/journal.pcbi.1005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib after platinum-based chemotherapy in patients with newly diagnosed advanced ovarian cancer and a Brca mutation: Efficacy by the timing of surgery and residual tumour status following upfront or interval cytoreductive surgery in the phase iii Solo1 trial Int J Gynecol Cancer 29A14–A152019 [Google Scholar]
- 12.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer N Engl J Med 3822091–21022020 [DOI] [PubMed] [Google Scholar]
- 13.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer N Engl J Med 381317–3272019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation (vol 377, pg 523, 2017) N Engl J Med. 2017;377:1700. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 15.Samstein RM, Krishna C, Ma X, et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy Nat Cancer 11188–12032021 [DOI] [PMC free article] [PubMed] [Google Scholar]