Abstract
Introduction There is an imminent need for faster-acting and more effective antidepressants beyond the monoaminergic hypothesis.
Methods We systematically searched the US Clinical Trials registry for antidepressant compounds with completed phase II and III trials. Compounds that demonstrated significant superiority over placebo in the primary outcome measure in the latest phase of phase II and III trials were identified. The collateral information was gathered via a PubMed search and press releases.
Results Nine compounds were identified. AXS-05 (a combination of dextromethorphan and bupropion) and ansofaxine hydrochloride showed a positive result over placebo in a phase III study for major depressive disorder or treatment-resistant depression. MIJ821, nitrous oxide, psilocybin, ayahuasca, facial injection of botulinum toxin A, prasterone, and casopitant demonstrated at least one positive result in phase II trials. Ayahuasca showed a greater response rate than placebo at week one, indicating the rapid antidepressant effect.
Discussion These new compounds with novel mechanisms of action are expected to provide a greater variety of treatment options for depression if preliminary positive results are confirmed.
Key words: antidepressant, depression, fast-acting, pipeline, treatment-resistant depression
Introduction
Antidepressants based on the monoamine hypothesis of depression have played a crucial role in treating major depressive disorder (MDD) for decades 1 . Current treatment guidelines recommend the use of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for initial treatment 2 3 4 . However, there are several challenges associated with the use of these antidepressants. First, the onset of antidepressant action is delayed; numerous double-blind, randomized controlled trials (DBRCTs) have demonstrated that the superiority of antidepressants to placebo generally occurs two or more weeks after initiating the intervention 5 . Second, approximately one-third of the patients with MDD do not achieve remission even after up to four antidepressant trials 6 . Furthermore, 40%–70% of those who initially responded to the treatment subsequently relapse within 1 year 7 . These shortcomings of conventional antidepressants emphasize the imminent need for faster-acting and more effective antidepressants beyond the monoaminergic hypothesis.
While SSRIs and SNRIs are still called “newer” antidepressants, the first SSRI, fluoxetine, was introduced to the market more than 40 years ago; much newer promising antidepressant compounds have recently been tested 8 9 10 . For example, multiple studies have demonstrated the effectiveness of ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, and (s)-ketamine, an S-enantiomer of ketamine, for treatment-resistant depression (TRD) and suicidal ideation 11 12 . Furthermore, there are several rapid-acting antidepressant compounds, such as α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) modulators, metabotropic glutamate receptor antagonists, γ-aminobutyric acid type A receptor modulators, muscarinic receptor antagonists, and psychedelic drugs 8 9 10 . However, despite their promising preliminary results, some of these compounds failed to show any significant benefit over placebo in phase II and III trials.
In this review, we conducted a systematic search of the US Clinical Trials registry for antidepressant compounds in the pipeline and reviewed the compounds that showed positive results in the most recent phase of phase II and III trials.
Methods
We performed a systematic search for DBRCTs of antidepressant compounds in the US Clinical Trials registry ( https://clinicaltrials.gov/ ), with the following filters: condition or disease, “depression”; recruitment, “completed” or “unknown”; and trial phase, “Phase II” and “Phase III” (last search: August 22nd, 2021). The trials were excluded if (1) they recruited patients with bipolar depression, depression due to physical or neurological disorders, or premenstrual dysphoric disorder; (2) compounds tested were already marketed for depression; (3) compounds tested as adjunctive therapy to ongoing antidepressants; (4) depressive symptomatology was not assessed as a primary outcome. After identifying clinical trials that fulfilled the selection criteria, test compounds that demonstrated statistically significant superiority over placebo in the primary outcome measure at the primary endpoint in the latest phase were identified. Collateral information on the included compounds was gathered from published articles via a PubMed literature search and press releases. The compounds were excluded from this review if the literature search revealed that they failed to obtain approval for depression or their development was terminated. TRD was defined as depression that failed to respond to one or more antidepressant trials in the present review. The superiority to placebo was defined as significant effectiveness over placebo in the primary outcome measure at the primary endpoint. Response was defined as a≥50% score reduction at primary endpoint from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS), 17-item or 21-item Hamilton Depression Rating Scale (HAMD 17 or HAMD 21 ), or 16-item Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR 16 ). Rapid-acting was defined as demonstrating superiority to placebo in response rate by the end of the first week of treatment.
Results
The search schematic is shown in Fig. 1 . A total of 176 antidepressant compounds were assessed for eligibility, and 167 compounds were excluded based on the exclusion criteria and negative study results. Thus, nine compounds fulfilled all selection criteria ( Table 1 ). The compounds which were excluded due to a lack of any significant positive result or termination of development are summarized in Table 2 .
Fig. 1.
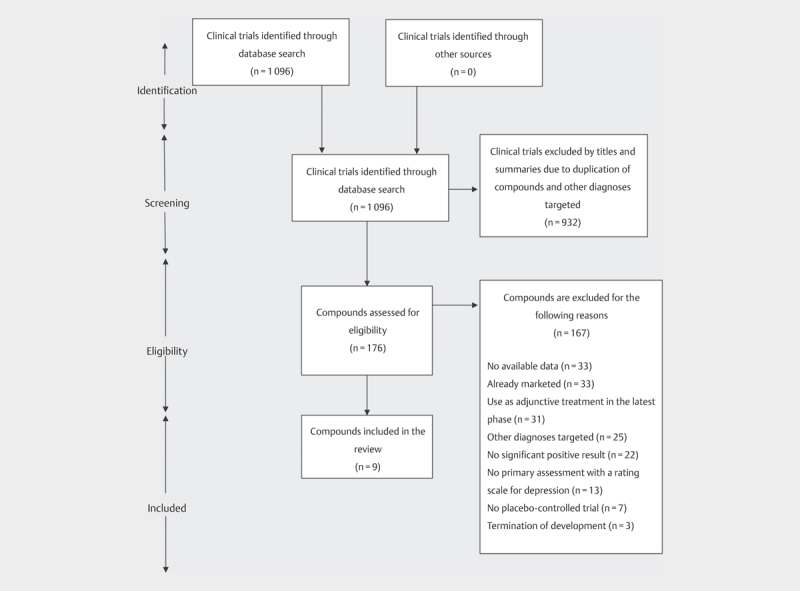
A flow diagram of search of clinical trials and antidepressant compounds.
Table 1 Novel antidepressant in the pipeline.
| Compound | Presumed mechanism of antidepressant action | Phase | Sponsor | Characteristics |
|---|---|---|---|---|
| AXS-05 | NMDAR antagonism | III | Axsome | AXS-05 showed rapid and durable improvement in the MADRS total score compared to placebo in one phase III DBRCT (NCT04019704). Another phase III DBRCT demonstrated its rapid antidepressant effects in the MADRS total score compared to bupropion (NCT02741791). One phase III open-label safety study has just been completed without any reported results (NCT04039022). One phase II trial is still in the recruitment stage on ClinicalTrials.gov (NCT04634669). |
| MIJ821 (CAD9271) | NMDAR subtype 2B negative allosteric modulation | II | Novartis | MIJ821 demonstrated greater improvements than placebo in the MADRS total score at 24 hours in a phase II DBRCT (NCT03756129). One phase II DBRCT is ongoing (NCT03756129). |
| Nitrous oxide | NMDAR antagonism | II | None | In a phase II crossover DBRCT, 50% nitrous oxide plus 50% oxygen was superior to that of placebo gas in the improvement of the HAMD 21 at 2 h and 24 h (NCT02139540). The results of other phase II studies examining 25% and 50% nitrous oxide have not been reported yet (NCT03283670, NCT03932825). There is one ongoing phase II DBRCT (NCT03869736). |
| Psilocybin | 5-hydroxytryptamine 2 A agonism | II | None | One phase II waiting list–controlled trial demonstrated that the HAMD 17 scores after two psilocybin sessions in the psilocybin group were significantly lower than those in the waiting-list group (NCT03181529). In another phase II DBRCT, two administrations of psilocybin were not inferior to the treatment of escitalopram in the QIDS-SR 16 (NCT03429075). Some phase-II DBRCTs are ongoing (NCT03775200, NCT03866174, NCT03715127). |
| Ayahuasca | 5-hydroxytryptamine 2 A agonism and Monoamine oxidase inhibition | II | None | Rapid antidepressant effects in the HAMD total score were observed in ayahuasca compared to placebo in one phase-II DBRCT (NCT02914769). No ongoing trial was registered on ClinicalTrials.gov. |
| Botulinum toxin A (BTA) | Positive effects of altered facial expression on emotional perception | II | None | In a phase II crossover DBRCT, there was a significant improvement in the HAMD 21 in the patients who received BTA compared with those who received placebo (NCT01392963). In another phase II DBRCT, BTA showed a significant score reduction in the MADRS compared to placebo (NCT02116361). The addition of BTA to an ongoing antidepressant also demonstrated greater efficacy in the HAMD 17 score reduction over placebo in a phase II DBRCT. In a phase IV DBRCT, BTA showed a greater response rate on the MADRS compared to placebo (NCT01556971). One clinical trial comparing two facial injection sites is ongoing (NCT03484754). |
| Prasterone (dehydroepiandrosterone, DHEA) | Sigma receptor agonism | II | None | In one phase II crossover DBRCT, the administration of DHEA resulted in a significant improvement in the HAMD 17 compared with placebo (NCT00001487). No ongoing trial was registered on ClinicalTrials.gov. |
| Casopitant (GW679769) | NK receptor antagonism | II | GlaxoSmithKline | In one phase II DBRCT, there was a significant difference in the HAMD 17 score change between casopitant and placebo (NCT00413023). Another phase II DBRCT reported that neither casopitant nor paroxetine achieved statistical separation from placebo on the HAMD 17 (NCT00102492). No active trial was registered on ClinicalTrials.gov. |
| Ansofaxine hydrochloride (LY03005, LPM570065) | Serotonin-norepinephrine-dopamine triple reuptake inhibition | III | Luye | Ansofaxine hydrochloride showed an antidepressant effect in the MADRS total score compared to placebo in a recent phase III DBRCT (NCT04853407). No ongoing trial was registered on ClinicalTrials.gov. |
Abbreviations: DBRCT, double-blind, randomized controlled trial; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; MDD major depressive disorder; NMDAR, N-methyl-D-aspartate receptor; QIDS-SR 16 , 16-item Quick Inventory of Depressive Symptomatology, Self-Report; TRD, treatment-resistant depression.
Table 2 Antidepressant drugs in the pipeline that failed to show any significant benefit over placebo in phase II and III trials.
| Compound | Phase | Patients | Characteristics |
|---|---|---|---|
| AZD2327 | II | MDD | No significant antidepressant effect was found in the HAMD 17 total score in AZD2327 compared with placebo in a phase II DBRCT for MDD (NCT00759395). |
| AZD7268 | II | MDD | No significant difference was demonstrated in the MADRS total score between AZD7268 and placebo in a phase II DBRCT for MDD (NCT01020799). |
| Decoglurant (RG1578, RO4995819) | II | MDD | In a phase II DBRCT for MDD, no significant differences were observed between decoglurant and placebo in the reduction in the MADRS total score and response and remission rates (NCT01457677). |
| GSK163090 | II | MDD | In a phase II DBRCT for MDD, GSK163090 showed no significant difference in the HAMD 17 total score compared with placebo (NCT00896363). |
| GSK372475 | II | MDD | No significant difference was found in the MADRS total score between GSK372475 and placebo in two phase II DBRCT for MDD (NCT00420641 and NCT00448058). |
| GSK561679 | II | MDD | GSK561679 did not show any significant difference in the HAMD 17 total score compared with placebo in a phase II DBRCT for MDD (NCT00733980). |
| GW856553 | II | MDD | No significant difference was found in the HAMD 17 total score between GW856553 and placebo in a phase II DBRCT for MDD (NCT00976560). |
| JNJ-18038683 | II | MDD | JNJ-18038683 demonstrated no statistically significant improvement over placebo in the MADRS total score in a phase II DBRCT for MDD (NCT00566202). |
| Lanicemine (AZD6765) | II | MDD | In a phase II DBRCT for MDD, lanicemine showed a significantly greater reduction in the MADRS total score within 80 minutes compared to placebo. However, the significant improvement remained only for 110 minutes (NCT00986479). The development of lanicemine was terminated in 2013. |
| Mecamylamine (TC-5214) | II | MDD | There was no statistically significant difference in terms of total score reduction in the MADRS between mecamylamine (2 mg/d or 8 mg/d) and placebo in a phase II DBRCT for MDD (NCT01288079). |
| MIN-117 | II | MDD | In a phase II DBRCT for MDD, no statistically significant difference was found between MIN-117 and placebo in terms of total score reduction in the MADRS (NCT03446846). |
| NSI-189 | II | MDD | There was no statistically significant difference in total score reduction in the MADRS between NSI-189 (40 mg/d or 80 mg/d) and placebo in a phase II DBRCT for MDD (NCT02695472). |
| PDC-1421 | II | MDD | In a phase II DBRCT for MDD, there was no statistically significant difference in terms of total score reduction in the MADRS between PDC-1421 and placebo (NCT02395978). |
| Serdaxin (Zoraxel, RX-10100) | II | MDD | While several ad-hoc analyses showed non-significant but a trend-level greater percent change in the MADRS total score in those treated with serdaxin compared to placebo, serdaxin did not demonstrate a pre-defined superiority to placebo in a phase II DBRCT for MDD (NCT00839176). |
| Traxoprodil (CP-101,606) | II | MDD | Traxoprodil demonstrated a greater decrease in the MADRS total score compared with placebo in a phase II DBRCT for MDD (NCT00163059). However, further development was not pursued due to a concern about potential cardiovascular risk via QT prolongation. |
| Yohimbine hydrochloride | II | MDD | In a phase II crossover DBRCT for MDD, there was no significant difference in the HAMD 6 total score between Yohimbine hydrochloride and placebo (NCT00078715). |
| Amibegron (SR58611A) | III | MDD | In a phase III DBRCT for MDD, amibegron was associated with a significantly greater decrease in the HAMD 17 total score compared with placebo (NCT00825058). The development of amibegron was terminated in 2008. |
| Aprepitant (MK0869) | III | MDD | No significant difference was found in the HAMD 17 total score change between aprepitant and placebo in a phase III DBRCT for MDD (NCT00042029). |
| Buprenorphine | III | TRD | Low-dose buprenorphine provided a rapid and sustained improvement for older adults with TRD in a phase II RCT. However, no significant difference was observed in the MADRS total score between buprenorphine and placebo in a phase III DBRCT for TRD (NCT01407575). |
| Edivoxetine (LY2216684) | III | MDD | In a phase III DBRCT for MDD, edivoxetine demonstrated a significant total score reduction in the MADRS compared with placebo (NCT00795821). However, edivoxetine did not separate from placebo in the MADRS total core reduction in three acute phase III DBRCTs (NCT01185340, NCT01173601, NCT01187407). |
| EPA | III | MDD | No significant difference was observed in the HAMD 17 total score reduction between EPA and placebo in a phase III DBRCT for MDD (NCT00361374). |
| Ethyl-EPA | III | MDD | Ethyl-EPA demonstrated a greater score reduction in the HAMD 17 total score compared with placebo; however, it did not reach statistical significance in a phase III DBRCT for MDD (NCT00096798). |
| Herbal extract | III | MDD | No significant difference in the HAMD 17 total score was observed between herbal extract and placebo in a phase III DBRCT for MDD (NCT01098318). |
| Rapastinel (GLYX-13, BV-102) | III | TRD | Rapastinel showed no greater improvement in the MADRS total score over placebo in a phase III DBRCT for TRD and its development was discontinued (NCT03560518). |
| Zuranolone (SAGE-217) | III | MDD | In a phase III DBRCT for MDD, zuranolone did not show any statistically significant reduction from baseline compared to placebo in the HAMD 17 total score (NCT03672175). |
Abbreviations: DBRCT, double-blind, randomized controlled trial; EPA, Eicosapentaenoic acid; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; MDD major depressive disorder; NMDAR, N-methyl-D-aspartate receptor; QIDS-SR 16 , 16-item Quick Inventory of Depressive Symptomatology, Self-Report; TRD, treatment-resistant depression.
AXS-05
AXS-05 is a combination of dextromethorphan, a non-selective NMDAR antagonist, and bupropion. Bupropion is compounded to protect dextromethorphan from being rapidly metabolized via cytochrome P450 2D6 and provides antidepressant effects. AXS-05 (45 mg dextromethorphan and 105 mg bupropion twice daily) demonstrated significant improvement in the MADRS total score reduction of 16.6 compared to 11.9 for placebo at week 6 (primary outcome) in one phase III DBRCT of 327 patients with TRD (p=0.002) (NCT04019704) 13 . A significant reduction in the MARDS total score was also observed at week 1 compared to placebo (p=0.007). While the response rate of AXS-05 at week 6 was different from that of placebo (54.0% versus 34.0%, p<0.001), there were no data at any other time points. While the most commonly reported adverse events in the AXS-05 group were dizziness, nausea, headache, diarrhea, somnolence, and dry mouth, their incidence rates were not reported. There was no information on dissociative symptoms, psychotic symptoms, or dependence. In another phase III DBRCT of 312 patients with TRD (NCT02741791), they were randomized to treatment with either AXS-05 (45 mg dextromethorphan and 105 mg bupropion) or 150 mg bupropion, twice daily for 6 weeks 14 . In this study, the antidepressant effect of AXS-05 was shown with significant MADRS total score reductions of 5.2 for AXS-05 versus 3.6 for bupropion at week 1 (p=0.02) and 8.0 for AXS-05 versus 6.1 for bupropion at week 2 (p=0.035). In contrast, there was no significant superiority in the MADRS score reduction at week 6 (primary outcome, 11.6 for AXS-05 versus 9.4 for bupropion, p=0.117) 14 . The response rates at these time points were not reported. The most commonly reported adverse events in AXS-05 were dizziness and nausea without any information on their frequency, while dissociative symptoms, psychotic symptoms, or dependence were not reported. One phase III open-label safety trial for 876 participants has been completed without any reported results (NCT04039022). In a recent phase II study for 44 patients with TRD in stable remission, AXS-05 twice daily significantly delayed the time to relapse up to 52 weeks as compared to placebo (primary outcome, p=0.002) (NCT04608396) 15 . There were no treatment-emergent adverse events reported in more than one patient in the AXS-05 group. The U.S. Food and Drug Administration granted fast track designation for TRD and breakthrough therapy designation for MDD to AXS-05 14 . One phase II trial was still in the recruitment stage on ClinicalTrials.gov (NCT04634669) as of August 22, 2021.
MIJ821
MIJ821 (CAD9271) is an NMDAR subtype 2B negative allosteric modulator. In a phase II DBRCT of 70 patients with TRD, MIJ821 at doses of 0.16 mg/kg and 0.32 mg/kg was administered via infusion on a weekly or bi-weekly basis for 6 weeks and compared with 0.5 mg/kg of ketamine and placebo administered weekly (i. e. 6 arms in total) (NCT03756129) 16 . There were greater reductions in the MADRS total score at 24 hours (primary outcome) in the pooled MIJ821 0.16 mg/kg group (−15.51, p=0.0013) and the pooled MIJ821 0.32 mg/kg group (−12.98, p=0.0196) than placebo group (−7.27). While the intravenous ketamine group demonstrated a score reduction of −12.9 in the MADRS, no comparison was made to MIJ821 or placebo. The significant antidepressant effects of MIJ821 were also observed at 48 hours but not 6 weeks. Response rate according to the MADRS was not reported in this study. The most frequent adverse event in MIJ821 was amnesia (10.0%). Dissociation was observed with an incidence rate of 5.0% in the pooled MIJ821 group. One phase II trial in patients with MDD who have suicidal ideation with intent was still in the recruitment stage on ClinicalTrials.gov (NCT04722666) as of August 22, 2021.
Nitrous oxide
Nitrous oxide is an inhalational anesthetic commonly used in dentistry, emergency centers, and ambulatory surgery centers 17 and acts as an NMDAR antagonist. In a phase II crossover DBRCT of 20 patients with TRD, 1-hour inhalation of 50% nitrous oxide plus 50% oxygen was superior to that of placebo gas in the improvement of the HAMD 21 at 2 hours (−4.8 versus −2.3, p<0.001) and 24 hours (primary endpoint, −5.5 versus −2.8, p<0.001) (NCT02139540) 18 . The response rates at 24 hours were 20.0% in the nitrous oxide group and 5.0% in the placebo group without any significance (Odds Ratio (OR)=4.0, 95% CI=0.5–35.8). The frequent adverse events included nausea/vomiting (15.0%), headache (10.0%), numbness/paresthesia (10.0%), and anxiety (10.0%). In another phase II crossover DBRCT of 24 patients with TRD, a single 1-hour inhalation of 50% and 25% nitrous oxide in oxygen did not demonstrate significant reduction in the HAMD 21 total score compared to placebo gas at hours 2, 24 and week 1, but did at week 2 (primary outcome, the estimated differences between 25% nitrous oxide and placebo: −0.75 at 2 hours (p=0.73), −1.41 at 24 hours (p=0.52), −4.35 at week 1 (p=0.05), and −5.19 at week 2 (p=0.02); the estimated differences between 50% nitrous oxide and placebo: −0.87 at 2 hours (p=0.69), −1.93 at 24 hours (p=0.37), −2.44 at week 1 (p=0.25), and −7.00 at week 2 (p=0.001) (NCT03283670) 19 . The response rates at 2 weeks were 41.7% in the 50% nitrous oxide group (Relative Risk (RR)=2.9; 95% CI=0.6–18.0), 33.3% in the 25% nitrous oxide group (RR=2.5; 95% CI=0.4–16.3), and 11.1% in the placebo group. The most frequently reported adverse event which occurred 24 hours after inhalation was common cold/strep throat (11.1%). Mild dissociative effects were reported during or immediately after inhalation session in some patients who were receiving nitrous oxide (feeling disconnected, 26.1% in the 50% nitrous oxide group; lightheadedness, 8.7% and 5.0% in the 50% and 25% nitrous oxide groups, respectively; feeling high, 13.0% in the 50% nitrous oxide group; and paranoia, 4.3% in the 50% nitrous oxide group). The result of another phase II study examining 1-hour inhalation of 50% nitrous oxide has not been reported, probably because it was only recently completed (NCT03932825). One phase II DBRCT is ongoing in which the participants have been randomized to receive 1-hour inhalation of 50% nitrous oxide, 25% nitrous oxide, or placebo gas (NCT03869736).
Psilocybin
Psilocybin is a psychedelic through serotonin 5-hydroxytryptamine type 2 A (5-HT 2A ) receptor agonist, which occurs naturally in the psychoactive psilocybe genus of mushrooms 20 . One phase II waiting list–controlled trial of 27 patients with MDD demonstrated that the HAMD 17 scores 1 (primary endpoint) and 4 weeks after two psilocybin sessions (session 1: 20 mg/70 kg; session 2: 30 mg/70 kg) in the psilocybin group (8.0±7.1 and 8.5±5.7, respectively) were significantly lower than those in the waiting-list group (23.8±5.4 and 23.5±6.0, respectively, both p-values<0.001) (NCT03181529) 20 . The response rates in the psilocybin group were 70.8% both at 1 and 4 weeks while data were not shown for the wait-list group. The adverse events in the psilocybin group included various emotional (e. g., fear and sadness) and physical (e. g., feeling body shake or tremble) experiences during psilocybin sessions, and headache. In another phase II DBRCT of 59 patients with MDD, two administrations of psilocybin (25 mg of psilocybin 3 weeks apart plus 6 weeks of daily placebo) were not inferior to the 6-week treatment of escitalopram (1 mg of psilocybin three weeks apart plus 10–20 mg escitalopram for 6 weeks) in the QIDS-SR 16 score changes (primary outcome, −8.0±1.0 and −6.0±1.0, respectively, p=0.17) (NCT03429075) 21 . The response rates at 6 weeks were 70.0% in the psilocybin group and 48.3% in the escitalopram group (difference: 22%, 95% CI: −3 to 48). The common adverse events were headache (66.7%), nausea (26.7%), and migraine (10.0%) in psilocybin. There was no report on dissociative symptoms, psychotic symptoms, or dependence. Other phase II DBRCTs targeting MDD are ongoing (NCT03775200, NCT03866174, and NCT03715127). Psilocybin received breakthrough therapy designation for the treatment of MDD in 2019.
Ayahuasca
Ayahuasca is a hallucinogenic botanical mixture that is traditionally used for healing and spiritual purposes in South America. Ayahuasca combines N, N-dimethyltryptamine (DMT), a psychedelic agent with 5-hydroxytryptamine 2 A agonist activity, with monoamine oxidase-inhibiting β-carboline alkaloids, such as harmine, harmaline, and tetrahydroharmine 22 . A single dose of ayahuasca (1 mL/kg adjusted to contain 0.36 mg/kg of N, N-DMT) demonstrated a significant improvement in the HAMD total score of 14.4 compared to 2.8 for placebo on day 7 in one phase II DBRCT of 29 patients with TRD (primary outcome, p=0.019, Cohen's d=1.49) (NCT02914769) 23 . The response rates at day 7 between the ayahuasca and placebo groups were significantly different (57.1% versus 20.0%, OR=5.3, 95% CI=1.1–22.6, p=0.04). In this study, most participants reported nausea and about 57% of them vomited. There were no data reported concerning other adverse events. No ongoing trial was registered on ClinicalTrials.gov as of August 22, 2021.
Botulinum toxin A
Local injections of botulinum toxin A (BTA; onabotulinumtoxinA) lead to muscle relaxation through a multistep mechanism 24 . BTA injections into the facial muscles in the glabellar region have been thought to have an antidepressant effect, possibly because facial expression influences emotional perception 25 . In a phase II crossover DBRCT of 30 patients with MDD, females received 29 units of BTA, and males received 39 units. In this study, there was a significant improvement in the HAMD 21 in the patients who received BTA compared with those who received placebo at week 6 (primary outcome, −12.7 versus −0.4, p<0.0001) (NCT01392963) 25 . The response rate at week 6 was significantly higher in the BTA group than in the placebo group (55.6% versus 0.0%, p<0.0001). There were no safety data reported in this study. In another phase II DBRCT for 255 female patients with MDD, 30 units of BTA, but not 50 units, showed a marginally significant score reduction in the MADRS compared to placebo at week 6 (primary endpoint, least-squares mean±standard error: −11.6±1.4 versus −7.9±1.4, p=0.053) (NCT02116361) 24 . Significant difference was also found at week 3 (−7.8±1.1 versus −3.6±1.1, p=0.005) and 9 (−13.7±1.3 versus −10.0±1.4, p=0.049) in this study. There was no available information on the response rate in this study. While headache commonly occurred in the BTA group (15.4%), there was no report on dissociative symptoms, psychotic symptoms, or dependence. The addition of BTA (29 units for females and 39 units for males) to an ongoing antidepressant also demonstrated greater efficacy in the HAMD 17 score reduction over placebo at week 6 in a phase II DBRCT of 30 patients with MDD (primary outcome, −10.1±8.2 versus −1.7±4.3, p=0.002) (NCT00934687) 26 . The response rate at week 6 was significantly higher in the additional BTA group than in the additional placebo group (60.0% versus 13.3%, p=0.02). Short episodes of headache were the only relevant adverse event (40.0% in the additional BTA group). In a phase IV DBRCT of 85 patients with MDD, BTA (29 units for females and 40 units for males) showed a greater rate of response compared to placebo at week 6 (primary outcome, 51.5% versus 14.6%, p<0.001) (NCT01556971) 27 . Safety data were not shown in this study. One clinical trial comparing two facial injection sites (i. e., in the corrugator and procerus and the lateral muscle orbicularis oculi) is ongoing (NCT03484754).
Prasterone
Prasterone (dehydroepiandrosterone, DHEA) is a steroid hormone precursor used to treat painful sexual intercourse due to vaginal atrophy. In one phase II crossover DBRCT of 46 participants with midlife-onset major or minor depression, the 6-week administration of DHEA (90 mg/d for 3 weeks and 450 mg/d for 3 weeks) resulted in a significant improvement in the HAMD 17 compared with the 6-week treatment with placebo (primary outcome, 13.3±0.9 to 7.5±1.2 versus 13.5±0.8 to 11.8±1.1, p<0.01) (NCT00001487) 28 . While 50.0% of the participants responded after the DHEA treatment, 28.3% responded after receiving a placebo (p=0.03). None reported adverse events in this study, except for one patient who experienced an increase in the skin oiliness. No ongoing trial was registered on ClinicalTrials.gov as of August 22, 2021.
Casopitant
Casopitant (GW679769) is an antagonist of the neurokinin (NK) receptor that is located in areas of the brain related to the regulation of affect and stress behaviors, including the amygdala hypothalamus, hippocampus, frontal cortex, raphe nucleus, and locus coeruleus 29 . In one phase II DBRCT of 356 patients with MDD, there was a significant difference in the HAMD 17 score change at week 8 (primary outcome) between casopitant 80 mg/d and placebo (difference=−2.7, p=0.023), but not between casopitant 30 mg/d and placebo (difference=−2.1, p=0.077) (NCT00413023) 29 . A significant difference between casopitant 80 mg/d and placebo was detected at week 1 (p=0.010). The response rates at week 8 in the casopitant groups (40% in the 80 mg/d group and 39% in the 30 mg/d group) were not significantly different from that in the placebo group (32%, both p’s>0.05). Another phase II DBRCT of 362 patients with MDD reported that neither casopitant 80–120 mg/d nor paroxetine 30 mg/d achieved statistical separation from placebo at week 8 on the HAMD 17 (primary outcome, difference for casopitant=−1.7, p=0.114 and difference for paroxetine=−1.2, p=0.282) (NCT00102492) 29 . The response rate at week 8 in neither the casopitant group (62%) nor the paroxetine group (60%) was not different from that in the placebo group (59%, both p’s>0.05). The adverse events that occurred≥10% in one or more casopitant groups in these two studies included headache, somnolence, nausea, diarrhea, and dry mouth. There was no report of dissociative symptoms, psychotic symptoms, or dependence. No active trial was registered on ClinicalTrials.gov.
Ansofaxine hydrochloride
Ansofaxine hydrochloride (LY03005, LPM570065) is a prodrug of desvenlafaxine which works as a serotonin–norepinephrine–dopamine reuptake inhibitor 30 . In a recent phase III DBRCT of 558 patients with MDD, the 8-week administration of ansofaxine hydrochloride (80 mg/d or 160 mg/d) resulted in a significant improvement in the MADRS compared with placebo although the detailed information was not reported (NCT04853407) 31 32 . The common adverse events were nausea, vomiting, headache, and drowsiness. No active trial was registered on ClinicalTrials.gov as of August 22, 2021.
Discussion
The latest report of a systematic search of not-yet-marketed antidepressant compounds showed positive results in phase II and III trials. The search in the US Clinical Trials registry identified nine compounds. AXS-05 provided a positive result over placebo in one phase III DBRCT for TRD and immediate antidepressant effect over bupropion in another phase III DBRCT for TRD. As TRD was targeted in these studies, AXS-05 may be considered to be a promising treatment option for those who did not respond to conventional antidepressants. Eight other compounds, including MIJ821, nitrous oxide, psilocybin, ayahuasca, facial injection of BTA, prasterone, casopitant, and ansofaxine hydrochloride demonstrated at least one positive result in phase II or III trials for TRD or MDD. Six compounds demonstrated the significant score reduction in the rating scale used for each primary outcome compared with placebo within one week (i. e., nitrous oxide, a few hours; MIJ821 and ayahuasca, one day; AXS-05, psilocybin, and casopitant, one week); of these, ayahuasca showed a greater response rate than placebo within one week. These new compounds with novel mechanisms of action beyond the conventional monoaminergic hypothesis are expected to provide a greater variety of treatment options in pharmacotherapy for depression in the near future if these preliminary results are confirmed.
AXS-05, MIJ821, and nitrous oxide have antagonistic or negative allosteric activity modulation in NMDAR like ketamine. The antidepressant effects of several NMDAR modulators have been examined since discovering the antidepressant effects of ketamine in 2000 33 . However, other than these three drugs, NMDAR modulators generally failed to show superiority to placebo (e. g., rapastinel [GLYX-13] 34 and L-4-chlorokynurenine [AV-101] 35 ), and the development of lanicemine (AZD6765, BHV-5000) seemed to be terminated 36 . The following mechanisms have been thought to underlie the antidepressant effects of ketamine: (1) inhibition of NMDARs localized to GABA inhibitory interneurons, (2) a cascade of intracellular changes due to transient inhibition of postsynaptic NMDAR activity, (3) activation of cellular plasticity cascades from inhibition of extrasynaptic NMDARs, and (4) inhibition of NMDAR-dependent high-frequency burst firing in the lateral habenula 37 . However, the antidepressant effects of ketamine are recently considered to be independent of NMDAR inhibition because of its more significant antidepressant-like effect and less potent inhibition of NMDAR in (R)-ketamine than (S)-ketamine 38 . Moreover, an NMDAR antagonist, MK-801, which binds to the same receptor site as ketamine, does not exert sustained antidepressant effects 39 . Instead, the antidepressant effects of ketamine require the acute activation of AMPAR. A single subanesthetic dose of ketamine produces a rapid increase in the expression levels of the AMPAR GluA1 subunit in the medial prefrontal cortex in a rodent model 40 . In addition, pretreatment with an AMPAR antagonist blocks the antidepressant-like behavioral effects of ketamine 41 . Altogether, these findings indicate that AMPARs likely play a pivotal role in the antidepressant actions of ketamine. As described above, AXS-05 is a combination of dextromethorphan and bupropion. Similar to ketamine, pretreatment with an AMPAR antagonist significantly blocks the antidepressant-like behavior of dextromethorphan, suggesting that AMPARs may also play a significant role in the antidepressant-like effects of dextromethorphan 42 . While the antidepressant effects of nitrous oxide and MIJ821 are unclear, the repeated exposure of nitrous oxide increases burst firing activity, which may lead to antidepressant-like effects 43 .
Increasing evidence suggests the safety and efficacy of psychedelic compounds as potential novel therapeutics in psychiatry 44 45 . Some clinical trials have demonstrated the safety, efficacy, and tolerability of (1) 3,4-methylenedioxymethamphetamine and lysergic acid diethylamide (LSD) for treatment-resistant post-traumatic stress disorder 46 47 48 ; (2) psilocybin for the obsessive-compulsive disorder 49 , (3) alcohol abuse 50 , (4) smoking cessation 51 , and (5) psilocybin and LSD for anxiety, depression, pain, and distress associated with a life-threatening illness 52 53 . As described in the present review, several clinical trials have shown the efficacy of psilocybin and ayahuasca for TRD. Most psychedelics, including psilocybin and ayahuasca, are 5-HT 2A receptor agonists, which are thought to mediate their psychedelic and hallucinogenic effects 54 55 . Psychedelic compounds may induce brain-derived neurotrophic factor-mediated AMPAR potentiation and enhancement of neural plasticity 56 57 , possibly resulting in antidepressant effects. Future studies are needed to dissect the mechanisms of these antidepressant effects in a compound- and dosage-specific manner 58 .
The safety and efficacy of BTA, prasterone, casopitant, and ansofaxine hydrochloride have also been demonstrated for the treatment of MDD. Treatment of the glabellar region with botulinum toxin produces a relative change in the facial expressions, from negative to positive, possibly impacting emotional experience 26 . Prasterone stimulates neurogenesis and increases hippocampal spine synapse density in the rat hippocampus 59 60 , potentially leading to neuroprotective and proliferative effects. Casopitant increases serotonin and norepinephrine neurotransmitters through the NK1 receptor system 61 . Ansofaxine hydrochloride blocks serotonin, norepinephrine, and dopamine transporters 30 .
This review has several limitations. First, only the US Clinical Trials registry was searched for relevant clinical trials and antidepressant compounds. However, this is the largest registry that contains more than 384,000 clinical trials, which is six times larger than the European registry. Second, the collection of collateral information via the PubMed search and press releases was not performed systematically. Third, the targeted populations (i. e., MDD or TRD), primary outcome measures (i. e., score reductions in the HAMD and MADRS and response rate), and primary endpoints (i. e., from 24 hours to 6 weeks) differed among individual studies. Those with TRD may be less likely to respond to treatment of interest compared to those with MDD since the lower response rate was observed in the later treatment step in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial 7 . Furthermore, the primary endpoint was not clarified in one study for nitrous oxide 19 . Fourth, the superiority/inferiority of each compound to ketamine cannot be examined because a direct comparison of only MIJ821 with ketamine is reported. Finally, it should be noted that the compounds demonstrated significant superiority to placebo in the primary outcome measure at the primary endpoint in the latest phase of clinical trials, regardless of their mechanisms of actions or presence/absence of rapid-acting property.
In conclusion, the present study identified nine promising antidepressant compounds (i. e., AXS-05, MIJ821, nitrous oxide, psilocybin, ayahuasca, facial injection of BTA, prasterone, casopitant, and ansofaxine hydrochloride) that demonstrated positive results in phase II or III studies for MDD or TRD. These new drugs with novel mechanisms of action can address unmet needs for the treatment of depression. At the same time, their efficacy and safety must be rigorously examined before they are marketed.
Funding Statement
Role of Funding This work was funded by Keio Next-Generation Research Project Program.
Footnotes
Conflict of Interest Dr. Sakurai reports grants from the Uehara Memorial Foundation and manuscript fees or speaker’s honoraria from Eisai, Meiji Seika Pharma, Shionogi Pharma, Sumitomo Dainippon Pharma, Takeda Pharma, and Yoshitomi Yakuhin. Dr. Yonezawa has nothing to declare. Dr. Tani received a fellowship from the Japanese Society of Clinical Neuropsychopharmacology and the Canadian Institutes of Health Research, a research grant from Eli Lilly, and manuscript fees from Dainippon Sumitomo Pharma, Otsuka Pharmaceutical, Wiley Japan, and Yoshitomi Yakuhin. Dr. Mimura has received speaker’s honoraria from Byer Pharmaceutical, Daiichi Sankyo, Dainippon-Sumitomo Pharma, Eisai, Eli Lilly, Fuji Film RI Pharma, Hisamitsu Pharmaceutical, Janssen Pharmaceutical, Kyowa Pharmaceutical, Mochida Pharmaceutical, MSD, Mylan EPD, Nihon Medi-physics, Nippon Chemipher, Novartis Pharma, Ono Yakuhin, Otsuka Pharmaceutical, Pfizer, Santen Pharmaceutical, Shire Japan, Takeda Yakuhin, Tsumura, and Yoshitomi Yakuhin within the past three years. Also, he received grants from Daiichi Sankyo, Eisai, Pfizer, Shionogi, Takeda, Tanabe Mitsubishi, and Tsumura within the past three years outside the submitted work. Dr. Bauer has received institutional funding/grant support from Deutsche Forschungsgemeinschaft (DFG), Bundesministeriums für Bildung und Forschung (BMBF), and the European Commission. He has received speaker honoraria and/or travel compensation from Aristo, Hexal AG, Janssen Pharmaceutica NV, Janssen-Cilag, and Sunovion. He has served on advisory boards or honoraria for consultancy from GH Research, Janssen-Cilag, Neuraxpharm, Novartis, Sandoz, Shire International GmbH, Sumitomo Dainippon, Sunovion, and Takeda. Dr. Uchida has received grants from Eisai, Otsuka Pharmaceutical, Dainippon-Sumitomo Pharma, Daiichi Sankyo Company, Mochida Pharmaceutical, and Meiji-Seika Pharma; speaker’s honoraria from Otsuka Pharmaceutical, Dainippon-Sumitomo Pharma, Eisai, and Meiji-Seika Pharma, and advisory panel payments from Dainippon-Sumitomo Pharma within the past 3 years.
References
- 1.Park L T, Zarate C A. Depression in the primary care setting. N Engl J Med. 2019;380:559–568. doi: 10.1056/NEJMcp1712493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Washington, DC: APA; 2010. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. [Google Scholar]
- 3.Kennedy S H, Lam R W et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological Treatments. Can J Psychiatry Rev Can Psychiatr. 2016;61:540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer M, Pfennig A, Severus E et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 5.Stassen H H, Angst J, Hell D et al. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry. 2007;68:1195–1205. doi: 10.4088/jcp.v68n0805. [DOI] [PubMed] [Google Scholar]
- 6.Gaynes B N, Warden D, Trivedi M H et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv Wash DC. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 7.Rush A J, Trivedi M H, Wisniewski S R et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 8.Witkin J M, Martin A E, Golani L K et al. Rapid-acting antidepressants. Adv Pharmacol San Diego Calif. 2019;86:47–96. doi: 10.1016/bs.apha.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson S T, Sanacora G. A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today. 2019;24:606–615. doi: 10.1016/j.drudis.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhard D M, Duman R S. Rapid-acting antidepressants: Mechanistic insights and future directions. Curr Behav Neurosci Rep. 2018;5:36–47. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson S T, Sanacora G. Ketamine: A potential rapid-acting antisuicidal agent? Depress Anxiety. 2016;33:711–717. doi: 10.1002/da.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson S T, Toprak M, Turner M S et al. A Survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174:695–696. doi: 10.1176/appi.ajp.2017.17020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axsome Therapeutics Inc. Axsome Therapeutics Announces AXS-05 Achieves Primary Endpoint in GEMINI Phase 3 Trial in Major Depressive Disorder2019https://www.globenewswire.com/en/news-release/2019/12/16/1960786/33090/en/Axsome-Therapeutics-Announces-AXS-05-Achieves-Primary-Endpoint-in-GEMINI-Phase-3-Trial-in-Major-Depressive-Disorder.html
- 14.Axsome Therapeutics Inc. Axsome Therapeutics Announces Topline Results of the STRIDE-1 Phase 3 Trial in Treatment Resistant Depression and Expert Call to Discuss Clinical Implications2020https://www.biospace.com/article/releases/axsome-therapeutics-announces-topline-results-of-the-stride-1-phase-3-trial-in-treatment-resistant-depression-and-expert-call-to-discuss-clinical-implications/
- 15.Axsome Therapeutics Inc. Axsome Therapeutics Announces AXS-05 Achieves Pprimary and Key Secondary Endpoints in the Merit Phase 2 Trial in Treatment Resistant Depression2021https://www.globenewswire.com/news-release/2021/08/09/2276951/33090/en/Axsome-Therapeutics-Announces-AXS-05-Achieves-Primary-and-Key-Secondary-Endpoints-in-the-MERIT-Phase-2-Trial-in-Treatment-Resistant-Depression.html
- 16.Proof of Concept Study Evaluating the Efficacy and Safety of MIJ821 in Patients With Treatment-resistant Depression2021https://clinicaltrials.gov/ct2/show/NCT03756129
- 17.Becker D E, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008;55:124–130. doi: 10.2344/0003-3006-55.4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagele P, Duma A, Kopec M et al. Nitrous oxide for treatment-resistant major depression: A proof-of-concept trial. Biol Psychiatry. 2015;78:10–18. doi: 10.1016/j.biopsych.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Nagele P, Palanca B J, Gott B et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci Transl Med. 2021;13:eabe1376. doi: 10.1126/scitranslmed.abe1376. [DOI] [PubMed] [Google Scholar]
- 20.Davis A K, Barrett F S, May D G et al. Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry. 2021;78:481–489. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carhart-Harris R, Giribaldi B, Watts R et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384:1402–1411. doi: 10.1056/NEJMoa2032994. [DOI] [PubMed] [Google Scholar]
- 22.Riba J, Valle M, Urbano G et al. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003;306:73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- 23.Palhano-Fontes F, Barreto D, Onias H et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol Med. 2019;49:655–663. doi: 10.1017/S0033291718001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brin M F, Durgam S, Lum A et al. OnabotulinumtoxinA for the treatment of major depressive disorder: A phase 2 randomized, double-blind, placebo-controlled trial in adult females. Int Clin Psychopharmacol. 2020;35:19–28. doi: 10.1097/YIC.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magid M, Reichenberg J S, Poth P E et al. Treatment of major depressive disorder using botulinum toxin A: A 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75:837–844. doi: 10.4088/JCP.13m08845. [DOI] [PubMed] [Google Scholar]
- 26.Wollmer M A, de Boer C, Kalak N et al. Facing depression with botulinum toxin: A randomized controlled trial. J Psychiatr Res. 2012;46:574–581. doi: 10.1016/j.jpsychires.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Finzi E, Rosenthal N E. Treatment of depression with onabotulinumtoxinA: A randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1–6. doi: 10.1016/j.jpsychires.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt P J, Daly R C, Bloch M et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 29.Ratti E, Bellew K, Bettica P et al. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:727–733. doi: 10.1097/JCP.0b013e31823608ca. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Li X, Shi Y et al. The effects of LPM570065, a novel triple reuptake inhibitor, on extracellular serotonin, dopamine and norepinephrine levels in rats. PloS One. 2014;9:e91775. doi: 10.1371/journal.pone.0091775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov. . A Study to Evaluate the Efficacy and Safety of Ansofaxine Hydrochloride Extended-release Tablets in the Treatment of Major Depressive Disorder (MDD) 2021. https://clinicaltrials.gov/ct2/show/NCT04853407 https://clinicaltrials.gov/ct2/show/NCT04853407
- 32.Luye Pharma . Luye Pharma’s Class 1 New Drug Anshufaxine Hydrochloride Extended-Release Tablets Meets Predefined Endpoints in Phase III Trial. 2021. https://www.luye.cn/lvye_en/view.php?id=1922 https://www.luye.cn/lvye_en/view.php?id=1922
- 33.Berman R M, Cappiello A, Anand A et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 34.Allergan . Allergan Announces Phase 3 Results for Rapastinel as an Adjunctive Treatment of Major Depressive Disorder (MDD) 2019. https://www.prnewswire.com/news-releases/allergan-announces-phase-3-results-for-rapastinel-as-an-adjunctive-treatment-of-major-depressive-disorder-mdd-300808044.html https://www.prnewswire.com/news-releases/allergan-announces-phase-3-results-for-rapastinel-as-an-adjunctive-treatment-of-major-depressive-disorder-mdd-300808044.html
- 35.VistaGen Therapeutics . VistaGen Reports Topline Phase 2 Results for AV-101 as an Adjunctive Treatment of Major Depressive Disorder. 2019. https://www.vistagen.com/news-media/press-releases/detail/130/vistagen-reports-topline-phase-2-results-for-av-101-as-an https://www.vistagen.com/news-media/press-releases/detail/130/vistagen-reports-topline-phase-2-results-for-av-101-as-an
- 36.Carroll J. AstraZeneca quietly sweeps out some notable mid-stage drug programs. 2014. https://www.fiercebiotech.com/r-d/astrazeneca-quietly-sweeps-out-some-notable-mid-stage-drug-programs https://www.fiercebiotech.com/r-d/astrazeneca-quietly-sweeps-out-some-notable-mid-stage-drug-programs
- 37.Gould T D, Zarate C A, Thompson S M. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–236. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Shirayama Y, Zhang J C et al. R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanos P, Moaddel R, Morris P J et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Lee B, Liu R-J et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeng S, Zarate C A, Du J et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen L, Matsumoto R R. Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behav Brain Res. 2015;295:26–34. doi: 10.1016/j.bbr.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Li Q, Ye B, Cao H, Shen F, Xu Z, Du W, Guo F, Liu J, Li T, Zhang B, Liu Z. Repeated Nitrous Oxide Exposure Exerts Antidepressant-Like Effects Through Neuronal Nitric Oxide Synthase Activation in the Medial Prefrontal Cortex. Front Psychiatry. 2020 Sep 3; 11: 837. doi: 10.3389/fpsyt.2020.00837. PMID: 33088274; PMCID: PMC7495238 [DOI] [PMC free article] [PubMed]
- 44.Nichols D E, Walter H. The history of psychedelics in psychiatry. Pharmacopsychiatry. 2021;54:151–166. doi: 10.1055/a-1310-3990. [DOI] [PubMed] [Google Scholar]
- 45.Gründer G. Psychedelics: A new treatment paradigm in psychiatry? Pharmacopsychiatry. 2021;54:149–150. doi: 10.1055/a-1520-5020. [DOI] [PubMed] [Google Scholar]
- 46.Mithoefer M C, Feduccia A A, Jerome L et al. MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology (Berl) 2019;236:2735–2745. doi: 10.1007/s00213-019-05249-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Mithoefer M C, Mithoefer A T, Feduccia A A et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5:486–497. doi: 10.1016/S2215-0366(18)30135-4. [DOI] [PubMed] [Google Scholar]
- 48.Schmid Y, Gasser P, Oehen P et al. Acute subjective effects in LSD- and MDMA-assisted psychotherapy. J Psychopharmacol. 2021;35:362–374. doi: 10.1177/0269881120959604. [DOI] [PubMed] [Google Scholar]
- 49.Moreno F A, Wiegand C B, Taitano E K et al. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:1735–1740. doi: 10.4088/jcp.v67n1110. [DOI] [PubMed] [Google Scholar]
- 50.Bogenschutz M P, Forcehimes A A, Pommy J A et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 51.Johnson M W, Garcia-Romeu A, Cosimano M P et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28:983–992. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasser P, Holstein D, Michel Y et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202:513–520. doi: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffiths R R, Johnson M W, Carducci M A et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols D E. Psychedelics. Pharmacol Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gründer G, Jungaberle H. The potential role of psychedelic drugs in mental health care of the future. Pharmacopsychiatry. 2021;54:191–199. doi: 10.1055/a-1486-7386. [DOI] [PubMed] [Google Scholar]
- 56.Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: Neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev. 2021;73:202–277. doi: 10.1124/pharmrev.120.000056. [DOI] [PubMed] [Google Scholar]
- 57.Ly C, Greb A C, Cameron L P et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–3182. doi: 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mertens L J, Preller K H. Classical psychedelics as therapeutics in psychiatry – current clinical evidence and potential therapeutic mechanisms in substance use and mood disorders. Pharmacopsychiatry. 2021;54:176–190. doi: 10.1055/a-1341-1907. [DOI] [PubMed] [Google Scholar]
- 59.Karishma K K, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 60.Hajszan T, MacLusky N J, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- 61.Gobbi G, Blier P. Effect of neurokinin-1 receptor antagonists on serotoninergic, noradrenergic and hippocampal neurons: Comparison with antidepressant drugs. Peptides. 2005;26:1383–1393. doi: 10.1016/j.peptides.2005.03.032. [DOI] [PubMed] [Google Scholar]


