Abstract
With approval of more COVID-19 vaccines for children, vaccine attributes may influence parental acceptance and choices. We aimed to assess effects of vaccine attributes and information on herd immunity on childhood COVID-19 vaccine acceptance. A survey experiment was conducted with caregivers of children aged 6 months to 11 years old and health care workers (HCWs) in China from September 14 to November 18, 2021. Respondents were randomly assigned to receive differing information on herd immunity (> 80% of the entire population must be vaccinated; or no information). Respondents then completed eight discrete choice tasks to assess vaccine acceptance based on attributes. 2331 (90.07%) of 2588 surveyed caregivers and 1576 (92.71%) of 1700 surveyed HCWs would accept COVID-19 vaccination for children, respectively. High Odds Ratios (OR) were found for acceptance of a vaccine with 90% over 50% efficacy (OR 6.70 [95% CI 6.11–7.35] for caregivers; 11.44 [10.12–12.95] for HCWs); and risk of adverse reactions to be 1 over 10 in 10,000 (3.96 [3.72–4.22] for caregivers; 2.98 [2.76–3.22] for HCWs). To achieve herd immunity target (> 80% vaccination coverage), vaccine efficacy should reach over 70% and risk of adverse reactions lower than 1 in 10,000. Knowledge on herd immunity target increased the odds of vaccine acceptance (1.82 [1.34–2.46] for caregivers; 2.42 [1.58–3.72] for HCWs). Childhood COVID-19 vaccine acceptance was high in China, independent of child's age, and depended on vaccine attributes.
Keywords: COVID-19 vaccine, Acceptance, Child, Survey experiment, China
1. Introduction
Vaccines are essential to control the global COVID-19 pandemic (Graham, 2020). Following widespread COVID-19 vaccination in adults and the elderly, vaccination of children and adolescents is recommended to protect them against COVID-19 (Nikolopoulou and Maltezou, 2022). In the United States, the COVID-19 vaccine was approved for emergency use in adolescents aged 12-to-15 years in May and children aged 5-to-11 years in October 2021 (FDA, 2021a, FDA, 2021b). In China, its emergency use was approved in children aged 3-to-17 years in June 2021 (The State Council of China, 2021a). China launched mass vaccination for children aged 12-to-17 years from late July, and for children aged 3-to-11 years from late October (The State Council of China, 2021b).
Parents may be more cautious about vaccinating their children than vaccinating themselves (Rane et al., 2022), especially when children appear to be less susceptible to COVID-19 than adults (Ludvigsson, 2020). A scoping review from 35 surveys found that, the median rate of parents' willingness to vaccinate their children against COVID-19 was 59.3% globally, lower than that for vaccinating themselves (Pan et al., 2021). Parental acceptance for childhood vaccination witnessed substantial variation across countries, ranging from 24% to 89% (Rane et al., 2022; Szilagyi et al., 2021; Bell et al., 2020; Kishore et al., 2021; Hetherington et al., 2021; Montalti et al., 2021; Aldakhil et al., 2021). Even in the same countries, parental acceptance of children's COVID-19 vaccines was significantly lower than their acceptance for routine childhood vaccines (Temsah et al., 2021).
COVID-19 vaccines have been developed worldwide (Ghasemiyeh et al., 2021). These vaccines differ in efficacy, safety, and the number of doses administered. With the approval of more COVID-19 vaccines for children, parents may have a choice among vaccines with various attributes. Discrete choice experiments (DCE) are a robust method for predicting preferences and behaviours (McPhedran and Toombs, 2021), and could estimate acceptance for a range of vaccine scenarios (Schwarzinger et al., 2021).
Our study investigates COVID-19 vaccine acceptance and its predictors in a representative childhood population in China. We also use a large-scale DCE survey experiment to assess the precise acceptance for hypothetical COVID-19 vaccines with varying attributes from repeated choice tasks. Both caregivers of children aged 6 months to 11 years and health care workers (HCWs) were surveyed in our study.
2. Method
2.1. Sampling
A survey was conducted with caregivers of children aged 6 months to 11 years old and HCWs in China from September 14 to November 18, 2021. The survey was fielded after the emergency use of COVID-19 vaccines was approved and before vaccination rollout began for children aged 3-to-11 years in China. A multi-stage sampling process was used to ensure the representativeness of the sample. Two provinces (Shaanxi and Anhui) and Shenzhen megacity were selected, located in Western, Central, and Eastern China, respectively. In each province, one urban city and one rural county were included. Note that Shaanxi province just started COVID-19 vaccination for children during the survey, whereas Anhui and Shenzhen had not started yet. Three communities were sampled in each city/county according to socioeconomic strata. In each sampled community, caregivers were recruited from its health center and kindergarten. Community health centers generally establish a WeChat online group for child health management which enrolls caregivers of all children registered in this community, mainly aged ≤ 3 years old. For all children from the WeChat group in sampled community health center and from the sampled kindergarten, one of their caregivers was invited to participate in our survey. Besides, in each sampled city/county, all HCWs who worked in a hospital and all sampled community health centers, and all routine vaccination service workers were also invited to join in the survey.
The questionnaire was pilot tested among 30 caregivers and 10 HCWs in a non-study community, and none of them were included in the data analysis. It took at least three minutes to complete the self-administered questionnaire, therefore we used three minutes as a cutoff point for valid questionnaires. Respondents signed the informed consent and would receive an electronic currency worth five Chinese Yuan (about 0.7 USD) as a reward of participant. The online questionnaire was distributed in WeChat groups of caregivers, and 2877 questionnaires were collected from caregivers. After excluding those completed in less than three minutes, questionnaires from 2588 caregivers (90.0%) were included in the analyses. Additionally, a total of 2225 HCWs were invited to participate in the survey, and 1887 completed questionnaires (84.8% response rate), of which 1700 (1700/1877, 90.6%) completing the questionnaire in more than three minutes were included in the data analysis. The Fudan University School of Public Health, and the London School of Hygiene & Tropical Medicine Ethics Committees approved the study protocol [FDU IRB#2018-10-0703, LSHTM Ethics Ref 16,016].
2.2. Survey experiment
All invited caregivers and HCWs completed a self-reported online questionnaire by mobile phone, which covered socio-demographic characteristics, history of COVID-19 vaccination themselves, perceived susceptibility and severity of COVID-19 infection for children, confidence in general vaccines, and knowledge on the coverage rate of COVID-19 vaccination to achieve herd immunity (presented in Appendix A).
The survey experiment consisted of three sections at the end of the questionnaire: information intervention, direct measure of childhood COVID-19 vaccine acceptance, and the elicitation of parental acceptance based on vaccine attributes through DCE experiment (presented in Appendix B).
In the first section, respondents were randomly assigned (1:1) to one of two groups to receive differing information on the concept of herd immunity and target: (1) introduce the term “herd immunity” and its collective benefits first, and then state that >80% of the entire population must be vaccinated against COVID-19 to achieve herd immunity; or (2) no information.
In the second section, respondents were asked, “when a COVID-19 vaccine is approved for use to children, would you have your children take it?” Each respondent was asked to rate the extent of acceptance on a five-point Likert scale: definitely yes, probably yes, unsure, probably not, definitely not. The responses were grouped into two categories for analysis: accept (“definitely yes” and “probably yes”) and hesitancy (“definitely not”, “probably not” and “unsure”). Those who would not accept COVID-19 vaccination for their children were further asked to choose reasons from eight options.
In the third section, a DCE was conducted in which respondents were provided with a series of choice tasks around hypothetical COVID-19 vaccines varying by vaccine attributes. According to clinical trials and real-world data of COVID-19 vaccines (World Health Organization, 2021), the following three attributes and corresponding levels were assigned in DCE: (1) vaccine efficacy to reduce the risk of infection (50%, 70%, or 90%); (2) vaccine safety in terms of the risk of adverse reactions (1 in 10,000 versus 10 in 10,000 vaccinated individuals); and (3) number of vaccine doses administered (1 versus 2 doses). The combination of the three attributes with each level resulted in 12 hypothetical vaccines and 66 possible choice tasks between any two hypothetical vaccines. A D-efficient experimental design was used to reduce these choice tasks down to eight tasks. In each choice task, respondents would choose between having one of two hypothetical vaccines for their children or neither. Respondents who would never choose to let children vaccinated regardless of vaccine attributes (those who chose non-vaccination for all eight choice tasks) were defined as outright vaccination refusers. The selection of vaccine attributes and levels, experiment design, and choice tasks are presented in detail in Appendix C.
2.3. Statistical analysis
Descriptive analyses were performed for socio-demographic characteristics, COVID-19 vaccination related behaviour and perceptions, childhood COVID-19 vaccine acceptance, and reasons of vaccine hesitancy. Univariate analyses were used to compare childhood COVID-19 vaccine acceptance through Chi-square tests for categorical variables and ANOVA for continuous variables. Logistic regressions were applied to investigate the predictors of vaccine acceptance and the effect of information on herd immunity on vaccine acceptance, with “hesitancy” as the reference group.
Preference for vaccine attributes and predicted probability of acceptance for hypothetical COVID-19 vaccines were analyzed under DCE framework (Appendix C). A main effect model with the three vaccine attributes were conducted first, and caregiver interactions were then added in the model to compare the preference differences between caregivers and HCWs. We also estimated probabilities and 95% confidence intervals (CIs) of vaccine acceptance, outright vaccination refusal, and vaccine hesitancy for 12 realistic vaccine scenarios in the whole sample, respectively. The probability of vaccine acceptance in the subsample excluding respondents with outright vaccination refusal (Pvaccine acceptance) was first predicted by a conditional logit model, and vaccine acceptance in the whole sample was then calculated as (1 - Poutright vaccination refusal) × Pvaccine acceptance.
Odds ratios (OR) or coefficient means and their 95% CIs were reported, with p < 0.05 considered to indicate statistical significance. All statistical analyses were performed using STATA 14.0.
3. Results
3.1. Childhood COVID-19 vaccine acceptance
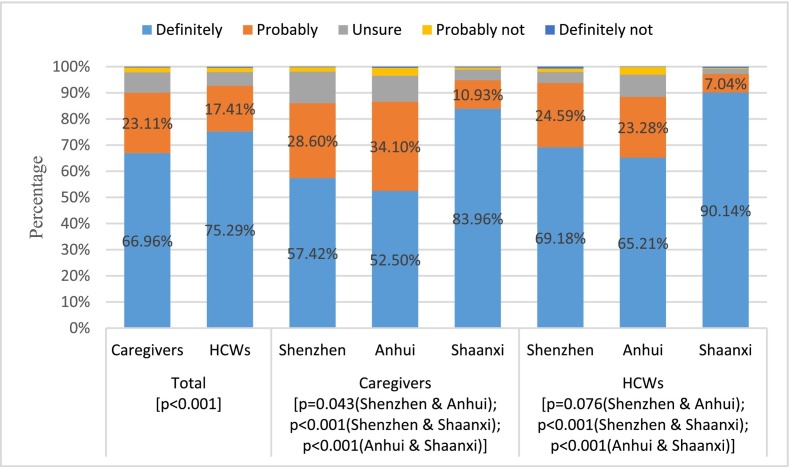
Questionnaires from 2588 caregivers and 1700 HCWs were included in the analyses. Among 2588 caregivers, 2386 (92.19%) had taken COVID-19 vaccination themselves, and 2331 (90.07%) would definitely or probably accept COVID-19 vaccination for their children (Fig. 1 ). Caregivers' acceptance was similar for children aged from 6 months to 11 years (Appendix Fig. 1). Among 1700 HCWs, 1658 (97.53%) had taken COVID-19 vaccination themselves, and 1576 (92.71%) would accept COVID-19 vaccination for children. More respondents from Shaanxi province would definitely have their children vaccinated against COVID-19 than those from Shenzhen and Anhui province (83.96% vs 57.42% and 52.50% for caregivers; 90.14% vs 69.18% and 65.21% for HCWs; all p < 0.001).
Fig. 1.
Childhood COVID-19 vaccine acceptance from caregivers and health care workers (HCWs) by region.
Notes: Chi-square tests with P values were used to compare vaccine acceptance between caregivers and HCWs; across regions for caregivers; and across regions for HCWs.
3.2. Preference for different COVID-19 vaccine attributes
Table 1 shows the associations between vaccine attributes and their acceptance for children using conditional logit models. All three vaccine attributes – efficacy, safety, and number of doses administered – were significantly associated with vaccine acceptance. Both caregivers and HCWs preferred two-dose vaccines with higher efficacy and less adverse reactions. The highest ORs were found for acceptance of a vaccine with 90% efficacy compared to 50% efficacy (OR 6.70 [95% CI 6.11–7.35] for caregivers; 11.44 [10.12–12.95] for HCWs); the risk of adverse reactions to be 1 in 10,000 over 10 in 10,000 (3.96 [3.72–4.22] for caregivers; 2.98 [2.76–3.22] for HCWs). Two-dose vaccine was preferred over one-dose vaccine (1.06 [1.03–1.09] for caregivers; 1.07 [1.03–1.11] for HCWs).
Table 1.
Main effect model with three COVID-19 vaccine attributes from Discrete Choice Experiment.
| Vaccine attributes | Caregivers |
Health care workers |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Vaccine efficacy | ||||
| 50% (ref) | ||||
| 70% | 2.92⁎⁎⁎ | (2.75,3.09) | 4.01⁎⁎⁎ | (3.73,4.32) |
| 90% | 6.70⁎⁎⁎ | (6.11,7.35) | 11.44⁎⁎⁎ | (10.12,12.95) |
| Risk of adverse reactions | ||||
| 10/10000 (ref) | ||||
| 1/10000 | 3.96⁎⁎⁎ | (3.72,4.22) | 2.98⁎⁎⁎ | (2.76,3.22) |
| Number of doses administered | ||||
| 1 dose (ref) | ||||
| 2 doses | 1.06⁎⁎⁎ | (1.03,1.09) | 1.07⁎⁎⁎ | (1.03,1.11) |
| Constant | 1.30⁎⁎⁎ | (1.24,1.36) | 1.32⁎⁎⁎ | (1.25,1.40) |
| Observations | 36,990 | 25,458 | ||
| Log likelihood | −8823.03 | −5782.38 | ||
| Wald χ2(df) | 2343.59(5)⁎⁎⁎ | 1726.59(5)⁎⁎⁎ | ||
Notes: ①OR, odds ratio; CI, confidence interval. ②Estimations are based on a conditional logit model. ③ ***P≦0.001.
Caregivers were more concerned than HCWs about the safety rather than the efficacy of vaccines when considering COVID-19 vaccination for children (Appendix Table 3). There was no significant difference between caregivers and HCWs on the preference for the number of administered doses.
3.3. Acceptance for hypothetical COVID-19 vaccine scenarios
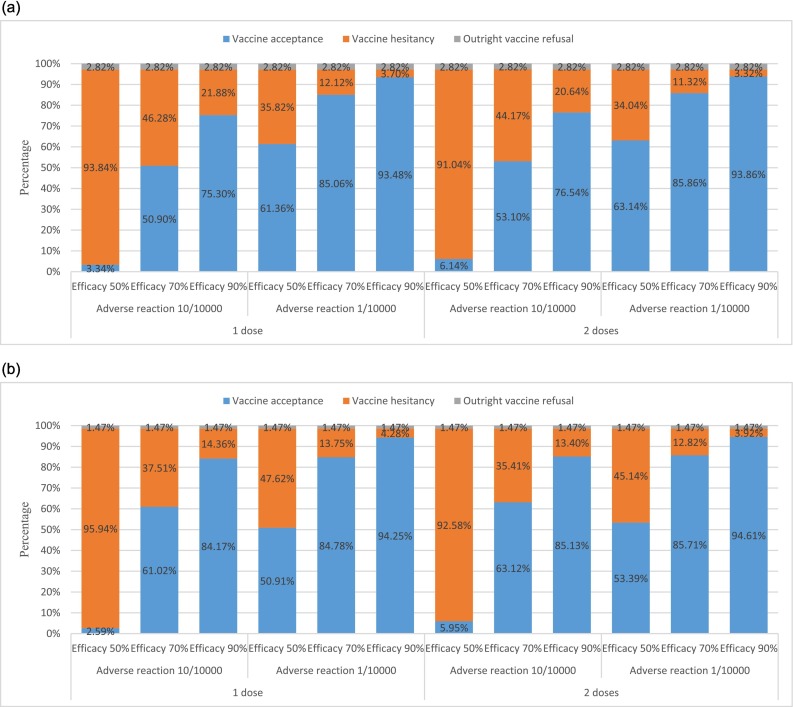
Fig. 2 and Appendix Table 4 and 5 show the predicted probability of acceptance for 12 hypothetical COVID-19 vaccines with various attributes by conditional logit models. Only 73 (2.82%) caregivers and 25 (1.47%) HCWs were identified as refusing COVID-19 vaccination outright for children. Vaccine acceptance reached the highest level for a vaccine with 90% efficacy, the risk of adverse reactions at 1 in 10,000, and two doses (93.86% for caregivers and 94.61% for HCWs). All else being equal, vaccine acceptance would drop to 85.86% for caregivers and 85.71% for HCWs if vaccine efficacy drops to 70%, and further drop to 63.14% for caregivers and 53.39% for HCWs if vaccine efficacy drops to 50%. All else being equal, vaccine acceptance would drop to 76.54% for caregivers and 85.13% for HCWs if the risk of adverse reactions increased to 10 in 10,000. However, there would be almost no change when the number of administered doses reduced to one dose.
Fig. 2.
a. Predicted probability of caregivers' acceptance of 12 hypothetical COVID-19 vaccines for children. b Predicted probability of health care workers' acceptance of 12 hypothetical COVID-19 vaccines for children.
3.4. Predictors of childhood COVID-19 vaccine acceptance
Logistic regressions (Table 2, Table 3 ) show that respondents from Shaanxi province were significantly more likely to accept COVID-19 vaccination for children than Anhui province (OR 2.59 [95% CI 1.79–3.73] for caregivers; 3.35 [1.91–5.88] for HCWs), but no significant difference was found between Shenzhen and Anhui.
Table 2.
Univariate analysis and logistic regression of childhood COVID-19 vaccine acceptance among caregivers.
| Variables | Total, no.(%) | Univariate analysis |
Logistic regression |
|||
|---|---|---|---|---|---|---|
| Acceptance, no.(%) | Hesitancy, no.(%) | P | OR(95%CI) | P | ||
| Total | 2588 | 2331(90.07) | 257(9.93) | NA | NA | NA |
| Region | ||||||
| Anhui(ref) | 1000(38.64) | 866(86.60) | 134(13.40) | <0.001 | NA | NA |
| Shenzhen | 472(18.24) | 406(86.02) | 66(13.98) | 1.18(0.80,1.74) | 0.403 | |
| Shaanxi | 1116(43.12) | 1059(94.89) | 57(5.11) | 2.59(1.79,3.73) | <0.001 | |
| Urban or rural | ||||||
| Rural(ref) | 962(37.17) | 910(94.59) | 52(5.41) | <0.001 | NA | NA |
| Urban | 1626(62.83) | 1421(87.39) | 205(12.61) | 0.55(0.36,0.82) | 0.004 | |
| Age | ||||||
| ≤30(ref) | 762(29.44) | 671(88.06) | 91(11.94) | 0.174 | NA | NA |
| ~35 | 1039(40.15) | 944(90.86) | 95(9.14) | 1.38(0.97,1.95) | 0.074 | |
| ~40 | 456(17.62) | 416(91.23) | 40(8.77) | 1.47(0.92,2.36) | 0.106 | |
| >40 | 331(12.79) | 300(90.63) | 31(9.37) | 0.95(0.54,1.67) | 0.848 | |
| Educational level | ||||||
| High school or below(ref) | 1154(44.59) | 1076(93.24) | 78(6.76) | <0.001 | NA | NA |
| Junior college | 685(26.47) | 617(90.07) | 68(9.93) | 0.77(0.51,1.17) | 0.225 | |
| Bachelor or higher degree | 749(28.94) | 638(85.18) | 111(14.82) | 0.52(0.33,0.81) | 0.004 | |
| Religious belief | ||||||
| None(ref) | 2332(90.11) | 2107(90.35) | 225(9.65) | 0.148 | NA | NA |
| Buddhism or others | 256(9.89) | 224(87.50) | 32(12.50) | 0.87(0.57,1.35) | 0.538 | |
| Occupation | ||||||
| Civil servant(ref) | 397(15.34) | 345(86.90) | 52(13.10) | 0.001 | NA | NA |
| Enterprise personnel | 608(23.49) | 538(88.49) | 70(11.51) | 1.43(0.94,2.18) | 0.098 | |
| Freelancer | 892(34.47) | 802(89.91) | 90(10.09) | 1.22(0.79,1.90) | 0.366 | |
| Self-employed | 250(9.66) | 227(90.80) | 23(9.20) | 1.51(0.83,2.76) | 0.174 | |
| Peasant | 441(17.04) | 419(95.01) | 22(4.99) | 1.18(0.61,2.28) | 0.626 | |
| Residence registration | ||||||
| Local resident(ref) | 2079(80.33) | 1883(90.57) | 196(9.43) | 0.084 | NA | NA |
| Internal migrant | 509(19.67) | 448(88.02) | 61(11.98) | 1.06(0.73,1.52) | 0.762 | |
| Annual household income (1000 Renminbi) | ||||||
| ≤20(ref) | 526(20.32) | 499(94.87) | 27(5.13) | <0.001 | NA | NA |
| 20–50 | 509(19.67) | 469(92.14) | 40(7.86) | 0.73(0.42,1.26) | 0.261 | |
| 50–100 | 667(25.77) | 597(89.51) | 70(10.49) | 0.57(0.34,0.95) | 0.031 | |
| 100–200 | 533(20.60) | 464(87.05) | 69(12.95) | 0.62(0.36,1.06) | 0.080 | |
| >200 | 353(13.64) | 302(85.55) | 51(14.45) | 0.67(0.37,1.21) | 0.182 | |
| Relationship with the child | ||||||
| Mother(ref) | 1990(76.89) | 1783(89.60) | 207(10.40) | 0.118 | NA | NA |
| Father | 420(16.23) | 380(90.48) | 40(9.52) | 1.17(0.78,1.73) | 0.450 | |
| Others | 178(6.88) | 168(94.38) | 10(5.62) | 1.63(0.73,3.67) | 0.237 | |
| Gender of the child | ||||||
| Boy(ref) | 1352(52.24) | 1225(90.61) | 127(9.39) | 0.339 | NA | NA |
| Girl | 1236(47.76) | 1106(89.48) | 130(10.52) | 0.88(0.67,1.16) | 0.370 | |
| Age of the child | ||||||
| ~11(ref) | 207(8.00) | 191(92.27) | 16(7.73) | 0.054 | NA | NA |
| ≤1 | 191(7.38) | 172(90.05) | 19(9.95) | 1.60(0.73,3.51) | 0.236 | |
| ~2 | 259(10.01) | 226(87.26) | 33(12.74) | 0.80(0.39,1.63) | 0.540 | |
| ~3 | 268(10.36) | 243(90.67) | 25(9.33) | 1.09(0.52,2.26) | 0.824 | |
| ~4 | 489(18.89) | 425(86.91) | 64(13.09) | 0.81(0.43,1.54) | 0.526 | |
| ~5 | 604(23.34) | 548(90.73) | 56(9.27) | 1.32(0.70,2.49) | 0.398 | |
| ~6 | 570(22.02) | 526(92.28) | 44(7.72) | 1.17(0.61,2.24) | 0.629 | |
| Family with only one child | ||||||
| No(ref) | 1460(56.41) | 1322(90.55) | 138(9.45) | 0.355 | NA | NA |
| Yes | 1128(43.59) | 1009(89.45) | 119(10.55) | 0.92(0.67,1.27) | 0.623 | |
| Confidence in general vaccines, mean(SD) | 4.32(0.82) | 4.36(0.81) | 3.92(0.88) | <0.001 | 1.58(1.37,1.81) | <0.001 |
| COVID-19 self-vaccination | ||||||
| No(ref) | 202(7.81) | 162(80.20) | 40(19.80) | <0.001 | NA | NA |
| Yes | 2386(92.19) | 2169(90.91) | 217(9.09) | 2.49(1.62,3.83) | <0.001 | |
| Perceived risk of COVID-19 infection for children | ||||||
| Low(ref) | 758(29.29) | 619(81.66) | 139(18.34) | <0.001 | NA | NA |
| High | 1830(70.71) | 1712(93.55) | 118(6.45) | 2.73(1.99,3.74) | <0.001 | |
| Perceived severity of COVID-19 if children infected | ||||||
| Low(ref) | 513(19.82) | 423(82.46) | 90(17.54) | <0.001 | NA | NA |
| High | 2075(80.18) | 1908(91.95) | 167(8.05) | 1.46(1.04,2.04) | 0.030 | |
| Knowledge on herd immunity | ||||||
| Not know(ref) | 742(28.67) | 635(85.58) | 107(14.42) | <0.001 | NA | NA |
| Know | 1846(71.33) | 1696(91.87) | 150(8.13) | 1.82(1.34,2.46) | <0.001 | |
| Information on herd immunity provided | ||||||
| No(ref) | 1295(50.04) | 1159(89.50) | 136(10.50) | 0.331 | NA | NA |
| Yes | 1293(49.96) | 1172(90.64) | 121(9.36) | 1.11(0.84,1.47) | 0.460 | |
Notes: OR, odds ratio; CI, confidence interval; NA, not applicable.
Table 3.
Univariate analysis and logistic regression of childhood COVID-19 vaccine acceptance among health care workers.
| Variables | Total, no.(%) | Univariate analysis |
Logistic regression |
|||
|---|---|---|---|---|---|---|
| Acceptance, no.(%) | Hesitancy, no.(%) | P | OR(95%CI) | P | ||
| Total | 1700 | 1576(92.71) | 124(7.29) | NA | NA | NA |
| Region | ||||||
| Anhui(ref) | 756(44.47) | 669(88.49) | 87(11.51) | <0.001 | NA | NA |
| Shenzhen | 305(17.94) | 286(93.77) | 19(6.23) | 1.66(0.89,3.10) | 0.112 | |
| Shaanxi | 639(37.59) | 621(97.18) | 18(2.82) | 3.35(1.91,5.88) | <0.001 | |
| Urban or rural | ||||||
| Rural(ref) | 659(38.76) | 626(94.99) | 33(5.01) | 0.004 | NA | NA |
| Urban | 1041(61.24) | 950(91.26) | 91(8.74) | 0.64(0.40,1.04) | 0.073 | |
| Gender | ||||||
| Male(ref) | 319(18.76) | 300(94.04) | 19(5.96) | 0.308 | NA | NA |
| Female | 1381(81.24) | 1276(92.40) | 105(7.60) | 0.78(0.45,1.36) | 0.387 | |
| Age | ||||||
| ≤30(ref) | 521(30.65) | 470(90.21) | 51(9.79) | 0.001 | NA | NA |
| ~35 | 350(20.59) | 325(92.86) | 25(7.14) | 0.96(0.51,1.80) | 0.899 | |
| ~40 | 314(18.47) | 285(90.76) | 29(9.24) | 0.94(0.45,1.96) | 0.867 | |
| >40 | 515(30.29) | 496(96.31) | 19(3.69) | 1.75(0.77,3.98) | 0.183 | |
| Educational level | ||||||
| High school or below(ref) | 109(6.41) | 101(92.66) | 8(7.34) | 0.415 | NA | NA |
| Junior college | 613(36.06) | 575(93.80) | 38(6.20) | 1.66(0.69,4.01) | 0.259 | |
| Bachelor or higher degree | 978(57.53) | 900(92.02) | 78(7.98) | 1.12(0.46,2.70) | 0.803 | |
| Religious belief | ||||||
| None(ref) | 1592(93.65) | 1481(93.03) | 111(6.97) | 0.050 | NA | NA |
| Buddhism or others | 108(6.35) | 95(87.96) | 13(12.04) | 0.79(0.40,1.55) | 0.488 | |
| Type of facility | ||||||
| Community health center(ref) | 1077(63.35) | 1019(94.61) | 58(5.39) | <0.001 | NA | NA |
| Hospital | 623(36.65) | 557(89.41) | 66(10.59) | 0.53(0.34,0.82) | 0.004 | |
| Vaccination services engagement | ||||||
| Routine vaccination(ref) | 592(34.82) | 564(95.27) | 28(4.73) | 0.012 | NA | NA |
| COVID-19 vaccination | 762(44.82) | 696(91.34) | 66(8.66) | 0.57(0.34,0.95) | 0.030 | |
| Not engaged at all | 346(20.35) | 316(91.33) | 30(8.67) | 0.56(0.31,1.01) | 0.055 | |
| Working years | ||||||
| ≤1(ref) | 153(9.00) | 142(92.81) | 11(7.19) | 0.235 | NA | NA |
| ~5 | 426(25.06) | 386(90.61) | 40(9.39) | 0.67(0.32,1.42) | 0.297 | |
| ~10 | 312(18.35) | 289(92.63) | 23(7.37) | 0.84(0.37,1.94) | 0.688 | |
| >10 | 809(47.59) | 759(93.82) | 50(6.18) | 0.99(0.41,2.40) | 0.992 | |
| Title | ||||||
| Junior(ref) | 787(46.29) | 719(91.36) | 68(8.64) | 0.041 | NA | NA |
| Intermediate | 758(44.59) | 707(93.27) | 51(6.73) | 1.11(0.65,1.89) | 0.700 | |
| Senior | 155(9.12) | 150(96.77) | 5(3.23) | 1.72(0.59,5.05) | 0.322 | |
| Confidence in general vaccines, mean(SD) | 4.46(0.84) | 4.48(0.85) | 4.24(0.67) | 0.002 | 1.22(0.99,1.51) | 0.059 |
| COVID-19 self-vaccination | ||||||
| No(ref) | 42(2.47) | 40(95.24) | 2(4.76) | 0.765 | NA | NA |
| Yes | 1658(97.53) | 1536(92.64) | 122(7.36) | 0.44(0.10,2.01) | 0.288 | |
| Perceived risk of COVID-19 infection for children | ||||||
| Low(ref) | 294(17.29) | 244(82.99) | 50(17.01) | <0.001 | NA | NA |
| High | 1406(82.71) | 1332(94.74) | 74(5.26) | 2.26(1.41,3.64) | 0.001 | |
| Perceived severity of COVID-19 if children infected | ||||||
| Low(ref) | 262(15.41) | 221(84.35) | 41(15.65) | <0.001 | NA | NA |
| High | 1438(84.59) | 1355(94.23) | 83(5.77) | 1.56(0.95,2.57) | 0.079 | |
| Knowledge on herd immunity | ||||||
| Not know(ref) | 318(18.71) | 271(85.22) | 47(14.78) | <0.001 | NA | NA |
| Know | 1382(81.29) | 1305(94.43) | 77(5.57) | 2.42(1.58,3.72) | <0.001 | |
| Information on herd immunity provided | ||||||
| No(ref) | 854(50.24) | 791(92.62) | 63(7.38) | 0.895 | NA | NA |
| Yes | 846(49.76) | 785(92.79) | 61(7.21) | 1.01(0.68,1.49) | 0.973 | |
Notes: OR, odds ratio; CI, confidence interval; NA, not applicable.
All child-related characteristics and most of respondents' socio-demographic characteristics were not significantly associated with childhood COVID-19 vaccine acceptance, except for urban/rural residence, educational level, and household income for caregivers, and type of working facility and whether engaging in vaccination work or not for HCWs. Caregivers from urban areas were less likely to accept COVID-19 vaccination for their children than rural areas (0.55 [0.36–0.82]). Educational level and household income were negatively associated with caregivers' acceptance for childhood vaccination. Caregivers with a bachelor degree or higher education had a significantly lower odds of childhood vaccine acceptance than those educated at high school level or below (0.52 [0.33–0.81]). Caregivers whose annual household income reached 50,000 to 100,000 RMB were significantly less likely to accept COVID-19 vaccination for children than those below 20,000 RMB (0.57 [0.34–0.95]). For HCWs, those working in hospitals had a significantly lower odds of childhood vaccine acceptance than community health centers (0.53 [0.34–0.82]). Both HCWs only engaging in COVID-19 vaccination services and not engaging in any vaccination services at all had a significantly lower odds of childhood vaccine acceptance than those engaging in routine vaccination services (0.57 [0.34–0.95] and 0.56 [0.31–1.01]).
For caregivers and HCWs, their own history of COVID-19 vaccination, perceived susceptibility and severity of COVID-19 infection for children, confidence in general vaccines, and knowledge on herd immunity were important predictors of childhood vaccine acceptance. Caregivers who had been vaccinated against COVID-19 were significantly more likely to accept vaccination for children (2.49 [1.62–3.83]). Respondents who perceived high susceptibility and severity of COVID-19 infection for children had significantly higher odds of childhood vaccine acceptance than those with perceived low susceptibility and severity (2.73 [1.99–3.74] and 1.46 [1.04–2.04] for caregivers; 2.26 [1.41–3.64] and 1.56 [0.95–2.57] for HCWs). Vaccine confidence was positively associated with childhood COVID-19 vaccine acceptance (1.58 [1.37–1.81] for caregivers; 1.22 [0.99–1.51] for HCWs). And 71.33% of caregivers and 81.29% of HCWs were aware of the needed herd immunity target. Those who already knew the coverage rate of COVID-19 vaccination to achieve herd immunity were more likely to accept vaccination for children than those who did not know (1.82 [1.34–2.46] for caregivers; 2.42 [1.58–3.72] for HCWs). However, childhood COVID-19 vaccine acceptance levels were unchanged by information on herd immunity.
3.5. Reasons for COVID-19 vaccine hesitancy
Among 257 caregivers and 124 HCWs who would not accept COVID-19 vaccination for their children (Appendix Fig. 2), the top three reasons for vaccine hesitancy were the lack of evidence on the safety and efficacy of COVID-19 vaccines for children (91.05% for caregivers and 82.26% for HCWs), concerns about the safety (risk of adverse reactions) of COVID-19 vaccines for children (89.88% for caregivers and 79.03% for HCWs), and the perception that children were too young to take COVID-19 vaccines (87.16% for caregivers and 87.10% for HCWs). Only a small proportion of respondents listed “unnecessary for children to be vaccinated against COVID-19” (24.51% for caregivers and 29.84% for HCWs), and “low risk of COVID-19 infection for children” (17.90% for caregivers and 17.74% for HCWs) as reasons of vaccine hesitancy.
4. Discussion
Our study assesses both caregivers' and HCWs' acceptance of COVID-19 vaccine for children aged 6 months to 11 years and predicts the acceptance for 12 hypothetical COVID-19 vaccines with varying attributes in China. Vaccine acceptance as measured in this study can be a good proxy of COVID-19 vaccination behaviour given the free vaccination policy and adequate vaccine supply in China. The overall acceptance of childhood vaccination reached >90% among Chinese caregivers and HCWs following its emergency use approval, and the definite acceptance further increased with vaccination rollout for children. Vaccine acceptance depended on the attributes of COVID-19 vaccines such as its efficacy and safety. Self-vaccination history, risk perception, vaccine confidence, and knowledge on herd immunity target had strong associations with their acceptance for childhood vaccination.
Consistent with a previous survey in China (Lin et al., 2021), we found a high level of universal acceptance among caregivers and HCWs to vaccinate their children against COVID-19, both over 90%. Parental acceptance for childhood vaccination in our study was higher than the median rate of 59.3% worldwide (Pan et al., 2021) and in countries such as the United States (61.9%) (Teasdale et al., 2021), Canada (60.4%) (Hetherington et al., 2021), and India (63.1%) (Kishore et al., 2021). The emergency use of COVID-19 vaccine was approved in children aged 3-to-17 years in June 2021 in China, earlier than that in other countries such as the United States (The State Council of China, 2021a). Chinese also expressed more supports for pandemic control measures including vaccination. Vaccine acceptance among adults themselves was also found to be high in China (Wang et al., 2020). Up to February 15, 2022, 1.27 billion people have been vaccinated against COVID-19, with an average of 211 doses per 100 people (Our World in Data, 2022). Caregivers' acceptance for childhood vaccination was independent of child's age. The undifferentiated high acceptance across children aged 6 months to 11 years provided social support for childhood COVID-19 vaccination.
The definite acceptance of childhood COVID-19 vaccine in Shaanxi province was much higher by around 30% than that in Anhui and Shenzhen. This large difference may be explained by two reasons. First, Shaanxi just launched COVID-19 vaccination for children aged 3–11 years prior to our survey, whereas Anhui and Shenzhen had not yet. The wide promotion of vaccination possibly improved vaccine acceptance among both caregivers and HCWs. Secondly, during our survey in October 2021, COVID-19 infection resurged and spread in Shaanxi, whereas no new local cases were found in other parts of mainland China. With the promotion of mass vaccination and increased risk of COVID-19 infection, the hesitant people may become accepting vaccination for their children.
Childhood vaccine acceptance heavily depended on vaccine attributes. We found that the efficacy and safety of vaccines were the most influential attributes in determining vaccine acceptance, confirming previous findings on COVID-19 vaccine preference (Huang et al., 2021; Leng et al., 2021). The likelihood of vaccine acceptance would increase substantially when the efficacy or safety was higher. Compared to HCWs, caregivers were more concerned about the safety of COVID-19 vaccines for children and were less influenced by vaccine efficacy. The number of doses administered was the least important attribute affecting vaccine acceptance, however, the result that 2-dose vaccine was preferred than a single dose is inconsistent to our expectation and other studies (McPhedran and Toombs, 2021; Hadjipanayis et al., 2018). One explanation may be that only 2-dose COVID-19 vaccine was approved and available at the time of this survey, while 1-dose vaccine was still in clinical trials. Our study also predicted uptake rate of COVID-19 vaccines with varying attributes and could have policy implications as more vaccines become available. The results showed that the efficacy of vaccines should reach over 70% and the risk of adverse reactions should be lower than 1 in 10,000 if we expect childhood COVID-19 vaccine acceptance to be above 80%.
COVID-19 vaccine hesitancy was more prevalent among caregivers with higher income and education and urban residence, consistent with studies on general vaccines in China (Du et al., 2021). Caregivers with better socioeconomic status may have better access to media sources and overexposure to disinformation on social media, which may negatively impact on vaccine confidence and acceptance (Du et al., 2021). For HCWs, vaccine hesitancy for children was more prevalent among those working in hospitals instead of community health centers and those who did not engaged in routine vaccination services. This may result from the facts that most routine vaccinations are provided in community health centers instead of hospitals in China, and that vaccination service workers are more familiar with the properties of vaccines. Similarly, another study found that parental acceptance of the COVID-19 vaccine was higher among physicians and nurses from infectious disease department than those from internal medicine department (Wang et al., 2021).
Importantly, risk perception, vaccine confidence and knowledge, and self-vaccination history were found to be key predictors of caregivers' and HCWs' acceptance of COVID-19 vaccination for children. We found that public confidence increased vaccine acceptance, and that concerns about the safety and efficacy of vaccines were also listed as the main reasons for vaccine hesitancy. Therefore, more clinical evidence and communication on vaccine safety and efficacy are essential to enhance public confidence and vaccine acceptance. In accordance with previous studies (Teasdale et al., 2021; Humble et al., 2021; Gendler and Ofri, 2021), we found that adults who were vaccinated against COVID-19 were more willing to vaccinate children against COVID-19. Another survey experiment in France verified our finding that during the early stage of COVID-19 pandemic, vaccine hesitancy significantly decreased when communicating the collective benefits of herd immunity (Schwarzinger et al., 2021).
This study has several limitations. First, as a cross-sectional study, our results could only reflect levels of COVID-19 vaccine acceptance at the time of the survey in China. Findings could be variable in other settings and other countries. Second, our study employed an online questionnaire to collect data and was on a voluntary basis, which may limit the representativeness of the respondents. Individuals who concerned more on our topic or with easier internet-access might have a higher probability to participate in the survey. Third, our data were collected from self-reported questionnaires, which may lead to report bias. Fourth, we only concern children aged 6 months to 11 years old in our study, and health conditions of the children and of their caregivers were not investigated although they are generally healthy. Finally, the vaccine attributes included in our experiment represent three salient attributes expected to be associated with COVID-19 vaccine acceptance (efficacy, safety, and number of doses). Other vaccine attributes such as duration of vaccine immunity and development platforms were not included in this study.
5. Conclusion
In summary, this survey and DCE experiment show high acceptance of childhood COVID-19 vaccine among Chinese caregivers and HCWs, which was undifferentiated across children aged 6 months to 11 years. Vaccine acceptance depends on its attributes such as efficacy and safety. To achieve herd immunity target with above 80% vaccination coverage, the efficacy of COVID-19 vaccine should reach over 70% and the risk of adverse reactions should be lower than 1 in 10,000.
Author contributions
Hou had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Hou.
Acquisition, analysis, or interpretation of data: Hou.
Drafting of the manuscript: Hou, Song.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: Song.
Obtained funding: Hou.
Administrative, technical, or material support: Hou.
Supervision: Hou.
Funding/Support
This research was funded by the National Natural Science Foundation of China (No. 71874034). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing
Data would be available on reasonable request by contacting Zhiyuan Hou (zyhou@fudan.edu.cn).
Declaration of Competing Interest
The Vaccine Confidence Project, which HL leads, receives collaborative grants with Astra Zeneca, GlaxoSmithKline, J&J, and Merck in addition to public sector grants. None of those research grants are related to this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107138.
Appendix A. Supplementary data
Supplementary methods and results
References
- Aldakhil H., Albedah N., Alturaiki N., et al. Vaccine hesitancy towards childhood immunizations as a predictor of mothers’ intention to vaccinate their children against COVID-19 in Saudi Arabia. J. Infect. Public Health. 2021;14(10):1497–1504. doi: 10.1016/j.jiph.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S., Clarke R., Mounier-Jack S., et al. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38(49):7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Chantler T., Francis M.R., et al. Access to vaccination information and confidence/hesitancy towards childhood vaccination: a cross-sectional survey in China. Vaccines (Basel). 2021;9(3):201. doi: 10.3390/vaccines9030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic. May 10. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use
- FDA FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. October 29. 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age
- Gendler Y., Ofri L. Investigating the influence of vaccine literacy, vaccine perception and vaccine hesitancy on Israeli parents’ acceptance of the COVID-19 vaccine for their children: a cross-sectional study. Vaccines (Basel). 2021;9(12):1391. doi: 10.3390/vaccines912139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemiyeh P., Mohammadi-Samani S., Firouzabadi N., et al. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021;100 doi: 10.1016/j.intimp.2021.108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- Hadjipanayis A., Efstathiou E., Michaelidou K., et al. Adherence to pneumococcal conjugate vaccination schedule and uptake rate as compared to the established diphtheria-tetanus-acellular pertussis vaccination in Cyprus. Vaccine. 2018;36(38):5685–5691. doi: 10.1016/j.vaccine.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Hetherington E., Edwards S.A., MacDonald S.E., et al. SARS-CoV-2 vaccination intentions among mothers of children aged 9 to 12 years: a survey of the all our families cohort. CMAJ Open. 2021;9(2):E548–E555. doi: 10.9778/cmajo.20200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Shao X., Wagner A.L., et al. COVID-19 vaccine coverage, concerns, and preferences among Chinese ICU clinicians: a nationwide online survey. Exp. Rev. Vaccin. 2021;20(10):1361–1367. doi: 10.1080/14760584.2021.1971523. [DOI] [PubMed] [Google Scholar]
- Humble R.M., Sell H., Dubé E., et al. Canadian parents’ perceptions of COVID-19 vaccination and intention to vaccinate their children: results from a cross-sectional national survey. Vaccine. 2021;39(52):7669–7676. doi: 10.1016/j.vaccine.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore J., Venkatesh U., Ghai G., et al. Perception and attitude towards COVID-19 vaccination: a preliminary online survey from India. J. Family Med. Prim. Care. 2021;10(8):3116–3121. doi: 10.4103/jfmpc.jfmpc_2530_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng A., Maitland E., Wang S., et al. Individual preferences for COVID-19 vaccination in China. Vaccine. 2021;39(2):247–254. doi: 10.1016/j.vaccine.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Hu Z., Zhao Q., et al. Chinese parents’ intentions to vaccinate their children against SARS-CoV-2 infection and vaccine preferences. Hum. Vaccin. Immunother. 2021;17(12):4806–4815. doi: 10.1080/21645515.2021.1999143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhedran R., Toombs B. Efficacy or delivery? An online discrete choice experiment to explore preferences for COVID-19 vaccines in the UK. Econ. Lett. 2021;200 doi: 10.1016/j.econlet.2021.109747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalti M., Rallo F., Guaraldi F., et al. Would parents get their children vaccinated against SARS-CoV-2? Rate and predictors of vaccine hesitancy according to a survey over 5000 families from Bologna, Italy. Vaccines (Basel). 2021;9(4):366. doi: 10.3390/vaccines9040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulou G.B., Maltezou H.C. COVID-19 in children: where do we stand? Arch. Med. Res. 2022 Jan;53(1):1–8. doi: 10.1016/j.arcmed.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our World in Data Coronavirus (COVID-19) Vaccinations. January 16, 2022. https://ourworldindata.org/covid-vaccinations
- Pan F., Zhao H., Nicholas S., et al. Parents’ decisions to vaccinate children against COVID-19: a scoping review. Vaccines (Basel). 2021;9(12):1476. doi: 10.3390/vaccines9121476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane M.S., Robertson M.M., Westmoreland D.A., et al. Intention to vaccinate children against COVID-19 among vaccinated and unvaccinated US parents. JAMA Pediatr. 2022;176(2):201–203. doi: 10.1001/jamapediatrics.2021.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzinger M., Watson V., Arwidson P., et al. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6(4):e210–e221. doi: 10.1016/S2468-2667(21)00012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi P.G., Shah M.D., Delgado J.R., et al. Parents’ intentions and perceptions about COVID-19 vaccination for their children: results from a national survey. Pediatrics. 2021;148(4) doi: 10.1542/peds.2021-052335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale C.A., Borrell L.N., Shen Y., et al. Parental plans to vaccinate children for COVID-19 in New York city. Vaccine. 2021;39(36):5082–5086. doi: 10.1016/j.vaccine.2021.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temsah M.H., Alhuzaimi A.N., Aljamaan F., et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front. Public Health. 2021 Oct 13;9:752323. doi: 10.3389/fpubh.2021.752323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The State Council of China Emergency Inactivated COVID-19 Vaccine Available for People Aged 3–17 years. June 12. 2021. http://www.gov.cn/xinwen/2021-06/12/content_5617313.htm
- The State Council of China The Epidemic is Still Developing Rapidly, and Many Places have Started COVID-19 Vaccination for People Aged 3–11 years. October 31. 2021. http://www.gov.cn/fuwu/2021-10/31/content_5647958.htm
- Wang J., Jing R., Lai X., et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines (Basel). 2020;8(3):482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., She R., Chen X., et al. Parental acceptability of COVID-19 vaccination for children under the age of 18 years among Chinese doctors and nurses: a cross-sectional online survey. Hum. Vaccin. Immunother. 2021;17(10):3322–3332. doi: 10.1080/21645515.2021.1917232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization COVID-19 vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines Accessed December 26, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and results