Abstract
Aim
To compare admission-blood-glucose (ABG) or stress-hyperglycemia-ratio (SHR) performs better in predicting mortality and worse outcomes in COVID-19 patients with (DM) and without known Type 2 Diabetes Mellitus (UDM).
Methods
ABG and SHR were tested for 451 patients with moderate-severe COVID-19 infection [DM = 216,47.9%; pre-diabetes = 48,10.6%, UDM = 187,41.4%],who were followed-up to look for in-hospital-mortality (primary outcome) and secondary outcomes (ICU stay or mechanical ventilation, hospital-acquired-sepsis and multiple organ dysfunction syndrome [MODS]). Those with and without SHR ≥ 1.14 were compared; logistic regression was done to identify predictors of outcomes, with subgroup analysis based on pre-existing DM status and COVID-19 severity.
Results
Those who died (n = 131) or developed ≥ 1 secondary outcomes (n = 218) had higher prevalence of SHR ≥ 1.14, ABG ≥ 180 mg/dl and higher median SHR (pall < 0.01). Those with SHR ≥ 1.14 had higher mortality (53.7%), higher incidence of ≥ 1 secondary outcomes (71.3%) irrespective of pre-existing diabetes status. SHR ≥ 1.14, but not ABG ≥ 180 was an independent predictor of mortality in the whole group (OR: 7.81,4.07–14.98), as also the DM (OR:10.51,4.34–25.45) and UDM (5.40 (1.57–18.55) subgroups. SHR ≥ 1.14 [OR: 4.41 (2.49–7.84)] but not ABG ≥ 180 could independently predict secondary outcomes AUROC of SHR in predicting mortality was significantly higher than ABG in all subgroups.
Conclusion
SHR better predicts mortality and adverse outcomes than ABG in patients with COVID-19, irrespective of pre-existing chronic glycemic status.
Keywords: Stress hyperglycemia, Stress hyperglycemia ratio, COVID-19, Mortality
1. Introduction
Transient hyperglycaemia is often observed during hospitalisation for serious medical illnesses, trauma or surgical emergencies and is known as stress hyperglycaemia (SH). The pathogenetic mechanisms driving the occurrence of SH include enhanced gluconeogenesis and glycogenolysis due to excessive catecholamines and cortisol secreted under stressful situations, increased glucagon secretion and transient insulinopenia.[1], [2] Historically, SH was believed to be a protective response of the body to acute injury by providing fuel to the brain and immune system during periods of stress. [3] However, several studies have now shown the association of SH with higher mortality or morbidity in patients with acute coronary syndromes, ischemic strokes, burns and trauma. [4], [5], [6], [7] Initial studies used absolute blood glucose values at admission (ABG) of over 180 as a marker for high risk in hospitalized patients, and most guidelines focus on strict glycemic control to keep blood glucose values during hospitalisation below this threshold. [8], [9] However, having the same threshold irrespective of the patient’s pre-existing glycemic status may lead to over or underestimation of the risk. In the past few years, there has been increased focus on novel parameters of stress hyperglycemia, like the stress hyperglycemia ratio (SHR), which provide a composite estimation of acute and chronic hyperglycemia. [10], [11], [12] The formula for SHR involves estimated average blood glucose (eAG) which is an index of chronic glycemic status from the previous 3 months, unaffected by short-term variability. SHR is calculated as admission blood glucose (ABG) divided by eAG, thus representing the relative difference from eAG. [7], [10]
SARS-CoV-2 enters the type II pneumocytes using ACE2 as a receptor. After binding to ACE2, serine proteases like TMPRSS2 cleaves the spike protein and facilitates viral entry into the cell by promoting fusion of the viral envelope with the host cell membrane and endosome formation, following which viral replication occurs in the presence of RNA dependent RNA polymerase. Following its entry into the cell, SARS-CoV2 degrades ACE2 leading to an overall downregulation of ACE2 mediated pathways. ACE2 is important to counter-regulate the pro-inflammmatory, pro-coagulant and pro-fibrotic pathways triggered by activation of the RAAS via Angiotensin (AngII) acting via AT1 and AT2 receptors. [13], [14] ACE2 degrades Ang II into Ang 1–7, which acts via the Mas receptor to exert potent vasodilatory, anti-inflammatory, anti-fibrotic, anti-thrombotic and anti- arrhythmogenic effects.. In patients with obesity, DM, hypertension, there is an inherent hyperactivation of the ACE1/Ang-II/AT1R arm of the RAAS and those with prolonged diabetes have low ACE2 activity in pneumocytes due to glycation. [15] Therefore, following COVID-19 infection in these individuals, elevated Ang-II levels act restrained through AT1R, promoting hyper-inflammation, fibrosis and oxidative damage and severe lung injury.
The bidirectional relationship between COVID-19 and diabetes mellitus (DM) is now well known. While DM is known to enhance the risk of mortality in patients with COVID-19, the SARS-CoV2 is known to affect the beta cells via ACE2 mediated pathways, as well as cause a surge of several pro-inflammatory cytokines, which leads to transient hyperglycaemia and new onset DM, often with ketosis in some patients. [16], [17], [18] Studies have described that those with diabetes as well as those with hyperglycemia detected at admission had higher risk of severe disease as compared to those without diabetes, and insulin infusion lowered the risk for severe disease. [19], [20] The higher propensity for patients with T2DM and COVID-19 to develop more severe disease and higher mortality rates might be related to hyperglycemia associated damage to pulmonary micro-vasculature as also hyperactivation of pro-inflammatory pathways, particularly IL-6 mediated. [21] The risk association of hypertension as also the effects of different antihypertensives in influencing mortality or morbidity in COVID-19 is however, less clearly evident. However, those with hypertension associated with cardiac systolic dysfunction portend a significantly higher risk. [22], [23] The use of ACE-inhibitors or Angiotensin-receptor-blockers (ARBs) have been shown to favorably affect COVID-19 outcomes in some studies. [22] Similarly, pre-existing cardiovascular disease, malignancies and several other co-morbidities have been shown to increase the risk for worse outcomes in COVID-19. [24], [25]Composite scores for co-morbidities have been found in studies to predict worse outcomes more precisely in patients with COVID-19. [26]
While the study by Fadini et al showed rapid respiratory deterioration and worse COVID-19 outcomes in those with newly diagnosed diabetes and/or hyperglycaemia at admission compared to those with known diabetes, a recent study by Ramon et al. found higher acute /chronic hyperglycaemic ratio to predict worse outcomes in COVID-19 patients.[12], [27] However, to the best of our knowledge, there has been no single study till date that has compared the effects of SH in COVID-19 in patients with and without pre-existing DM or pre-diabetes. Utilising the stress hyperglycemia ratio (SHR), a novel index providing combined evaluation of acute and chronic hyperglycemia, we aimed to evaluate the influence of SH on mortality and other adverse outcomes in hospitalized COVID-19 patients, with or without type 2 diabetes mellitus or prediabetes. Further, we aimed to compare the performance of admission SHR to absolute admission blood glucose (ABG) levels, in predicting outcomes in this group of COVID-19 patients.
2. Subjects
The current study was a prospective observational study conducted over a period of 12 months (January 2021 to December 2021). The study population included all patients with moderate, severe or critical COVID-19 infection, aged between 20 and 80 years, who were admitted to a tertiary care hospital in West Bengal, India. We excluded patients with mild COVID-19 infection, those with type 1 DM, DM arising from pancreatic disorders or other forms of secondary DM, those who were on chronic glucocorticoid therapy or who had received oral or parenteral glucocorticoids prior to admission, pregnant women, patients on dialysis or post-renal transplant patients, those with a recent hypoglycaemia or hypoglycaemia (ABG < 70 mg/dl) at admission, those with recent blood transfusion in the preceding three months and those who were admitted for non-COVID-19 related illness.
3. Materials and methods
3.1. Primary and secondary outcomes
The primary outcome was in-hospital mortality and the secondary outcomes included the need for ICU admission, need for mechanical ventilation, development of hospital acquired sepsis, multiple organ dysfunction syndrome (MODS) and the length of ICU stay (for those in ICU). Need for ICU admission was decided by pulmonologists and critical care medicine specialists as per national guidelines. [28], [29], [30] The criteria for ICU admission included the need for mechanical ventilation, respiratory rate (RR) exceeding 25 breaths per minute (/min), partial pressure of oxygen (PaO2) < 50 mm Hg on room air or capillary oxygen saturation (SpO2) < 90% on supplemental oxygen therapy at 6 Litres/min, hypotension requiring fluid resuscitation or the need for vasopressors, confusion, leukopenia or thrombocytopenia, hypothermia and /or a quick sequential organ failure assessment (qSOFA) score of > 2.[31], [32], [31], [32]Those initially admitted in non-ICU wards were shifted to ICU if they were found to develop any of these criteria during stay. All patients with acute respiratory distress syndrome (ARDS) were put on mechanical ventilation, ARDS being defined as new onset breathlessness (<1 week) with bilateral opacities in chest X-ray in the absence of heart failure or volume overload, and the ratio of PaO2 / fraction of inspired oxygen (FiO2) being less than or equal to 300. [31], [33] Mechanical ventilation could be either non-invasive (NIV) using BiPAP (for those with PaO2/FiO2 > 150) or invasive ventilation using lung-protective strategy (for those with PaO2/FiO2 〈1 5 0) with early prone ventilation.[31]
Hospital acquired sepsis was defined as a new-onset infection with sepsis at least 48 h following admission for COVID-19, in which sepsis was defined as having two or more features of systemic inflammatory response syndrome (SIRS) (core body temperature > 380 or < 36 °C), heart rate > 90 beats/min, RR > 20 breaths/min or PaCo2 < 32 mm Hg, leukocyte count > 12,000 cells/ul or < 4000 cells/ul or > 10% band forms) [34]. MODS was defined as dysfunction involving 2 or more organ systems requiring intervention. [34]Length of ICU stay was determined for those who required ICU admission at any point of time during hospitalisation from the day of ICU admission till the patient was shifted to a step-down unit or ward. The decision regarding the discharge from ICU to step-down ward was taken when the patient was conscious, oriented and hemodynamically stable with heart rate < 90 /min, systolic blood pressure (SBP) > 120 mm Hg without vasopressor support, RR < 20/min, tolerating oral feeds and not requiring any organ support treatment or monitoring. [31]
3.2. Definitions used in the study
Classification of severity of COVID-19, definition of MODS, need for ICU stay and need for mechanical ventilation were determined as per national COVID-19 guidelines. [35]. The diagnosis of DM and pre-diabetes (PDM) was made as per ADA 2021 criteria for HbA1c% or a known pre-existing history of type 2 DM, PDM or treatment for the same. [36]. Thus, DM was diagnosed if at admission, HbA1c was ≥ 6.5% or patient has a known history of having DM or taking or having taken anti-diabetic agents. Pre-diabetes (PDM) was diagnosed when the Hba1c was between 5.7 and 6.4%. All patients fiitting the inclusion and exclusion criteria and having HbA1c < 5.7% and not affirming a pre-existing history of DM or treatment for the same were considered in a group of unknown DM status (UDM). At least one documented HbA1c report within last three months prior to admission was considered to determine pre-existing glycemic status. Estimated average blood glucose (eAG) was calculated from HbA1c% using the formula eAG = 28.7 X HbA1c% – 46.7. [37] Absolute blood glucose at admission (ABG) was tested for all patients and SHR was calculated using the formula SHR = ABG / eAG. Cut-offs for ABG and SHR used to define SH were determined as ABG ≥ 180 mg/dl and SHR ≥ 1.14 respectively. [7]
3.3. Study design
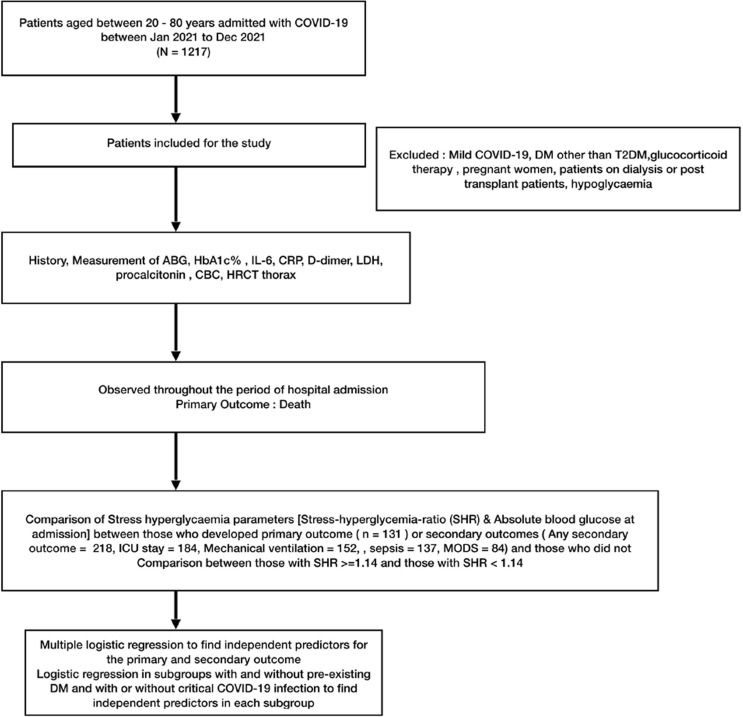
The study design is outlined in Fig. 1 . Patients were enquired regarding their age, pre-existing diabetes status and relevant history required for the calculation of Charlston co-morbidity index (CCI). [26], [38] For all the patients included in the study, SpO2 and severity of COVID-19 at the time of admission and 25-point CT severity index (CTSI) on the first HRCT of thorax done at admission were documented. Following admission, blood samples were sent for ABG, glycated Hb (HbA1c) and serum levels of IL-6, CRP, D-dimer, LDH, procalcitonin as well as complete blood count, urea, creatinine and liver function tests. All patients received standard of care for COVID-19 as per local and national guidelines. [29], [31], [28] All patients with breathlessness, RR > 20 breaths/min or mild hypoxemia (SpO2 < 92%) were given supplemental oxygen with target SpO2 above 94%. Thromboprophylaxis with LMWH (Enoxaparin 40 mg sc or Unfractionated Heparin for those with eGFR 〈3 0) was given to all. All patients received parenteral dexamethasone or Methylprednisolone as per recommended doses during their stay. Antiviral agent in the form of Remdesvir injection (200 mg iv on Day 1 followed by 100 mg iv for 5 days) was given to cases presenting within 10 days of symptom onset. The interleukin-6 (IL-6) inhibitor Tocilizumab was considered only in those who had features of cytokine storm and very high IL-6 levels.
Fig. 1.
Study design.
3.4. Assay methods
Diagnosis of COVID-19 infection was based on a positive result on qualitative detection by Truenat Real-Time polymerase chain reaction (PCR) based on the amplification of specific regions of the pathogen genome od SARS-CoV2. Oropharyngeal or nasopharyngeal swab specimens were collected as per standard procedures using a standard nylon flocked swab. Swab with the specimen was inserted into the Transport Medium and mixed well by repeatedly twirling the swab in the buffer solution. The handle of the nylon swab was gently broken at the break point, leaving the swab containing the specimen in the Transport Medium and the cap was tightly closed. Testing was done on the same day in the hospital laboratory. Assay was observed to be linear over the range of dilutions tested and PCR efficiency was found to be 100.8% for Orf1a and 101.87% for Egene, with Limit of Detection (LoD) of Orf1a and E gene being was estimated to be 480 (C.I. 410–628) and 487 (C.I. 419–631) genome copies/mL respectively.
Serum CRP was measured using particle-enhanced immunoturbidimetry by Integra 400 + analyser (Roche Diagnostics, Rotkreuz, Switzerland, CV: 6.6%), serum IL-6 and procalcitonin by electrochemiluminiscence assay using Cobas e411 analyser (Roche Diagnostics, Rotkreuz, Switzerland, CV 10% and 7.6% for IL-6 and procalcitonin respectively) and D – dimer using quantitative ELISA by VIDAS® D-Dimer Exclusion™ (Biomerieux, India, CV: 8.2%). Capillary blood glucose measurements were done before and 2 h after every meals using Accu-Check Active glucometer and strips following standard measures and precautions. SpO2 was measured using battery operated AccuSure fingertip pulse oximeter. HRCT of thorax was done using the Somatom Emotion 16-slice CT scan 16 by Siemens AG, Germany, and 25 point CT severity score was reported using the formula by Saeed et al [39]. CBC was analysed using Sysmex 6 part cell counter. HbA1c% was measured using HPLC via BIORAD D10 analyser (BIO-RAD, India, CV: 2.8%) and expressed both as HbA1c% (NGSP) and in mmol/mol (IFCC). Blood glucose values were measured using Roche Integra 400 plus analyser (Roche Diagnostics, Rotkreuz, Switzerland, CV 3.6%). Ethical clearance for the current project was obtained from the institutional ethical committee reference no HWH/IEC-BMHR/001/2020.
3.5. Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc.,Chicago,Illinois). Groups were compared using unpaired t-test or Mann-Whitney’s U test for quantitative variables and Chi-square test, with Fisher’s correction where appropriate, for categorical variables. Multiple group comparisons for DM, PDM and UDM were done using one way ANOVA test followed by Tukey’s post hoc analysis with comparison of harmonic means to correct for unequal sample sizes in the three groups. A p value < 0.05 was considered significant. Correlation co-efficient is expressed using Spearman’s rho for parameters with significant linear correlation. Binomial logistic regression was done to identify the independent predictors of death and the secondary outcomes in two steps — univariate analysis (step 1) followed by multivariate analysis using independent variables which were found to be significant (p < 0.05) or suggestive of significance (p < 0.100) in step 1. Multicollinearity was tested between the multiple predictors and parameters with VIF>5 were not included in the same logistic regression model. All the logistic models were validated using the Hosmer- Lemeshow test As sub-group analysis, logistic regression analysis for independent predictors of death and the secondary outcomes were conducted separately in the DM, PDM and UDM sub-groups. ROC curves were constructed and analysed for differences in AUROC for the different parameters.
4. Results
4.1. Baseline demographic characteristics
Out of 1217 patients admitted to the hospital with COVID-19 infection during the study period, a total of 451 patients with moderate, severe or critical COVID-19 were finally included in the study based on the study inclusion criteria. The mean age of the population was 58.84 (±13.86) years and 62% (n = 279) were males. The primary outcome of in-hospital mortality was seen in n = 131 (29%), whereas any one or more of the secondary outcomes were seen in n = 218 (48.3%) of the patients Amongst the secondary outcomes, ICU admission was required in n = 184 (40.8%) with a mean duration of ICU stay of 6.5 days (SD: 6.8), mechanical ventilation was needed in n = 152 (33.7%), hospital-acquired sepsis occurred in n = 137 (30.4%) and MODS developed in 84 (18.6%) patients. A total of n = 224 (49.7%) patients were admitted with severe COVID-19 infection and n = 125 (27.7%) had critical COVID-19, while the remaining n = 102 (22.6%) patients had moderate COVID-19 infection.
4.1.1. Anti-diabetic medication use and discontinuation for those with pre-existing DM
Out of the 216 patients with pre-existing DM, 166 (76.9%) were receiving Metformin, 96 (44.4%) were on sulfonylureas, 33 (15.3%) were on pioglitazone, 47 (21.9%) were on alpha-glucosidase inhibitors (AGI), 134 (62%) were on DPP4 inhibitors (DPP4i), 89 (41.2%) were on SGLT2 inhibitors (SGLT2i) and two patients were on injectable GLP1Ra and 57 (26.4%) were on insulin, alone or in combination with other agents, prior to admission. During admission, all non-insulin anti-diabetic agents except DPP4 inhibitors were discontinued for those with severe or critical COVID-19, those requiring ICU admission, those with sepsis/MODS or those requiring intensive insulin therapy, according to existing local and national policies. DPP4i were withheld for those with MODS. Accordingly, metformin was discontinued in 156 (93.9% of those previously on metformin) patients, sulfonylureas in 89 of 96 patients (92.7%), pioglitazone in all 33 patients (100%), AGI in all the 47 patients (100%), SGLT2i discontinued in 83 of 89 patients (93.3%).DPP4-i were discontinued in 41 out of the 134 patients (30.4%).
4.1.2. Vaccination status
A total of 41 patients (9.1%) had completed two doses of COVID-19 vaccines prior to admission while 80 patients (17.8%) had received single dose while the majority (n = 330,73.2%) were unvaccinated.
4.2. Treatment received during hospitalization
All the patients in this study received parenteral glucocorticoids (Dexamethasone or Methylprednisolone) in their recommended doses for COVID-19 and thromboprohylaxis with heparin. Remdesvir was given to 148 patients (32.8%) and Tocilizumab to 24 (5.3%) of the patients.
4.2.1. Glycemic optimization for those with hyperglycemia at admission/during hospitalization
All patients with ABG ≥ 180 mg/dl at admission were started on insulin either as subcutaneous basal bolus (>2 pre-meal bolus/day) or basal-plus (<2 pre-meal bolus/day) regimen or intravenous infusion (if ABG > 250 mg/dl). Strict glycemic control was maintained for all patients during hospital stay with intensive insulin regimen to keep target BG between 80 and 180 mg/dl throughout the day. The average BG on the day at discharge was 176.5 mg/dl (±28).
4.3. Comparison between those with and without elevated SHR (SHR ≥ 1.14)
SH, as per SHR ≥ 1.14 criterion was seen in 164 (36.4%) cases. There were significant differences in the in-hospital mortality as well as the presence of at least one of the secondary outcomes between those with and without SHR ≥ 1.14 (n = 88, 53.7% vs n = 43, 15% for death; and n = 117, 71.3% vs n = 101, 35.2% for any secondary outcome, pboth < 0.001). There were also significantly greater occurrences of most of the secondary outcomes in those with elevated SHR, though there were no differences in the severity of COVID-19, or mean HbA1c at admission between the two groups (Table 1 ). There was a higher prevalence of pre-existing T2DM in those with SHR ≥ 1.14 (53.7% vs 44.6%, p = 0.02). Inteerstingly, among those with T2DM, those who were on sulfonylureas had lesser incidence of SH defined by SHR ≥ 1.14 (n = 67, 50% vs n = 29, 33% of those with SHR ≥1.14 and < 1.14 respectively were on sulfonylureas prior to admission).
Table 1.
Comparison between those with and without elevated Stress Hyperglycaemia Ratio (SHR ≥1.14).
| Patients with SHR ≥ 1.14 (n = 164) | Patients with SHR < 1.14 (n = 287) | p | |
|---|---|---|---|
| Age in years | 60.77 (12.51) | 57.75 (13.73) | 0.02 |
| Gender | M: 108F: 56 | M: 171F: 115 | 0.23 |
| Severity of COVID-19 at admission | Moderate = 30 (18.0.3%) Severe = 85 (51.8%) Critical = 49 (29.9%) |
Moderate = 72 (25.1%) Severe = 139 (48.4%) Critical = 76 (26.5%) |
0.25 |
| Known Type 2 Diabetes Mellitus (DM) or Prediabetes | DM = 88 (53.7%) Prediabetes = 9 (5.5%) None = 67 (40.9%) |
DM = 128 (44.6%) Prediabetes = 39 (13.6%) None = 120 (41.8%) |
0.02 |
| SpO2 (%) at admission | 83.46 (7.86) * | 86.27 (6.92) | <0.001 |
| Charlston comorbidity index | 4.67 (3.50) | 4.42 (3.46) | 0.46 |
| 25-point CT Severity score | 11.95 (5.31) | 11.08 (5.09) | 0.08 |
| IL-6 (pg/ml) | 135.26 (266.12) | 151.90 (536.32) | 0.71 |
| CRP (mg/L) | 152.61 (163.61)* | 113.20 (112.73) | 0.003 |
| LDH (mg/dl) | 585.93 (448.22)* | 456.51 (304.26) | <0.001 |
| D – dimer (ng/ml) | 3945.2(3605.90) | 2984.13 (3304.02) | 0.003 |
| Procalcitonin (mcg/L) | 2.97 (5.36) | 3.09 (11.74) | 0.901 |
| Neutrophil:Lymphocyte ratio | 36.23 (29.89( | 29.88 (48.72) | 0.131 |
|
HbA1c%(NGSP) HbA1c in mmol/mol (IFCC) |
6.68 (1.84) 49.5 (6) |
6.44 (1.57) 46(5) |
0.135 |
| ABG (mg/dl) | 194.68 (71.97)* | 145.83 (50.08) | <0.001 |
| SHR | 1.36 (0.13)* | 1.06 (0.09) | <0.001 |
| In hospital mortality | 88 (53.7%) * | 43 (15%) | <0.001 |
|
Any secondary outcome Required ICU stay Required mechanical ventilation Hospital acquired sepsis MODS |
117 (71.3%)* 105 (64%) * 85 (51.8%) * 81 (49.4%) * 39 (23.9%) * |
101 (35.2%) 79 (27.5%) 67 (23.3%) 56 (19.5%) 45 (15.7%) |
<0.001 <0.001 <0.001 <0.001 0.02 |
| Duration of ICU stay in days | 10.81 (7.59) | 5.04 (5.97) | 0.67 |
|
Prior medication for DM Metformin Sulfonylureas Thiazolidinediones α-glucosidase inhibitor DPP4 inhibitors GLP1Ra SGLT2i |
70 (72.2%) 29 (33%) 13 (14.8%) 14(15.9%) 58 (65.9%) 1 38 (43.2%) |
98 (58.7%) 67 (50%) 20 (15.5%) 32(25.2%) 77 (60.2%) 1 51 (39.8%) |
0.03 0.01 0.98 0.13 0.48 0.67 |
*Denotes significant differences between the two groups with and without SHR ≥ 1.14 (p < 0.05 All values are expressed as mean (SD) or number (percentage) except SHR which is expressed as median (IQR). ABG = Absolute blood glucose at admission, SHR = Stress hyperglycemia ratio, SHR was derived from the formula (Blood glucose a admission) / estimated Average Glucose (eAG) derived from HbA1c% at admission. IL-6 = Interleukin-6, CRP = C-Reactive Protein, LDH = Lactate dehydrogenase, MODS = Multiple organ dysfunction syndrome.
4.4. Comparison between those with and without primary and secondary outcomes
4.4.1. Primary outcome (In-hospital mortality)
In our study, in-hospital mortality was seen in 29% (n = 131) of the study population. Those who died during hospital admission were older in age, had higher severity of COVID-19 infection and higher prevalence of DM, with significantly greater rise in inflammatory markers than those who survived (n = 342, 71%). Significantly higher proportion of those who survived had completed double-dose COVID-19 vaccination compared to those who died (11.3% vs 3.8%,p = 0.01). (Table 2 ). Compared to those who survived, there was a significantly higher prevalence of SH among those who died, as defined by SHR ≥ 1.14 (n = 88,67.2% vs n = 76,23.8%) or ABG ≥ 180 (n = 74,56.5% vs n = 77,24.1%). Further, the median SHR was significantly higher in those who died (1.16 vs 1.07, p < 0.01).
Table 2.
Comparison between those with and without the primary outcome of in-hospital mortality.
| Parameter | All (n = 451) | Died (n = 131) | Survived (n = 320) | p |
|---|---|---|---|---|
| Age (yrs)* | 58.84 (13.36) | 62.62 (12.14) | 57.3(13.55) | 0.001 |
| Gender | M: 279F: 171 | M: 90F: 41 | M: 189F: 130 | 0.07 |
|
Severity of COVID 19 * Moderate Severe Critical |
112 (32.6%) 224 (49.7%) 125 (27.7%) |
7(5.3%) 75(57.3%) 49(37.4%) |
95(29.7%) 149(46.6%) 76(23.8%) |
0.04 |
| SpO2%* | 85.25 (7.39) | 80.67 (7.14) | 87.12 (6.65) | <0.001 |
|
Type 2 Diabetes Mellitus * Pre-diabetes |
216 (47.9%) 48 (10.6%) |
104(79.4%) 6 (4.6%) |
112(35%) 42(13.1%) |
<0.001 |
| Charlston comorbidity index* | 4.51 (3.47) | 5.53 (3.62) | 4.09(3.32) | <0.001 |
| 25 point CT severity index* | 11.40 (5.19) | 13.37(4.84) | 10.59 (5.11) | <0.001 |
|
HbA1c%(NGSP)* HbA1c in mmol/mol (IFCC)* |
6.53 (1.68) 48 (5) |
7.35 (2) 56 (6) |
6.19 (1.40) 43.5 (5) |
<0.001 |
| ABG* | 163.59 (63.43) | 208.60 (77.16) | 145.17 (45.57) | <0.001 |
| SHR* | 1.11 (0.27) | 1.16 (0.32) | 1.07 (0.11) | <0.001 |
|
Prevalence of SH* ABG ≥ 180 * SHR ≥ 1.14 * |
151 (33.5%) 164 (36.4%) |
74 (56.5%) 88 (67.2%) |
77 (24.1%) 76 (23.8%) |
<0.001 <0.001 |
| CRP(mg/L)* | 127.53 (134.66) | 197.71 (170.91) | 98.80 (104.04) | <0.001 |
| D-Dimer(ng/ml)* | 3310.70 (3446.51) | 4974.55 (3739.53) | 2629.56(3074.95) | <0.001 |
| LDH(mg/dl)* | 503.57 (368.09) | 714.31 (352.61) | 417.30 (338.76) | <0.001 |
| NLR* | 32.2 (42.88) | 53.70(50.55) | 23.34(35.79) | <0.001 |
| Procalcitonin(mcg/L)* | 3.05 (9.91) | 6.09 (12.88) | 1.80 (8.09) | 0.001 |
| IL-6 (pg/ml) | 145.85 (456.64) | 199.84 (327.84) | 123.74(498.67) | 0.11 |
|
Prior medication for DM Metformin Sulfonylureas Thiazolidinediones α-glucosidase inhibitor DPP4 inhibitors GLP1Ra SGLT2i Insulin |
(DM subgroup, n = 216) 166 (76.9%) 96 (44.4%) 33 (15.3%) 47 (21.9%) 134 (62%) 2 89 (41.2%) 57 (26.4%) |
(DM subgroup, n = 104) 74 (71.2%) 47 (45.2%) 13 (12.4%) 20 (19.2%) 68 (65.4%) 2 37 (35.6%) 23 (22.1%) |
(DM subgroup, n = 112) 92 (82.1%) 49 (43.8%) 20 (17.9%) 27 (24.3%) 66 (58.9%) 0 52 (46.4%) 34 (30.4%) |
0.08 0.89 0.34 0.41 0.4 0.13 0.22 |
|
Discontinued medication for DM Metformin Sulfonylureas Thiazolidinediones α-glucosidase inhibitor DPP4 inhibitors GLP1Ra SGLT2i |
156 (72.2%) 89 (41.2%) 33 (15.2%) 47 (21.8%) 41 (19%) 2 83 (38.4%) |
75 (72.1%) 45 (43.3%) 13 (12.4%) 20 (19.2%) 15 (14.4%) 2 37 (35.6%) |
81 (72.3%) 44 (39.3%) 20 (17.9%) 27 (24.1%) 26 (23.2%) 0 46 (41.1%) |
1 0.58 0.57 0.41 0.12 0.48 |
| Started on Insulin during admission * | 151 (33.5%) | 74 (56.5%) | 77 (24.1%) | <0.001 |
| Hypertension | 185 (41%) | 54 (41.2%) | 131 (40.9%) | 1 |
| On ACEi/ARB | 132 (29.3%) | 36 (27.5%) | 96 (30%) | 0.65 |
| Remdesvir | 148 (32.8%) | 49 (37.4%) | 99 (30.9%) | 0.19 |
| Tocilizumab | 24 (5.3%) | 10 (7.6%) | 14 (4.4%) | 0.17 |
| Received single dose Vaccine prior to admission | 84 (18.6%) | 17 (13%) | 67 (20.9%) | 0.06 |
| Received double dose Vaccine prior to admission* | 41 (9.1%) | 5(3.8%) | 36(11.3%) | 0.01 |
*denotes parameter with significant differences (p < 0.05) between those who died vs those who survived. All values are expressed as mean (SD) or number (percentage)except SHR which is expressed as median (IQR). SH = Stress hyperglycemia, ABG = Absolute blood glucose at admission SHR = Stress hyperglycemia ratio, SHR = Blood glucose at admission / estimated Average Glucose (eAG) derived from HbA1c% at admission] IL-6 = Interleukin-6, CRP = C-Reactive Protein, LDH = Lactate dehydrogenase, MODS = Multiple organ dysfunction syndrome,ACEi = Angiotensin converting Enzyme inhibitor, ARB = Angiotensin receptor blockers.
There were no differences in the rates of use or of discontinuation of any of the anti-hyperglycemic agents or of single-dose vaccination between those who died vs those who survived (Table 2). Need for intensive insulin therapy during admission was significantly higher for those who died (56.5% vs 24.1%, p < 0.001). No differences in the rates of remdesvir or tocilizumab use were noted in the two groups (Table 2). However, the total number of patients receiving Tocilizumab was small in our study (n = 24).
4.4.2. Secondary outcomes
Comparing those who had at least one of the secondary outcomes (n = 218, 48.33%)with those without any of the secondary outcomes (n = 233, 51.6%), the former had significantly higher prevalence of DM, prevalence of stress hyperglycemia (defined by ABG or SHR criteria), greater degree of COVID-19 severity and higher levels of SHR and ABG, as well as rise in inflammatory biomarkers. Similar findings were observed for the subgroups requiring ICU admission (n = 184) or mechanical ventilation (n = 152), compared to those not requiring either (pall < 0.03). (Suppl Table 1). Significantly lower proportion of patients with one or more secondary outcomes had taken at least one dose of COVID-19 vaccines (n = 43, 19.7% vs 82, 35.2% for those with and without secondary outcomes respectively, p < 0.001). Paradoxically, higher proportion of those with secondary outcomes had received Tocilizumab (8.3% vs 2.1%, p = 0.004), but this was likely due to the fact that those receiving Tocilizumab had more severe disease and higher IL-6 levels and therefore, greater probability of having received Tocilizumab.
Amongst those who developed hospital acquired sepsis (n = 137) or MODS (n = 84, 18.6%) significantly higher levels of HbA1c, ABG and SHR were observed compared to those who did not [6.85% vs 6.4% (50.5 vs 46 mmol/mol), 184.84 0.7 vs 154.32 and 1.24 vs 1.12 respectively, pall < 0.003). However, severity of COVID-19 infection at admission as well as levels of inflammatory markers failed to show significant differences between these two sub-groups.
4.5. Comparison of differences between groups with and without elevated SHR (≥ 1.14) depending on baseline DM status
Overall, pre-existing type 2 Diabetes Mellitus (DM) was found in 47.9% (n = 216) and pre-diabetes (PDM) in 10.6% (n = 48) of the patients, while the rest were without diabetes mellitus (UDM, n = 187, 58.5%). ANOVA and post-hoc Tukey’s analysis among the three groups yielded significant differences between the parameters due to differences between the DM and UDM group, while there were no significant differences between either DM or UDM with PDM group. Apart from higher HbA1c% and ABG values, those with DM had lower mean SpO2 levels and higher CRP, D-dimer and LDH levels at admission, indicating a greater severity of COVID-19 infection at admission . The prevalence of in-hospital mortality, hospital acquired sepsis and MODS were highest in those with DM, followed by those with PDM and lowest in UDM, with significant differences between the groups for hospital-acquired-sepsis, MODS and need for ICU stay (p < 0.05). (Suppl Table 2). The median SHR values and the prevalence of SH was similar in DM, PDM and UDM groups.
In-hospital mortality, for those with elevated SHR ≥ 1.14, was greater than those without elevated SHR, irrespective of glycemic status. Similarly, majority of the secondary outcomes in those with elevated SHR, were significantly greater for both DM and UDM subgroups. (pall < 0.03, Suppl Table 2). Interestingly in the UDM group, those with elevated SHR had significantly higher HbA1c at admission compared to those without [5.24% vs 5.05% (33.5 vs 31.5 mmol/mol), p = 0.01].
4.6. Results of logistic regression analysis
4.6.1. Predictors for the primary outcome
Following multiple logistic regression, in a model with independent variables of age, SpO2, severity of COVID-19 infection, pre-existing DM status, HbA1c%, vaccination status, Charlston comorbidity index scores (CCI) and SH defined by ABG ≥ 180 and SHR ≥ 1.14, we found that SHR≥ 1.14 [OR: 7.81 (4.07–14.98), p < 0.001] was an independent predictor for in-hospital mortality. The other independent predictors of mortality were admission SpO2 [OR: 0.77 (0.72–0.83)], CCI [OR: 1.17 (1.07–1.28)], critical COVID-19 [OR: 12.05 (4.34–33.43)], double-dose vaccination status [OR: 0.19 (0.05–0.71)], HbA1c% [OR: 1.004 (1.001–1.008)] and pre-existing DM/pre-diabetes status [OR: 4.88 (2.06–11.57)]. However, SH defined by ABG ≥ 180 was not found to be an independent predictor for mortality (Table 3 ).
Table 3.
Logistic regression analysis of admission glucose metrics as predictors of in-hospital mortality.
| Whole group [n = 451] |
Subgroup with Diabetes Mellitus [n = 216] |
Subgroup without Diabetes Mellitus /pre-diabetes [n = 187] |
|
|---|---|---|---|
| Glucose metric thresholds at admission* | O.R. (C.I.) | ||
| Stress hyperglycemia ratio (SHR) ≥ 1.14 | 7.81 (4.07–14.98)# | 10.51 (4.34–25.45)# | 5.40 (1.57–18.55)# |
| Absolute glucose at admission (ABG) ≥ 180 mg/dl | 0.37 (0.13–1.01) | 4.55 (1.33–15.59)# | 0 |
*Results from logistic regression of a model adjusted for age, severity of COVID-19, pre-existing Type 2 Diabetes Mellitus status, Charlston comorbidity index, SpO2, HbA1c%,vaccination status in addition to the admission glucose metrics SHR ≥ 1.14 and ABG ≥ 180.O.R. = Odds’ ratio, C.I. = Confidence Intervals # represents significant results,p < 0.05.
Upon subgroup analysis by preexisting DM status, both SHR ≥ 1.14 and ABG ≥180 were independent predictors of in-hospital mortality in the DM subgroup [OR: 10.51 (4.34–25.45) and OR: 4.55 (1.33–15.59) for SHR ≥ 1.14 and ABG ≥ 180 respectively,pboth < 0.02). However, in the UDM subgroup SHR ≥ 1.14 remained an independent mortality predictor [OR: 5.40 (1.57–18.55), p = 0.01] but ABG ≥ 180 was not. Notably, HbA1c% as a continuous variable [OR: 1.005 (1.001–1.009),p = 0.02] and double-dose vaccination status [OR: 0.14 (0.03–0.77),p = 0.02] were independent positive and negative predictors, respectively, of mortality in the DM subgroup but not in the UDM subgroup.
Considering severity of COVID-19 at admission, SHR ≥ 1.14 was an independent predictor of mortality in those with critical as well as non-critical COVID-19 infection though the Odds for mortality was much higher in those with critical COVID-19 [OR: 26.28 (5.98–115.38) and 5.33 (2.47–11.51) for critical and non-critical COVID-19 respectively, pboth < 0.001], On the contrary, ABG ≥ 180 was found to significantly predict mortality only in those with critical COVID-19 [OR: 16.62 (1.64–168.51),p = 0.02]. Interestingly, double-dose vaccination status was a significant negative predictor of mortality in those with critical COVID-19 [OR: 0.08 (0.01–0.81), p = 0.03] but not in those with non-critical COVID-19.
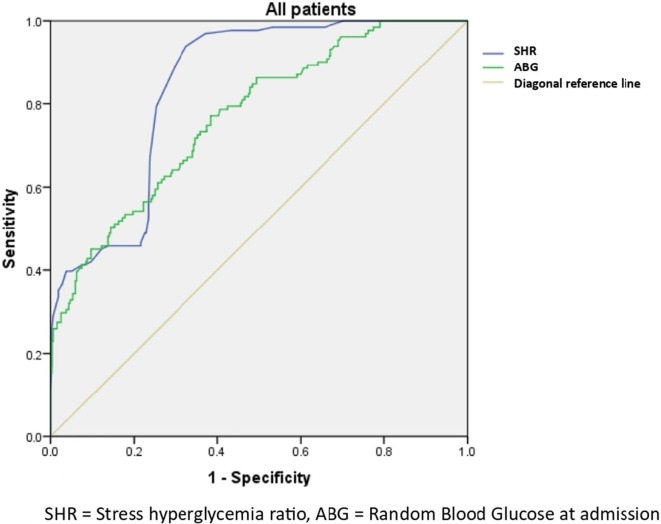
Due to strong collinearity between themselves, admission ABG and SHR values could not be used as continuous independent variables in the same model. When they were analysed in separate prediction models with age, SpO2, critical COVID-19, pre-existing DM status and double-dose vaccination status as the other independent variables, both ABG and SHR emerged to be independent predictors for in-hospital mortality in the entire cohort [OR: 1.04 (1.03–1.06) and OR: 1.06 (1.04–1.08) for ABG and SHR respectively, pboth < 0.001). AUROC curves were established for both and the AUROC was higher for SHR (84.2%) than for ABG (76.8%) (Fig. 2 ). When analysed separately for the DM and UDM subgroups, the AUROC persisted to be higher for SHR than ABG in both the DM (87.8% and 64.1% respectively, pboth < 0.001) and the UDM groups (79.7% and 71.6% respectively, pboth < 0.001). (Table 4 ) (Suppl Fig. 1a and 1b).
Fig. 2.
ROC curves for Stress hyperglycemia ratio (SHR) and absolute blood glucose levels at admission (ABG) in predicting in-hospital mortality in the whole cohort.
Table 4.
AUROC with C.I for Stress hyperglycemia Ratio and Absolute Blood Glucose at admission in the whole group and subgroups according to pre-existing chronic glycemic status.
| Whole group | Subgroup with Diabetes Mellitus | Subgroup without Diabetes Mellitus/pre-diabetes | |
|---|---|---|---|
| Stress hyperglycemia ratio (SHR) | 0.842 (0.806–0.878) | 0.878 (0.833–0.924) | 0.797 (0.719–0.874) |
| Absolute glucose at admission (ABG) | 0.768 (0.721–0.815) | 0.641 (0.564–0.718) | 0.716 (0.625–0.807) |
*AUROC = Area under Receiver operating characteristic curve, C.I. = Confidence intervals, p < 0.001 for all.
4.6.2. Predictors for the secondary outcome
Following multiple logistic regression analysis, we found that elevated SHR ≥ 1.14 [OR: 4.41 (2.49–7.84), p < 0.001] was an independent predictor for the occurrence of one or more secondary outcomes, along with low admission SpO2 [OR: 0.79 (0.74–0.84)], critical COVID-19 [OR: 6.15 (2.37–15.97)], age [OR: 1.06 (1.04–1.09)] and HbA1c [OR: 1.004 (1.001–1.008)]. SHR ≥ 1.14 remained to be an independent predictor for one or more secondary outcomes in both DM and UDM subgroups [OR: 4.60 (1.77–12.01) and OR: 6.46 (2.68–15.58) respectively in DM and UDM subgroups, pboth < 0.002]. However, ABG ≥ 180 failed to act as independent predictors for secondary outcomes in either the entire cohort or any of its subgroups. The protective effect of double dose vaccination status against one or more secondary outcomes was seen in the DM subgroup [OR: 0.16 (0.03–0.75, p = 0.02] but not in the UDM sub-group.
5. Discussion
In the current study, we analysed the role of stress hyperglycemia in patients with moderate (49.7%), severe (29.7%) or critical (22.6%) COVID-19 infection admitted in a tertiary care hospital, of whom 43.5% were having pre-existing type 2 diabetes mellitus. The primary outcome of in-hospital mortality after admission occurred in 29% of cases, and other adverse outcomes including the need for ICU stay or mechanical ventilation, hospital acquired infections and MODS were seen in 40.8%, 33.7%, 8.8%, 30.4% and 18.6% respectively. Our study period included the months which coincided with the second wave in India and we excluded patients with mild COVID-19 patients, which explains the high mortality rates in our study than that reported in some other studies. [40], [41]
In our cohort, more than one-third had developed SH, defined either by admission blood glucose ≥ 180 mg/dl (35.6%) or SHR ≥ 1.14(40.3%). Prior studies considering an admission glucose values above 140 mg/dl as a diagnosis of SH reported rates of 35–40% [42], [43]. In the past five years, several studies have shown that HbA1c-adjusted glycemic variables, like the stress hyperglycemia ratio, which have an adjustment factor for the chronic hyperglycaemic state, could be more appropriate biomarkers for predicting worse outcomes in higher risk of certain critical illnesses[4], [7], [11], [44], [45] [The high prevalence of SH seen in our study could be related to the combined effects of SARS-CoV2 mediated beta cell dysfunction, effect of pro inflammatory cytokines and hypoxia in addition to other stress induced factors. Hypoxia has been shown to cause acute rise in blood glucose levels, possibly mediated by increased release of epinephrine [46] This is borne out by the fact that greater degrees of hypoxia and higher levels of pro-inflammatory cytokines were seen in those with SHR ≥ 1.14 in our study.
We found elevated SHR ≥ 1.14 to be an independent predictor of in-hospital mortality and other secondary outcomes, including hospital acquired sepsis and MODS, irrespective of their background chronic glycemic status. In those without DM, only SHR ≥ 1.14 could predict mortality but admission blood glucose > 180 couldn’t. In those with pre-existing type 2 DM, both SHR ≥ 1.14 as well as ABG ≥ 180 were mortality predictors but the Odd’s ratio and AUROC for SHR was higher than admission blood glucose. Some older studies claimed SH to be prognostically important only in those without DM whereas more recent studies have focussed on the importance of SH in patients with DM admitted with different critical illnesses. [47] In older studies, SH was believed to be a protective mechanism developed due to creation of a high blood glucose diffusion gradient, thus enhancing cellular glucose uptake in the setting of disturbed microvascular flow. [48] However, this overload of glucose and oxidative phosphorylation leads to oxidative stress and ROS production in the mitochondria, and consequently the endoplasmic reticulum, leading to cellular dysfunction and worsening the course of the disease.[49] In acute hypoxia, there is increased production of ROS by alteration of the cytochrome chain activity, further decreasing the normal cellular antioxidant response. [50] Additionally, direct infection by the SARS-CoV2 has been shown to trigger oxidative stress by a number of mechanisms, including ACE2 down regulation leading to decreased activity of the AT2-ACE2-MapK pathway, increased neutrophil:lymphocyte ratio with neutrophil activation and the role of pro inflammatory cytokines. [51], [52] Therefore, SH occurring in the setting of COVID-19 can lead to overwhelming ROS production. In patients with pre-existing DM, particularly those with poor control, there is reduced compensatory anti-oxidative capacity.[8] This might explain the damaging effects of SH being more prominently seen in those with pre-existing type 2 DM. Mortality rates were lower in those who received two doses of COVID-19 vaccination but not in those having received singly vaccination. Double-dose vaccination had a protective effect from COVID-19 mortality in those with DM but not in those without pre-existing DM/pre-diabetes in our study. This is interesting to note given that some studies have shown that patients with DM and poor glycemic control at the time of vaccination could dampen the immunological response to COVID-19 vaccination.[53]
We found no differences in the use of oral anti-diabetic agents or insulin prior to admission in those who died and those who survived. However, there was a higher need for insulin therapy during admission in those who died, this being driven by the higher rates of admission hyperglycemia in the former. In another study on COVID-19 patients with admission hyperglycemia, the rates of oral anti-diabetic agents and insulin was not found to affect disease severity or mortality. [19] In our study, we had not initiated oral anti-diabetic agents in any patient with admission hyperglycemia and used insulin therapy in all of them while discontinuing many of the pre-hospital oral agents according to national COVID-19 management guidelines. The use of Remdesvir and Tocilizumab also were not found to affect mortality, though, the overall number of patients receiving Tocilizumab was less in our cohort due to financial constraints. To account for multiple co-morbidities that might affect. COVID-19 outcomes, we used the Charlston-comorbidity index which has been validated as a predictor for mortality, ICU stay and other adverse outcomes in multiple settings including COVID-19 and is particularly suitable for small studies.
In a recent study on COVID-19 patients with type 2 DM, the authors have concluded that hyperglycaemia at admission is associated with poor outcomes but not mortality, while acute: chronic hyperglycaemia has a U shaped mortality curve with high mortality rates at both the lowest and highest tertile.[12]In our study, the values for SHR, which is essentially the same as the acute: chronic hyperglycemia ratio, are higher than those reported by the authors. The reasons for the higher SHR might be the use of non-fasting blood glucose levels at admission for SHR calculation in our study, unaccounted and unrecorded use of glucocorticoids prior to admission in some of the patients and a higher severity of COVID-19 with greater degree of hypoxia and pro-inflammatory markers in our patients. Also, the study by Raman et al was conducted on a predominantly elderly population (mean age 75 years, range 61 to 85 years), some of whom had chronic kidney disease, which might have contributed to an attenuated stress response and release of cortisol and catecholamines, which in turn would elicit a lesser degree of stress hyperglycaemia. In our study, we included patients without diabetes mellitus in addition to those with pre-existing type 2 DM and pre-diabetes, while carefully excluding those with documented hypoglycaemia or advanced CKD at admission. In another study, authors found that in COVID-19 patients with stress hyperglycemia defined as admission glucose above 180 mg/dl, decrease in blood glucose within 24 hrs from from baseline was associated with lower rates of mortality and progress to severe disease in both nondiabetic and diabetic patients. [19] Thus, early and intensive glycemic control must be ensured in all patients with COVID-19.
Our results suggest that SH plays an important role in determining outcomes in hospitalised moderate-to-severe COVID-19 patients with or without pre-existing type 2 DM. Further, SH as elicited by an elevated admission SHR ≥ 1.14, proves to be a consistent and better predictor for in-hospital mortality and other adverse outcomes than absolute blood glucose at admission, irrespective of baseline glycemic status. Almost all existing guidelines focus on intensive glycemic control when the ABG rises above 180 mg/dl. However, novel indices like the SHR, which factor-in the both acute and chronic glycemic status, might be a more clinically useful parameter to identify COVID-19 patients at higher risk of adverse outcomes, and hence be considered as treatment targets for intensive glycemic monitoring and treatment.
Our study has some limitations. The subjects in our study were followed up till discharge from the hospital or death. Since the follow-up period was not a uniform pre-determined time duration in days or months for all, we could not analyse the data using a Cox-proportional hazards model but used the multivariate regression instead. While use of fasting blood glucose values alone may have been ideal to calculate SHR at admission, getting an adequate number of moderate-to-severely ill COVID-19 patients presenting to the hospital in the fasted state may not have been practically feasible. Since many of our patients with severe illness could not stand properly at admission, BMI could not be calculated for all, while arterial blood gas parameters were not available for comparison in all. Since our study emcompassed a period in which vaccines were being rolled out across the country, the proportion of vaccinated patients were low. The type of vaccine received was not taken into account. We used the Charlston-comorbidity index to give a single quantitative value adjusting for multiple co-morbidities in our patients and didn’t consider individual comorbidities for statistical analysis. The details regarding the type of vaccines was also not taken into account. The findings of our study will need further validation in larger, multicenter prospective studies.
In conclusion, the presence of stress hyperglycaemia, can adversely affect mortality and morbidity in patients hospitalised with COVID-19, and this effect is seen both those with type 2 diabetes mellitus and those without diabetes mellitus. An elevated SHR ≥ 1.14 proves to be a better predictor of in-hospital mortality and morbidity in COVID 19 patients, irrespective of baseline chronic glycemic status, when compared with absolute admission blood glucose values. While results of our study underscore the importance of strict glycemic monitoring for all hospitalised patients with COVID-19, irrespective of their baseline glycemic status, elevated SHR can be used a as a novel index at admission to identify at-risk populations who requires closer supervision and early intervention to control blood glucose levels.
CRediT authorship contribution statement
Sunetra Mondal: Data curation, Formal analysus, Methodology, Writing - Original draft. Riddhi DasGupta: Conceptualisation, Formal analysis,Writing-review and editing. Moushumi Lodh: Investigation. Ramprasad Garai: Methodology. Brojen Choudhury: Methodology. Arindam Kumar Hazra: Methodology. Aniket Mondal: Methodology. Arunangshu Ganguly: Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors thank all the clinical and paraclinical staff, nurses, technicians working in COVID-19 dedicated wards and ICUs, radiologists and laboratory personnel working in the critical care settings and emergency wards dedicated to management of COVID-19 infected patients in our hospital.
Funding
None.
Data availability
The raw data file and analysed datasets can be available from the corresponding author o reasonable request by e mail to riddhi_dg@rediffmail.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2022.109974.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Shamoon H., Hendler R., Sherwin R.S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981 Jun;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- 2.Harp J.B., Yancopoulos G.D., Gromada J. Glucagon orchestrates stress-induced hyperglycaemia. Diabetes Obes Metab. 2016 Jul;18(7):648–653. doi: 10.1111/dom.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik P.E., Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capes S.E., Hunt D., Malmberg K., Gerstein H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000 Mar 4;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 5.Capes S.E., Hunt D., Malmberg K., Pathak P., Gerstein H.C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001 Oct;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 6.J S, Gv B, M J, K B, K T, Tm S. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma [Internet] 2005 Jul [cited 2022 Jun 2];59(1). Available from: https://pubmed.ncbi.nlm.nih.gov/16096543/. [DOI] [PubMed]
- 7.Yang Y., Kim T.H., Yoon K.H., Chung W.S., Ahn Y., Jeong M.H., et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017 Aug;15(241):57–63. doi: 10.1016/j.ijcard.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 8.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002 Oct;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 9.Jacobi J., Bircher N., Krinsley J., Agus M., Braithwaite S.S., Deutschman C., et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012 Dec;40(12):3251–3276. doi: 10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 10.Roberts G.W., Quinn S.J., Valentine N., Alhawassi T., O’Dea H., Stranks S.N., et al. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J Clin Endocrinol Metab. 2015 Dec;100(12):4490–4497. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 11.Liao W.I., Wang J.C., Chang W.C., Hsu C.W., Chu C.M., Tsai S.H. Usefulness of Glycemic Gap to Predict ICU Mortality in Critically Ill Patients With Diabetes. Medicine (Baltimore) 2015 Sep 11;94(36) doi: 10.1097/MD.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramon J., Llauradó G., Güerri R., Climent E., Ballesta S., Benaiges D., et al. Acute-to-Chronic Glycemic Ratio as a Predictor of COVID-19 Severity and Mortality. Diabetes Care. 2022 Jan 1;45(1):255–258. doi: 10.2337/dc21-1321. [DOI] [PubMed] [Google Scholar]
- 13.Matarese A., Gambardella J., Sardu C., Santulli G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines. 2020 Oct 30;8(11):E462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Onofrio N., Scisciola L., Sardu C., Trotta M.C., De Feo M., Maiello C., et al. Glycated ACE2 receptor in diabetes: open door for SARS-COV-2 entry in cardiomyocyte. Cardiovascular Diabetol. 2021 May 7;20(1):99. doi: 10.1186/s12933-021-01286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Apr;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Can COVID-19 cause diabetes? | Nature Metabolism [Internet]. [cited 2022 Jun 2]. Available from: https://www.nature.com/articles/s42255-020-00339-7.
- 17.Gupta R.D., Atri A., Mondal S., Bhattacharjee A., Garai R., Hazra A.K., et al. Characterizing progressive beta-cell recovery after new-onset DKA in COVID-19 provoked A-β+ KPD (ketosis-prone diabetes): A prospective study from Eastern India. J Diabetes Complications. 2022 Mar;36(3) doi: 10.1016/j.jdiacomp.2021.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.S M, R D, M L, R G, B C, Ak H, et al. Predictors of new-onset diabetic ketoacidosis in patients with moderate to severe COVID-19 receiving parenteral glucocorticoids: A prospective single-centre study among Indian type 2 diabetes patients. Diabetes Metabolic Syndrome [Internet]. 2021 Jun [cited 2022 Jun 2];15(3). Available from: https://pubmed.ncbi.nlm.nih.gov/33839639/. [DOI] [PMC free article] [PubMed]
- 19.Sardu C., D’Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020;63(11):2486. doi: 10.1007/s00125-020-05216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sardu C., D’Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., et al. Outcomes in Patients With Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care. 2020 Jul;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol 2020 Dec;19(1):76, s12933-020-01047-y. [DOI] [PMC free article] [PubMed]
- 22.Clark C.E., McDonagh S.T.J., McManus R.J., Martin U. COVID-19 and hypertension: risks and management. A scientific statement on behalf of the British and Irish Hypertension Society. J Hum Hypertens. 2021 Apr;35(4):304–307. doi: 10.1038/s41371-020-00451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardu C., Maggi P., Messina V., Iuliano P., Sardu A., Iovinella V., et al. Could Anti-Hypertensive Drug Therapy Affect the Clinical Prognosis of Hypertensive Patients With COVID-19 Infection? Data From Centers of Southern Italy. J Am Heart Assoc. 2020 Sep;9(17) doi: 10.1161/JAHA.120.016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020 Dec;13(12):1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A I, Ma R, F T, Ma LM, C A, A B, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PloS one [Internet]. 2020 Nov 17 [cited 2022 Jun 12];15(11). Available from: https://pubmed.ncbi.nlm.nih.gov/33201896/. [DOI] [PMC free article] [PubMed]
- 26.Kim D.H., Park H.C., Cho A., Kim J., Yun K.S., Kim J., et al. Age-adjusted Charlson comorbidity index score is the best predictor for severe clinical outcome in the hospitalized patients with COVID-19 infection. Medicine (Baltimore) 2021 May 7;100(18) doi: 10.1097/MD.0000000000025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini G.P., Morieri M.L., Boscari F., Fioretto P., Maran A., Busetto L., et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020 Oct;168 doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Management_Protocol_for_COVID-19_-_WB_2nd_Edition.pdf [Internet]. [cited 2022 Jun 12]. Available from: https://www.wbhealth.gov.in/uploaded_files/corona/Management_Protocol_for_COVID-19_-_WB_2nd_Edition.pdf.
- 29.CLINICAL GUIDANCE FOR MANAGEMENT OF ADULT COVID-19 PATIENTS | AIIMS Covid Information Portal [Internet]. [cited 2022 Jun 12]. Available from: https://covid.aiims.edu/clinical-guidance-for-management-of-adult-covid-19-patients/.
- 30.Admin. CME INDIA COVID-19 Management Protocol 2022 [Internet]. CME INDIA. 2022 [cited 2022 Jun 12]. Available from: https://cmeindia.in/cme-india-covid-19-management-protocol-april-2021/.
- 31.1587101879731_ijccm23395.pdf [Internet]. [cited 2022 Jun 3]. Available from: https://isccm.org/pdf/1587101879731_ijccm23395.pdf.
- 32.Koch C., Edinger F., Fischer T., Brenck F., Hecker A., Katzer C., et al. Comparison of qSOFA score, SOFA score, and SIRS criteria for the prediction of infection and mortality among surgical intermediate and intensive care patients. World J Emerg Surg. 2020 Nov 25;15(1):63. doi: 10.1186/s13017-020-00343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acute respiratory distress syndrome: the Berlin Definition - PubMed [Internet]. [cited 2022 Jun 3]. Available from: https://pubmed.ncbi.nlm.nih.gov/22797452/.
- 34.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992 Jun;101(6):1644–55. [DOI] [PubMed]
- 35.96997299691580715786.pdf [Internet]. [cited 2022 Jun 12]. Available from: https://ncdc.gov.in/WriteReadData/l892s/96997299691580715786.pdf.
- 36.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021 Jan;44(Suppl 1):S15–33. [DOI] [PubMed]
- 37.Nathan D.M., Kuenen J., Borg R., Zheng H., Schoenfeld D., Heine R.J., et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008 Aug;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen D.M., Strange J.E., Gislason G., Torp-Pedersen C., Gerds T., Fosbøl E., et al. Charlson Comorbidity Index Score and Risk of Severe Outcome and Death in Danish COVID-19 Patients. J Gen Intern Med. 2020 Sep;35(9):2801–2803. doi: 10.1007/s11606-020-05991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed G.A., Gaba W., Shah A., Al Helali A.A., Raidullah E., Al Ali A.B., et al. Correlation between Chest CT Severity Scores and the Clinical Parameters of Adult Patients with COVID-19 Pneumonia. Radiol Res Pract. 2021;2021:6697677. doi: 10.1155/2021/6697677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar G., Mukherjee A., Sharma R.K., Menon G.R., Sahu D., Wig N., et al. Clinical profile of hospitalized COVID-19 patients in first & second wave of the pandemic: Insights from an Indian registry based observational study. Indian J Med Res. 2021 May;153(5 & 6):619–628. doi: 10.4103/ijmr.ijmr_1628_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain V.K., Iyengar K., Vaish A., Vaishya R. Differential mortality in COVID-19 patients from India and western countries. Diabetes Metab Syndr. 2020 Oct;14(5):1037–1041. doi: 10.1016/j.dsx.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umpierrez G.E., Isaacs S.D., Bazargan N., You X., Thaler L.M., Kitabchi A.E. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002 Mar;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 43.Swanson C.M., Potter D.J., Kongable G.L., Cook C.B. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011 Dec;17(6):853–861. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 44.Liao W.I., Lin C.S., Lee C.H., Wu Y.C., Chang W.C., Hsu C.W., et al. An Elevated Glycemic Gap is Associated with Adverse Outcomes in Diabetic Patients with Acute Myocardial Infarction. Sci Rep. 2016 Jun;13(6):27770. doi: 10.1038/srep27770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Luzio R., Dusi R., Barbanti F.A., Calogero P., Marchesini G., Bianchi G. Prognostic Value of Stress Hyperglycemia in Patients Admitted to Medical/Geriatric Departments for Acute Medical Illness. Diabetes Ther. 2022 Jan;13(1):145–159. doi: 10.1007/s13300-021-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oltmanns K.M., Gehring H., Rudolf S., Schultes B., Rook S., Schweiger U., et al. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004 Jun 1;169(11):1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- 47.Kerby J.D., Griffin R.L., MacLennan P., Rue L.W. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg. 2012 Sep;256(3):446–452. doi: 10.1097/SLA.0b013e3182654549. [DOI] [PubMed] [Google Scholar]
- 48.Choi S.W., Benzie I.F.F., Ma S.W., Strain J.J., Hannigan B.M. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008 Apr 1;44(7):1217–1231. doi: 10.1016/j.freeradbiomed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Burgos L.G., Ebert T.J., Asiddao C., Turner L.A., Pattison C.Z., Wang-Cheng R., et al. Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology. 1989 Apr;70(4):591–597. doi: 10.1097/00000542-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 50.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018 Sep;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 51.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and Oxidative Stress. Biochemistry (Mosc) 2020 Dec;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020 Sep;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marfella R., D’Onofrio N., Sardu C., Scisciola L., Maggi P., Coppola N., et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes Metab. 2021 Oct 1 doi: 10.1111/dom.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data file and analysed datasets can be available from the corresponding author o reasonable request by e mail to riddhi_dg@rediffmail.com.