Abstract
Despite that clinical trials have been examining the safety profile of coronavirus disease 2019 (COVID-19) vaccines, there are concerns about long-term side effects as the number of vaccinations increases. Herein, we report a case of new-onset renal-limited anti-myeloperoxidase (MPO) antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis after booster vaccination with the mRNA 1273 (Moderna) vaccine. A 72-year-old woman with no specific past history, and who had a normal renal function, developed ANCA-associated vasculitis following heterologous booster with mRNA1273 (Moderna) vaccine. After a kidney biopsy, she was diagnosed with ANCA-associated pauci-immune crescentic glomerulonephritis. Her renal function and constitutional symptoms have been improved with treatment with plasmapheresis, intravenous cyclophosphamide and steroid pulse therapy (intravenous 500 mg of methylprednisolone sodium succinate for 3 days) followed by a reduced steroid regimen.
Keywords: ANCA-Vasculitis, Heterologous, mRNA1273 COVID-19 Vaccine
Graphical Abstract
INTRODUCTION
There have been shreds of evidence about coronavirus disease 2019 (COVID-19) vaccines that can trigger antibody production through activation of T helper cells.1 The mRNA vaccines appear to induce autoimmune diseases in susceptible individuals by activating the production and secretion of proinflammatory cytokines.1 There have been case reports and reviews about the causality of various vaccines and autoimmune diseases, however, no definite relationship with vaccines has been clearly elucidated.2 Although further studies should be continued, it was identified that myeloperoxidase (MPO) titer has been increased after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a pilot study.3 Despite that the immune responses after natural infection with SARS-CoV-2 and mRNA vaccination could differ, there have been concerns about antineutrophil cytoplasmic antibody (ANCA) vasculitis after COVID-19 vaccines.4
CASE DESCRIPTION
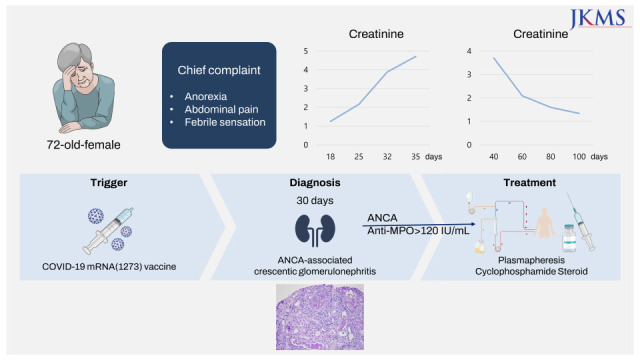
A 72-year-old woman with no specific past medical history presented with tinnitus, nasal congestion, and bloody rhinorrhea. Her constitutional symptoms developed after she received the first dose of the ChAdOx1 nCoV-19 (Oxford AstraZeneca) COVID-19 vaccine on 12 July 2021, and her symptoms were aggravated after she received the second dose of the ChAdOx1 nCoV-19 vaccine on 16 August. She underwent tympanostomy tube insertion and received antibiotic therapy for 2 weeks. However, after she received her third (booster) dose, the mRNA1273 (Moderna) vaccine, on 28 December 2021, she experienced anorexia, abdominal pain, and febrile sensation. Therefore, she was admitted to the gastroenterology department of our hospital. Her endoscopic findings revealed erosive gastritis, and she was treated with intravenous hydration because of her constitutional symptoms of poor oral intake and abdominal pain. She had normal body temperature (36.7°C), pulse rate (78/min), and respiratory rate (18/min) but high blood pressure (152/83 mmHg). A routine laboratory test performed 18 days after vaccination with the mRNA1273 (Moderna) vaccine revealed a serum creatinine (Cr) level of 1.25 mg/dL, and microscopic findings of urinalysis showed microscopic hematuria, occult blood (2+), 10–29 red blood cells (RBCs) per high-power field, and proteinuria (2+). The last previous laboratory test was performed in 2018 and was notable for a serum Cr level of 0.81 mg/dL. On the 25th day after vaccination, her Cr level elevated to 2.16 mg/dL. The spot urine protein to Cr ratio was 0.85 mg/g·Cr, and the spot urine albumin to Cr ratio was 0.19 mg/g·Cr. Despite intravenous hydration, the creatine level remained elevated, and with this finding along with persistent hematuria with proteinuria, we were prompted to perform serological testing. Serological tests revealed positive ANCA titers (> 120 IU/mL) and antibodies against MPO. Anti-nuclear antibody (ANA), anti-phospholipase A2 receptor (PLA2R) antibody, and anti-glomerular basement membrane antibody were all negative. The rheumatoid factor titer was 221 IU/mL and the C3 and C4 levels were 121 and 18 mg/dL, while immunoglobulins levels were all within normal limits (immunoglobulin [Ig] A 197 mg/dL, IgG 1,121 mg/dL, and IgM 96 mg/dL). Kidney imaging showed a left kidney cyst; otherwise, no abnormal findings were present. A kidney biopsy (Fig. 1) showed fibrocellular crescents (42.9%) and global sclerosis (14.3%) in glomeruli, with focal moderate tubular atrophy found 30 days after vaccination with the mRNA1273 (Moderna) vaccine. Immune complex-mediated deposits were not seen by electron microscopy. ANCA-associated pauci-immune crescentic glomerulonephritis was diagnosed based on the serological test and kidney biopsy findings. After kidney biopsy, intravenous pulse steroid therapy was initiated; 500 mg of methylprednisolone sodium succinate was injected intravenously over 1 hour once daily for three days. Two weeks after admission, her serum Cr level was elevated from 1.25 mg/dL (glomerular filtration rate [GFR], 42.1 mL/min/1.73 m2) to 4.71 mg/dL (GFR, 9.1 mL/min/1.73 m2) (Table 1). The plasmapheresis is considered when a patient’s serum Cr level is above 5.7 mg/dL as the guideline,5 however, it was initiated considering the patient’s rapid deterioration of renal function and biopsy findings showing crescents in nearly 50% of glomeruli. Intravenous cyclophosphamide 2.5 mg/kg was administered because of severe glomerulonephritis, and a reduced dose was given based on the patient’s age (> 70 years) and GFR less than 30 mL/min/1.73 m2. We tapered oral steroids based on the PEXIVIAS trial (oral prednisolone 60 mg per day on 1st week, 30 mg per day on 2nd, 25 mg per day on 3rd and 4th, 20 mg per day on 5th and 6th, 15 mg per day on 7th and 8th, 12.5 mg per day on 9th and 10th, 10 mg per day on 11th and 12th, 7.5 mg per day on 13th and 14th, 6 mg per day on 15th and 16th, and 5 mg per day afterwards).5 After the third dose of intravenous cyclophosphamide, her renal function improved, and induction therapy with oral cyclophosphamide was administered. Her Cr level improved to 1.34 mg/dL after two months of diagnosis, and her constitutional symptoms improved.
Fig. 1. Kidney biopsy showing (A) cellular crescent (white arrow) noted in a glomerulus. Inflammatory cell infiltration and fibrin deposit are also found. Tubules reveal protein and cellular casts in lumina (PAS stain ×200). (B) Ultrastructural examination shows diffuse effacement of foot processes (black arrow) with distorted and fused cytoplasms of podocytes (TEM ×2,000). Scale bar = 10 µm.
Table 1. Clinical laboratory findings after vaccination.
| Days after vaccination | +18 | +25 | +32 | +35 | Reference | |
|---|---|---|---|---|---|---|
| Serum Cr, mg/dL | 1.25 | 2.16 | 3.89 | 4.71 | 0.5–0.9 | |
| Serum sodium, mEq/L | 132 | 131 | 133 | 136 | 135–145 | |
| Serum potassium, mEq/L | 3.9 | 4.3 | 3.6 | 4.5 | 3.5–5.1 | |
| Serum chloride, mEq/L | 96 | 96 | 106 | 105 | 98–107 | |
| Urinalysis | ||||||
| Specific gravity | 1.015 | 1.016 | - | - | 1.010–1.030 | |
| Protein | 2+ | 1+ | - | - | Negative | |
| Glucose | Trace | Trace | - | - | Negative | |
| Occult blood | 2+ | 3+ | - | - | Negative | |
| Nitrite | Negative | Positive | - | - | Negative | |
| RBCs/HPF | 0–29 | 5–9 | - | - | 0–5 | |
| WBCs/HPF | Numerous | Many | - | - | 0–6 | |
Cr = creatinine, RBCs = red blood cells, HPF = high-power field, WBCs = white blood cells.
DISCUSSION
After the rapid authorization of COVID-19 vaccines worldwide, there have been concerns about de novo ANCA-associated glomerulonephritis following COVID-19 vaccination.6 There has been debate about the causality and relationship between vaccination and new-onset vasculitis. Among the patients who developed ANCA-associated vasculitis, symptoms appeared not only after the first dose but also after the second dose, and in some patients, symptoms worsened with serial doses.6,7,8 Given that ANCA vasculitis is a rare disease, with an incidence of 1.5–16 per 1 million person-year and a prevalence of 9–95 per 1 million person-year,9 it is noteworthy that the reported cases of ANCA-vasculitis after COVID vaccination increase. Notably, this patient’s symptoms began after she received the second dose of ChAdOx1 nCoV-19 (Oxford AstraZeneca) COVID-19 vaccine. Although there are certain possibilities that the immune cascades may have started on her initial doses of Oxford AstraZeneca vaccines, we believe her third dose with mRNA1273 (Moderna) played a critical role in developing ANCA-associated vasculitis. The evidence that there have been more reported cases of ANCA vasculitis following mRNA vaccines than following mRNA vector vaccines supports the idea.4 Furthermore, the lipid-nanoparticle-formulated mRNA vaccines coding for the SARS-CoV-2 full-length spike protein can induce immunogenic pathways.10 It has been previously demonstrated that mRNA vaccines can promote more robust adaptive and native immune reactions via prolonged S-specific germinal center B-cell responses and antigen-specific Th1-skewed immunity.11,12 It is hypothesized that these promoted immune responses can provide an effective defense mechanism against SARS-CoV-2; however, they might also provoke glomerular diseases in predisposed individuals. Moreover, a recent comparative study on using the same vaccine as the primary series (homologous boosters) and using a different vaccine (heterologous boosters) showed that the immunogenicity of heterologous boosting was generally similar to or greater than those of homogenous boosting.13 Further studies are required to confirm whether the increased immune response of heterologous injection of COVID-19 vaccinations contributes to a higher incidence of ANCA-associated vasculitis. Therefore, clinicians should be aware of de novo ANCA vasculitis following COVID-19 vaccination in this pandemic situation.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim BC, Jo HA.
- Data curation: Kim BC.
- Formal analysis: Kim BC, Kim HS, Jo HA.
- Investigation: Kim BC, Kim HS, Jo HA.
- Visualization: Kim HS.
- Writing-original draft: Kim BC, Jo HA.
- Writing - review & editing: Han KH, Han SY.
References
- 1.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41(3):509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295–311. doi: 10.1007/s13167-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelzo M, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Scialò F, et al. A Transient increase in the serum ANCAs in patients with SARS-CoV-2 infection: a signal of subclinical vasculitis or an epiphenomenon with no clinical manifestations? A pilot study. Viruses. 2021;13(9):1718. doi: 10.3390/v13091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhahar A, Naidu GS, Chauhan P, Sekar A, Sharma A, Sharma A, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022;42(4):749–758. doi: 10.1007/s00296-021-05069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(4):611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderegg MA, Liu M, Saganas C, Montani M, Vogt B, Huynh-Do U, et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100(2):474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidovic T, Schimpf J, Sprenger-Mähr H, Abbassi-Nik A, Soleiman A, Zitt E, et al. Novo and relapsing glomerulonephritis following SARS-CoV-2 mRNA vaccination in microscopic polyangiitis. Case Rep Nephrol. 2021;2021:8400842 [Google Scholar]
- 9.Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford) 2020;59(Suppl 3):iii42–iii50. doi: 10.1093/rheumatology/keaa089. [DOI] [PubMed] [Google Scholar]
- 10.Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021;100(5):959–965. doi: 10.1016/j.kint.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]



