Abstract
Objective
Dementia with Lewy bodies (DLB) is the second-most common form of neurodegenerative dementia after Alzheimer's disease (AD). Falls are a vital prognostic factor in patients with dementia and are a characteristic feature of DLB. This study investigated the screening potential of the fall risk evaluation for DLB and compared it with that of AD to facilitate an accurate diagnosis.
Methods
We enrolled patients diagnosed with DLB (n=410) and AD (n=2,683) and categorized the participants into 3 groups depending on their physical ability, age, cognitive function, and fall events. Using the Fall Risk Index-21 (FRI-21) questionnaire, we evaluated and comparatively analyzed the fall risk between DLB and AD patients in three defined groups of participants.
Results
The FRI-21 score was significantly higher in DLB patients than in AD patients in every group. Using this score, we were able to distinguish between DLB and AD patients in each group. Among the three groups, the group with a young age, relatively mild cognitive dysfunction, and no fall events exhibited the best specificity for DLB (0.895).
Conclusions
The FRI-21 is a useful tool for screening for DLB and differentiating it from AD. This questionnaire can be used at a relatively early stage of the disease in young patients with mild cognitive dysfunction and no history of falling. These preliminary results need to be validated in an interventional study to evaluate the effectiveness of rehabilitative measures and daily environmental changes carried out to prevent falls using this tool.
Keywords: falls, Alzheimer's disease, Lewy body disease
Introduction
Falls are a major cause of a decline in daily activities and an increase in the rate of institutionalization and mortality in older people, especially those with dementia (1,2). People with dementia are more likely to fall frequently and recover poorly after a falling event than those without (3-5). Dementia with Lewy body disease (DLB) is prevalent in approximately 25% of patients diagnosed with dementia (6). Patients with DLB are affected by falls three times more frequently than patients with Alzheimer's disease (AD) (7), the most common type of dementia.
The characteristic features of DLB include recurrent visual hallucinations, parkinsonism, and rapid eye movement-sleep behavior disorder (8). However, an accurate diagnosis of DLB is often difficult, especially in the early stages of the disease due to variable clinical presentation (9,10). DLB is often misdiagnosed as AD and only confirmed as DLB on a postmortem pathological evaluation (11-13). From a clinical viewpoint, the progression and prognosis of DLB are worse than those of AD (14). For example, patients with DLB have a shorter survival time (by nearly two years) than those with AD (15). Furthermore, pharmacotherapy also differs between DLB and AD patients. DLB is treated using anti-Parkinson's drugs with due consideration of the medication response because of the risk of severe hypersensitivity reactions to neuroleptic drugs. Therefore, an accurate and early diagnosis of DLB is vital for a detailed examination and improved outcomes.
The differential diagnosis of DLB can be arrived at using dopamine transporter (DAT) imaging via single-photon emission computed tomography (16) or 123I-metaiodobenzylguanidine myocardial scintigraphy (17,18). However, these tests are relatively expensive and not readily available at all centers. Therefore, doctors should effectively screen and differentiate DLB from AD when diagnosing a patient with cognitive impairment. An easy-to-use and efficient tool for screening for DLB is thus necessary to achieve an early diagnosis.
Given the above, we focused on fall events and risks in the present analysis, as the tendency to fall is a characteristic feature of DLB (8) that considerably affects the lives of patients. It can be difficult to determine the cause of a fall based on clinical symptoms, as there are often various underlying conditions. Physical strength measurement tests, such as the 1-foot standing test and 10-m walking speed, are generally performed to evaluate the fall risk. However, these are ineffective in DLB patients because of the characteristic feature of intraday symptom variations in such patients (8). Hence, a questionnaire can more appropriately ascertain the actual presenting condition. An ideal tool should help clinicians diagnose DLB early and develop a preventive strategy by evaluating the risk of a fall before an actual fall occurs.
In the present study, we analyzed and evaluated the possibility of screening and differentiating DLB from AD using a validated fall risk questionnaire to identify factors associated with fall risk in patients with DLB.
Materials and Methods
Ethical approval
This study was approved by the Medical Ethics Committee of the National Center for Geriatrics and Gerontology, Obu, Japan. All data were analyzed while preserving the anonymity of the subjects. Participants' consent was obtained in the form of opt-out on the institutional website. Individuals who opted out were excluded from the study.
Participants and the diagnosis
We analyzed outpatients with AD and DLB registered at the Center for Comprehensive Care and Research for Memory Disorders in the National Center for Geriatrics and Gerontology, Aichi, Japan, between January 2010 and June 2020. All patients were newly diagnosed, which ensured that their responses and assessments were unaffected by various medications. For an accurate diagnosis, we standardized the protocol. All participants underwent the following assessments: 1) laboratory analyses - complete blood test, including vitamin B12/folate levels, syphilis serology, and thyroid function tests; 2) neuroimaging - 3-T magnetic resonance imaging and single-photon emission computed tomography; 3) psychological assessments - the Mini-Mental State Examination (MMSE) (19) and Geriatric Depression Scale (GDS) (20); additional neuropsychological tests were performed, if needed; and 4) the level of daily living skills was evaluated using the Barthel Index (BI) (21) and the Instrumental Activities of Daily Living (IADL) scale (22). To assess physical and mental decline, we evaluated physical frailty based on the standard phenotype model of the Cardiovascular Health Study established by Fried et al. (23). These examinations were reviewed by experienced neurologists, psychiatrists, and/or geriatricians. Cases that could not be diagnosed clearly or had a possible overlap of multiple dementia diseases were excluded.
For this study, all patients were evaluated again retrospectively for the presence of probable AD per the criteria defined by the National Institute on Aging - Alzheimer's Association (24) or possible and probable DLB per the criteria specified in the Fourth Report of the DLB Consortium (8).
Evaluation of the fall risk
We measured the height and weight of all patients and calculated their body mass index (BMI) for the general physical evaluation. All patients were asked to answer the Fall Risk Index-21 (FRI-21) questionnaire (25) and report any fall events that had occurred within the last year. The caregivers of the participants were asked to ensure the accuracy of the responses.
Table 1 lists the questions in the FRI-21 (25). The questionnaire evaluates the risk factors for falls and was developed by the Working Group of Fall Prevention commissioned by the Japanese Ministry of Health, Labor, and Welfare. It comprises 21 items, including previously established risk factors for falls, such as the physical function, presence of geriatric syndromes, and environmental factors. The interclass coefficient (ICC) of the 1-month test-retest reproducibility of the scores of this questionnaire was satisfactory [ICC, 0.74; 95% confidence interval (CI), 0.46-0.89]. The questionnaire significantly correlated with physical function tests, such as the timed up and go, one-foot standing, and functional reach tests, which are also used to predict the risk of falls (26).
Table 1.
The Fall Risk Index-21 (FRI-21).
| Q1. Tripping or stumbling |
| Q2. Inability to ascend or descend stairs without help |
| Q3. Decreased walking speed |
| Q4. Inability to cross a road during a green signal interval |
| Q5. Inability to walk 1 km without a break |
| Q6. Inability to stand on one leg for 5 seconds (eyes open) |
| Q7. Using a cane |
| Q8. Inability to wring a towel |
| Q9. Dizziness or faintness |
| Q10. Stooped or rounded back |
| Q11. Knee joint pain |
| Q12. Visual trouble |
| Q13. Hearing trouble |
| Q14. Decline in cognitive functioning |
| Q15. Fear of falling |
| Q16. Taking five or more prescribed drugs |
| Q17. Sensation of darkness at home |
| Q18. Obstacles inside the house |
| Q19. Barriers on the carpet or floor |
| Q20. Using steps daily at home |
| Q21. Steep slopes around home |
Grouping for analyses
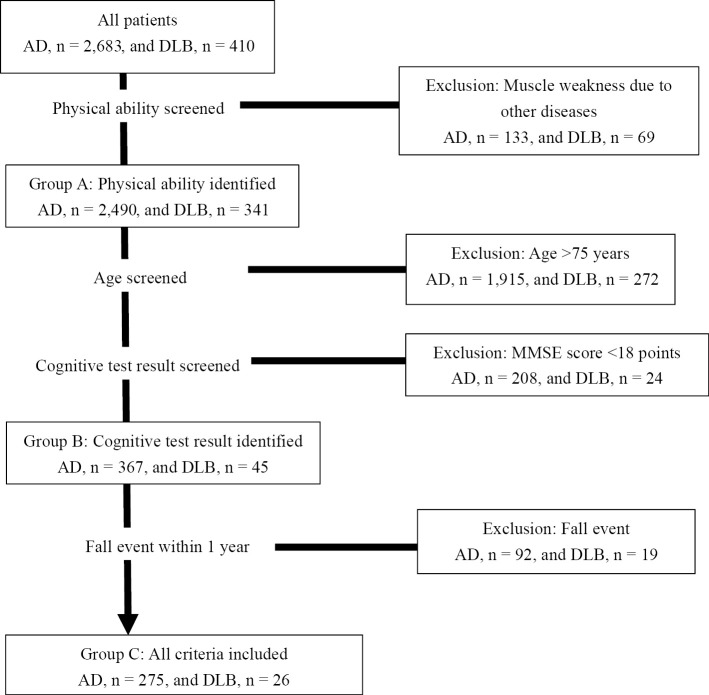
We conducted several analyses to evaluate the data according to the demographics of the patients. Figure shows the study flowchart. To decrease the bias of other diseases, we first excluded participants with muscle weakness due to other diseases, such as brain stroke or spinal cord injury, based on a doctor's evaluation and medical history (AD, n=133; DLB, n=69). We defined this population as group A. Next, we set the age criteria, since older people already have various reasons they might fall, as well as the MMSE score criteria, since the accuracy of the reproducibility of the results would be lower in patients with severe dementia than mild stage. Group B was screened for their age and cognitive function; patients older than 75 years (AD, n=1,915; DLB, n=272) and those with an MMSE score <18 points (AD, n=208; DLB, n=24) were excluded, based on previously reported cut-off levels between mild and severe AD (27, 28). The remaining patients were selected for the group B evaluation. Finally, because it is ideal to be able to conduct screening before a fall event occurs, we excluded participants with a history of falls in the past year in order to rule out the impact of a fall event (AD, n=92; DLB, n=24). The remaining patients progressed to group C screening.
Figure.
Study flowchart. AD: Alzheimer's disease, DLB: dementia with Lewy bodies, MMSE: Mini-Mental State Examination
We thus categorized the study participants based on a set of criteria into three groups: (A) physical ability; (B) physical ability, age, and cognitive function; and (C) physical ability, age, cognitive function, and fall events.
Statistical analyses
We performed descriptive analyses using the demographic data of the patients. We derived the mean scores and 95% CIs for quantitative variables, numbers, and percentages for qualitative variables. The two groups of DLB and AD were compared; quantitative data were evaluated using the t-test, and qualitative variables were evaluated using Fisher's exact test. Next, we determined a cut-off point that would differentiate DLB from AD. For a revised evaluation, the sensitivity, specificity, and area under the receiving operating characteristic (ROC) curve (AUC) were calculated at each point for the diagnosis of DLB.
All data management and statistical analyses were performed using the SPSSⓇ Statistics software program, version 26.0 (IBM, Armonk, USA). P<0.05 was considered statistically significant.
Results
Table 2 shows the demographic data of all participants; a total of 2,683 patients were diagnosed with AD and 410 with DLB. There was no significant difference between the two groups with respect to the age, BMI, or MMSE score. The GDS score was significantly worse in patients with DLB than in those with AD. The scores of daily living ability, BI and IADL, showed significant group differences, and the ratio of frailty was also higher in the DLB group than in the AD group.
Table 2.
Demographic Data of All Study Participants.
| Total (n=3,093) | AD (n=2,683) | DLB (n=410) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Sex, female (%) | 69.6 | 70.8 | 61.5 | |||||
| Age (years) | 79.05 (6.95) | 78.99 (7.03) | 79.43 (6.41) | N.S. | ||||
| BMI (kg/m2) | 21.80 (3.53) | 21.80 (3.48) | 21.82 (3.84) | N.S. | ||||
| MMSE | 17.93 (5.04) | 17.91 (5.00) | 18.05 (5.35) | N.S. | ||||
| GDS | 4.14 (3.01) | 3.98 (2.94) | 5.25 (3.22) | <0.01 | ||||
| BI | 92.19 (14.88) | 93.27 (13.45) | 85.06 (20.80) | <0.01 | ||||
| IADL | ||||||||
| Male | 2.87 (1.43) | 2.94 (1.42) | 2.50 (1.42) | <0.01 | ||||
| Female | 4.99 (2.14) | 5.09 (2.10) | 4.16 (2.27) | <0.01 | ||||
| Frailty (%) | 19.90 | 18.60 | 28.80 | <0.01 |
Data are presented as the mean (standard deviation) or %. p<0.01: statistically significant difference between the AD and DLB groups. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant
Supplementary materials 1-3 present the demographic data of the patients in groups A, B, and C, respectively. The MMSE score was significantly better in DLB patients than in AD patients in groups B and C. Regarding the abilities of daily living, both the BI and IADL showed significant differences between DLB and AD patients in group A but not in groups B or C. The ratio of frailty was higher in patients with DLB than in those with AD in all three groups.
Table 3 shows the FRI-21 scores and percentage of fall events within one year for the patients in each group. Overall, the FRI-21 score was significantly worse in the DLB group than in the AD group. In group A, the DLB patients had more fall events; however, in group B, there was no significant difference in fall events between DLB and AD patients.
Table 3.
FRI-21 Scores and Percentages of Fall Events within 1 Year for Patients with AD and DLB in Each Group.
| Group A | Group B | Group C | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | DLB | p value | AD | DLB | p value | AD | DLB | p value | ||||||||||
| FRI-21 | 8.80 (3.96) | 11.43 (3.66) | <0.01 | 6.31 (3.31) | 9.65 (3.64) | <0.01 | 5.49 (2.80) | 8.58 (3.78) | <0.01 | |||||||||
| Fall event (%) | 35.7 | 56.9 | <0.01 | 23.2 | 35 | N.S. | ||||||||||||
Participants are grouped into three groups according to physical ability (group A); physical ability, age, and cognitive test results (group B); and physical ability, age, cognitive test results, and fall events (group C). Data are presented as the mean (standard deviation) or %. p<0.01: statistically significant difference between the AD and DLB groups. AD: Alzheimer’s disease, DLB: dementia with Lewy bodies, FRI-21: Fall Risk Index-21, N.S.: not significant
Using the FRI-21 score, we calculated the ROC curves (Table 4) to differentiate between AD and DLB in each group. The AUC was 0.688 (95% CI, 0.678-0.834) for group A. We then determined the cut-off for discrimination. The maximum cut-off of the Youden Index was 10/11 (sensitivity, 0.641; specificity, 0.816). The AUCs were 0.756 (95% CI, 0.678-0.834) and 0.746 (95% CI, 0.647-0.845) for groups B and C, respectively. The maximum cut-off for the Youden Index was 9/10 in both groups (group B: sensitivity, 0.575; specificity, 0.816; group C: sensitivity, 0.462; specificity, 0.895). Among the 3 groups, group C had the highest specificity, indicating that the probable ratio to distinguish DLB from AD was 0.895.
Table 4.
Results of the ROC Analysis (Cutoff, Sensitivity, Specificity, and AUC).
| Cutoff | Sensitivity | Specificity | AUC (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Group A | 10/11 | 0.641 | 0.665 | 0.688 (0.678-0.834) | ||||
| Group B | 9/10 | 0.575 | 0.816 | 0.756 (0.678-0.834) | ||||
| Group C | 9/10 | 0.462 | 0.895 | 0.746 (0.647-0.845) |
Cutoff: cutoff score of the Fall Risk Index-21 (FRI-21), CI: confidence interval, ROC: receiver operating characteristics, AUC: area under the curve
Discussion
The FRI-21 questionnaire evaluates the fall risk in daily situations and may help caregivers provide appropriate support to patients in order to prevent falls. Frequent falling is considered a characteristic feature of DLB, and it would be ideal to diagnose DLB and prepare a prevention strategy before any fall occurs. As patients and their caregivers can answer the FRI-21 questionnaires themselves, it is a convenient and time-saving tool for doctors.
The novelty of our study is the practical application of the FRI-21 questionnaire. For a definitive diagnosis of DLB, a detailed examination is necessary. Therefore, previous reports have indicated the use of a specialized instrument or elaborated evaluation to distinguish between DLB and AD. However, a screening tool for a routine clinical evaluation for DLB in patients with cognitive deficits has not yet been established. We therefore focused on one of DLB's characteristic symptoms - falling - and analyzed the risk with a tool that can be used in actual daily clinical situations.
Another feature of our study is that we were able to further categorize participants into subgroups to analyze the cognitive function, age, and percentage of fall events. As shown in Table 2 and Supplementary materials 1-3, all groups maintained an average score of >85 points in BI, indicating that the included patients were independent in terms of their basic activities of daily living. Furthermore, in groups B and C, there was no significant difference between the AD and DLB groups in terms of the BI and IADL scores. By contrast, the frailty score was always higher in the DLB group than in the AD group, even in the groups with the most basic activities of daily living level, indicating an increased likelihood of falling. This suggests that the FRI-21 questionnaire is useful for evaluating the possibility of fall at a stage without significant differences in the activities of daily living, illustrating the effectiveness of this tool at a relatively early stage of the disease.
Another novel point of our study is that we analyzed several parameters, including the 1) age, 2) cognitive function, and 1) fall event itself. First, in elderly patients, any number of factors can be associated with falls, such as eyesight deterioration and muscle weakness. These factors might have generated a bias in our analysis. In addition, patients younger than 75 years old have a higher possibility of actively participating in various therapies, such as occupational therapy, than older patients. As DLB is a disease that can occur at a relatively young age, and falling events can severely reduce the quality of life of patients, fall prevention strategies have a greater impact on younger patients than older ones. Second, the group with a low MMSE score reportedly did not respond well to a fall prevention program (29). We hypothesized that patients in the mild stage of cognitive impairment (27,28) would respond better to improvements in the daily environment and interventions, such as rehabilitation, than others. Furthermore, the medical costs for DLB were calculated to be 70% greater than those for AD due to differences in the cognitive and functional status of the patients (30,31). Therefore, the early diagnosis of DLB and a fall risk evaluation and prevention will help reduce medical costs as well. Third, the evaluation of the fall risk in these patients (Table 3) denoted a higher risk in patients with DLB than in those with AD in all groups, as fall events were significantly more frequent in group A than in group B. This implies that the history of a fall event alone is inadequate to evaluate the risk, and the use of the FRI-21 questionnaire may be more useful. Group B showed better specificity (0.815) than group A (0.665), but group C showed the highest specificity (0.895). There was no significant difference with regard to fall events in group B between patients with DLB and AD (Table 3). The increasing specificity from group A to group C in Table 4 demonstrates the utility of this tool at a relatively early phase in young patients with mild cognitive decline and no falls. To support the interpretation of these results, we also evaluated the incidence rate of main parkinsonism, which is characterized by tremor, rigidity, bradykinesia, akinesia, and retropulsion, in each group; the incidence rates were 51% (174/341 patients) in group A, 60% (27/45 patients) in group B, and 50% (13/26 patients) in group C. The rate of parkinsonism was not increased in group C. This suggests the possibility of using this tool to evaluate latent symptoms of parkinsonism and the risk of falls.
Several limitations associated with the present study warrant mention. First, as the results depend on the answers to the questionnaire, the accuracy of these answers may be affected by the level of cognitive dysfunction of the participant; although a previous study evaluated its validity and reproducibility (27), this is a common problem with such studies among patients with dementia. A longitudinal study is thus necessary to ascertain the reliability of this assessment. Second, we used only the MMSE as a scale for evaluating the cognitive function, as the purpose of this study was to establish an analysis during routine clinical examinations. Therefore, it is important to examine the cognitive function using other scales in further evaluations of this tool.
The findings of this study will help formulate preventive interventions for patients at risk of falls and alert patients and their caregivers on the risk of future falls. These findings are particularly applicable to young patients with mild cognitive dysfunction who have a high score on this scale.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by Research and Development Grants for Longevity Science from the Japan Agency for Medical Research and Development (research grant 19dk0207027h0004) and a research grant (30-18) from the Research Funding of the Longevity Science Program of the National Center for Geriatrics and Gerontology, Japan.
Supplementary Materials
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and DLB groups.
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and the DLB groups.
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and the DLB groups.
References
- 1. Tinetti ME, Williams CS. The effects of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci 53: M112-M119, 1998. [DOI] [PubMed] [Google Scholar]
- 2. Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 337: 1279-1284, 1997. [DOI] [PubMed] [Google Scholar]
- 3. Komatsu T. [Fall risk and fracture. Falls in patients with dementia]. Clin Calcium 23: 731-738, 2013(in Japanese). [PubMed] [Google Scholar]
- 4. van Doorn C, Gruber-Baldini AL, Zimmerman S, et al. Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc 51: 1213-1218, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One 4: e5521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 44: 673-683, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Hanyu H, Sato T, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer's disease. Eur J Neurol 16: 212-217, 2009. [DOI] [PubMed] [Google Scholar]
- 8. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89: 88-100, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain 129: 729-735, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet 386: 1683-1697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60: 1586-1590, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 257: 359-366, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy bodies. Lancet Neurol 3: 19-28, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Boström F, Jönsson L, Minthon L, Londos E. Patients with dementia with Lewy bodies have more impaired quality of life than patients with Alzheimer disease. Alzheimer Dis Assoc Disord 21: 150-154, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Rongve A, Vossius C, Nore S, Testad I, Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer's dementia. Int J Geriatr Psychiatry 29: 392-398, 2014. [DOI] [PubMed] [Google Scholar]
- 16. McCleery J, Morgan S, Bradley KM, Noel-Storr AH, Ansorge O, Hyde C. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev 1: CD010633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology 66: 1850-1854, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Chung EJ, Kim SJ. 123I-metaiodobenzylguanidine myocardial scintigraphy in Lewy body-related disorders: a literature review. J Mov Disord 8: 55-66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198, 1975. [DOI] [PubMed] [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17: 37-49, 1983. [DOI] [PubMed] [Google Scholar]
- 21. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 14: 61-65, 1965. [PubMed] [Google Scholar]
- 22. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9: 179-186, 1969. [PubMed] [Google Scholar]
- 23. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146-M156, 2001. [DOI] [PubMed] [Google Scholar]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging - Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toba K, Okochi J, Takahashi T, et al. [Development of a portable fall risk index for elderly people living in the community]. Nihon Ronen Igakkai Zasshi 42: 346-352, 2005(in Japanese). [DOI] [PubMed] [Google Scholar]
- 26. Kikuchi R, Kozaki K, Iwata A, Hasegawa H, Toba K. Evaluation of risk of falls in patients at a memory impairment outpatient clinic. Geriatr Gerontol Int 9: 298-303, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40: 922-935, 1992. [DOI] [PubMed] [Google Scholar]
- 28. Forsell Y, Fratiglioni L, Grut M, Viitanen M, Winblad B. Clinical staging of dementia in a population survey: comparison of DSM-III-R and the Washington University clinical dementia rating scale. Acta Psychiatr Scand 86: 49-54, 1992. [DOI] [PubMed] [Google Scholar]
- 29. Jensen J, Nyberg L, Gustafson Y, Lundin-Olsson L. Fall and injury prevention in residential care - effects in residents with higher and lower levels of cognition. J Am Geriatr Soc 51: 627-635, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Murman DL, Kuo SB, Powell MC, Colenda CC. The impact of parkinsonism on costs of care in patients with AD and dementia with Lewy bodies. Neurology 61: 944-999, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Zhu CW, Scarmeas N, Stavitsky K, et al. Comparison of costs of care between patients with Alzheimer's disease and dementia with Lewy bodies. Alzheimers Dement 4: 280-284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and DLB groups.
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and the DLB groups.
Data are presented as the mean (standard deviation) or %. AD: Alzheimer’s disease, BI: Barthel Index, BMI: body mass index, DLB: dementia with Lewy bodies, GDS: Geriatric Depression Scale, IADL: Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination, N.S.: not significant. P > 0.01: statistically significant difference between the AD and the DLB groups.