Abstract
We herein report a 74-year-old man who developed Lambert-Eaton myasthenic syndrome (LEMS) during atezolizumab treatment for extensive-stage small-cell lung cancer. He was started on maintenance immunotherapy with atezolizumab every three weeks after four cycles of atezolizumab plus carboplatin plus etoposide combination therapy. After 13 cycles of maintenance atezolizumab therapy, he complained of muscular weakness and fatigue. Findings from a nerve conduction study and positive findings for anti-P/Q-type voltage-gated calcium channel antibody resulted in a diagnosis of LEMS. This was a rare case of LEMS as a neurological immune-related adverse event induced by atezolizumab therapy.
Keywords: Lambert-Eaton myasthenic syndrome, small-cell lung cancer, atezolizumab, immune checkpoint inhibitor, immune-related adverse events
Introduction
Lambert-Eaton myasthenic syndrome (LEMS) is a disease of the neuromuscular junction, characterized by proximal muscle weakness, areflexia, and autonomic dysfunction, and it is also known as a representative paraneoplastic neurological syndrome (PNS) generally related to small-cell lung cancer (SCLC). It is caused by antibodies targeting voltage-gated calcium channels (VGCCs) in the pre-synaptic nerve terminal (1). Autoimmunization by the tumor causes LEMS because the same VGCCs are expressed in SCLC.
SCLC accounts for 15% of all lung malignancies. Despite a high response rate to existing chemotherapy, the duration of response obtained was insufficient; furthermore, limited progress in treatment has been made in more than two decades. Recently, the addition of anti-programed cell death-ligand 1 (PD-L1) antibody to chemotherapy in the first-line treatment of extensive disease (ED) SCLC has resulted in a significantly longer overall survival than with chemotherapy alone (2,3). Atezolizumab is an anti-PD-L1 antibody approved for ED-SCLC treatment in Japan. Immune checkpoint inhibitors (ICIs), such as anti-PD-L1 antibody, are known to cause a wide spectrum of immune-related adverse events (irAEs), including neurological events. However, LEMS is rare as an irAE.
We herein report a case of LEMS that was likely an irAE triggered by atezolizumab therapy.
Case Report
A 74-year-old man had been diagnosed with ED-SCLC with pulmonary metastasis (c-stage IV, cT4N2M1a in the 8th TNM classification) 12 months prior to this event (Fig. 1). The patient was started on combination therapy with atezolizumab (dose of 1,200 mg) plus carboplatin (area under the curve: 5 mg/mL/min) and etoposide (100 mg/m2 body surface area). Four cycles of combination therapy resulted in a partial response (PR). Subsequently, he received a total of 13 cycles of maintenance atezolizumab therapy. During this period, he had no adverse events or symptoms. Interval computed tomography (CT) showed stable disease compared with the previous CT scan.
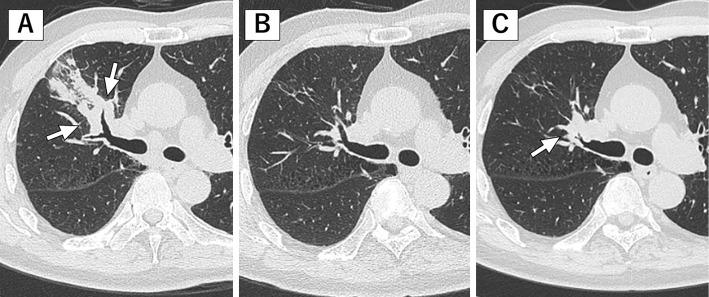
Figure 1.
Chest computed tomography (CT) shows a right upper lobe lung mass (white arrows) at SCLC diagnosis (A), and this lesion has continued to shrink at LEMS onset (B). About two months later, the CT scan shows an increase in the size of the primary tumor (white arrow) in the right lung (C).
At the start of the 14th cycle of maintenance atezolizumab therapy (12 months after starting atezolizumab), the patient complained of dry mouth (grade 1 CTCAE v4). Anti-SS-A antibody and anti-SS-B antibody tests performed for suspected Sjögren's syndrome were negative. The 14th cycle of atezolizumab was administered as scheduled. Ten days later, he felt lower limb weakness and fatigue, and these symptoms then gradually worsened. On a follow-up visit three weeks after the last atezolizumab administration, he complained of difficulty climbing stairs and arm weakness. However, he did not report any double vision, ptosis, dyspnea, swallowing difficulties, bladder problems, or bowel problems. The thyroid function test results and cortisol level were normal. Initially, there was concern about myositis as an atezolizumab-induced irAE. However, the creatinine phosphokinase (CPK) level was normal. There were no other irAEs, such as a rash. Other neurological disorders, such as myasthenia gravis, were therefore suspected. Atezolizumab therapy was discontinued. He was referred to the neurology department and admitted to our hospital for a diagnosis and treatment of his neurological symptoms.
On a neurological examination, cranial nerves 2-12 were grossly normal. He was oriented to person, place, and time. Language was fluent with normal comprehension and repetition. While weakness of the iliopsoas and quadriceps was noted bilaterally on a motor examination, the muscle tone, bulk, and strength in other muscle groups were normal. Sensation was normal. The patellar tendon reflexes were absent bilaterally, whereas other tendon reflexes were almost normal. Hoffmann's sign and Babinski's sign were absent. He had a slightly waddling gait.
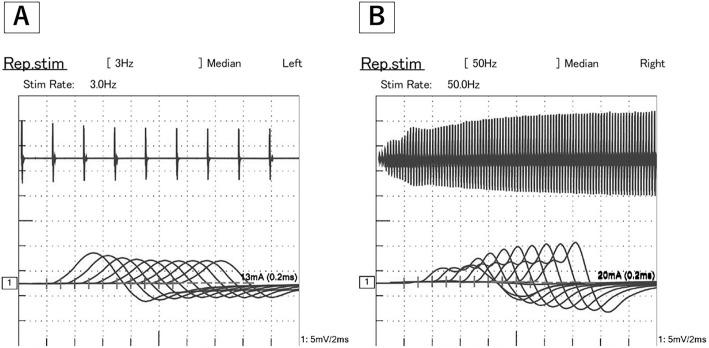
Magnetic resonance imaging (MRI) of the brain showed no pathological intracranial enhancement, mass effect, or recent infarct. Acetylcholine receptor binding antibody was not detected. Anti-P/Q type VGCC antibody was elevated (Table). Repetitive nerve stimulation of the right medial nerve and ulnar nerve showed the waning phenomenon on 3-Hz repetitive stimulation and a waxing phenomenon on 50-Hz repetitive stimulations (Fig. 2). Based on these results, LEMS was diagnosed. He was started on steroid pulse therapy (methylprednisolone 1 g/day for 3 days). However, difficulty walking and weakness in the lower limbs were exacerbated further. Therefore, he received intravenous immunoglobulin (IVIg) therapy. After that, his symptoms improved gradually. Three weeks after starting steroid therapy he was able to walk independently indoors without a cane.
Table.
Results of Blood Tests at LEMS Onset.
| Hematology | Biochemistry | Serology | |||||||||||
| White blood cells | 6,680 | /µL | TP | 7.5 | g/dL | C-reactive protein | 0.3 | mg/dL | |||||
| Neutrophil | 65.7 | % | Alb | 4.4 | g/dL | IgG | 1,265 | mg/dL | |||||
| Lymphocyte | 23.4 | % | AST | 20 | U/L | IgA | 347 | mg/dL | |||||
| Monocyte | 7.0 | % | ALT | 16 | U/L | IgM | 103 | mg/dL | |||||
| Eosinophil | 3.3 | % | LDH | 234 | U/L | ProGRP | 56.6 | pg/mL | |||||
| Basophil | 0.6 | % | CPK | 134 | U/L | NSE | 15.9 | ng/mL | |||||
| Red blood cells | 458×104 | /µL | BUN | 18.5 | mg/dL | anti VGCC Ab | 30 | pmol/L | |||||
| Hemoglobin | 14.9 | g/dL | Cre | 0.93 | mg/dL | anti ARS Ab | (-) | ||||||
| Hematocrit | 42.9 | % | Ca | 9.8 | mg/dL | anti Jo-1 Ab | (-) | ||||||
| Platelets | 23.5×104 | /µL | Na | 136 | mEq/L | anti Ach receptor Ab | <0.3 | nmol/L | |||||
| K | 4.4 | mEq/L | anti MuSK antibody | <0.02 | nmol/L | ||||||||
| Endocrine | Cl | 98 | mEq/L | anti GM1 Ab | (-) | ||||||||
| TSH | 0.5 | µIU/mL | anti GQ1b Ab | (-) | |||||||||
| Free T3 | 2.8 | pg/mL | |||||||||||
| Free T4 | 1.0 | ng/dL | |||||||||||
Ab: antibody, Ach: acetylcoline, ARS: aminoacyl transfer RNA synthetase, MuSK: muscle specific tyrosin kinase, NSE: neuron specific enolase, ProGRP: pro gastrin releasing peptide, TSH: thyroid-stimulating hormone, VGCCs; voltage-gated calcium channels
Figure 2.
The 3-Hz repetitive nerve stimulation test of the medial nerve shows the waning phenomenon. The seventh amplitude of the compound muscle action potentials (CMAPs) is 26.9% smaller than that of the first CMAP (A). In addition, the 50-Hz repetitive stimulation test shows the waxing phenomenon (B). The 100th amplitude of CMAPs is 16.3 times larger than that of the first CMAP.
About two months after the onset of LEMS, CT showed an increase in the size of the primary tumor in the right lung (Fig. 1). He was started on palliative chemotherapy with amrubicin. Although tumor shrinkage was observed after the administration of amrubicin, the improvement of symptoms was slight, and the feeling of weakness in the lower limbs remained. Therefore, steroid administration was continued with slight tapering after amrubicin therapy.
Discussion
ICIs are known to often cause various types of neurological irAEs. Encephalitis, meningitis, Guillain-Barré syndrome, and myasthenia gravis have been mainly reported as neurological irAEs (4). However, a case of LEMS associated with ICI use was extremely rare in our literature review. To our knowledge, there have been four case reports of ICIs associated with LEMS in patients with lung cancer (5-8). In three of these case reports, nivolumab was used, and one of them was in combination with ipilimumab. In the remaining case report, atezolizumab was used, as in the present case. However, this reported case did not achieve sufficient relief despite several treatments, unlike the present case.
Whether LEMS was an irAE or a PNS is difficult to distinguish. The onset time of LEMS is a point of interest. Typically, the clinical symptoms of LEMS as a PNS are nearly always present before SCLC is detected. According to a previous study, the diagnosis of SCLC preceded the recognition of LEMS in only 7% of LEMS patients (9). Neurologic irAEs typically occur during the induction phase, after a median 5.5 cycles of ICIs (10). In the present case, however, the onset time was about 13 months after the diagnosis of SCLC and 12 months after the induction of atezolizumab. In addition, CT images obtained around the onset time showed that each lesion had continued to shrink. This does not correlate with PNS, since it is known that the clinical symptoms of a PNS improve in parallel with the remission of the malignant tumor.
Given that the tumor regrew about two months after the onset of LEMS, it can also be regarded as a PNS induced by atezolizumab. In the present case, the therapeutic effect was better with steroids and IVIg than with the anticancer drug amrubicin. In general, the treatment strategy for irAEs is to discontinue the ICI first and then consider steroid therapy; however, that for PNS is to shrink the tumor. A neurological irAE thus appears to be the more likely cause of LEMS than a PNS in the present case. However, the possibility of PNS induced by atezolizumab cannot be completely excluded. Therefore, if LEMS develops during ICI therapy, careful monitoring for subsequent tumor progression may be required.
An effective treatment of ICI-induced LEMS has not yet been established. The general principle of LEMS treatment involves the detection of SCLC and its treatment with chemotherapy, radiotherapy, or surgery. This strategy can improve the symptoms of LEMS patients with massive tumors. However, it is potentially difficult in ICI-induced LEMS patients, as the tumor has already shrunk in most cases. Inevitably, medication will be considered. According to the guideline of the European Federation of Neurological Societies, the first-line drug for patients with LEMS is 3,4-diaminopyridine (11). This drug blocks potassium channels in nerve terminals, resulting in an increase in acetylcholine release, and its efficacy has been demonstrated in a randomized, double-blinded, placebo-controlled trial. However, this drug is currently not approved for use in Japan. In the present case, steroid and IVIg were administered and were somewhat effective. Steroids suppress the immune system and prevent the formation of abnormal auto-immune antibodies. Based on our experience, these treatments are worth trying. In addition, approval for the use of 3,4-diaminopyridine in Japan is desirable.
Anti-PD-L1 antibodies were an important breakthrough in SCLC treatment. As a result of anti-PD-L1 antibody plus chemo combination therapy becoming standard, the prognosis of SCLC patients is expected to improve. According to past reports, SCLC is almost always the tumor type that occurs in patients with LEMS. As the number of SCLC patients undergoing anti-PD-L1 antibody therapy increases, the prevalence of ICI-induced LEMS may increase as well. Once a neurological irAE occurs, patients will suffer from persistent symptoms despite discontinuation of ICIs and adequate therapy. Therefore, the prevention and early detection of neurological irAEs are very important. Oncologists should be alert for LEMS induced by ICI, especially in the treatment of SCLC.
In conclusion, a rare case of LEMS related to atezolizumab was presented. LEMS should be considered as a possible neurological irAE. Physicians need to be aware of the possibility of LEMS arising in SCLC patients receiving anti-PD-L1 antibodies.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol 10: 1098-1107, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379: 2220-2229, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase3 trial. Lancet 394: 1929-1939, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Möhn N, Beutel G, Gutzmer R, Ivanyi P, Satzger I, Skripulets T. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy - review of the literature and future outlook. J Clin Med 8: 1777, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakatani Y, Tanaka N, Enami T, Minami S, Okazaki T, Komuta K. Lambert-Eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol 10: 346-352, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agrawal K, Agrawal N. Lambert-Eaton myasthenic syndrome secondary to nivolumab and ipilimumab in a patient with small-cell lung cancer. Case Rep Neurol Med 2019: 5353202, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill A J, Gandhy S, Lancaster E. Nivolumab-associated Lambert-Eaton myasthenic syndrome and cerebellar dysfunction in a patient with a neuroendocrine tumor. Muscle Nerve 63: E18-E21, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Krishnan GS, Wilson WA. Immunotherapy related myasthenia gravis and Lambert Eaton syndrome case reports and review of literature. J Immunother 3: 007, 2020. [Google Scholar]
- 9. Titulaer MJ, Wirtz PW, Willems LN, van Kralingen KW, Smitt PA, Verschuurenn JJ. Screening of small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol 26: 4276-4281, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 74: 1216-1222, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neural 17: 893-902, 2010. [DOI] [PubMed] [Google Scholar]