Abstract
Objective
Smoking is a known risk factor for the development and progression of autoimmune diseases. Previous studies in ocular myasthenia gravis (MG) patients showed that smoking is associated with the severity of symptoms and progression to generalized MG. However, whether smoking affects MG symptoms in patients with a broader clinical spectrum of presentations is unknown. Therefore, in this study, the associations of smoking with the clinical characteristics of MG were analyzed in a cohort of patients including those with generalized, seronegative, and thymoma-associated MG.
Methods
The smoking history was investigated in a cross-sectional study of 187 patients with MG followed in a referral hospital for neurology. The association of smoking with MG-activities of daily living score at survey, the presence of generalized manifestations, and the age of onset was assessed using multiple regression models.
Results
Neither current nor prior smoking habit was associated with the MG-activities of daily living score at survey. However, smoking exposure after MG onset was significantly associated with the presence of generalized manifestations during the disease course (odds ratio, 3.57; 95% confidence interval, 1.04, 12.3). The smoking history before or at onset of MG was not associated with the age of onset.
Conclusion
Smoking exposure after the onset is associated with generalized manifestations of MG in our cohort of patients with a broad clinical spectrum of presentations.
Keywords: activities of daily living, age of onset, generalization, myasthenia gravis, smoking
Introduction
Myasthenia gravis (MG) is an autoimmune disease of the neuromuscular junction, clinically characterized by early fatigable weakness of the ocular and extraocular muscles (1,2). Development of MG results from complex interactions between genetic factors, such as polymorphisms of human leukocyte antigens, and environmental factors, such as viral infections (1). Smoking is a known risk factor for the development and/or progression of autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus (3,4). A recent study demonstrated an association between smoking and MG severity, as assessed by the MG-activities of daily living (ADL) scale in ocular MG patients (5). Another recent study showed smoking to be an independent risk factor for progression to generalized MG in anti-acetylcholine receptor (AChR) antibody-positive ocular MG patients (6). However, whether smoking affects MG symptoms in patients with a broader clinical spectrum of presentations is not yet known. Therefore, in this study, we analyzed the association of smoking history with severity of MG symptoms, generalized manifestations, and age of onset in our cohort of MG patients, including those with generalized, seronegative, and thymoma-associated MG.
Materials and Methods
Settings and participants
This cross-sectional study was conducted at Hokkaido Medical Center, which is a referral hospital for neurology covering approximately 2 million populations. Our clinic is specialized for neuroimmunological diseases including MG, and follows patients including relatively severe cases. The smoking prevalence of the general population in Hokkaido prefecture was 22.6% (14.8% in women and 31.7% in men) in 2019 (7). Patient data was collected from 187 consecutive cases of MG at the outpatient clinic and neurology department between August 2019 and November 2019. The enrolled patients comprise 85% of the total MG patients followed in our clinic. MG was diagnosed based on medical history and clinical findings, including improvement after intravenous edrophonium, decremental response to repetitive nerve stimulation, and the presence of antibodies against AChR or muscle-specific receptor tyrosine kinase (MuSK) (2). All participants provided their written informed consent, and the study was approved by the ethics review board of Hokkaido Medical Center.
Data collection and variables
A questionnaire was used to investigate the smoking history of the MG patients. The questionnaire included current smoking status (never-smoker, ex-smoker, or current smoker), the age at which the patient started smoking, and the age of smoking cessation in ex-smokers. Severity of MG symptoms was assessed by one of the authors (YM, IA, SA, or NM) using the MG-ADL scale. The MG-ADL scale is a measure of patient-reported symptoms of MG, shown to have good correlation with objective measures of MG symptoms (8,9). The score ranges from 0-24, with higher score indicating more severe symptoms (8). Generalized MG was defined by the presence of any extra-ocular symptoms due to MG, including dysphagia, dysarthria, neck and/or limb weakness, and respiratory dysfunction, at any time during the disease course (2). Therefore, patients who had presented with generalized symptoms in the past but had only ocular symptoms at the time of survey were classified as generalized MG. Baseline information, including patient demographics, past medical history, and medications, were collected from medical records.
Statistical analyses
Descriptive summaries of patient characteristics are reported as the mean and standard deviation for continuous variables, and as the number and percentage of patients for categorical variables. In univariate analyses, the associations between MD-ADL score and variables were assessed using Mann-Whitney U test or Kruskal-Wallis test for categorical variables, and Spearman's rank correlation for continuous variables. The associations between the presence of generalized manifestations and variables were assessed using Fisher's extract test for categorical variables, and Student's t-test for age at survey. The difference in age of onset was analyzed using Student's t-test. In multivariate analyses, associations of smoking with MG-ADL score and age of MG onset were analyzed using linear regression models. The association between smoking and presence of generalized manifestations was assessed using a logistic regression model. Statistical analyses were performed using the R software program version 3.6.3 (10), and p values less than 0.05 were considered to be statistically significant.
Results
Smoking and severity of symptoms
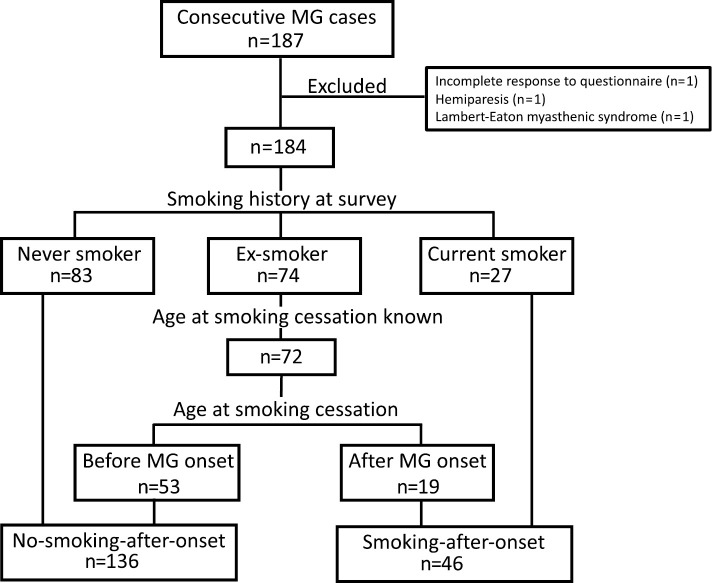
Of the 187 participants, 3 were excluded from the analysis because 1 did not complete the questionnaire, 1 had hemiparesis due to stroke and her MG-ADL score could not be appropriately assessed, and 1 turned out to have Lambert-Eaton myasthenic syndrome after the survey. The characteristics of the remaining 184 participants are shown in Table 1. At the time of survey, 83 participants had never smoked, 74 were ex-smokers, and 27 were current smokers (Fig. 1). Among the 184 participants, 141 (76.6%) and 3 (1.6%) were positive for anti-AChR or anti-MuSK antibodies respectively, 147 (80.0%) had generalized MG, 55 (30.0%) had thymoma, and 159 (86.4%) were being treated with steroids and/or immunosuppressants. The never-smoker group had a significantly higher proportion of women compared with the ex-smoker and current-smoker groups. In contrast, the current-smoker group was significantly younger and had a significantly younger age of onset compared with the other two groups. The other clinical characteristics did not significantly differ among the three groups.
Table 1.
Patient Characteristics.
| Never-smoker (n=83) |
Ex-smoker (n=74) |
Current smoker (n=27) |
p value† | |||||
|---|---|---|---|---|---|---|---|---|
| Female (%) | 66 (79.5) | 26 (35.0) | 10 (37.0) | <0.0001 | ||||
| Age at survey, years (SD) | 60.7 (16.8)‡ | 63.1 (12.0)§ | 50.4 (13.2) | <0.0001 | ||||
| Age of onset, years (SD) | 51.3 (20.1)‡ | 52.4 (14.0)‡ | 42.3 (13.8) | 0.028 | ||||
| Disease duration, years (SD) | 9.45 (10.1) | 10.7 (9.90) | 8.11 (6.70) | 0.449 | ||||
| Anti-AChR antibody (%) | 62 (74.1) | 61 (82.4) | 18 (66.7) | 0.204 | ||||
| Generalized (%) | 65 (78.3) | 60 (81.1) | 22 (81.5) | 0.889 | ||||
| Thymoma (%) | 27 (32.5) | 21 (28.9) | 7 (25.9) | 0.779 | ||||
| Steroids or immunosuppressants (%) | 69 (83.1) | 64 (86.5) | 26 (96.3) | 0.218 |
SD: standard deviation, AChR: acetylcholine receptor, †p value by one-way analysis of variance or Fisher’s extract test, ‡p<0.05, compared with the current-smoker group (Bonferroni’s correction), §p<0.01 compared with the current-smoker group (Bonferroni’s correction).
Figure 1.
A schematic diagram showing the classification of patients according to the smoking history at survey and smoking status after MG onset. MG: myasthenia gravis
First, the association of the smoking status with subjective severity of symptoms at survey was assessed. According to the univariate analysis, the MG-ADL score did not differ among the three groups (Table 2). In addition, no differences were observed in MG-ADL score when only patients with ocular MG were analyzed (not shown). A multivariate analysis revealed that the smoking status was not associated with MG-ADL score even when adjusted for other variables, including sex, age, disease duration, the presence of anti-AChR antibody, the presence of thymoma, generalized manifestations, and use of steroids or immunosuppressants (Table 2).
Table 2.
Univariate and Multivariate Analyses for Factors Associated with MG-ADL Score.
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| MG-ADL [median (IQR)] | p value | Regression coefficient (95%CI) | p value | |||||
| (Intercept) | 1.77 (-0.43, 3.97) | 0.114 | ||||||
| Never-smoker | 4 (2-6) | |||||||
| Ex-smoker | 4 (2-6) | 0.21 (-0.69, 1.12) | 0.641 | |||||
| Current-smoker | 4 (3-5) | 0.825† | 0.22 (-1.01, 1.46) | 0.720 | ||||
| Male | 3 (2-6) | |||||||
| Female | 4 (3-6) | 0.024 | 0.71 (-0.20, 1.61) | 0.124 | ||||
| Age at survey | -0.048‡ | 0.515 | 0.01 (-0.02, 0.03) | 0.697 | ||||
| Disease duration (years) | -0.0597‡ | 0.421 | 0.02 (-0.02, 0.06) | 0.322 | ||||
| Anti-AChR antibody-negative | 5 (3-7) | |||||||
| Anti-AChR antibody-positive | 4 (2-6) | 0.036 | -1.12 (-2.09, -0.15) | 0.024 | ||||
| Ocular | 3 (1-4) | |||||||
| Generalized | 4 (2-6) | 0.010 | 0.77 (-0.23, 1.77) | 0.129 | ||||
| Non-thymoma | 4 (2-6) | |||||||
| Thymoma | 4 (2-5) | 0.730 | -0.26 (-1.14, 0.62) | 0.559 | ||||
| Without steroid or immunosuppressant | 2 (1-4) | |||||||
| With steroid or immunosuppressant | 4 (3-6) | <0.001 | 2.13 (0.94, 3.31) | 0.001 | ||||
MG: myasthenia gravis, ADL: activities of daily living, IQR: interquartile range, CI: confidence interval, AChR: acetylcholine receptor, †p value by Kruskal-Wallis test, ‡Spearman’s rank correlation coefficient
Smoking and generalized manifestations
Next, we assessed the association of smoking exposure after MG onset with the presence of generalized symptoms during the disease course. In this analysis, the ex-smoker group was subclassified according to the chronological order of MG onset and smoking cessation (Fig. 1). Two subjects were excluded from the analysis because they did not provide information about their age of smoking cessation. Among the 72 ex-smokers, 53 had quit smoking before the onset of MG, and 19 had quit smoking after the onset. The 83 participants in the never-smoker group and the 53 participants who had stopped smoking before MG onset were classified into the no-smoking-after-onset group. The 27 participants in the current-smoker group and the 19 participants who had stopped smoking after MG onset were classified into the smoking-after-onset group. According to the univariate analysis, smoking-after-onset was not associated with generalized manifestations of MG (Table 3). In logistic regression analysis, smoking-after-onset was significantly associated with the presence of generalized MG symptoms (Table 3). A subgroup analysis in anti-AChR antibody positive subjects showed a significant association between smoking-after-onset and the presence of generalized symptoms [logistic regression model adjusting for gender, age, presence of thymoma, use of steroids or immunosuppressants; odds ratio, 5.71; 95%confidence interval (CI), 1.82, 21.6, p=0.005].
Table 3.
Univariate and Multivariate Analyses for Factors Associated with Generalized Manifestations.
| Variables | Ocular | Generalized | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95%CI) | p value | OR (95%CI) | p value | |||||||
| (Intercept) | 0.107 (0.0102, 1.12) | 0.062 | ||||||||||
| Smoking-after-onset | 4 (12.5) | 42 (28.0) | 2.71 (0.871, 11.3) | 0.076 | 3.57 ( 1.04, 12.3) | 0.043 | ||||||
| Female | 11 (34.4) | 90 (60.0) | 2.85 (1.21, 7.05) | 0.011 | 4.92 (1.83, 13.2) | 0.002 | ||||||
| Age at survey | 60.6 (14.8)† | 60.0 (15.2)† | 0.851 | 1.01 ( 0.981, 1.04) | 0.436 | |||||||
| Anti-AChR antibody | 23 (71.9) | 117 (78.0) | 1.38 (0.513, 3.48) | 0.490 | 1.51 (0.525, 4.34) | 0.445 | ||||||
| Thymoma | 5 (15.6) | 50 (33.3) | 2.69 (0.942, 9.48) | 0.057 | 1.37 (0.436, 4.31) | 0.590 | ||||||
| Steroids or immunosuppressants | 19 (59.4) | 138 (92.0) | 7.73 (2.81, 21.7) | <0.0001 | 8.16 (2.81, 23.7) | <0.001 | ||||||
OR: odds ratio, CI: confidence interval, SD: standard deviation, AChR: acetylcholine receptor, †mean (SD)
Given that age at smoking cessation is prone to recall bias, the presence of generalized manifestations was compared between never-smokers and ever-smokers (ex-smokers plus current-smokers). Ever-smoking was significantly associated with the presence of generalized manifestations when adjusted for sex, age, presence of anti-AChR antibody, presence of thymoma, and use of steroids or immunosuppressants (odds ratio, 2.90; 95%CI, 1.04, 8.09, p=0.042).
Smoking and the age of onset
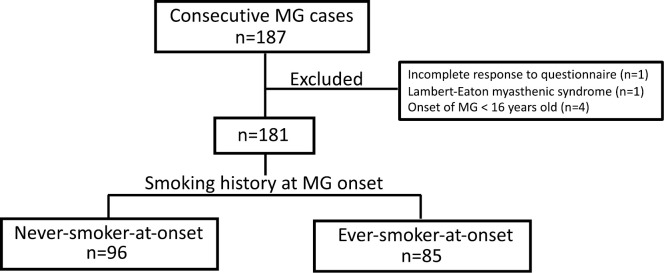
Finally, the association of smoking with age of MG onset was assessed. The 4 participants who had developed MG before the age of 16 were excluded from the analysis. This analysis included one participant who was excluded from the earlier analyses due to the presence of hemiparesis that developed after the onset of MG. Therefore, a total of 181 participants were analyzed (Fig. 2): 96 who had never smoked before MG onset (never-smoker-at-onset group), and 85 who had smoked or were smoking at MG onset (ever-smoker-at-onset group). According to a univariate analysis, the mean age of onset was comparable between the never- and ever-smoker-at-onset groups (Table 4). A linear regression analysis showed that ever-smoking at onset was not associated with age of onset when adjusted for sex, the presence of anti-AChR antibody, and the presence of thymoma (Table 4).
Figure 2.
A schematic diagram showing the classification of patients according to the smoking history at MG onset. MG: myasthenia gravis
Table 4.
Univariate and Multivariate Analyses for Factors Associated with Age at Onset.
| Variables | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | p value | Regression coefficient (95%CI) | p value | ||||||
| (Intercept) | 50.0 (42.9, 57.0) | <0.0001 | |||||||
| Never-smoker-at-onset | 51.7 (19.0) | ||||||||
| Ever-smoker-at-onset | 51.1 (13.4) | 0.788 | -3.67 (-8.82, 1.48) | 0.162 | |||||
| Male | 54.3 (14.1) | ||||||||
| Female | 49.0 (17.5) | 0.028 | -4.87 (-10.3, 0.535) | 0.077 | |||||
| Anti-AChR antibody-negative | 44.4 (14.1) | ||||||||
| Anti-AChR antibody-positive | 53.5 (16.3) | 0.001 | 9.28 (3.54, 15.0) | 0.002 | |||||
| Non-thymoma | 52.2 (16.6) | ||||||||
| Thymoma | 49.5 (15.4) | 0.313 | -3.65 (-8.96, 1.66) | 0.176 | |||||
SD: standard deviation, CI: confidence interval, AChR: acetylcholine receptor
Discussion
This study analyzed the association of smoking history with three clinical aspects of MG: subjective severity of symptoms at the time of survey, the presence of generalized manifestations during the disease course, and age of onset. First, we did not find an association between the smoking status and the subjective severity of symptoms at the time of the survey, as assessed on the MG-ADL scale. However, smoking exposure after MG onset was significantly associated with the presence of generalized manifestations during the disease course. Finally, we did not find an association of smoking before or at the time of MG onset with age of onset.
In contrast to the present results, a previous study showed a significant association between smoking and the subjective severity of MG symptoms, where current smokers had the highest and never-smokers had the lowest MG-ADL score (5). The reason for this discrepancy is not clear but may include differences in patient characteristics between the two studies. The previous study was performed at a neuro-ophthalmological clinic and recruited patients whose initial diagnosis was ocular MG (5). Although patients who developed generalized manifestations between the initial diagnosis and the survey were also included, the proportion of such patients was substantially lower in that study than in our study (23% vs. 80%). However, our subgroup analysis in ocular MG patients did not find an association between smoking and the MG-ADL score. In addition, differences in other patient characteristics might also be relevant, including disease duration, presence of thymoma, and use of immunosuppressants, although our multivariate analysis, which adjusted for these factors, was still negative. The nature of the MG-ADL scale may also have contributed to this inconsistency. The MG-ADL scale is a well-validated tool for assessing subjective severity of MG symptoms (8,9), and has been used as a major outcome in multiple randomized controlled trials as well as observational studies. However, it is not an objective scale for muscle fatigue or a direct measure of the neuromuscular junction function, and its sensitivity for detecting differences in muscular function might differ between ocular and systemic muscles.
In contrast, the significant association between smoking exposure after MG onset and generalized manifestations observed in our study was in line with a recent study showing smoking as a risk factor for generalization in ocular MG patients (6). These results suggest a detrimental role of smoking in the development of extraocular manifestations, although no conclusion can be drawn regarding causality from these observational studies. Smoking has been shown to be associated with the development and/or progression of several autoimmune diseases (3,4). Multiple pathways, including alterations of the cytokine levels, modulation of cellular and humoral immunity, induction of tissue damage, and anti-estrogenic effect, have been proposed as underlying mechanisms (4). In rheumatoid arthritis and systemic lupus erythematosus, smoking was shown to be associated with positivity of autoantibodies (4). Likewise, smoking might augment humoral immunity and lead to the development of generalized manifestations in MG. Another possible underlying mechanism is the pharmacological effect of nicotine on AChR. It has been suggested that prolonged exposure to nicotine causes desensitization or even permanent inactivation of AChR (11). Interestingly, an exacerbation of MG by transdermal application of nicotine has been reported (12). Thus, it is reasonable to speculate that chronic systemic exposure to nicotine by smoking might manifest as abnormal neuromuscular transmission in extraocular muscles, which would have remained subclinical without smoking. Whether smoking cessation leads to an amelioration of generalized manifestations in MG is an important issue to be investigated in future interventional studies. In addition, since MG patients with prolonged exposure to steroids generally have increased risks for cardiovascular diseases, these patients should be strongly advised to quit smoking.
The observed significant association of smoking with generalized manifestations might seem discrepant with the negative finding in the analysis of MG-ADL score. We believe that this is due to the difference in the time frame in which these 2 outcomes were assessed: generalized symptoms present at any time during the disease course was taken into account, while MG-ADL score was assessed at the time of survey.
We analyzed the association between smoking and age at MG onset, under the assumption that if smoking could trigger MG development, patients with a history of smoking would develop MG at younger ages. Although we could not find such an association in the present study, the relationship between smoking and MG development remains a valid hypothesis to be investigated in future studies because it can be inferred from other autoimmune diseases (3,4). Indeed, recent studies have pointed out that smoking was more prevalent in MG patients compared to the general population (13,14).
As an observational study, this study is not free from limitations. First, as study performed in a referral hospital, our cohort may not be a representative of MG patients in general, since it contains not a few numbers of intractable cases, thymoma associated MG, and those with comorbidities. Second, the severity of MG symptoms was evaluated by a subjective scale and were not confirmed by other scales that include objective assessments, such as quantitative MG score, MG composite scale, or post-intervention status. In addition, because the smoking history was obtained from patients via questionnaire, the information might be subject to some recall bias. In particular, uncertainty about the age at smoking cessation might have affected our results showing the association between smoking and generalized manifestations. However, the consistent finding in the analysis comparing never and ever smokers supports the robustness of our results. Third, because we do not have reliable information about the amount of tobacco consumption for each patient, dose responsiveness could not be evaluated, and therefore this point needs to be assessed in future studies. Finally, the influence of potential confounders associated with smoking habit such as alcohol, diet, sleep habits, sports and other leisure activities could not be evaluated in our study. On the other hand, the strength of our study derives from its relatively large number of participants compared with previous studies, as well as the inclusion of patients with various clinical presentations, which contributes to the generalizability of our results.
In conclusion, smoking exposure after the onset of MG was significantly associated with generalized manifestations. However, we did not find an association between the smoking history and the subjective severity of symptoms at the time of the survey or the age of onset in our cohort.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by a Health and Labor Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan (20FC1030).
References
- 1. Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 52: 90-100, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Gilhus NE. Myasthenia gravis. New Engl J Med 375: 2570-2581, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neuro 14: 263-273, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol 3: 707-715, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Gratton SM, Herro AM, Feuer WJ, Lam BL. Cigarette smoking and activities of daily living in ocular myasthenia gravis. J Neuroophthalmol 36: 37-40, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apinyawasisuk S, Chongpison Y, Thitisaksakul C, Jariyakosol S. Factors affecting generalization of ocular myasthenia gravis in patients with positive acetylcholine receptor antibody. Am J Ophthalmol 209: 10-17, 2020. [DOI] [PubMed] [Google Scholar]
- 7. Cancer Information Service, National Cancer Center, Japan. Cancer Registry and Statistics [Internet]. [cited 2021 May 27]. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html#smoking (in Japanese).
- 8. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology 52: 1487-1489, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Muppidi S, Wolfe GI, Conaway M, Burns TM; the MG Composit and MG-QOL15 Study Group. MG-ADL: still a relevant outcome measure. Muscle Nerve 44: 727-731, 2011. [DOI] [PubMed] [Google Scholar]
- 10. R Core Team. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria [Internet]. [cited 2019 Dec 1]. Available from: http://www.r-project.org/
- 11. Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol 15: 193-222, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Moreau T, Depierre P, Burdon F, Confavreux C. Nicotine-sensitive myasthenia gravis. Lancet 344: 548-549, 1994. [DOI] [PubMed] [Google Scholar]
- 13. Maniaol AH, Boldingh M, Brunborg C, Harbo HF, Tallaksen CME. Smoking and socio-economic status may affect myasthenia gravis. Eur J Neurol 20: 453-460, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Westerberg E, Landtblom AM, Punga AR. Lifestyle factors and disease-specific differences in subgroups of Swedish Myasthenia Gravis. Acta Neurol Scand 138: 557-565, 2018. [DOI] [PubMed] [Google Scholar]