Abstract
PURPOSE
The aim of this study is to summarize various biomechanical aspects in evaluating the long-term stability of dental implants based on finite element method (FEM).
MATERIALS AND METHODS
A comprehensive search was performed among published studies over the last 20 years in three databases; PubMed, Scopus, and Google Scholar. The studies are arranged in a comparative table based on their publication date. Also, the variety of modeling is shown in the form of graphs and tables. Various aspects of the studies conducted were discussed here.
RESULTS
By reviewing the titles and abstracts, 9 main categories were extracted and discussed as follows: implant materials, the focus of the study on bone or implant as well as the interface area, type of loading, element shape, parts of the model, boundary conditions, failure criteria, statistical analysis, and experimental tests performed to validate the results. It was found that most of the studied articles contain a model of the jaw bone (cortical and cancellous bone). The material properties were generally derived from the literature. Approximately 43% of the studies attempted to examine the implant and surrounding bone simultaneously. Almost 42% of the studies performed experimental tests to validate the modeling.
CONCLUSION
Based on the results of the studies reviewed, there is no "optimal" design guideline, but more reliable design of implant is possible. This review study can be a starting point for more detailed investigations of dental implant longevity.
Keywords: Bone remodeling, Fatigue, Optimization, Sensitivity analysis, Reliability
INTRODUCTION
The use of dental implants has become a common procedure in dentistry. However, there is always a risk of failure due to structural and functional defects. The success of an implant depends on its stability, which is influenced by the biomechanical conditions of the bone-implant interface.1 With the advent of computer-aided modeling technology and software packages such as ABAQUS and ANSYS, the design and analysis techniques of implants have undergone a new approach.2 Fracture resistance is the most important property of implant components.3 Static fracture tests are usually simulated to determine the strength of implant components. However, verification of the actual performance of implants should be investigated with intermittent and prolonged loading.4
An important aspect to consider in implant biomechanics is the size of the implant and its components. Considering the anatomy of the jawbone in terms of geometry and the mechanical and physiological properties of the cortical and cancellous bone, it can be concluded that there are fundamental limitations to implant design, particularly diameter and length.5 After implantation, bone adapts its structure to the mechanical loads through remodeling processes.6 In order to achieve optimal longevity of dental implants, careful selection of the implant structure considering the bone characteristics is recommended.7 The choice of material and geometric design of other components such as abutment, screws, and threads also influence the stress distribution in the implant and the surrounding bone and thus the results of long-term stability. Therefore, attempts to improve stress distribution have been the subject of some research in this area.8 Some studies have evaluated stress distribution patterns in the bone-implant interface by investigating stress shielding and remodeling to estimate implant function and potential defects.9 However, many studies have investigated the durability of implants rather than the bone-implant complex.10
Loading of implants is based on replicating the in vivo loading conditions in the oral cavity and includes the magnitude and direction of the applied load. Different loading angles are set with respect to the longitudinal axis of the implant, resulting in a combination of axial, bending, and torsional loads.11
Studies show that stress concentration leads to bone tissue resorption. Moreover, if the load is too low, bone loss will occur. Therefore, the issue of uniform stress distribution during long-term operation is very important and requires the proper design of implants.12 It can be concluded that a partial and asymmetric type of bone repair around the implant poses a potential risk to implant reliability.13
After implant placement, the initial stability of the implant depends on the amount of surrounding cortical and trabecular bone.14 However, predicting the long-term stability of dental implants is more complicated. The long-term stability of dental implants can be divided into two main aspects: 1) stability of the implant-bone complex, 2) fatigue resistance of the implant and its components.15 First, implants are subjected to different masticatory loads over time, and the remodeling process simultaneously changes the surrounding medium. This affects the stability of the implant-bone complex. Secondly, the long-term stability of the dental implant itself (rather than the implant-bone complex) is highly dependent on the fracture strength under cyclic masticatory forces and is usually evaluated by fatigue testing. Studies on the fatigue behavior of dental implants are conducted using the criteria of Goodman, Soderberg, and Gerber. The results show that these criteria can be effective in evaluating the failure of dental prostheses.16 Experimental tests conducted by researchers in this field are often based on ISO 14801 (Dynamic fatigue test for endosseous dental implants).4,5 The loading and boundary conditions of many finite element method (FEM) are also derived from this standard.17
Accurate investigation of dental implants embedded in the jawbone under oral cavity conditions such as temperature fluctuations, humidity, and masticatory loading require in vivo testing. However, due to the difficulties involved, researchers often use the finite element method to simulate implants and bone. However, there are limitations when it comes to modeling all conditions in the oral cavity in the software. In conclusion, the finite element method allows the simulation of real conditions of biomechanical structures with an acceptable approximation.18
So far, many studies have been presented in the field of dental implant design and analysis. This has led to the creation of geometric diversity in order to obtain implants with long-term functions.19 Due to the variety of geometric parameters involved in implant design such as length, diameter, thread form, helix angle, etc., the importance of statistical methods such as sensitivity analysis and reliability to achieve optimal geometry in different implant models seems greater than before.20 In the problems of geometric optimization of dental implants, the mentioned geometric parameters of the model are selected as input variables. The stress distribution pattern in the form of maximum Von-Mises stress and/or strain or other failure criteria as output is included to optimize the geometry and increases the reliability of dental implants.21
Another recently proposed solution to improve stress distribution is the use of porous structures. Porous implants can act as a porous scaffold and improve ossification around the implant.22 Moreover, if geometric parameters are defined for a porous geometric pattern, it would be possible to use optimization methods.23
In this study, a summary of the efforts to evaluate the long-term stability of dental implants is given and different categories are extracted and classified in the comparison tables. The details of experimental data and statistical analysis are also tabulated. Then various aspects of these studies including methodology and shortcomings are discussed. Here, the use of statistical approaches and comparison of modeling with experimental results are considered as qualitative measurement criteria in the study of the long-term stability of dental implants. Now it is possible to compare the methods and results of the studies and to get an overview of the different approaches of the articles to each extracted category.
MATERIALS AND METHODS
Search strategy
In order to obtain a comprehensive biomechanical overview of dental implant stability, the first step of the careful review involved a thorough search of articles published in databases such as PubMed, Scopus, and Google Scholar over the last 20 years up to November 2021. Terms such as “dental implant + longevity + FEM”, “dental implant + reliability + FEM”, “dental implant + stability + FEM”, and “dental implant + fatigue + FEM” were searched. These terms represent the study of various biomechanical aspects of dental implant stability by researchers. A manual search was also performed through the reference list of studies found. A total of 174 articles were found.
Screening the title
The initial screening was based on the titles of the found articles. The titles of the articles were studied in detail by both authors to confirm the purpose and relevance of each investigation to the content of our manuscript (long-term stability of dental implants from a biomechanical point of view). After reading their titles, those that met the qualification were selected for a more accurate processing.
Inclusion and exclusion criteria
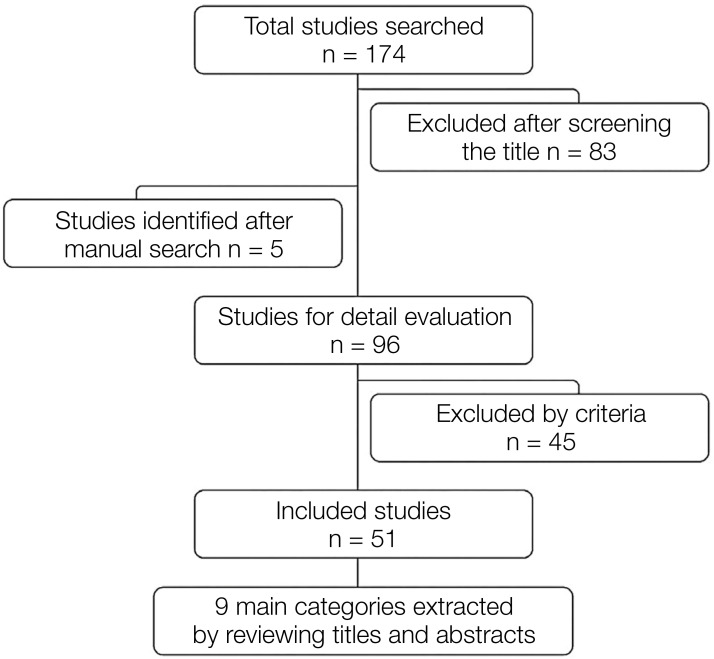
In this study, the main criteria for evaluating dental implant stability methods are long-term evaluations (dynamic tests) using the finite element method. Therefore, studies that satisfied the initial stability and/or static tests to predict the long-term behavior of dental implants without performing dynamic loading were excluded. Finally, 51 articles that were most relevant to the main content were selected. Figure 1 shows the review process in the form of a flow chart.
Fig. 1. Flow chart of the review procedure.
Category selection
In the second step, the authors carefully reviewed the abstracts of the selected papers to examine the similarities. Nine main categories were selected including, implant materials, bone-implant interface, type of loading, element shape, parts in the finite element model, boundary conditions, failure criteria, statistical analyses, and comparison of results with experimental data. By comparing the studies using these categories, it is possible to understand the strengths and weaknesses of each study, which finally led to determine the necessary future attempts in this area. The collected articles are compared in Table 1 based on the date of publication, and then each category was discussed separately.
Table 1. Summary of articles reviewed: implant materials, studies focusing on bone or implant, type of loading, element shape, boundary conditions, failure criteria, parts of the finite element model, statistical analyses, and comparison with experimental results.
| Author (Year) | Implant Material | Study Focus on Bone and/or Implant | Type of Loading | Element Shape | Boundary Condition | Failure Criteria | Parts of Finite Element Model | Statistical Analyses | Comparison with Experimental |
|---|---|---|---|---|---|---|---|---|---|
| Kunavisarut et al.24 (2002) | Titanium | Both | Static Overload | - | - | Von misses | 7 Parts: Crown, Gold Screw, Abutment, Screw, Implant, Cortical Bone, Cancellous Bone | - | No |
| Perriard et al.25 (2002) | Titanium | Implant | Static, Dynamic (Fatigue) | Tetrahedron | - | - | 5 Parts: Implant, Abutment, Screw, Loading Cap, Resin Block | Yes | Yes |
| Genna26 (2003) | Titanium | Implant | Dynamic (Fatigue) | - | - | - | 4 Parts: Abutment, Implant, Screw, Epoxy Resin as Fixture | - | Yes |
| Kayabasi et al.27 (2006) | Ti-6Al-4V | Both | Static, Dynamic (Fatigue) | 4-Node Tetrahedron | Bottom Surface of Mandible Fixed | Von Mises | 6 Parts: Implant, Abutment, Metal Framework, Occlusal Material, Cortical Bone, Trabecular Bone | - | No |
| Wierszycki et al.28 (2006) | Titanium | Implant | Dynamic (Fatigue) | - | Different Levels of Osseointegration | The factor of strength | 4 Parts: Implant, Abutment, Screw, Bone | - | Yes |
| Yang et al.29 (2007) | Functionally Graded Biomaterial | Both | Static and Harmonic Occlusal | 10-Node Tetrahedron | - | Von Mises | 5 Parts: Abutment, Screw, Cortical Bone, Trabecular Bone, FGM Implant | - | No |
| Kong et al.30 (2009) | Titanium | Both | Immediate Loading | 10-Node Tetrahedron/20-Node Hexahedron | - | Von Mises | 4 Parts: Implant, Cortical Bone, Cancellous Bone, Porcelain | Yes | No |
| Hasan et al.31 (2010) | Titanium | Both | Immediate Loading | 4-Node Tetrahedron | End Faces Constrained | Von Mises | 2 Parts: Implant, Bone Block | - | No |
| Pérez32 (2012) | Titanium | Implant-bone Interface | Dynamic | Tetrahedron | Bone Sides and Bottom Fixed | Von Mises | 2 Parts: Implant, Bone | Yes | No |
| Hasan et al.33 (2012) | Titanium | Implant | Immediate Loading | Tetrahedron | Bone Sides and Bottom Fixed | Equivalent stress | 3 Parts: Implant, Cortical Bone, Cancellous Bone | Yes | Yes |
| Tsai et al.34 (2012) | Titanium | Implant | Static, Dynamic (Fatigue) | - | - | Von Mises, Soderberg, Goodman & Gerber | 4 Parts: Implant, Abutment, Loading Cap, Holder | Yes | Yes |
| Lee et al.35 (2012) | Titanium | Both | Static, Dynamic | Tetrahedron | Bone Fixed at Mesio-Distal Ends/Implant Fully Bonded Bone | Strain Energy Density/Von Mises | 4 Parts: Implant, Abutment, Cortical Bone, Trabecular Bon | - | No |
| Ali et al.36 (2013) | Titanium | Both | Dynamic/Dynamic (Over Load) | - | Bottom Surface of Jaw Fixed | Von Mises | 5 Parts: Implant, Crown, Abutment, Cancellous Bone, Cortical Bone | - | No |
| Covani et al.37 (2013) | Titanium | Implant | Dynamic | Tetrahedral | Bone Constrained | Von Mises | 4 Parts: Implant, Abutment, Bone, Screw | - | No |
| Geringer et al.38 (2014) | Titanium | Both | Static, Fatigue | Tetrahedral | Base Displacements Set to Zero in 3 Dimensions | Von Mises | 4 Parts: Implant, Crown, Abutment, Resin Block | - | No |
| Ayllón et al.39 (2014) | Titanium | Implant | Dynamic (Fatigue) | 10-Node Tetrahedron | Null Displacement on The Crests of External Thread | Von Mises | 5 Parts: Implant, Screw, Pillar, Loading Cap, Fixed Support | - | Yes |
| Bulaqi et al.40 (2015) | Titanium | Both | Dynamic (Fatigue) | Tetrahedral | Bone Bounded | Von Mises | 8 Parts: Implant, Direct Abutment, Screw, Metal Frame, Porcelain, Resin, Cortical Bone, Trabecular Bone | - | No |
| Hernandez et al.41 (2015) | Cobalt-Chrome Alloy | Implant | Dynamic (Fatigue) | 10-Node SOLID 187 3 Degrees of Freedom | Down Base and Lateral Bone Faces Restricted in 3 Longitudinal and Rotational Directions | Von Mises, Goodman, Soderberg, and Gerber | 8 Parts: Crown Inner, Crown Outer, Fixation's Screw, Abutment's Screw, Abutment, Single Implant, Cancellous Bone, Cortical Bone | - | No |
| Hernandez-Rodriguez et al.42 (2015) | Ti-6Al-4V | Implant | Static, Dynamic (Fatigue) | Tetrahedron | Holder Constrained by a Fixed Support | Von Mises | 5 Parts: Loading Cap, Abutment, Implant, Screw, Holder | - | Yes |
| Prados-Privado et al.43 (2015) | Ti-6Al-4V | Implant | Static, Dynamic (Fatigue) | - | Displacements Restrained, Only Normal to The Surface Allowed | Goodman, Soderberg, Gerber | 1 Part: Implant | Yes | No |
| Toyoshima et al.44 (2015) | Ti-6Al-4V | Implant | Static, Dynamic (Fatigue) | 8-Node Tetrahedron | Holder Constrained by a Fixed Support | Von Mises | 3 Parts: Implant, Cancellous Bone, Cortical Bone | - | Yes |
| Bicudo et al.45 (2016) | Titanium | Both | Static, Dynamic (Fatigue) | SOLID187 | Constrained Lateral Faces of Epoxy and Saw bone | Von Mises | 3 Parts: Implant, Epoxy Resin layer, Saw Bone | - | Yes |
| Szajek et al.46 (2016) | Titanium | Implant | Static, Dynamic (Fatigue) | 8-Node Linear Brick | Fixed Implant Root | Von Mises | 3 Parts: Abutment, Screw, Fixture | - | No |
| Bicudo et al.47 (2016) | Titanium | Both | Dynamic (Fatigue) | SOLID187 | Constrained Lateral Faces of Epoxy and Saw Bone | Von Mises | 3 Parts: Implant, Epoxy Resin layer, Saw bone | - | Yes |
| Prados-Privado et al.48 (2016) | Titanium | Implant | Dynamic (Fatigue) | - | - | Von Mises | 3 Parts: Implant, Abutment, Crown | Yes | No |
| Wu et al.49 (2017) | Titanium, Ti-6Al-4V | Both | Dynamic (Fatigue) | - | Constrained Cuboid Bone Surface | Von Mises | 6 Parts: Implant, Abutment, Fixation Screw, Crown, Cortical Bone, Cancellous Bone | Yes | Yes |
| Geramizadeh et al.50 (2017) | Titanium | Both | Static Dynamic (Fatigue) | 4-Node Tetrahedron | Mandible Base Fixed | Von Mises, Goodman | 4 Parts: Implant, Abutment, Cortical Bone, Cancellous Bone | - | No |
| Bordin et al.51 (2017) | Titanium | Implant | Dynamic (Fatigue) | Tetrahedron | Full Constrain of Model | Von Mises | 4 Parts: Implant, Abutment, Crown, PVC Tube Holder | - | Yes |
| Prados-Privado et al.52 (2017) | Titanium | Implant | Dynamic (Fatigue) | - | Inferior Border of Cortical Bone Restrained/Mesial/Distal Borders of End, Bone Section Was Constrained | Von Mises | 3 Parts: Implant, Cortical Bone, Trabecular Bone | Yes | No |
| Castolo et al.53 (2018) | Titanium | Implant | Static, Dynamic (Fatigue) | Triangular Elements for Surface/Tetrahedral Elements For Volume | Implant Root Embedded in Fixture | Von Mises | 5 Parts: Dental Implant, Connecting Screw, Hemispherical Member, Connecting Part, Holding Element | - | Yes |
| Yamaguchi et al.54 (2018) | Titanium | Implant | Dynamic (Fatigue) | - | - | - | 5 Parts: Implant, Abutment, Screw, Cortical Bone, Trabecular Bone | Yes | Yes |
| Cinel et al.55 (2018) | Titanium/Titanium-Zirconium | Both | Static, Dynamic (Fatigue) | 8-Node Tetrahedral | Mandible Lower Border, Maxilla Upper Border Fixed | Von Mises, Goodman, Soderberg, Gerber | 7 Parts: Implant, Crown, Metal Framework, Screw, Abutment, Cortical Bone, Trabecular Bone | - | No |
| Cervino et al.56 (2018) | Titanium, Ti-6Al-4V | Both | Dynamic | SOLID 186/SOLID 187 | Ideal Osseointegration with Total Contact Surface Between Implant/Bone | Von Mises | 5 Parts: Implant, Abutment, Screw, Cortical Bone, Trabecular Bone | - | No |
| Geramizadeh et al.57 (2018) | Titanium | Both | Static, Dynamic (Fatigue) | 4-Node Tetrahedron | Mandible Base Fixed | Von Mises | 4 Parts: Implant, Abutment, Cortical Bone, Trabecular Bone | Yes | No |
| Topkaya et al.58 (2018) | Ti-6Al-4V | Implant | Dynamic (Fatigue) | 10-node Tetrahedron 6 Degrees of Freedom | All Edges, Area of Substructure Constrained | Von Mises | 5 Parts: Implant, Abutment, Screw, Cortical Bone, Trabecular Bone | - | Yes |
| Abasolo et al.59 (2018) | Ti-6Al-4V Cobalt-Chrome Alloy | Implant | Dynamic (Fatigue) | High/low order Hexahedral 3 Degrees of Freedom | Both Ends of The Mandible Are Clamped | Goodman | 8 Parts: Implant, Abutment, Lower Screw, Cortical and Trabecular Bone, Lower-Upper Screw, Framework | - | No |
| Duan et al.60 (2018) | Titanium | Implant | Dynamic (Fatigue) | 4- Nodes Tetrahedron | Constrained Bottom Surface of The Holder Block | Von Mises | 5 Parts: Implant Body, Abutment, Abutment Screw, Cylindrical Base, Hemispherical Loading Cap | Yes | Yes |
| Bayata et al.61 (2018) | Titanium | Both | Dynamic (Fatigue) | 10-Node Tetrahedron | Lateral Surface of Holder Fixed | Von Mises, Goodman | 3 Parts: Implant, Abutment, Holder Block | - | No |
| Prados-Privado et al.62 (2018) | Ti-6Al-4V | Implant | Dynamic (Fatigue) | - | Bottom/Lateral Surface of Bone Restrained | Von Mises | 4 Parts: Implant, Loading Cap, Abutment, Cylindrical Bone | - | |
| Lee et al.63 (2019) | Titanium | Both | Dynamic (Fatigue) | 4-Nodes Tetrahedron | Both Ends of Bone Block Fixed in All Directions | Von Mises | 8 Parts: Crown, Cement layer, Abutment, Screw, Implant, Cortical Bone, Trabecular Bone, Nerve Canal | - | No |
| Wang et al.64 (2019) | Commercially pure Titanium | Both | Dynamic (Fatigue) | - | Bone/Implant Perfectly Bonded | Von Mises | 5 Parts: Implant, Loading Cap, Abutment, Cortical Bone, Cancellous Bone | - | Yes |
| Bataineh et al.65 (2019) | Ti-6Al-4V | Both | Dynamic (Fatigue) | - | Bone Fixed | Von Mises, Maximum Principle Stress/Strain | 5 Parts: Implant, Crown, Abutment, Cortical Bone, Cancellous Bone | - | No |
| Manea et al.66 (2019) | Ti-6Al-4V | Implant | Static Dynamic (Fatigue) | Tetrahedron | Complete Immobilization of Implant Exterior Part/Outer Surface Blocked | Von Mises | 8 Parts: Implant, Cushioning Mechanism, Abutment, Locking Pins, Implant Screw, O-Ring, Loading Cap, Bone Block | Yes | Yes |
| Prados-Privado et al.67 (2019) | Titanium | Implant | Dynamic (Fatigue) | - | Degrees of Freedom in Bottom/Lateral Surfaces in Resin Block Restrained | - | 3 Parts: Implant, Metallic Crown, Resin Block | - | No |
| Zhang et al.68 (2020) | Ti-6Al-4V | Implant | Static Dynamic (Fatigue) | - | Good Osseointegration Between Implant/Alveolar Bone | Von Mises | 6 Parts: Implant, Abutment, Central Screw, Loading Cap, Cortical Bone, Cancellous Bone | - | Yes |
| Sahin69 (2020) | Titanium/Titanium-Zirconium | Both | Static Dynamic (Fatigue) | Tetrahedron | Inferior Edge of Mandible, Superior Edge of Maxilla, Lateral Region of Jaw Fixed | Von Mises | 7 Parts: Implant, Crown, Substructure Part, Abutment, Screw, Cortical Bone, Cancellous Bone | - | No |
| Nokar et al.70 (2020) | Ti-6Al-4V | Both | Static Dynamic (Fatigue) | Tetrahedron | Bone Segment Fixed From The Mesial/Distal | Von Mises | 7 Parts: Implant, Crown, Abutment, Frame Work, Porcelain, Cortical, Cancellous Bone | - | No |
| Armentia et al.71 (2020) | Commercially Pure Titanium Ti-6Al-4V | Implant | Static Dynamic (Fatigue) | - | Holder Fixed | - | 4 Parts: Implant, Abutment, Screw, Specimen Holder | - | Yes |
| Bayata et al.72 (2020) | Ti-6Al-4V | Implant | Dynamic (Fatigue) | 10-Node Tetrahedron | Lateral Surface of Implant Holder Fixed | Von Mises | 3 Parts: Implant, Abutment, Holder Block | - | No |
| Lee et al.73 (2021) | Ti-6Al-4V | Implant | Dynamic (Fatigue) | Tetrahedron | Bone Block Fixed Along All Axes | Von Mises | 9 Parts: Crown, Cement, Screw, Abutment, Implant, Cortical Bone, Cancellous Bone, Cylindrical Part, Nerve Canal | Yes | No |
| Bergamo et al.74 (2021) | Ti-6Al-4V | Implant | Dynamic (Fatigue) | - | Bottom Surface of Abutment Fixed | Maximum Principal Stress/Strain | 4 Parts: Implant, Abutment, Resin Matrix, Ceramic Crown | Yes | Yes |
Cells marked with ((-)) indicate that the case was not mentioned in the article.
*Ti-6Al-4V refers to the chemical composition of the alloy of almost 90% titanium, 6% aluminum, 4% vanadium.
RESULTS
Implant material
It was found that most of the studies mainly used titanium (Ti) and its alloys such as Ti-6Al-4V (the alloy of almost 90% titanium, 6% aluminum, 4% vanadium) as implant material. Due to the biocompatibility of titanium and its physical and mechanical properties, implants are highly successful. Various studies have shown that titanium has considerable long-term stability.75 The main alloys used in the manufacture of commercial implants are pure titanium and Ti-6Al-4V. The fatigue strength of Ti-6Al-4V has been reported to be better. However, there are concerns about the biological effects of the very small amounts of aluminum and vanadium that enter the body. Despite these concerns, there is ample empirical evidence that both alloys have good biocompatibility and the ability to osseointegrate.76
Other implant parts such as the abutments or abutment screws are made of different alloys such as gold, cobalt, or chromium.77 In biomechanical simulations of dental implants, the main focus is usually on the mechanical behavior of material, alloys, and structural components. Other biocompatibility parameters such as surface modification and roughness and their effects on bone remodeling and physiology are not a priority in such studies.78 Based on the reviewed studies, Titanium and its alloys are commonly used in the evaluation of long-term stability when selecting materials, and the variety of materials used in this field is small.
The focus of the study on bone and/or implant
In 53% of the articles reviewed, the focus was only on the implant and the components embedded in a restrained fixture, but other studies considered the surrounding bone as an important part to be studied under different loading conditions, such as a fatigue test. In the studies investigated, the surrounding bone was modeled as a continuous medium, taking into account the different mechanical properties of cortical and cancellous bone, without focusing on the complicated geometry of cancellous bone. However, three-dimensional reconstructed models of bone based on micro-computed tomography (micro CT scanning) provide more accurate geometry for FE analyses.79,80
In the reviewed studies, the contact surface between the bone and the implant is often considered as a complete connection. 47% of the reviewed studies investigated the distribution of stress in this area. The biomechanical modeling of the bone-implant interface is of particular importance. Recent studies have been performed on the biomechanical aspects of this area.81
Loading
Implant and bone must be considered under the following loading conditions: static and dynamic. Dynamic loading is usually derived from the standard ISO 14801,17 which represents the loading conditions for the implant during mastication. The loading should represent a simple case, but the worst possible case. The oblique orientation is often considered biologically justified because it causes an accumulation of bending and pressure on the implant components.82 Many modeling studies in the field of dental implants have only investigated implants under static loading, while fewer studies have investigated implants under dynamic loading.83 Since satisfactory static behavior is not necessarily a guarantee of long-term implant performance, this review focused on articles that investigated the dynamic test as a representative of long-term implant behavior.84
Recent studies show that the success rate of dental implants in immediate loading depends on factors such as bone quality, implant dimensions, implant components, and force.85
Shape of elements
To perform FE analysis, the model must be discretized into small elements in the spatial dimension so that the software can construct a mesh of the objects. In the studied articles, a frequently used element is tetrahedron (in some studies the element shape was not mentioned). The accuracy of the result in finite element analysis depends on the element shape, the number of nodes and the degrees of freedom. These factors were explicitly mentioned in some studies. Higher-order elements were used for complex geometries.86 Some studies only specified the element type. The degrees of freedom in each element are also an important feature in FEM. However, very few articles mentioned the number of degrees of freedom of elements. The most widely used software packages for FE analysis are ANSYS and ABAQUS, although not all studies mentioned their software.
The property of the FEM to obtain an accurate solution by mesh improvement and corrections for error minimization is called “convergence”. After appropriate meshing and achieving suitable finite element network, one will obtain the desired number of elements and degrees of freedom. The main variable to be considered for the solution convergence is the field variable in FEA (e.g. the maximum stress or displacement, etc.). Usually, by increasing the number of elements to an optimum number, an agreement will be grasped between the convergence time and the number of elements. It is important to realize that increasing the number of elements does not necessarily conclude to higher accuracy of the solution, but increases the computational cost.87
Although optimization of the mesh size and convergence of the results is one of the most important issues in the application of the FEA and it is necessary to gain acceptable solution accuracy, most of the reviewed studies mention the number of elements, the type of elements and, in some cases, the number degrees of freedom.
Boundary conditions
Due to the complexity of the bone-implant interface area, modeling this region is difficult and always involves assumptions. The structure of the bone changes over time due to the remodeling process. Moreover, due to the different elastic modulus of the implant and the surrounding bone, as well as the mechanical behavior of the individual parts, the local occurrence of complex multiaxial stresses is predictable. On the other hand, the rate of bone repair varies in different parts of the bone-implant interface (BII) region.88 In some studies, different stages of bone repair were considered as contact boundary conditions. In the present study, based on paper reviewed, different boundary conditions were investigated and considered in different modeling cases. Most of the boundary conditions aim to restrain the displacements of the jaw bone or the implant root attachment.
Failure criteria
In the studies reviewed, various criteria were considered to evaluate the risk of failure of the implant and/or surrounding bone. In the evaluation of stress distribution, the Von-Mises stress criterion was considered in the analysis of the models. The Goodman, Gerber, and Soderberg criteria were used in the fatigue studies.
Although these criteria are mainly used in articles, recent studies show that the onset of bone remodeling is more closely related to strain energy density. Mechanical parameters that are closely related to the onset of the remodeling response are strain energy density, longitudinal shear stress, and principal tensile stress.89
Parts of the model
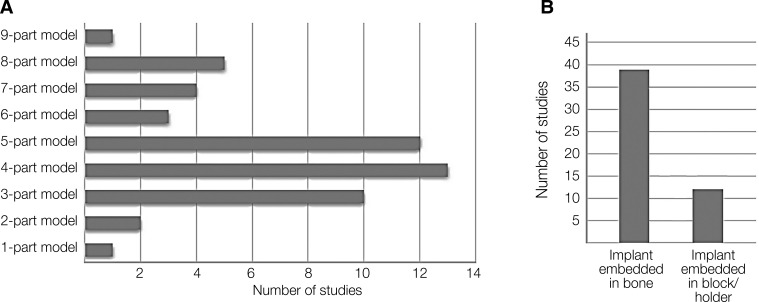
As shown in Table 1, in the reviewed studies, the modeling related to dental implants varies in terms of the components of the finite element model. Modeling from one-part to nine-part modeling is given in the comparison table with details. For example, one of the models includes implants, abutments, screw abutments, and a model of the jaw bone containing cortical and cancellous bone. In some of the reviewed studies, dental implants are embedded in resin blocks (holders). As shown in Figure 2, the authors are more inclined to 4-part and 5-part modeling.
Fig. 2. Classification of modeling based on the number of model components and the tendency of researchers for each (A), number of models with implant embedded in block/holder or bone (B).
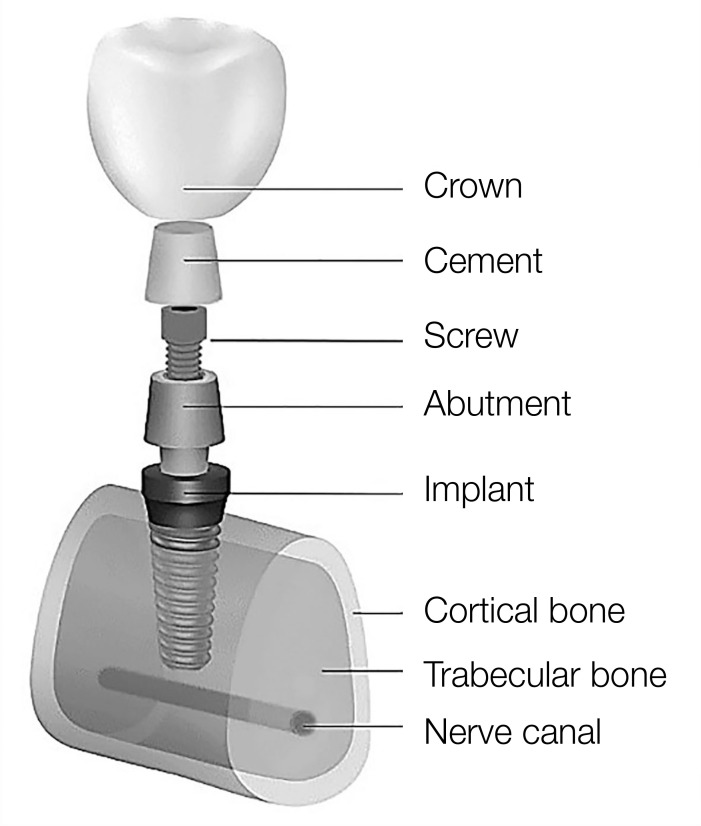
The number of components of the finite element model can determine the accuracy of modeling and its greater adaptation to real conditions. For this reason, in some models, more details including implants, abutments, screw abutments, crowns, cement layer, and even the nerve canal inside the jaw bone have been modeled (Fig. 3). In modeling, the crown (ceramic) is often modeled as a rigid body. As can be seen in Figure 2, in most models (77%), the implant is embedded in the bone, while in a number of studies (23%), the implant is placed inside a resin block (or holder).
Fig. 3. Schematic representation of the different parts of a finite element model.
Aside from discussing the quality and accuracy of dental implant modeling and components of each finite element model, it is important to examine which component(s) are specially targeted. Table 2 shows the main topic of each of the studies regarding the component of the implant set. Since dental implants are made up of a number of components that are in contact and interconnected, when discussing the long-term stability of the implant, the strength and durability of each component affect the success of the implant placement. In addition, the stress distribution between the components and in the bone-implant set should be optimal. The study of long-term stability has been conducted in several areas, including the study of the effects of component material, geometric optimizations, the study and comparison of the stability of commercial implants, sudden or overloaded implants, and the study of fatigue. These topics are summarized in Table 2.
Table 2. Summary of the main themes of the studies and topics.
| Author | Main Issue of study | Study Subject | |||||
|---|---|---|---|---|---|---|---|
| Crown | Metal Framework | Abutment Screw | Abutment | Implant | Bone | ||
| Kunavisarut et al.24 | * | * | * | * | * | Misfit of Parts | |
| Perriard et al.25 | * | * | Abutment Connectors Fatigue | ||||
| Genna26 | * | * | * | Cyclic Transversal Force | |||
| Kayabasi et al.27 | * | * | * | * | Dental Implant Fatigue | ||
| Wierszycki et al.28 | * | * | Dental Implant Fatigue | ||||
| Yang et al.29 | * | * | Functionally Graded Material | ||||
| Kong et al.30 | * | * | * | Immediate Load | |||
| Hasan et al.31 | * | * | Short Dental Implant | ||||
| Pérez32 | * | Dental Implant Fatigue | |||||
| Hasan et al.33 | * | Design Abutment Influence | |||||
| Tsai et al.34 | * | * | Dental Implant Fatigue | ||||
| Lee et al.35 | * | Dental Implant Fatigue | |||||
| Ali et al.36 | * | * | * | * | Overloading In Mastication | ||
| Covani et al.37 | * | * | * | Implant-Abutment Connect | |||
| Geringer et al.38 | * | Zirconia Abutment | |||||
| Ayllón et al.39 | * | Fatigue Life Estimation | |||||
| Bulaqi et al.40 | * | * | * | * | * | * | Stress In Bone-Implant |
| Hernandez et al.41 | * | * | * | * | * | Dental Implant Fatigue | |
| Hernandez-R et al.42 | * | * | Failure Analysis | ||||
| Prados-Privado et al.43 | * | Dental Implant Fatigue | |||||
| Toyoshima et al.44 | * | Load Limit Mini-Implant | |||||
| Bicudo et al.45 | * | * | Mechanical Behavior | ||||
| Szajek et al.46 | * | * | * | Fatigue life | |||
| Bicudo et al.47 | * | * | Performance Evaluation | ||||
| Prados-Privado et al.48 | * | Random Fatigue | |||||
| Wu et al.49 | * | Effect of Lubricant | |||||
| Geramizadeh et al.50 | * | * | Dental Implants Threads | ||||
| Bordin et al.51 | * | * | Narrow Dental Implants | ||||
| Prados-P et al.52 | * | Dental Implant Fatigue | |||||
| Castolo et al.53 | * | * | Mechanical Strength | ||||
| Yamaguchi et al.54 | * | * | * | Fixture/Abutment Joint | |||
| Cinel et al.55 | * | * | Narrow Diameter Implants | ||||
| Cervino et al.56 | * | * | * | Structural Components | |||
| Geramizadeh et al.57 | * | Optimization, Sensitivity | |||||
| Topkaya et al.58 | * | Implant Dimension/Fatigue | |||||
| Abasolo et al.59 | * | Misfit/Screw Fatigue | |||||
| Duan et al.60 | * | * | * | * | Fatigue Lifetime Prediction | ||
| Bayata et al.61 | * | * | Mechanical Behaviors | ||||
| Prados-Privado et al.62 | * | External Hexagonal | |||||
| Lee et al.63 | * | * | * | Short Dental Implants | |||
| Wang et al.64 | * | * | Porous Dental Implant | ||||
| Bataineh et al.65 | * | * | Biocompatible Implant | ||||
| Manea et al.66 | * | Shock Absorbers | |||||
| Prados-P et al.67 | * | Fatigue Behavior | |||||
| Zhang et al.68 | * | * | * | Statics/Fatigue Analysis | |||
| Sahin69 | * | * | Narrow Diameter Implants | ||||
| Nokar et al.70 | * | * | * | Stress in Bone/Abutment | |||
| Armentia et al.71 | * | * | * | Fatigue Design | |||
| Bayata et al.72 | * | * | Design Parameters Effects | ||||
| Lee et al.73 | * | * | * | Diameter, Connection Type | |||
| Bergamo et al.74 | * | Survival of Implant | |||||
*Means that the study contains the items listed.
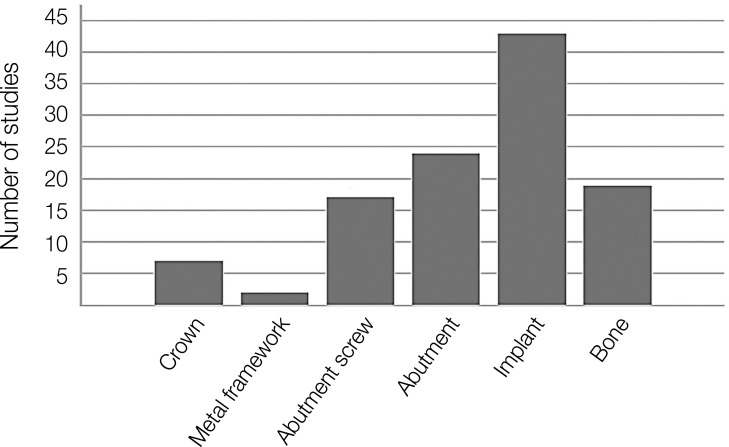
From the graph in Figure 4, it can be seen that implants (roots) and abutments have received the most attention from researchers over the past twenty years when it comes to long-term stability. Many studies have investigated the contact properties and stress distribution between implants and abutments as a part of the discussion on long-term stability. However, the role of the screw connection is also important and the subject of several studies in this field.
Fig. 4. The extent of each part of the implant-bone set as the main subject of the studies.
As shown in Table 2 and Figure 4, the study of components such as the crown or the metal framework has received the least attention. In 97% of the reviewed studies, the framework was ignored. Due to the fact that the crown is made of ceramic, it is modeled as a rigid element in most studies. Furthermore, in many models, a rigid spherical metal cap was used to apply force instead of the implant crown.
Statistical analyses
A few studies used statistical methods in which multiple statistical analyses were performed to assess the significance of the difference between the modeling approach and the results obtained. The characteristics of these analyses, including sample size, methodologies, mean and variance of stresses resulting from modeling, reliability, and sensitivity analyses are summarized in Table 3.
Table 3. The summary of the statistical analyses performed to assess results.
| Author | Number of Samples | Details |
|---|---|---|
| Perriad et al.25 | 9 Specimens to Adjust Machine Settings, 20 Specimens for O-O Combination 10 For Both The S-S and O-S Pairs | Standard Deviation Computed |
| Kong et al.30 | 9 Samples | Response Surface Construction and Sensitivity Analysis |
| Pérez32 | - | Mean Value and Monte Carlo Sampling |
| Hasan et al.33 | 30 Samples | Differences of Mean Implant Displacements and Rotations Analysed Using Mann-Whitney Test |
| Tsai et al.34 | 4 Samples | Regression Analysis Method |
| Prados-Privado et al.43 | Several Taylor Expansions Done Around Every Random Variable, Instead of Generating Samples | PFEM have been chosen (perturbation method) |
| Prados-Privado et al.48 | - | Mean Value and Variance, Perturbation Method |
| Wu et al.49 | One-way analysis of variance (ANOVA) with Fisher's PLSD test was used to compare the differences (P < .05) | |
| Prados-Privado et al.52 | - | Mean Value and Variance to Estimate The Fatigue Life and Probability of Failure |
| Yamaguchi et al.54 | Mild: n = 3 Moderate: n = 2 Aggressive: n = 1 |
Two-Way ANOVA P < .001 |
| Geramizadeh et al.57 | 3 Candidate Points of 1000 Iteration Extracted | Sensitivity Analysis |
| Duan et al.60 | - | Fatigue Lifetime Statistics of Physical Specimens Were Estimated in a Reliability Analysis Software (ALTA PRO) |
| Manea et al.66 | 10 Samples | U Test, Mann-Whitney, and Wilcoxon Test For Paired Samples |
| Lee et al.73 | 12 Samples | Statistical Analysis Performed with SPSS 20.0 Software (IBM)/Analysis for Normal Distribution With The Shapiro-Wilk Test. Kruskal-Wallis One-Way And Mann-Whitney U Tests |
| Bergamo et al.74 | n = 9 in the mild, n = 6 in the moderate, n = 3 in the aggressive loading |
Reliability Analyses, Weibull Beta Parameter Calculation |
The cells indicated by ((-)) means that the case has not been mentioned in the article.
One of the most important applications of statistics to represent the behavior of phenomena is found in “reliability theory”. This theory is mainly used in cases such as engineering and determining the lifetime of systems. Reliability can be considered as the ability to determine the lifetime of a system based on its components. Therefore, based on probability theory, the degree of reliability can be considered as the probability of failure (probability of longevity).90 In recent studies, the method of sensitivity and reliability analysis is considered as a new approach to estimate the lifetime of implants and their sensitivity to changes in various parameters as input.57
Comparison between FEM and experimental results
Comparing the results of FEM with the experimental results, one could conclude that some results of FEM have been validated. About 42% of the studied articles have performed experimental studies to compare with the computational results, while 5% of the articles have performed this comparison using the experimental results from the literature. The rest of the studied articles have been satisfied with computational analyses. The summary of the experimental methods performed by some authors is summarized in Table 4.
Table 4. The summary of the experimental results performed to validate finite element modeling.
| Author | Test Details |
|---|---|
| Perriad et al.25 | Implant Under Servohydraulic Fatigue Tester |
| Genna26 | Implant Immersed in Resin Cylinder |
| Transversal Cyclic Test | |
| Wierszycki et al.28 | Radiograph Case Report of Patient |
| Hasan et al.33 | Immediate Loading Condition on The Implant |
| Tsai et al.34 | Fatigue Test/5*106 Cycles |
| Ayllón et al.39 | Implant Locked in Fixture/Test According to ISO 14801/5*106 Cycles |
| Hernandez-Rodriguez et al.42 | Dental Implant with Fixed Clamping Device |
| Visual Inspection, Chemical Analysis, Metallography, Micro Hardness | |
| Toyoshima et al.44 | Fatigue Test According to ISO 14801/5*106 Cycles |
| Bicudo et al.45 | Two Types of Implants Embedded in Polyurethane Foam/Fatigue Test According to ISO 14801 |
| Bicudo et al.47 | Two Types of Implants Embedded in Polymeric Foam/Fatigue Test According to ISO 14801 |
| Wu et al.49 | Fatigue Test According to ISO-FDIS 14801 /5*106 Cycles |
| Bordin et al.51 | Implant Embedded in Cylinder PVC Tube/105 Cycles |
| Castolo et al.53 | Fatigue Test According to ISO 14801/106 Cycles |
| Yamaguchi et al.54 | Implants Embedded in Resin/Fatigue Test |
| Topkaya et al.58 | Fatigue Test According to ISO 14801/103 – 106 Cycles |
| Duan et al.60 | Fatigue Test According to ISO 14801/5*106 Cycles |
| Prados-Privado et al.62 | Static and Fatigue Tests |
| Wang et al.64 | Fatigue Test According to ISO 14801/5*106 Cycles |
| Manea et al.66 | Static and Dynamic Test/2*105 Cycles |
| Zhang et al.68 | Fatigue Test of Dental Implant/6*105 Cycles |
| Armentia et al.71 | 46 Experimental Tests According to ISO 14801 |
| Bergamo et al.74 | Stereomicroscope Micrographs of Cracks in Loading Areas |
DISCUSSION
In this study, various research aspects of the biomechanical evaluation of long-term stability of dental implants were investigated including modeling and/or laboratory methods. To understand the significance of studying the long-term stability of dental implants using the finite element method, it is worth stating that, in addition to the variety of modeling, this method also allows the application of different boundary conditions.1 Since several factors play a role in the study of the long-term stability of dental implants, it is possible to apply a variety of boundary and loading conditions simultaneously. Another advantage of the FEM is that it is inexpensive compared to laboratory methods. It is possible to identify the pattern of stress distribution and stress shielding in complex geometric structures.88 The ability to use higher-order elements to improve the accuracy of the results can increase the accuracy of the modeling solutions.86 It is also possible to study the stress pattern at the implant-bone interface region, which can predict the mechanical and physiological response of bone to implant over time. Evaluating the bone biological response to the implant required in vivo testing, which is limited, expensive, and time-consuming. Finally, FEA has the capability to adapt to new approaches such as optimization and reliability analysis to achieve optimal geometric structures and increase the long-term stability of dental implants.57
Although many studies have been conducted on the design and fabrication of dental implants, less attention has generally been paid in the literature to long-term evaluation, including fatigue test analysis and reliability. The evaluation of the long-term stability of dental implants is not limited to biomechanical aspects. Other researchers have attempted to investigate various aspects of this topic from other perspectives such as biology, tissue engineering, and the interaction between implants and biological tissues.91
It has been shown that the structure of the implant strongly influences the stress distribution at the implant-bone interface. Therefore, proper choice of the implant could allow more realistic stress transmission at the interface and stress distribution in the surrounding bone.92 This avoids stress shielding and stress concentration, which is beneficial for the long-term performance and stability of the implant.93
The choice of material as the first step in selecting a dental implant is of particular importance. Although most dental implants are made of titanium, other alloys can be modeled in this manner. Based on the fact that titanium alloys have acceptable fatigue strength, in most studies reviewed, these alloys were used as the basis for implants. However, less attention has been paid to the properties of other materials, such as functionally graded biomaterials, when it comes to long-term stability.75,78
It is necessary that dental implants to be examined separately according to existing standards (ISO 14801, 2007). However, there is always a need to examine the implant and bone collection to observe stress distribution, stress concentration, and shielding after implant placement.
Static loading is commonly used to evaluate the initial stability of a dental implant. But simulating the loading in masticatory mode can be an accurate assessment of the loading status of the implant. When applying FEA to the model, it is important to consider not only axial loads and horizontal forces (moment-inducing loads) but also a combined load (oblique occlusal force). Although the study of static and dynamic loads is performed according to the existing standards (ISO 14801, 2007), the modeling of excessive and impact loads in implant performance can be performed as an approach in the discussion of long-term stability.
In finite element modeling, the choice of element type depends on the geometric complexity of the model structure. The type of elements, the number of nodes per element, and the degrees of freedom of each node directly affect the accuracy and precision of the results. For more complex geometries, elements with higher orders such as 10-node tetrahedron are recommended.58
Usually, the cancellous mandibular bone is considered as a continuum and uniform. Considering that the structure of cancellous bone is heterogeneous and has a complex geometry, the three-dimensional reconstruction of the mandibular bone based on micro-CT scans seems to give a more accurate model.94
Several assumptions were made in the development of the finite element models in the studies reviewed. For example, the model components are homogeneous and isotropic and have linear elastic properties. In several studies, the thickness of the applied cement layer was ignored. In addition, the bone-implant interface region is generally assumed to be fully osseointegrated, which does not necessarily correspond to clinical conditions. Despite all the advantages of using FEM to evaluate dental implants, studies do not consider the physiology of bone as an important factor in modeling. As the bone heals after implant placement, the environment surrounding the implant changes. Therefore, some studies have considered different stages of osseointegration.28 Because bone density differs between men and women, the validity of modeling must be categorized by gender.95
In reviewed studies, the Von Mises stress criterion is often used to discuss the quality of stress distribution in the structures. Criteria such as Goodman are also used to study the fatigue behavior of structures. The choice of failure criterion is one of the most important issues when analyzing the results of FEM. Depending on the nature of the question, consideration of other failure criteria such as strain energy density may be helpful in modeling implants and bone remodeling.89
In most of the studies, the focus of the investigation was on the implant, whereas the presence of other components and the diversity of their material and geometry may affect the overall distribution of stress in the bone and implant complex and may impact long-term stability. Given the differences in modeling and case studies, it was pointed out that each model consists of several parts. For example, some of the modeling studies have investigated one-piece implants,39 while others have investigated implants that contain some components such as abutment, abutment screw, crown, and implant, as shown in Figure 3.
None of the studies reviewed discuss how much the number of components in a model affects the accuracy of the responses. The physical parameters involved in the design of each component, the material of each part, and their effects on the long-term stability of the implant may be the subject of case studies. New methods such as sensitivity analysis can be used to accurately determine the effects of the individual components.57
In the studies reviewed, the diversity of modeling in terms of the number of components of the model and the subject matter of each study has led to different outcomes in the discussion of the long-term stability of dental implants, making it difficult to compare the results. Due to the breadth of the discussion, there is no consistent trend in the studies examined regarding the long-term stability of dental implants.
In the reviewed studies, there is little literature that deals with the statistical analysis of dental implants. Moreover, the studies in this field have a limited sample size and more attention has been paid to modeling aspects such as the number of fatigue cycles, loading devices, loading variables. Obviously, this is a matter of selecting an appropriate sample size depending on the problem variables. For example, implant geometry and mechanical and physical properties, and the simultaneous effect of these variables need many case studies that require accurate statistical analysis to investigate the significance of the differences.57 The method of sensitivity and reliability analysis is recommended as a new approach to evaluating the long-term performance of dental implants, which is less common in the reviewed studies. In other words, there is a way to change the inputs of a statistical model in an organized manner that can predict more realistic effects of these changes on the output of the model. Design sensitivity analysis plays an important role in inverse studies and identification as well as numerical optimization and reliability analysis. The main purpose of sensitivity analysis is to inform about the significance of the input parameters.90
The accuracy of the computational results of FEM depends on several factors, such as material properties, boundary conditions, bone-implant interface conditions, and modeling precision. The finite element models presented in previous studies always represent an approximation of the clinical condition. Therefore, laboratory and clinical tests are always required to evaluate the agreement of the results with the model. As can be seen in Table 1, of the studies conducted, fewer experimental tests were performed to validate the data obtained from FEM. A few experimental tests were performed based on ISO 14801,17 where the implant is placed in a metal fixator and not in the bone. Importantly, the exact level of stress that triggers biological responses such as bone resorption and remodeling is not fully known.6 Therefore, loading data obtained from finite element analysis must be compared with data from clinical studies. In vivo tests can be used to evaluate dental implants over time.
One of the methods used in engineering to validate the results of finite element analysis is the photoelastic test. In this method, by placing the dental implant in a transparent block made of resin, a load is applied, and light radiation creates a colored contour in the interface areas of the implant and the holder. By interpreting the colors and the relevant calculations, the amount and pattern of stress distribution can be obtained. This method has been used in only one study.57
Recently, another solution has been proposed to increase the long-term stability of dental implants based on the use of porous implants. By reducing the effects of the mismatch between bone and implant hardness, more adaptive biomechanical properties are created compared to conventional screws. The size, morphology, and porosity of the medium that cause bone ingrowth directly affect biological adaptability. This leads to an overall mechanical interlock, resulting in higher reliability of the bone-implant complex.96 In addition, more regular pores exhibited a higher elastic modulus than pores in a random arrangement.97 In addition, the porous structure within a certain range of porosity and pore size can create strong adhesions between bone and implants, resulting in better ossification.98
CONCLUSION
This study could be a starting point for a more detailed investigation of the fatigue life of dental implants. In general, finite element models have their limitations because the mechanical properties and nonlinear behavior of biological tissues cannot be accurately and exactly modeled. The evaluation of implant stability is difficult because it depends on several factors, such as the mechanical design and the topography and the geometry of the implant, bone quality, applied loads, and the implant-bone interface. Also, a common problem with fatigue testing is that it must be performed in multidimension, over a long period of time which makes it very costly. The use of porous implants can be an effective way to improve the bone-implant interaction and increase the biological adaptivity of dental implants. New methods such as geometry optimization based on sensitivity analysis and dental implant reliability can be used for optimal implant designs at a reasonable time and cost.
References
- 1.Chen LJ, He H, Li YM, Li T, Guo XP, Wang RF. Finite element analysis of stress at the implant-bone interface of dental implants with different structures. Trans Nonferrous Met Soc Chin. 2011;21:1602–1610. [Google Scholar]
- 2.Chang CL, Chen CS, Huang CH, Hsu ML. Finite element analysis of the dental implant using a topology optimization method. Med Eng Phys. 2012;34:999–1008. doi: 10.1016/j.medengphy.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Chang HS, Chen YC, Hsieh YD, Hsu ML. Stress distribution of two commercial dental implant systems: a three-dimensional finite element analysis. J Dent Sci. 2013;8:261–271. [Google Scholar]
- 4.Omori M, Sato Y, Kitagawa N, Shimura Y, Ito M. A biomechanical investigation of mandibular molar implants: reproducibility and validity of a finite element analysis model. Int J Implant Dent. 2015;1:10. doi: 10.1186/s40729-015-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu AY, Hsu JT, Chee W, Lin YT, Fuh LJ, Huang HL. Biomechanical evaluation of one-piece and two-piece small-diameter dental implants: in-vitro experimental and three-dimensional finite element analyses. J Formos Med Assoc. 2016;115:794–800. doi: 10.1016/j.jfma.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:319–338. doi: 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paracchini L, Barbieri C, Redaelli M, Di Croce D, Vincenzi C, Guarnieri R. Finite element analysis of a new dental implant design optimized for the desirable stress distribution in the surrounding bone region. Prosthesis. 2020;2:225–236. [Google Scholar]
- 8.Ueda N, Takayama Y, Yokoyama A. Minimization of dental implant diameter and length according to bone quality determined by finite element analysis and optimized calculation. J Prosthodont Res. 2017;61:324–332. doi: 10.1016/j.jpor.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Hussein MO. Stress-strain distribution at bone-implant interface of two splinted overdenture systems using 3D finite element analysis. J Adv Prosthodont. 2013;5:333–340. doi: 10.4047/jap.2013.5.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Zubaidi SM, Madfa AA, Mufadhal AA, Aldawla MA, Hameed OS, Yue X-G. Improvements in clinical durability from functional biomimetic metallic dental implants. Front Mater. 2020;7:106 [Google Scholar]
- 11.Marcián P, Wolff J, Horáčková L, Kaiser J, Zikmund T, Borák L. Micro finite element analysis of dental implants under different loading conditions. Comput Biol Med. 2018;96:157–165. doi: 10.1016/j.compbiomed.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira H, Brizuela Velasco A, Ríos-Santos JV, Sánchez Lasheras F, Lemos BF, Gil FJ, Carvalho A, Herrero-Climent M. Effect of different implant designs on strain and stress distribution under non-axial loading: a three-dimensional finite element analysis. Int J Environ Res Public Health. 2020;17:4738. doi: 10.3390/ijerph17134738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim T, See CW, Li X, Zhu D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng Reg. 2020;1:6–18. [Google Scholar]
- 14.Javed F, Romanos GE. The role of primary stability for successful immediate loading of dental implants. A literature review. J Dent. 2010;38:612–620. doi: 10.1016/j.jdent.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Hong HC, Chang Y, Pan YH. The stability of implant-abutment complex with different implant-abutment connection designs, review of literature. J Oral Maxillofac Surg. 2015;26:262–286. [Google Scholar]
- 16.Shemtov-Yona K, Rittel D. Fatigue of dental implants: facts and fallacies. Dent J (Basel) 2016;4:16. doi: 10.3390/dj4020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dentistry-implants-dynamic fatigue test for end osseous dental implants. Geneva; Switzerland: International Standards Organization (ISO); 2007. ISO 14801. [Google Scholar]
- 18.Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. J Prosthet Dent. 2001;85:585–598. doi: 10.1067/mpr.2001.115251. [DOI] [PubMed] [Google Scholar]
- 19.Steigenga JT, al-Shammari KF, Nociti FH, Misch CE, Wang HL. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003;12:306–317. doi: 10.1097/01.id.0000091140.76130.a1. [DOI] [PubMed] [Google Scholar]
- 20.Niroomand MR, Arabbeiki M. Effect of the dimensions of implant body and thread on bone resorption and stability in trapezoidal threaded dental implants: a sensitivity analysis and optimization. Comput Methods Biomech Biomed Engin. 2020;23:1005–1013. doi: 10.1080/10255842.2020.1782390. [DOI] [PubMed] [Google Scholar]
- 21.Jadhav L, Kapole S, Dhatrak P, Palange A. Design of experiments (DoE) based optimization of dental implants: a review. AIP Conf Proc. 2021;2358:1–13. [Google Scholar]
- 22.Bandyopadhyay A, Espana F, Balla VK, Bose S, Ohgami Y, Davies NM. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010;6:1640–1648. doi: 10.1016/j.actbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji F, Zhang C, Chen X. Structure optimization of porous dental implant based on 3D printing. IOP Conf Ser Mater Sci Eng. 2018;324:012060 [Google Scholar]
- 24.Kunavisarut C, Lang LA, Stoner BR, Felton DA. Finite element analysis on dental implant-supported prostheses without passive fit. J Prosthodont. 2002;11:30–40. doi: 10.1111/j.1532-849x.2002.00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Perriard J, Wiskott WA, Mellal A, Scherrer SS, Botsis J, Belser UC. Fatigue resistance of ITI implant-abutment connectors - a comparison of the standard cone with a novel internally keyed design. Clin Oral Implants Res. 2002;13:542–549. doi: 10.1034/j.1600-0501.2002.130515.x. [DOI] [PubMed] [Google Scholar]
- 26.Genna F. On the effects of cyclic transversal forces on osseointegrated dental implants: experimental and finite element shakedown analyses. Comput Methods Biomech Biomed Engin. 2003;6:141–152. doi: 10.1080/1025584031000091696. [DOI] [PubMed] [Google Scholar]
- 27.Kayabasi O, Yüzbasıoğlu E, Erzincanlı F. Static, dynamic and fatigue behaviors of dental implant using finite element method. Adv Eng Softw. 2006;37:649–658. [Google Scholar]
- 28.Wierszycki M, Kakol W, Lodygowski T. Fatigue algorithm for dental implant. Found Civ Environ Eng. 2006;7:363–380. [Google Scholar]
- 29.Yang J, Xiang HJ. A three-dimensional finite element study on the biomechanical behavior of an FGBM dental implant in surrounding bone. J Biomech. 2007;40:2377–2385. doi: 10.1016/j.jbiomech.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Kong L, Gu Z, Li T, Wu J, Hu K, Liu Y, Zhou H, Liu B. Biomechanical optimization of implant diameter and length for immediate loading: a nonlinear finite element analysis. Int J Prosthodont. 2009;22:607–615. [PubMed] [Google Scholar]
- 31.Hasan I, Heinemann F, Aitlahrach M, Bourauel C. Biomechanical finite element analysis of small diameter and short dental implant. Biomed Tech (Berl) 2010;55:341–350. doi: 10.1515/BMT.2010.049. [DOI] [PubMed] [Google Scholar]
- 32.Pérez M. Life prediction of different commercial dental implants as influence by uncertainties in their fatigue material properties and loading conditions. Comput Methods Programs Biomed. 2012;108:1277–1286. doi: 10.1016/j.cmpb.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Hasan I, Röger B, Heinemann F, Keilig L, Bourauel C. Influence of abutment design on the success of immediately loaded dental implants: experimental and numerical studies. Med Eng Phys. 2012;34:817–825. doi: 10.1016/j.medengphy.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Tsai YT, Wang KS, Woo JC. Fatigue life and reliability evaluation for dental implants based on computer simulation and limited test data. J Mech Eng Sci. 2012;227:554–564. [Google Scholar]
- 35.Lee WT, Koak JY, Lim YJ, Kim SK, Kwon HB, Kim MJ. Stress shielding and fatigue limits of poly-ether-etherketone dental implants. J Biomed Mater Res B Appl Biomater. 2012;100:1044–1052. doi: 10.1002/jbm.b.32669. [DOI] [PubMed] [Google Scholar]
- 36.Ali B, Chikh EBO, Meddah HM, Merdji A, Bouiadjra BAB. Effects of overloading in mastication on the mechanical behaviour of dental implants. Mater Des. 2013;47:210–217. [Google Scholar]
- 37.Covani U, Ricci M, Tonelli P, Barone A. An evaluation of new designs in implant-abutment connections: a finite element method assessment. Implant Dent. 2013;22:263–267. doi: 10.1097/ID.0b013e318292625f. [DOI] [PubMed] [Google Scholar]
- 38.Geringer A, Diebels S, Nothdurft FP. Influence of superstructure geometry on the mechanical behavior of zirconia implant abutments: a finite element analysis. Biomed Tech (Berl) 2014;59:501–506. doi: 10.1515/bmt-2013-0088. [DOI] [PubMed] [Google Scholar]
- 39.Ayllón JM, Navarro C, Vázquez J, Domínguez J. Fatigue life estimation in dental implants. Eng Fract Mech. 2014;123:34–43. [Google Scholar]
- 40.Bulaqi HA, Mousavi Mashhadi M, Safari H, Samandari MM, Geramipanah F. Effect of increased crown height on stress distribution in short dental implant components and their surrounding bone: A finite element analysis. J Prosthet Dent. 2015;113:548–557. doi: 10.1016/j.prosdent.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez BA, Paterno A, Sousa EAC, De Oliveira Freitas JP, Foschini CR. Fatigue analysis of dental prostheses by finite element method (FEM) IMECE. 2015;3 [Google Scholar]
- 42.Hernandez-Rodriguez MAL, Contreras-Hernandez GR, Juarez-Hernandez A, Beltran-Ramirez B, Garcia-Sanchez E. Failure analysis in a dental implant. Eng Fail Anal. 2015;57:236–242. [Google Scholar]
- 43.Prados-Privado M, Prados-Frutos JC, Manchón Á, Rojo R, Felice P, Bea JA. Dental implants fatigue as a possible failure of implantologic treatment: the importance of randomness in fatigue behaviour. Biomed Res Int. 2015;2015:825402. doi: 10.1155/2015/825402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toyoshima Y, Wakabayashi N. Load limit of mini-implants with reduced abutment height based on fatigue fracture resistance: experimental and finite element study. Int J Oral Maxillofac Implants. 2015;30:e10–e16. doi: 10.11607/jomi.3653. [DOI] [PubMed] [Google Scholar]
- 45.Bicudo P, Reis J, Deus AM, Reisa L, Vaza MF. Mechanical behaviour of dental implants. Procedia Struct Integr. 2016;1:26–33. [Google Scholar]
- 46.Szajek K, Wierszycki M. Numerical verification of two-component dental implant in the context of fatigue life for various load cases. Acta Bioeng Biomech. 2016;18:103–113. [PubMed] [Google Scholar]
- 47.Bicudo P, Reis J, Deus AM, Reisa L, Vaza MF. Performance evaluation of dental implants: An experimental and numerical simulation study. Theor Appl Fract Mech. 2016;85:74–83. [Google Scholar]
- 48.Prados-Privado M, Prados-Frutos JC, Calvo-Guirado JL, Bea JA. A random fatigue of mechanize titanium abutment studied with Markoff chain and stochastic finite element formulation. Comput Methods Biomech Biomed Engin. 2016;19:1583–1591. doi: 10.1080/10255842.2016.1170124. [DOI] [PubMed] [Google Scholar]
- 49.Wu T, Fan H, Ma R, Chen H, Li Z, Yu H. Effect of lubricant on the reliability of dental implant abutment screw joint: An in vitro laboratory and three-dimension finite element analysis. Mater Sci Eng C Mater Biol Appl. 2017;75:297–304. doi: 10.1016/j.msec.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 50.Geramizadeh M, Katoozian H, Amid R, Kadkhodazadeh M. Finite element analysis of dental implants with and without microthreads under static and dynamic loading. J Long Term Eff Med Implants. 2017;27:25–35. doi: 10.1615/JLongTermEffMedImplants.2017020007. [DOI] [PubMed] [Google Scholar]
- 51.Bordin D, Bergamo ETP, Fardin VP, Coelho PG, Bonfante EA. Fracture strength and probability of survival of narrow and extra-narrow dental implants after fatigue testing: In vitro and in silico analysis. J Mech Behav Biomed Mater. 2017;71:244–249. doi: 10.1016/j.jmbbm.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Prados-Privado M, Bea JA, Rojo R, Gehrke SA, Calvo-Guirado JL, Prados-Frutos JC. A new model to study fatigue in dental implants based on probabilistic finite elements and cumulative damage model. Appl Bionics Biomech. 2017;2017:3726361. doi: 10.1155/2017/3726361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de la Rosa Castolo G, Guevara Perez SV, Arnoux PJ, Badih L, Bonnet F, Behr M. Mechanical strength and fracture point of a dental implant under certification conditions: a numerical approach by finite element analysis. J Prosthet Dent. 2018;119:611–619. doi: 10.1016/j.prosdent.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi S, Yamanishi Y, Machado LS, Matsumoto S, Tovar N, Coelho PG, Thompson VP, Imazato S. In vitro fatigue tests and in silico finite element analysis of dental implants with different fixture/abutment joint types using computer-aided design models. J Prosthodont Res. 2018;62:24–30. doi: 10.1016/j.jpor.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Cinel S, Celik E, Sagirkaya E, Sahin O. Experimental evaluation of stress distribution with narrow diameter implants: A finite element analysis. J Prosthet Dent. 2018;119:417–425. doi: 10.1016/j.prosdent.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Cervino G, Romeo U, Lauritano F, Bramanti E, Fiorillo L, D’Amico C, Milone D, Laino L, Campolongo F, Rapisarda S, Cicciù M. FEM and von Mises analysis of OSSTEM® dental implant structural components: evaluation of different direction dynamic loads. Open Dent J. 2018;12:219–229. doi: 10.2174/1874210601812010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geramizadeh M, Katoozian H, Amid R, Kadkhodazadeh M. Three-dimensional optimization and sensitivity analysis of dental implant thread parameters using finite element analysis. J Korean Assoc Oral Maxillofac Surg. 2018;44:59–65. doi: 10.5125/jkaoms.2018.44.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topkaya H, Kaman MO. Effect of dental implant dimensions on fatigue behaviour: a numerical approach. Uludağ Üniversitesi Mühendislik Fakültesi Dergisi. 2018;23:249–260. [Google Scholar]
- 59.Abasolo M, Aguirrebeitia J, Vallejo J, Albizuri J, Coria I. Influence of vertical misfit in screw fatigue behavior in dental implants: a three-dimensional finite element approach. Proc Inst Mech Eng H. 2018;232:1117–1128. doi: 10.1177/0954411918806325. [DOI] [PubMed] [Google Scholar]
- 60.Duan Y, Gonzalez JA, Kulkarni PA, Nagy WW, Griggs JA. Fatigue lifetime prediction of a reduced-diameter dental implant system: numerical and experimental study. Dent Mater. 2018;34:1299–1309. doi: 10.1016/j.dental.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayata F, Yildiz C. The mechanical behaviors of various dental implant materials under fatigue. Adv Mater Sci Eng. 2018:5047319 [Google Scholar]
- 62.Prados-Privado M, Sergio G, Rojo R, Prados-Frutos JC. Complete mechanical characterization of an external hexagonal implant connection: in vitro study, 3D FEM and probabilistic fatigue. Med Biol Eng Comput. 2018;56:2233–2244. doi: 10.1007/s11517-018-1846-8. [DOI] [PubMed] [Google Scholar]
- 63.Lee H, Park S, Noh G. Biomechanical analysis of 4 types of short dental implants in a resorbed mandible. J Prosthet Dent. 2019;121:659–670. doi: 10.1016/j.prosdent.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Chen X, Zhang C, Feng W, Zhang P, Chen Y, Huang J, Luo Y, Chen J. Studies on the performance of selective laser melting porous dental implant by finite element model simulation, fatigue testing and in vivo experiments. Proc Inst Mech Eng H. 2019;233:170–180. doi: 10.1177/0954411918816114. [DOI] [PubMed] [Google Scholar]
- 65.Bataineh K, Al Janaideh M. Effect of different biocompatible implant materials on the mechanical stability of dental implants under excessive oblique load. Clin Implant Dent Relat Res. 2019;21:1206–1217. doi: 10.1111/cid.12858. [DOI] [PubMed] [Google Scholar]
- 66.Manea A, Baciut G, Baciut M, Pop D, Comsa DS, Buiga O, Trombitas V, Colosi H, Mitre I, Bordea R, Manole M, Lenghel M, Bran S, Onisor F. New dental implant with 3d shock absorbers and tooth-like mobility-prototype development, finite element analysis (FEA), and mechanical testing. Materials (Basel) 2019;12:3444. doi: 10.3390/ma12203444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prados-Privado M, Ivorra C, Martínez-Martínez C, Gehrke SA, Calvo-Guirado JL, Prados-Frutos JC. A finite element analysis of the fatigue behavior and risk of failure of immediate provisional implants. Metals. 2019;9:535 [Google Scholar]
- 68.Zhang X, Mao J, Zhou Y, Ji F, Chen X. Study on statics and fatigue analysis of dental implants in the descending process of alveolar bone level. Proc Inst Mech Eng H. 2020;234:843–853. doi: 10.1177/0954411920926080. [DOI] [PubMed] [Google Scholar]
- 69.Sahin SC. Static and dynamic stress analysis of standard- and narrow-diameter implants: a 3D finite element analysis. Int J Oral Maxillofac Implants. 2020;35:e58–e68. doi: 10.11607/jomi.8037. [DOI] [PubMed] [Google Scholar]
- 70.Nokar S, Jalali H, Nozari F, Arshad M. Finite element analysis of stress in bone and abutment-implant interface under static and cyclic loadings. Front Dent. 2020;17:1–8. doi: 10.18502/fid.v17i21.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armentia M, Abasolo M, Coria I, Albizuri J. Fatigue design of dental implant assemblies: a nominal stress approach. Metals. 2020;10:744 [Google Scholar]
- 72.Bayata F, Yildiz C. The effects of design parameters on mechanical failure of Ti-6Al-4V implants using finite element analysis. Eng Fail Anal. 2020;110:104445 [Google Scholar]
- 73.Lee H, Jo M, Noh G. Biomechanical effects of dental implant diameter, connection type, and bone density on microgap formation and fatigue failure: A finite element analysis. Comput Methods Programs Biomed. 2021;200:105863. doi: 10.1016/j.cmpb.2020.105863. [DOI] [PubMed] [Google Scholar]
- 74.Bergamo ETP, Yamaguchi S, Coelho PG, Lopes ACO, Lee C, Bonfante G, Benalcázar Jalkh EB, de Araujo-Júnior ENS, Bonfante EA. Survival of implant-supported resin-matrix ceramic crowns: In silico and fatigue analyses. Dent Mater. 2021;37:523–533. doi: 10.1016/j.dental.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Nicholson JW. Titanium alloys for dental implants: a review. Prosthesis. 2020;2:100–116. [Google Scholar]
- 76.Koike M, Lockwood PE, Wataha JC, Okabe T. Initial cytotoxicity of novel titanium alloys. J Biomed Mater Res B Appl Biomater. 2007;83:327–331. doi: 10.1002/jbm.b.30799. [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Chen S, Tsoi JKH, Matinlinna JP. Binary titanium alloys as dental implant materials-a review. Regen Biomater. 2017;4:315–323. doi: 10.1093/rb/rbx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saini M, Singh Y, Arora P, Arora V, Jain K. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3:52–57. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Augat P, Schorlemmer S. The role of cortical bone and its microstructure in bone strength. Age Ageing. 2006;35:27–31. doi: 10.1093/ageing/afl081. [DOI] [PubMed] [Google Scholar]
- 80.Ovesy M, Voumard B, Zysset P. A nonlinear homogenized finite element analysis of the primary stability of the bone-implant interface. Biomech Model Mechanobiol. 2018;17:1471–1480. doi: 10.1007/s10237-018-1038-3. [DOI] [PubMed] [Google Scholar]
- 81.Gao X, Fraulob M, Haïat G. Biomechanical behaviours of the bone-implant interface: a review. J R Soc Interface. 2019;16:20190259. doi: 10.1098/rsif.2019.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallucci GO, Hamilton A, Zhou W, Buser D, Chen S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin Oral Implants Res. 2018;29:106–134. doi: 10.1111/clr.13276. [DOI] [PubMed] [Google Scholar]
- 83.Gapski R, Wang HL, Mascarenhas P, Lang NP. Critical review of immediate implant loading. Clin Oral Implants Res. 2003;14:515–527. doi: 10.1034/j.1600-0501.2003.00950.x. [DOI] [PubMed] [Google Scholar]
- 84.Colomina LE. Immediate loading of implant-fixed mandibular prostheses: a prospective 18-month follow-up clinical study-preliminary report. Implant Dent. 2001;10:23–29. doi: 10.1097/00008505-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Horiuchi K, Uchida H, Yamamoto K, Sugimura M. Immediate loading of Brånemark system implants following placement in edentulous patients: a clinical report. Int J Oral Maxillofac Implants. 2000;15:824–830. [PubMed] [Google Scholar]
- 86.Longva A, Löschner F, Kugelstadt T, Fernandez-Fernandez JA, Bender J. Higher-order finite elements for embedded simulation. ACM Trans Graph. 2020;39:1–14. [Google Scholar]
- 87.Lopez B, Arruda MRT, Almeida-Fernandez L, Castro L, Silvestre N, Correia JR. Assessment of mesh dependency in the numerical simulation of compact tension tests for orthotropic materials. Composite Part C: Open Access. 2020;1:100006 [Google Scholar]
- 88.Flanagan D. Osseous remodeling around dental implants. J Oral Implantol. 2019;45:239–246. doi: 10.1563/aaid-joi-D-18-00130. [DOI] [PubMed] [Google Scholar]
- 89.Haase K, Rouhi G. Prediction of stress shielding around an orthopedic screw: using stress and strain energy density as mechanical stimuli. Comput Biol Med. 2013;43:1748–1757. doi: 10.1016/j.compbiomed.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 90.Tortorelli DA, Michaleris P. Design sensitivity analysis: overview and review. Inverse Probl Sci Eng. 1994;1:71–105. [Google Scholar]
- 91.Blázquez-Hinarejos M, Ayuso-Montero R, Jané-Salas E, López-López J. Influence of surface modified dental implant abutments on connective tissue attachment: a systematic review. Arch Oral Biol. 2017;80:185–192. doi: 10.1016/j.archoralbio.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 92.Ding X, Zhu XH, Liao SH, Zhang XH, Chen H. Implant-bone interface stress distribution in immediately loaded implants of different diameters: a three-dimensional finite element analysis. J Prosthodont. 2009;18:393–402. doi: 10.1111/j.1532-849X.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 93.Raffa ML, Nguyen VH, Hernigou P, Flouzat-Lachaniette CH, Haiat G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J Orthop Res. 2021;39:1174–1183. doi: 10.1002/jor.24840. [DOI] [PubMed] [Google Scholar]
- 94.Kim JE, Shin JM, Oh SO, Yi WJ, Heo MS, Lee SS, Choi SC, Huh KH. The three-dimensional microstructure of trabecular bone: analysis of site-specific variation in the human jaw bone. Imaging Sci Dent. 2013;43:227–233. doi: 10.5624/isd.2013.43.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Martinis M, Sirufo MM, Polsinelli M, Placidi G, Di Silvestre D, Ginaldi L. Gender differences in osteoporosis: a single-center observational study. World J Mens Health. 2021;39:750–759. doi: 10.5534/wjmh.200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carpenter RD, Klosterhoff BS, Torstrick FB, Foley KT, Burkus JK, Lee CSD, Gall K, Guldberg RE, Safranski DL. Effect of porous orthopaedic implant material and structure on load sharing with simulated bone ingrowth: a finite element analysis comparing titanium and PEEK. J Mech Behav Biomed Mater. 2018;80:68–76. doi: 10.1016/j.jmbbm.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aguilar C, Arancibia M, Alfonso I, Sancy M, Tello K, Salinas V, De Las Cuevas F. Influence of porosity on the elastic modulus of Ti-Zr-Ta-Nb foams with a low Nb content. Metals. 2019;9:176 [Google Scholar]
- 98.Abbasi N, Hamlet S, Love RM, Nam-Trung N. Porous scaffolds for bone regeneration. J Sci Adv Mater Dev. 2020;5:1–9. [Google Scholar]