Abstract
Background:
Evidence indicates that cytokines are associated with cognitive deficits in schizophrenia; however, the underlying brain–behaviour mechanisms remain unclear. We hypothesized that aberrations in brain structural connectivity mediate the cytokine effect in schizophrenia.
Methods:
In this study, we recruited patients with first-episode schizophrenia (n = 75, average illness duration 12.3 months, average medication period 0.6 days) and healthy controls (n = 44) of both sexes. We first conducted whole-blood RNA sequencing to detect differentially expressed genes. We also explored transcriptomic data on the dorsal lateral prefrontal cortices (dlPFC) retrieved from the CommonMind Consortium for gene functional clustering; we measured plasma transforming growth factor β1 (TGF-β1) levels by enzyme-linked immunosorbent assay; we acquired high-resolution T1-weighted MRI data on cortical thickness MRI; and we assessed cognitive function using the validated Chinese version of the MATRICS Consensus Cognitive Battery. We compared these parameters in patients with schizophrenia and controls, and analyzed their associations.
Results:
Patients with schizophrenia had higher TGF-β1 at both the mRNA level (log2 fold change = 0.24; adjusted p = 0.026) and the protein level (12.85 ± 6.01 μg/mL v. 8.46 ± 5.15 μg/mL, adjusted p < 0.001) compared to controls. Genes coexpressed with TGFB1 in the dlPFC were less abundant in patients with schizophrenia compared to healthy controls. In patients with schizophrenia, TGF-β1 protein levels were inversely correlated with cortical thickness, especially of the lateral occipital cortex (r = −0.47, adjusted p = 0.001), and with the MATRICS Consensus Cognitive Battery visual learning and memory domain (r = −0.50, adjusted p < 0.001). We found a complete mediation effect of the thickness of the lateral occipital cortex on the negative relationship between TGF-β1 and visual cognition (p < 0.05).
Limitations:
We did not explore the effect of other blood cytokines on neurocognitive performance and cortical thickness. Participants from the CommonMind Consortium did not all have first-episode schizophrenia and they were not all antipsychotic-naive, so we could not exclude an effect of antipsychotics on TGF-β1 signalling in the dlPFC. The sample size and cross-sectional design of our study were additional limitations.
Conclusion:
These findings highlighted an association between upregulated blood levels of TGF-β1 and impairments in brain structure and function in schizophrenia.
Introduction
Schizophrenia is a complex neurodevelopmental disorder. Accumulating evidence suggests that dysregulation of the immune system may be involved in the pathogenesis of schizophrenia, with changes occurring in both the peripheral blood and brain tissue.1–3 Multiple recent studies have observed dysregulation of inflammatory markers in the prefrontal cortex4–6 and blood of people with schizophrenia,7–9 which may converge to (and propagate through) microglia-dependent neuroinflammation in association with other neuropathological hallmarks in the brains of people with schizophrenia.10 Most cytokines are synthesized and secreted by astrocytes and microglia in the brain. Transforming growth factor β1 (TGF-β1) is one such brain cytokine.11,12 The expression of TGF-β1 is regulated by neurons in an activity-dependent manner,13 and its receptors are expressed on multiple brain cell types.14 TGF-β1 signalling is critical for a range of biological processes during brain development and regeneration via the regulation of neurotransmission, synaptic remodelling and brain connectivity.11,15–18 Accumulated evidence suggests that TGF-β1 may also be involved in disease onset and cognitive deficits in schizophrenia.11,19 Notably, in a previous genetic study by the Psychiatric Genomics Consortium, TGF-β signalling was included among the enriched biological pathways for schizophrenia.20
Although immune genes including TGFB1 have been implicated in schizophrenia, how they cause cerebral abnormalities and behavioural deficits in schizophrenia remains elusive.21,22 We have recently observed that immune-related factors negatively affect cognition in association with brain structural deficits in patients with first-episode23–25 and treatment-resistant schizophrenia.26 In the present study, we hypothesized that changes in blood immune genes may be linked to alterations in cerebral cortical structures (such as cortical thinning) and their connectivity, which in turn may lead to cognitive decline in schizophrenia. We used RNA sequencing to examine the blood transcriptomes of patients with first-episode schizophrenia and healthy controls, and to identify differentially expressed genes from which TGF-β1 family members were surfaced during pathway analysis (Appendix 1, Tables S1 and S2, available at www.jpn.ca/lookup/doi/10.1503/jpn.210121/tab-related-content). We further explored cortical transcriptomic data from the CommonMind Consortium to identify biological pathways associated with TGF-β1 the brains of people with schizophrenia. Moreover, we measured participants’ plasma TGF-β1 and cerebral cortical thickness and evaluated the relationships between TGF-β1, cognition and cerebral cortical thickness.
Methods
Participants
We recruited patients with first-episode schizophrenia (n = 105) from the Beijing Huilongguan Hospital. Patients met the diagnostic criteria for schizophrenia according to the Structured Clinical Interview for DSM-IV, and confirmed by 2 psychiatrists. Inclusion criteria were as follows: 18–45 years old and Han Chinese; an illness duration of 3 years or less (less than 1 year on average); and taking antipsychotic medication for less than 2 weeks at the time of the blood draw and cognitive testing.
We recruited age- and sex-matched healthy controls (n = 52) from the local community by advertisement.
Among the participants, 75 patients with schizophrenia (average illness duration 12.3 months, average medication period 0.6 days) and 44 healthy controls had mRNA samples that passed quality control for RNA sequencing and were selected for further clinical evaluation. Among the patients with schizophrenia, 29 had been hospitalized for first-episode psychosis and were not taking antipsychotic medication at the time of admission and blood sample collection; 41 had received a first- or second-generation antipsychotic for less than 3 days; and 5 had been medicated for longer (8 d on average). Antipsychotic medication doses were calculated as chlorpromazine equivalents.
We collected complete medical histories of the healthy controls and conducted physical examinations for all participants to identify those with chronic medical or psychiatric conditions. Exclusion criteria for potential healthy controls were as follows: previous diagnosis of an Axis I psychiatric disorder; substance abuse or dependence in the previous 6 months; a history of autoimmune disorders or other significant medical conditions; or receiving anti-inflammatory medications.
All participants provided written informed consent. The study was approved by the institutional ethics committee of Beijing Huilongguan Hospital.
Psychological assessment
We assessed psychopathology in the patients with schizophrenia using the Positive and Negative Syndrome Scale,27 administered independently by 2 psychiatrists on the day blood samples were collected. The intraclass correlation coefficient for the 2 raters was greater than 0.80.
Neurocognition assessment
We assessed cognitive function in both patients and controls using the validated Chinese version of the MATRICS Consensus Cognitive Battery (MCCB).28 The MCCB contains assessments of 7 cognitive domains: speed of processing, attention and vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. Initial scores were transformed to Chinese-normalized t scores. We calculated domain scores and total scores for the MCCB.
Whole blood collection and RNA sequencing
We collected whole blood samples for RNA sequencing (5 mL) between 7 and 9 am after overnight fasting using PAXgene blood RNA tubes (Applied Biosystems). Tubes were shaken vigorously for at least 10 seconds after sampling and immediately stored at −80°C. We extracted total RNA using the Mag-MAX for Stabilized Blood Tubes RNA Isolation Kit (Applied Biosystems), following the manufacturer’s instructions.
We quantified RNA and assessed it for purity using NanoDrop spectrophotometry and optical density ratios of 260/280 nm and 260/230 nm. The RNA samples (1 μg per sample) were immediately sent to the Beijing Genomics Institution) for mRNA sequencing (after globin mRNA removal) on the BGIseq-500 platform. The Beijing Genomics Institution confirmed quality controls on RNA samples (RNA integrity number/RNA quality number ≥ 7.0; 28S/18S ≥ 1.0). They collected clean data of at least 4 Gb (20 M clean reads) per sample.
RNA sequencing data analysis
We first conducted quality control checks of the fastq files and used bam files to construct an mRNA sequencing count table. We conducted gene expression analysis using DESeq2 on the NetworkAnalyst platform.29 Data with a variance percentile rank of less than 15% and counts of less than 4 were filtered out. Counts per million reads were transformed and normalized, and fold changes (log2FC) were calculated for differentially expressed genes. Those with adjusted p values of less than 0.05 were further analyzed in Panther and DAVID for their biological pathways and molecular function using Gene Ontology (GO) terms (Appendix 1, Tables S1 to S4).
CommonMind Consortium brain RNA sequencing data
We obtained brain tissues for the CommonMind Consortium study from the following brain bank collections: the Mount Sinai National Institutes of Health Brain and Tissue Repository; the University of Pennsylvania Alzheimer’s Disease Research Center; the University of Pittsburgh NeuroBioBank and Brain and Tissue Repositories; and the National Institute of Mental Health Human Brain Collection Core.30 We downloaded gene coexpression data from the CommonMind Consortium portal (https://synapse.org). To compare mRNA expression levels of TGF-β1 in the dorsolateral prefrontal cortex (dlPFC) between patients with schizophrenia (n = 258) and controls (n = 279), we also explored the SZDB database (www.szdb.org),31,32 which uses RNA sequencing data published by the CommonMind Consortium.30
Functional clustering analyses of the TGF-β1 coexpression gene network in the dlPFC
We constructed coexpression networks using weighted gene coexpression network analysis. Details of the statistical analysis are provided by the CommonMind Consortium.30 We further analyzed TGFB1-coexpressed genes in the schizophrenia and control samples for functional clustering and protein–protein interactions using String version 11 (https://string-db.org). We set the clustering stringency threshold at 0.4 and the k-means value at 3. We used GO biological pathways for functional annotation analysis, and we corrected enrichment scores using false discovery rate (Appendix 1, Tables S5 to S8).
Measurement of plasma TGF-β1
We collected blood samples for measurement of plasma TGF-β1 (5 mL) between 7 and 9 am after overnight fasting and centrifuged them at 5000 rpm for 10 minutes. Plasma was immediately separated and stored at −80 °C until assayed.
We determined plasma TGF-β1 concentrations using an enzyme-linked immunosorbent assay kit (Biosensis) and following the manufacturer’s instructions. Intra- and interassay variation coefficients were approximately 5%. TGF-β1 concentrations were expressed in micrograms per millilitre.
MRI acquisition and processing
We acquired brain structural MRI data using a Siemens Prisma 3.0 T MRI scanner with a 64-channel head coil, located at the Beijing Huilongguan Hospital Magnetic Resonance Scanner Centre. We used foam pads to minimize head motion. We used a sagittal, 3-dimensional, magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence to collect anatomic data. Scan parameters were as follows: echo time 2.98 ms, inversion time 1100 ms, repetition time 2530 ms, flip angle 7°, field of view 256 × 224 mm, matrix size 256 × 224, thickness/gap = 1/0 mm. After the scan, 2 radiologists evaluated image quality, and if there were any artifacts, images were collected again.
Following the ENIGMA protocol (http://enigma.ini.usc.edu/), we used FreeSurfer33,34 software for data processing (http://surfer.nmr.mgh.harvard.edu). Based on the Desikan–Killiany atlas, FreeSurfer software automatically divides the occipital cortex of both hemispheres into 4 parts: the cuneiform leaf, the lateral occipital leaf, the lingual gyrus and the peridistal sulcus. After imputing the corresponding internal instructions, we obtained anatomic parameters for cortical thickness. We measured the thickness of 34 cerebral cortical features from each hemisphere and calculated averages.
Statistical analysis
We then examined data distributions using the Shapiro–Wilk test. We compared demographic and clinical variables between groups using analysis of variance for continuous variables and χ2 tests for categorical variables. For TGF-β1 levels and MCCB total score and subscores, we used analysis of covariance, using age, sex, education and body mass index as covariates. We evaluated the relationships among TGF-β1, MCCB scores and cortical structures using Pearson partial correlation and linear regression, controlled by age, sex, education and body mass index. We performed statistical analyses in SPSS version 23.0 (IBM). For the MCCB and MRI data, we corrected p values for multiple comparisons using the Bonferroni method (e.g., adjusted p values = p values × 7 for MCCB data and p values × 34 for MRI data).
We conducted principal component analysis for MRI features to separate the patient and control groups and identify clustering trends with 95% confidence intervals; we conducted heatmap analysis on correlational data using online tools (www.bioinformatics.com.cn). Data are presented as mean ± standard deviation. All statistical tests were 2-tailed, and an adjusted p < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
Participants’ demographic and clinical characteristics are listed in Table 1. Patients and controls were not statistically different in terms of age, sex or years of education, but patients had a lower mean body mass index than controls (20.90 ± 2.91 kg/m2 v. 23.35 ± 3.67 kg/m2; p = 0.001). We used these variables as covariates in subsequent analyses. Whole blood cell counts did not differ between patients and controls (data not shown).
Table 1.
Demographic characteristics of patients with early-stage schizophrenia and healthy controls
| Characteristic | Patients with schizophrenia (n = 75)* | Healthy controls (n = 44)* | t or χ2 | p value |
|---|---|---|---|---|
| Sex, M/F | 34/41 | 24/20 | 1.20 | 0.27 |
| Age, yr | 28.61 ± 6.90 | 30.07 ± 7.49 | −1.07 | 0.29 |
| Education, yr | 13.01 ± 3.77 | 14.28 ± 2.18 | −2.02 | 0.05 |
| BMI, kg/m2 | 20.90 ± 2.91 | 23.35 ± 3.67 | −3.92 | 0.001 |
| Illness duration, mo | 12.25 ± 17.85 | NA | NA | NA |
| Positive and Negative Syndrome Scale | ||||
| Positive symptom score | 21.79 ± 4.91 | NA | NA | NA |
| Negative symptom score | 17.56 ± 6.00 | NA | NA | NA |
| General psychopathological symptom score | 36.92 ± 6.98 | NA | NA | NA |
| Total score | 76.26 ± 12.81 | NA | NA | NA |
NA = not applicable.
Data are mean ± standard deviation unless otherwise indicated.
The MCCB total and domain index scores are summarized in Table 2. Compared with controls, patients with schizophrenia had worse cognitive performance on all MCCB indices (all adjusted p < 0.001).
Table 2.
MCCB scores and TGF-β1 levels
| Characteristic | Patients with schizophrenia* | Healthy controls* | t or χ2 | p value |
|---|---|---|---|---|
| MCCB domain score | ||||
| Speed of processing | 42.86 ± 13.06 | 59.00 ± 6.93 | −8.36 | < 0.001 |
| Attention/vigilance | 39.19 ± 13.33 | 58.05 ± 11.58 | −7.33 | < 0.001 |
| Working memory | 46.65 ± 10.94 | 58.62 ± 5.48 | −7.50 | < 0.001 |
| Verbal learning and memory | 46.74 ± 14.06 | 55.76 ± 7.36 | −4.36 | < 0.001 |
| Visual learning and memory | 44.20 ± 12.47 | 56.21 ± 6.17 | −6.66 | < 0.001 |
| Reasoning and problem solving | 46.14 ± 12.92 | 57.84 ± 5.04 | −6.66 | < 0.001 |
| Social cognition | 44.55 ± 13.44 | 54.47 ± 9.73 | −4.01 | < 0.001 |
| Composite score | 44.83 ± 9.86 | 59.83 ± 4.40 | −10.52 | < 0.001 |
| TGF-β1, μg/mL | 12.85 ± 6.01 | 8.46 ± 5.15 | 3.94 | < 0.001 |
MCCB = MATRICS Consensus Cognitive Battery; TGF-β1 = transforming growth factor-β1.
Data are mean ± standard deviation.
TGFB1 mRNA higher in the whole blood of patients than controls
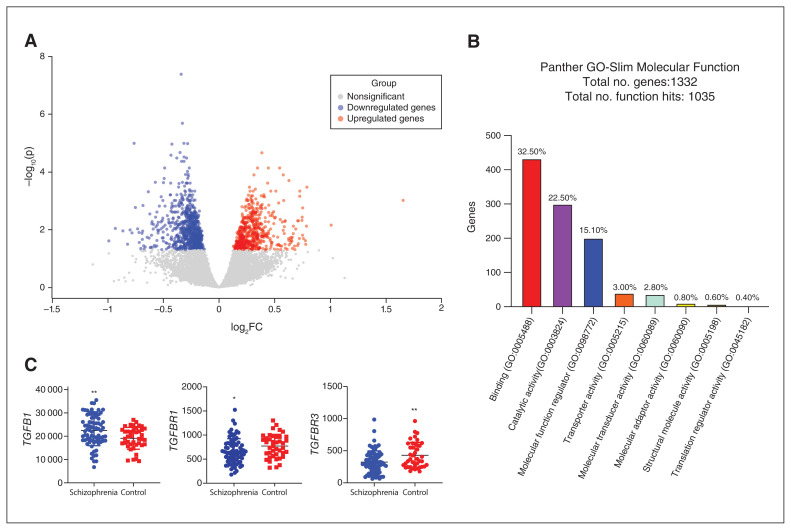
A total of 1332 differentially expressed genes were detected by the RNA sequencing of blood samples from patients with schizophrenia and controls; of those, 567 were downregulated and 755 were upregulated (adjusted p < 0.05; Figure 1A and Appendix 1, Table S1). Panther pathway analysis of molecular function showed that these differentially expressed genes mainly regulated molecular binding (32.50% hit) and catalytic activity (22.50% hit; Figure 1B). The top 3 rankings for Panther biological process were cellular process (GO:0009987, 53.8% hit), metabolic process (GO:0008152, 34.3% hit) and biological regulation (GO:0065007, 33.8% hit). Gene clusters enriched in the GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses done in DAVID are listed in Appendix 1, Table S2. Notably, functional clustering analysis of these differentially expressed genes by DAVID molecular function high-lighted the top-ranking genes involved in TGF-β receptor binding (Appendix 1, Table S2). Of these, TGFB1 was significantly higher in patients with schizophrenia than in controls (log2FC = 0.24, adjusted p = 0.026), and its receptors TGFBR1 (log2FC= −0.24, adjusted p = 0.019) and TGFBR3 (log2FC = −0.45, adjusted p = 0.004) were both lower in patients with schizophrenia than in controls (Figure 1C and Appendix 1, Table S3 and S4).
Figure 1.
RNA sequencing analysis in the peripheral blood of patients with schizophrenia and healthy controls. (A) Volcano plot representing differentially expressed genes among patients with schizophrenia and healthy controls. Only differentially expressed genes with adjusted p < 0.05 are shown in colour. (B) Functional enrichment analysis of the list of differentially expressed genes in the Panther database. Enriched Gene Ontology terms (GO-Slim Molecular Function) are presented. (C) RNA sequencing counts for TGFB1 and its receptors TGFBR1 and TGFBR3 in patients with schizophrenia and controls. TGFB1 was upregulated and TGFBR1 and TGFBR3 were downregulated in patients with schizophrenia compared to controls. One-way analysis of covariance. *p < 0.05; **p < 0.01; log2FC = log2 fold change.
TGFB1-coexpressed genes in the dlPFC involved in different biological pathways in patients than controls
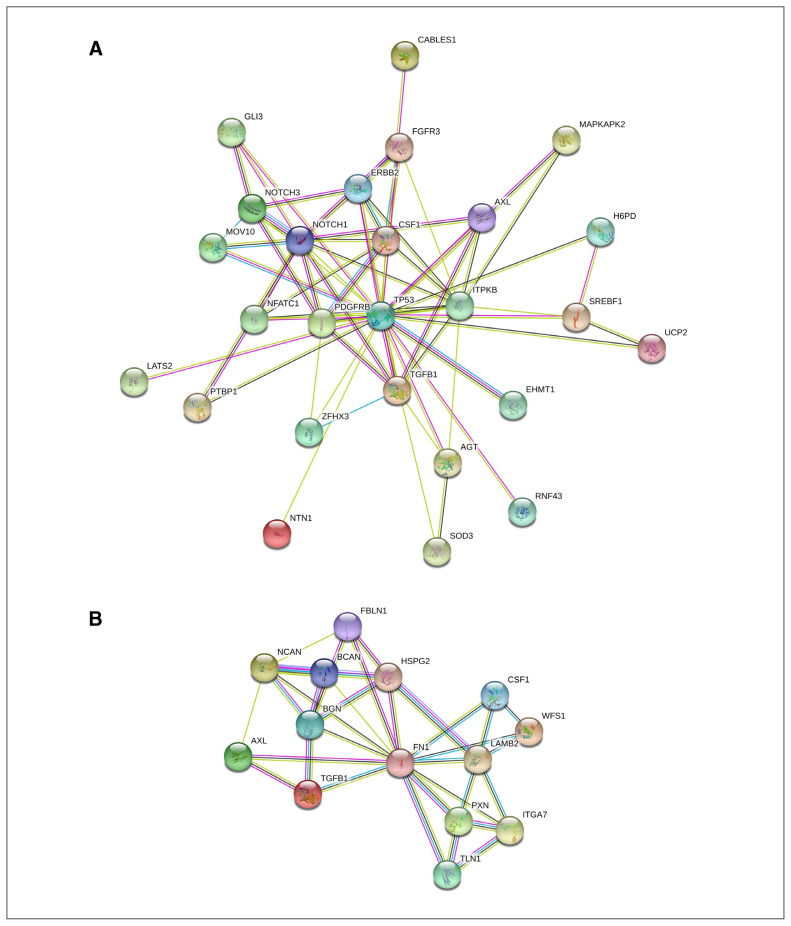
To better understand the function of TGF-β1 in the human brain, we further explored RNA sequencing data from the dlPFC of deceased people with schizophrenia (n = 258) and control participants (n = 279) provided by the CommonMind Consortium.30 We retrieved a weighted gene coexpression network analysis list containing TGFB1-coexpressed genes and conducted gene functional clustering and protein–protein interaction analyses to compare the characteristics of TGF-β1- associated gene networks between patients with schizophrenia and controls. The analyses showed that TGFB1 was coexpressed with a different and smaller number of genes in patients with schizophrenia than in controls (Appendix 2, Table S5), constituting a less prominent interaction network (Figure 2). These TGF-β1-associated genes were also enriched in different biological pathways, showing that the top-ranked GOs in the control group were involved in the regulation of receptor signalling and developmental process (Appendix 1, Tables S6 and S8), but the top-ranked GO in patients with schizophrenia was for extracellular matrix organization (Appendix 1, Tables S7 and S8).
Figure 2.
TGF-β1–interacting protein networks in the dorsolateral prefrontal cortex of patients with schizophrenia and healthy controls. Protein–protein interaction network of TGFB1 coexpressed genes in (A) healthy controls and (B) patients with schizophrenia. We analyzed TGFB1-coexpressed genes retrieved from the CommonMind Consortium portal using String (version 11). Clustered molecules are represented as nodes tagged with their gene symbols. The interacting strength between 2 nodes is represented by the thickness of the line.
TGF-β1 plasma levels higher in patients versus controls
As shown in Table 2, TGF-β1 levels were significantly upregulated in the plasma of patients with schizophrenia relative to controls (12.85 ± 6.01 μg/mL v. 8.46 ± 5.15 μg/mL; p < 0.001). We found no significant difference in TGF-β1 levels between women and men with schizophrenia. To evaluate the potential effect of antipsychotic medication on TGF-β1 levels, we analyzed the regression between chlorpromazine dosages and TGF-β1 levels and found no significant correlation. We also compared TGF-β1 levels between drug-naive and medicated patients, as well as between patients with fewer than 3 days of antipsychotic medication and those with a longer period of treatment, but we found no group differences in these separate analyses (data not shown).
We also explored dlPFC transcriptomic data from the CommonMind Consortium,30 but brain mRNA levels of TGF-β1 did not differ between the schizophrenia and control groups this cohort (adjusted p > 0.05; Appendix 1, Figure S1).
Reduced thickness of the lateral occipital cortex and positive association with visual cognition in schizophrenia
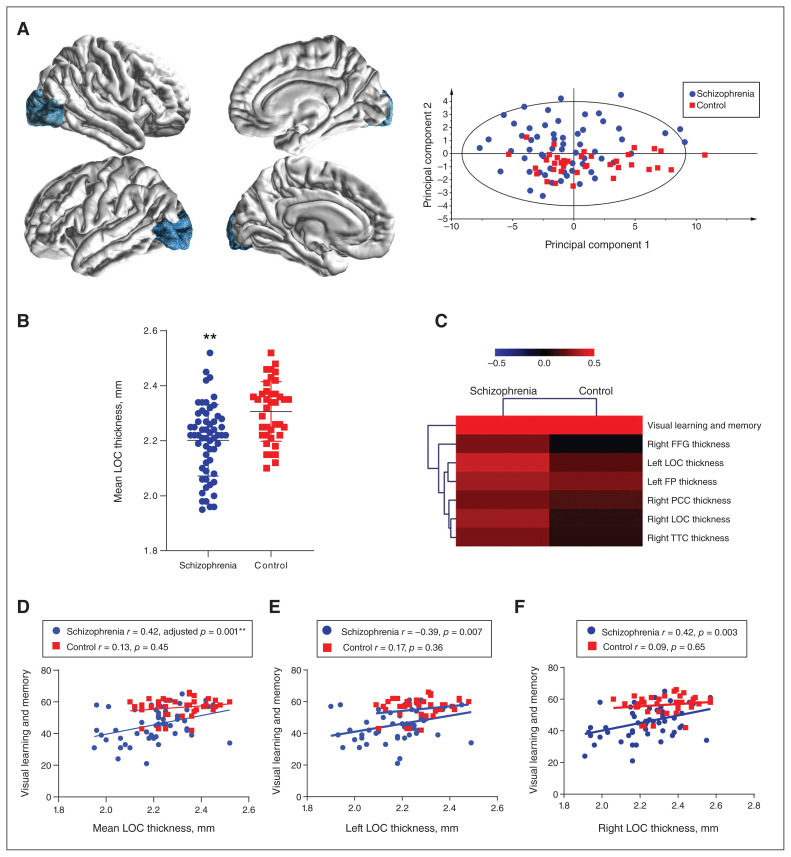
We next compared cerebral cortical structures in 34 brain regions measured by MRI, including the visual cortex and associated areas (Brodmann areas 17 to 19) of patients with schizophrenia and healthy controls. As summarized in Table 3 and Figure 3A, multiple cortical regions showed significantly reduced thickness in patients with schizophrenia compared with controls. The reduction was most striking in the mean thickness of the lateral occipital cortex (LOC; adjusted p < 0.001, Table 3 and Figure 3B). Both the left and right LOC were thinner in patients with schizophrenia compared to controls (Appendix 1, Table S9). Other cortical regions related to visual cognition and working memory, such as the inferior parietal cortex and the supramarginal gyrus, were also decreased in patients versus controls (Table 3 and Appendix 1, Table S9). However, we found no change in cortical thickness of the dlPFC (adjusted p > 0.05).
Table 3.
Average thickness of the visual cortex and associated cortical areas
| Region | Thickness, mm* | t | Adjusted p value | |
|---|---|---|---|---|
|
| ||||
| Patients with schizophrenia | Healthy controls | |||
| Lateral occipital cortex | 2.20 ± 0.13 | 2.31 ± 0.11 | −4.12 | < 0.001 |
| Inferior parietal cortex | 2.49 ± 0.15 | 2.62 ± 0.10 | −4.64 | < 0.001 |
| Supramarginal gyrus | 2.57 ± 0.13 | 2.69 ± 0.11 | −4.74 | < 0.001 |
Data are mean ± standard deviation.
Figure 3.
Positive associations between visual brain cortical structures and cognitive performance in patients with schizophrenia. (A) An exemplar graph showing the location and shape of the LOC and a principal component analysis scatter plot showing the separation of the schizophrenia and healthy control groups by all 34 cerebral cortical MRI measures. In the imaging graphs, blue represents a decrease in cortical thickness. (B) The mean LOC thickness was significantly lower in patients with schizophrenia than in controls (2.20 ± 0.13 mm v. 2.31 ± 0.11 mm, adjusted p < 0.001). Data are expressed as mean ± standard deviation. (C) A heatmap of MRI features, which bears significant correlations with MATRICS Consensus Cognitive Battery visual scores in the schizophrenia group, shows hierarchical clustering of the features. (D–F) Visual learning and memory scores were positively associated with mean LOC thickness (adjusted p. = 0.001), left LOC thickness (unadjusted p = 0.007) and right LOC thickness (unadjusted p. = 0.003) in patients with schizophrenia but not controls. **Adjusted p < 0.01. FFG = fusiform gyrus; FP = frontal pole; LOC = lateral occipital cortex; PCC = pericalcarine cortex; TTC = transverse temporal cortex.
We also studied the relationship between visual cognition and brain cortical structures and found that it was positively correlated with multiple cortical regions in patients with schizophrenia, but not in healthy controls (Figure 3C). Specifically, the mean thickness of the LOC (r = 0.42, adjusted p = 0.001; Figure 3D), as well as the thickness of the left LOC (r = −0.39, unadjusted p = 0.007; Figure 3E) and the right LOC (r = 0.42, unadjusted p = 0.003; Figure 3F) were most significantly related to visual learning and memory scores in patients with schizophrenia. These relationships were non-significant in healthy controls.
Scores on the Positive and Negative Syndrome Scale were not significantly correlated with MCCB scores, TGF-β1 levels or LOC thickness in patients with schizophrenia.
Negative association between TGF-β1 and visual cognition in schizophrenia
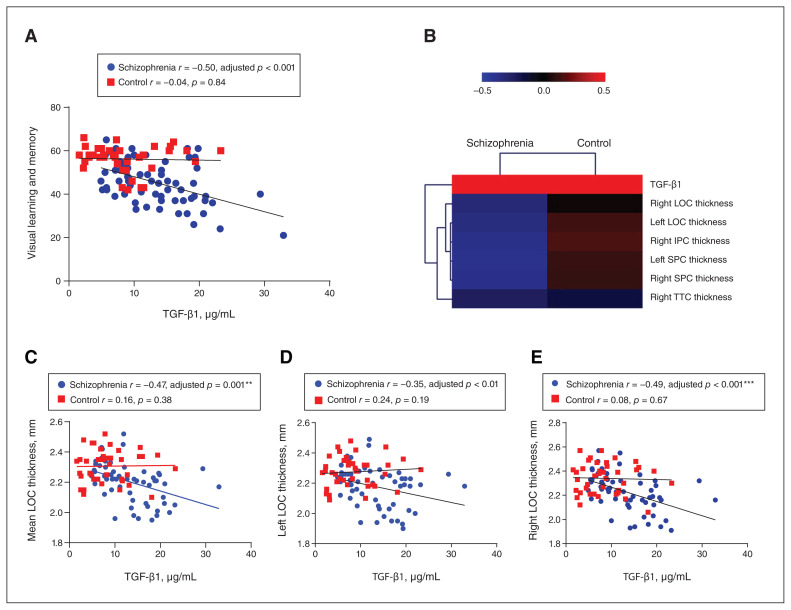
We further observed associations between plasma TGF-β1 levels and MCCB total score and subscores. TGF-β1 plasma levels were negatively associated with MCCB total scores (r = −0.48, adjusted p = 0.001) in the schizophrenia group alone and in the schizophrenia and healthy control groups combined (r = −0.40, adjusted p < 0.001). After Bonferroni correction for multiple comparisons, the most impressive correlations were for visual learning and memory (r = −0.50, adjusted p < 0.001; Figure 4A) and for social cognition (r = −0.41, adjusted p = 0.004) in patients with schizophrenia (Appendix 1, Figure S2). These correlations remained significant when we evaluated the subgroup of patients with fewer than 3 days of anti-psychotic treatment for visual learning and memory (r = −0.45, adjusted p = 0.002, other data not shown). Other measurements of cognition, including speed of processing, attention and vigilance, working memory, verbal learning, reasoning and problem solving were nonsignificant in patients with schizophrenia. In the control group, TGF-β1 levels were not correlated with MCCB total or subscale scores.
Figure 4.
Negative correlations of TGF-β1 with visual cognitive performance and LOC thickness in schizophrenia. (A) TGF-β1 levels were negatively associated with visual learning and memory scores in patients with schizophrenia, but not in healthy controls. (B) A heatmap of TGF-β1, which bears significant correlations with MRI features, shows hierarchical clustering of the features. (C–E) TGF-β1 levels were negatively correlated with the mean thickness of the LOC and with the thickness of the left LOC (unadjusted p = 0.01) and right LOC in patients with schizophrenia, but not healthy controls. **Adjusted p < 0.01; ***adjusted p < 0.001. IPC = inferior parietal cortex; LOC = lateral occipital cortex; SPC = superior parietal cortex; TGF-β1 = transforming growth factor β1; TTC = transverse temporal cortex.
Negative association between TGF-β1 and LOC thickness in schizophrenia
When we explored the associations between plasma TGF-β1 levels and brain cortical structures, we found multiple negative associations in patients with schizophrenia, but healthy controls showed only trends of positive correlations in most cases (Figure 4B). In particular, TGF-β1 levels showed the strongest negative correlations with the mean thickness of the LOC (r = −0.47, adjusted p = 0.001; Figure 4C), as well as the thickness of the left LOC (r = −0.35, unadjusted p < 0.01; Figure 4D) and the right LOC (r = −0.49, adjusted p < 0.001; Figure 4E). These findings remained significant when we analyzed a subgroup of patients with fewer than 3 days of antipsychotic treatment (r = −0.45, adjusted p = 0.002; r = −0.34, adjusted p = 0.02; r = −0.46, adjusted p = 0.001).
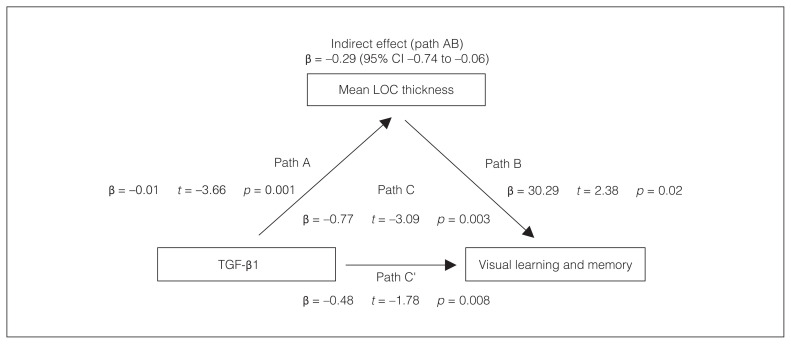
Effect of TGF-β1 on visual cognition mediated via LOC thickness
We assessed whether the LOC mediated the association between TGF-β1 and MCCB visual cognition score. The negative effect of TGF-β1 on the visual learning and memory score was fully mediated by mean LOC thickness in patients with schizophrenia (path AB: β = −0.29 [95% confidence interval −0.74 to −0.06], p < 0.05; Figure 5). In healthy controls, none of the mediation analyses showed significance.
Figure 5.
The LOC mediates the negative effect of TGF-β1 on visual cognitive performance in schizophrenia. We performed mediation analyses to examine the effect of the LOC on the correlation between TGF-β1 and visual learning and memory in patients with schizophrenia. Path A represents a direct relationship between TGF-β1 and mean LOC thickness; path C’ represents a direct relationship between TGF-β1 and visual learning and memory; path B represents a direct relationship between mean LOC thickness and visual learning and memory; and path C represents a relationship between TGF-β1 and visual learning and memory, considering mean LOC thickness. Path AB reflects an indirect mediating effect of LOC thickness between TGF-β1 and visual learning and memory; p < 0.05. CI = confidence interval; LOC = lateral occipital cortex; TGF-β1 = transforming growth factor β1
Discussion
The present study showed the following main findings for patients with first-episode schizophrenia: increases in blood TGF-β1 mRNA levels and plasma TGF-β1 protein levels; decreases in the thicknesses of multiple cortical regions, particularly the LOC, and reduced neurocognitive performance; negative association between plasma TGF-β1 levels and both visual cognition and LOC thickness, as well as a positive association between LOC thickness and visual cognition; a full mediation effect of the LOC on the relationship between TGF-β1 and visual cognition.
Cortical thinning or grey matter loss in schizophrenia may be caused by multiple mechanisms, including neuronal maldevelopment and neurodegeneration. To the best of our knowledge, this study is the first to assess the correlations between the thickness of the LOC and other associated cortical regions and TGF-β1 levels in patients with schizophrenia and healthy controls. We showed that increased TGF-β1 levels in patients with schizophrenia were associated with significant LOC thinning, leading to worsened performance on a visual learning and memory scale.
Our finding of higher blood cell mRNA and plasma protein levels of TGF-β1 in patients with schizophrenia was in line with most previous studies.19,35–37 A meta-analysis by Miller and colleagues has also confirmed a significant increase in TGF-β1 in the blood during acute exacerbations of schizophrenia, which were normalized with antipsychotic treatment. 38 Upregulation of TGF-β1 in peripheral blood mononuclear cells was also correlated with positive symptoms in drug-free patients.39 However, some other studies have found no change or a reduction in TGF-β1 in the cerebrospinal fluid21 or blood of patients with schizophrenia.22,40,41 The inconsistency in these results is likely influenced by sample size, illness process, antipsychotic medications or a combination of these.
The upregulation of blood TGF-β1 may imply a biased immune function in patients with schizophrenia. Interestingly, we observed opposite changes in TGFB1 (upregulation) and its receptors TGFBR1 and TGFBR3 (downregulation) in our blood RNA sequencing data. The cause of such an opposite relationship is intriguing but may reflect a compensational feedback mechanism to balance pro- and anti-inflammatory cytokine production via TGF-β1-receptor-mediated signalling in target cells. However, we did not observe a broad change in pro- or anti-inflammatory cytokines in blood RNA sequencing, except for a minor population (2.3% hit) representing the immune system process (GO_BP:0002376) that involved some chemokine and TNF superfamily members (Appendix 1, Table S1 and S2), the significance of which warrants further investigation.
TGF-β1 levels in the peripheral blood and brain may be aligned, because it has been reported that TGF-β1 can pass the blood–brain barrier via P-glycoprotein efflux transporters expressed on brain capillary endothelial cells,42,43 and TGF-β1 concentrations in the blood and the cerebrospinal fluid were parallel with each other.21 Furthermore, studies have shown that TGF-β1 plays a very important role in blood–brain barrier function and angiogenesis. For instance, the formation and maturation of the blood–brain barrier relies on interactions between endothelial cells and astrocytes via TGF-β–mediated activation of integrin avβ8.44 Given that changes in blood–brain barrier structure and permeability have been identified in psychiatric disorders, allowing peripheral inflammatory effects on the brain,45 this may also explain the influence of peripheral TGF-β1 in schizophrenia.
In the brain, aberrant TGF-β1 expression affects the formation of cellular and subcellular structures as well as their remodelling. TGF-β is a potent inducer of astrocytes derived from radial glia and is one of the principal factors for microglial development. Astrocytes may sustain a noninflammatory microglial phenotype through TGF-β, and astrocytic TGF-β overdrive pushes microglia to dysfunctional synaptic pruning.11 At the embryonic stage, inactivation of TGF-β signalling impairs neuronal development as well.15–17
To further investigate the underlying mechanisms of TGF-β1 function in the brains of people with schizophrenia, we analyzed TGF-β1 coexpressed genes in postmortem dlPFC samples of people with schizophrenia using the CommonMind Consortium data resource.30 The frontal cortex — particularly the prefrontal cortex — is known to orchestrate the hierarchical processing of visual and spatial working memory, including visual recognition memory and scene-specific memory for objects, as well as the associated emotional memory.46,47 We observed that although TGF-β1 mRNA levels in the dlPFC did not differ between patients with schizophrenia and healthy controls, coexpressed genes that mainly regulate cell surface receptor signalling and developmental processes were compromised in patients with schizophrenia. The schizophrenia-specific biological process was driven instead toward extracellular matrix remodelling, which may be related to changes in neuropil and synaptic remodelling in schizophrenia, as described below. Nevertheless, the overlapping coexpressed genes AXL and CSF1, conserved in both healthy controls and patients with schizophrenia, are inflammatory response regulators, implying that brain developmental or metabolic processes could intercalate with glia-mediated inflammatory processes, and these may all involve TGF-β1. However, the dlPFC gene expression data from the CommonMind Consortium may have been affected by antipsychotic treatment or other medications.
We have also provided evidence that peripheral TGF-β1 may negatively affect cognitive performance in patients with schizophrenia, particularly visual learning and memory, implying that increased circulating levels of TGF-β1 may affect synaptic connectivity and cognition in the brains of patients with schizophrenia via the aforementioned biological pathways. Indeed, evidence has shown that increased TGF-β1–modulated glutamate receptor expression in the hippocampus15 and neuronal C1q expression and synaptic pruning in the developing visual system18 promoted radial glia–astrocyte differentiation in the cerebral cortex in mice.48 Mice lacking
TGF-β signalling in dopaminergic neurons were hyperactive and exhibited deficits in reversal learning and memory formation. 16,17 However, other studies have revealed that over-expressed TGF-β1 can cause aberrant synaptic function49–51 and reduced neurogenesis,48 likely detrimental for cognition. A study also showed that people with schizophrenia who displayed poor verbal fluency and decreased volumes in the Broca area had elevated peripheral cytokine levels, including TGF-β1.52
The above evidence indicates plural potencies of TGF-β1 in steering the cognitive impairment that is a core component of the symptomatology of schizophrenia and accounts for related poor functional outcomes. Cytokines, either synthesized locally by brain cells or infiltrated from the blood, affect cognition depending on their type, concentration and spatiotemporal expression patterns.53 On one hand, our findings reinforce the hypothesis of inflammatory influence on the pathogenesis of schizophrenia. On the other hand, they reveal the complicated functions of pleiotropic cytokines (in this case anti-inflammatory TGF-β1) in regulating synaptic function and cognition.11
Our results also suggest that TGF-β1 levels in the peripheral blood may be a useful biomarker for detecting cortical or cognitive impairment in patients with early-stage schizophrenia. We observed a significant decrease in the thickness of the LOC, along with several other related regions, and this decrease had a significant positive association with visual learning and memory scores, indicating that these regions are probably the substrate of deficits in early visual processing. Consistent with this finding, altered structure and function of the occipital cortex has been implicated in schizophrenia by several other imaging studies.54–56 For instance, an MRI study55 suggested that patients with schizophrenia had relatively intact grey matter volume in the primary visual area but reduced bilateral grey matter volume in vision-associated areas. Excessive grey matter loss in the occipital lobe, accompanied by severe perceptual impairments after visual stimulus in some patients with schizophrenia, has also been reported.56 Earlier studies of postmortem tissues in the primary visual cortex in schizophrenia found reduced cortical thickness but an abnormally high density of small neurons, implicating reduced neuropils (axonal and dendritic tissues) as a likely cause of cortical thinning.57–59 Such reduction in neuropils could be related to a decrease in synaptic density, long thought to be a contributor to the pathophysiology of schizophrenia.60 However, another study has suggested decreased thickness in the occipital cortex was less consistent in people with schizophrenia, and that the temporal and frontal cortices were more consistently different.61
Limitations
There were 4 major limitations in our study that should be acknowledged. First, although TGF-β1 levels were associated with LOC thickness and visual cognition, these observations were only correlational, not causative. Second, we did not explore the effect of other cytokines on neurocognitive performance and cortical thickness. The role of cytokines in the pathogenesis of schizophrenia requires better elucidation in future studies. Third, participants from the CommonMind Consortium did not all have first-episode psychosis, and they were not all antipsychotic-naive; therefore, we cannot exclude the possible effect of antipsychotics on TGF-β1 signalling in the dlPFC data. Finally, the heterogeneity of region-specific cortical thinning in patients with schizophrenia may have caused wide confidence intervals as a result of our small sample size and decreased the precision of results.
Conclusion
Overall, our results provide deeper insight into the potential role of TGF-β1 in influencing cortical structure (especially the occipital lobe) and cognition in patients with schizophrenia. This better understanding may provide new clues for the prevention or treatment of cognitive deficits in patients with schizophrenia.
Supplementary Material
Acknowledgements
This work was supported by the National Natural Science Foundation of China grants 81761128021 and 81771452, the National Institute of Health grants R01MH112180, the Estonian Research Council-European Union Regional Developmental Fund Mobilitas Pluss Program No. MOBTT77 and the Beijing Municipal Administration of Hospitals Incubating Program, PX2021074. We would like to thank all the study participants and staff.
Footnotes
Competing interests: No competing interests declared.
Contributors: S. Pan, Y. Li, Y. Cui, F. Yang, S. Tan, Z. Wang, L.E. Hong, Y. Tan and L. Tian designed the study. Y. Zhou, J. Tong, J. Huang, W. Feng and S. Chen acquired the data, which L. Yan, F. Xuan and B. Tian analyzed. S. Pan, Y. Zhou, L. Yan, F. Xuan, J. Tong, J. Huang and S. Chen wrote the article, which Y. Li, W. Feng, Y. Cui, F. Yang, S. Tan, Z. Wang, B. Tian, L.E. Hong, Y. Tan and L. Tian reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry 2020;25:761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mondelli V, Vernon AC, Turkheimer F, et al. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017;4:563–72. [DOI] [PubMed] [Google Scholar]
- 3.Fraguas D, Diaz-Caneja CM, Ayora M, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull 2019;45:742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado-Torres L, Burke EE, Peterson A, et al. Regional heterogeneity in gene expression, regulation, and coherence in the frontal cortex and hippocampus across development and schizophrenia. Neuron 2019;103:203–216.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum R, Jaffe AE, Chen Q, et al. Investigating the neuroimmunogenic architecture of schizophrenia. Mol Psychiatry 2018;23: 1251–60. [DOI] [PubMed] [Google Scholar]
- 6.Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013;18:206–14. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Sun J, Chen J, et al. RNA-Seq analysis implicates dysregulation of the immune system in schizophrenia. BMC Genomics 2012; 13(Suppl 8):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sainz J, Mata I, Barrera J, et al. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry 2013;18:1056–7. [DOI] [PubMed] [Google Scholar]
- 9.Wu JQ, Green MJ, Gardiner EJ, et al. Altered neural signaling and immune pathways in peripheral blood mononuclear cells of schizophrenia patients with cognitive impairment: a transcriptome analysis. Brain Behav Immun 2016;53:194–206. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry 2010;15: 326–36. [DOI] [PubMed] [Google Scholar]
- 11.Corsi-Zuelli F, Deakin B. Impaired regulatory T cell control of astroglial overdrive and microglial pruning in schizophrenia. Neurosci Biobehav Rev 2021;125:637–53. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Sloan SA, Clarke LE, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 2016;89:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon VY, Choi S, Park M. Growth factors in synaptic function. Front Synaptic Neurosci 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Canabal A. Potential neuroprotective role of transforming growth factor beta1 (TGFbeta1) in the brain. Int J Neurosci 2015; 125:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Bae JJ, Xiang YY, Martinez-Canabal A, et al. Increased transforming growth factor-beta1 modulates glutamate receptor expression in the hippocampus. Int J Physiol Pathophysiol Pharmacol 2011;3: 9–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Luo SX, Timbang L, Kim JI, et al. TGF-beta signaling in dopaminergic neurons regulates dendritic growth, excitatory-inhibitory synaptic balance, and reversal learning. Cell Rep 2016;17:3233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkhipov VI, Pershina EV, Levin SG. Deficiency of transforming growth factor-beta signaling disrupts memory processes in rats. Neuroreport 2018;29:353–5. [DOI] [PubMed] [Google Scholar]
- 18.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 2013;16:1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Frydecka D, Misiak B, Pawlak-Adamska E, et al. Sex differences in TGFB-beta signaling with respect to age of onset and cognitive functioning in schizophrenia. Neuropsychiatr Dis Treat 2015;11: 575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 2015;18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vawter MP, Dillon-Carter O, Issa F, et al. Transforming growth factors beta 1 and beta 2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology 1997;16:83–7. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Lv P, Li F, et al. Association of peripheral cytokine levels with cerebral structural abnormalities in schizophrenia. Brain Res 2019;1724:146463. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YF, Huang JC, Zhang P, et al. Choroid plexus enlargement and allostatic load in schizophrenia. Schizophr Bull 2020;46:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Huang J, Zhang P, et al. Allostatic load effects on cortical and cognitive deficits in essentially normotensive, normoweight patients with schizophrenia. Schizophr Bull 2021;47:1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong J, Huang J, Luo X, et al. Elevated serum anti-NMDA receptor antibody levels in first-episode patients with schizophrenia. Brain Behav Immun 2019;81:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Zhou Y, Huang J, et al. N-methyl-D-aspartate receptor antibody and white matter deficits in schizophrenia treatment-resistance. Schizophr Bull 2021;47:1463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell M, Milstein R, Beam-Goulet J, et al. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale. Reliability, comparability, and predictive validity. J Nerv Ment Dis 1992;180: 723–8. [DOI] [PubMed] [Google Scholar]
- 28.August SM, Kiwanuka JN, McMahon RP, et al. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res 2012;134:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou G, Soufan O, Ewald J, et al. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res 2019;47:W234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fromer M, Roussos P, Sieberts SK, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 2016;19:1442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Li X, Liu J, et al. SZDB2.0: an updated comprehensive resource for schizophrenia research. Hum Genet 2020;139:1285–97. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Yao YG, Luo XJ. SZDB: A database for schizophrenia genetic research. Schizophr Bull 2017;43:459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischl B. FreeSurfer. Neuroimage 2012;62:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- 35.Amoli MM, Khatami F, Arzaghi SM, et al. Over-expression of TGF-beta1 gene in medication free schizophrenia. Psychoneuroendocrinology 2019;99:265–70. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Zhang Q, Li N, et al. Plasma levels of Th17-related cytokines and complement C3 correlated with aggressive behavior in patients with schizophrenia. Psychiatry Res 2016;246:700–6. [DOI] [PubMed] [Google Scholar]
- 37.Kim YK, Myint AM, Lee BH, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:1129–34. [DOI] [PubMed] [Google Scholar]
- 38.Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011;70:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobis J, Rykaczewska-Czerwinska M, Swietochowska E, et al. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep 2015;67: 353–9. [DOI] [PubMed] [Google Scholar]
- 40.Lin CC, Chang CM, Chang PY, et al. Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gung Med J 2011;34:375–81. [PubMed] [Google Scholar]
- 41.Hong W, Zhao M, Li H, et al. Higher plasma S100B concentrations in schizophrenia patients, and dependently associated with inflammatory markers. Sci Rep 2016;6:27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides 2007;28:1317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C, Hawkins KE, Dore S, et al. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 2019;316:C135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diniz LP, Matias I, Siqueira M, et al. Astrocytes and the TGF-beta1 pathway in the healthy and diseased brain: a double-edged sword. Mol Neurobiol 2019;56:4653–79. [DOI] [PubMed] [Google Scholar]
- 45.Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry 2018;5:79–92. [DOI] [PubMed] [Google Scholar]
- 46.Khan ZU, Martin-Montanez E, Baxter MG. Visual perception and memory systems: from cortex to medial temporal lobe. Cell Mol Life Sci 2011;68:1737–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proverbio AM, De Benedetto F. Auditory enhancement of visual memory encoding is driven by emotional content of the auditory material and mediated by superior frontal cortex. Biol Psychol 2018;132:164–75. [DOI] [PubMed] [Google Scholar]
- 48.Stipursky J, Francis D, Dezonne RS, et al. TGF-beta1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 2014;8:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chin J, Angers A, Cleary LJ, et al. TGF-beta1 in Aplysia: role in long-term changes in the excitability of sensory neurons and distribution of TbetaR-II-like immunoreactivity. Learn Mem 1999;6:317–30. [PMC free article] [PubMed] [Google Scholar]
- 50.Chin J, Liu RY, Cleary LJ, et al. TGF-beta1-induced long-term changes in neuronal excitability in aplysia sensory neurons depend on MAPK. J Neurophysiol 2006;95:3286–90. [DOI] [PubMed] [Google Scholar]
- 51.Feng Z, Ko CP. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J Neurosci 2008;28:9599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fillman SG, Weickert TW, Lenroot RK, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry 2016;21:1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kogan S, Ospina LH, Kimhy D. Inflammation in individuals with schizophrenia — implications for neurocognition and daily function. Brain Behav Immun 2018;74:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tohid H, Faizan M, Faizan U. Alterations of the occipital lobe in schizophrenia. Neurosciences (Riyadh) 2015;20:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onitsuka T, McCarley RW, Kuroki N, et al. Occipital lobe gray matter volume in male patients with chronic schizophrenia: a quantitative MRI study. Schizophr Res 2007;92:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitelman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry 2007;19: 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995;52:805–18, discussion 819–20. [DOI] [PubMed] [Google Scholar]
- 58.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 1998;55:215–24. [DOI] [PubMed] [Google Scholar]
- 59.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999;45: 17–25. [DOI] [PubMed] [Google Scholar]
- 60.Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016;530: 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Erp TGM, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry 2018;84:644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.