Abstract
Background:
Mechanical ventilation is an important component of patient critical care, but it adds expense to an already high-cost setting. This study evaluates the cost-utility of 2 modes of ventilation: proportional-assist ventilation with load-adjustable gain factors (PAV+ mode) versus pressure-support ventilation (PSV).
Methods:
We adapted a published Markov model to the Canadian hospital-payer perspective with a 1-year time horizon. The patient population modelled includes all patients receiving invasive mechanical ventilation who have completed the acute phase of ventilatory support and have entered the recovery phase. Clinical and cost inputs were informed by a structured literature review, with the comparative effectiveness of PAV+ mode estimated via pragmatic meta-analysis. Primary outcomes of interest were costs, quality-adjusted life years (QALYs) and the (incremental) cost per QALY for patients receiving mechanical ventilation. Results were reported in 2017 Canadian dollars. We conducted probabilistic and scenario analyses to assess model uncertainty.
Results:
Over 1 year, PSV had costs of $50 951 and accrued 0.25 QALYs. Use of PAV+ mode was associated with care costs of $43 309 and 0.29 QALYs. Compared to PSV, PAV+ mode was considered likely to be cost-effective, having lower costs (−$7642) and increased QALYs (+0.04) after 1 year. In cost-effectiveness acceptability analysis, 100% of simulations would be cost-effective at a willingness-to-pay threshold of $50 000 per QALY gained.
Interpretation:
Use of PAV+ mode is expected to benefit patient care in the intensive care unit (ICU) and be a cost-effective alternative to PSV in the Canadian setting. Canadian hospital payers may therefore consider how best to optimally deliver mechanical ventilation in the ICU as they expand ICU capacity.
Clinicians have a medical and fiscal responsibility to choose wisely in spending health care dollars, and to optimize patient access to hospital resources and flow through the hospital from admission to discharge. This requires discerning which treatments are most likely to be clinically effective and most cost-effective for patient-important outcomes. As hospitals look to increase intensive care unit (ICU) capacity during the global COVID-19 pandemic, health economic models provide a means of modelling the economic and clinical impact of a change in intervention before hospitals commit to implementation.
Mechanical ventilation is an important component of patient critical care, with 33% of patients in Canadian ICUs in 2013–2014 requiring invasive ventilation.1 In Ontario alone, about 125 000 patients required mechanical ventilation in ICUs between 2006 and 2012, which amounted to around 570 000 days of ventilation.2 Since the onset of the COVID-19 pandemic, awareness of mechanical ventilation has increased, with ventilator support becoming an essential component of care.3 A 2020 global review of published studies showed that 29%–90% of patients with COVID-19 admitted to ICUs received invasive mechanical ventilation.4 With COVID-19’s disrupting the availability of critical care resources,5 including mechanical ventilation, and straining hospital finances owing to such factors as cancellation of elective operations,6 there is an increasing need for strategies to optimize use of health care resources.
Mechanical ventilation can be delivered via a variety of modes. Constant-pressure ventilation, in the form of pressure-support ventilation (PSV), is the most common mode of ventilator support after the acute phase of critical illness.2 It works by delivering airflow until a target pressure is reached, which is then held constant for a duration that is influenced by the patient’s respiratory system mechanics. Since the ventilator support is independent of the patient’s work of breathing, patient–ventilator interaction with PSV can be suboptimal,7 and overassistance may promote respiratory muscle weakness and asynchrony between patient and ventilator. Patient–ventilator asynchrony has been associated with increased requirements for tracheostomy,8 longer duration of mechanical ventilation9 and higher ICU mortality rates.9,10
To improve patient–ventilator interaction, adaptive modes of ventilation were developed whereby the volume or pressure of air is modulated within each breath.11 One such adaptive mode is proportional-assist ventilation with load-adjustable gain factors (PAV+ mode). This allows for measurement and control of the level of respiratory muscle work to ensure it remains in an optimal range,12–14 and helps improve the coupling of patient and ventilator inspiratory and expiratory times.15 Some studies suggest a clinical benefit of PAV+ mode over PSV;15–17 however, it is unknown whether increasing the use of PAV+ mode in Canadian ICUs would result in resource use savings and be cost-effective.
In this study, we synthesized available evidence to explore the cost-utility of PAV+ mode versus PSV from the Canadian hospital-payer perspective.
Methods
Study design
We performed a cost-utility analysis in line with guidance from the International Society for Pharmacoeconomics and Outcomes Research on good practice and research ethics.18–21
Setting and population
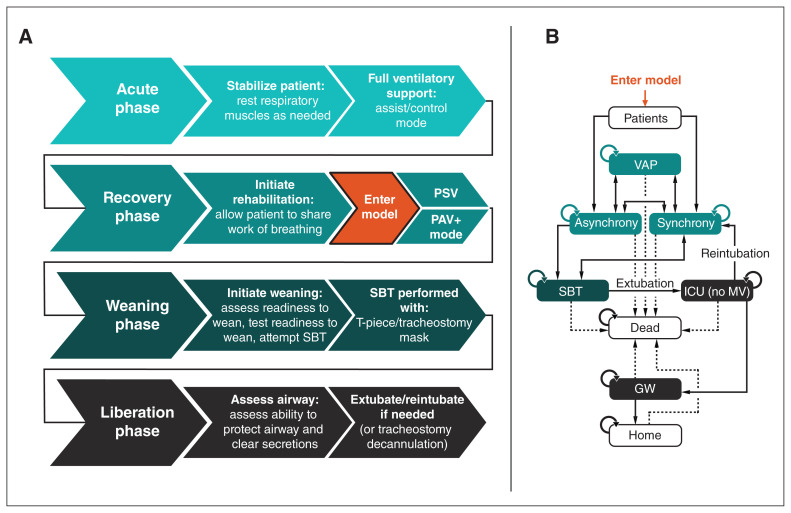
The setting for this analysis is a Canadian ICU using mechanical ventilation. The model includes all patients receiving invasive mechanical ventilation who have completed the acute phase of ventilatory support and have entered the recovery phase (Figure 1).
Figure 1:
Structure of adapted Markov cohort cost-utility model. The clinical stages of mechanical ventilation (MV) are shown in (A). The model begins once the patient has completed the acute phase of ventilatory support and enters the recovery phase. The model is shown in (B). Patients receiving MV are either synchronous or asynchronous with the ventilator. Those who are synchronous can become asynchronous and vice versa. Patients receiving MV are at risk for ventilator-associated pneumonia (VAP). From MV, patients undergo a spontaneous breathing trial (SBT), which, if successful, results in liberation (extubation and removal from invasive MV). After liberation, patients are transferred to lower-acuity care (general ward [GW]) and later discharged home. If there is patient compromise after extubation (extubation failure), the endotracheal tube is reinserted and MV reinstituted. At any stage, patients may die. Note: ICU = intensive care unit, PAV+ mode = proportional-assist ventilation with load-adjustable gain factors, PSV = pressure-support ventilation.
Model structure
Ventilation patient care pathway
Original published model
The published Markov cohort cost-utility model22 considered patient care from the point of initiating invasive mechanical ventilation. While receiving mechanical ventilation, patients could be synchronous or asynchronous with the ventilator. Patients who were synchronous had a higher probability of being stable during a spontaneous breathing trial and progressing to extubation (removal of the ventilator).23 After successful weaning, patients were in the ICU without mechanical ventilation before transfer to the general ward and subsequent discharge.
The model has daily cycles, such that a patient can move between health states (e.g., from asynchronous to synchronous mechanical ventilation, or from ICU to general ward) once per day. At any stage of the pathway, patient death was possible. Adverse events considered during care were tracheostomy, ventilator-associated pneumonia, other nosocomial infection and reintubation.
Adapted model
We adapted the published Markov cohort cost-utility model22 to the Canadian setting. It contains 8 health states (Figure 1). The adapted model considers patients who have been admitted to the ICU and require mechanical ventilation in Canada. Where the published model contained a single health state for weaning (i.e., a spontaneous breathing trial and extubation as 1 step),22 for the Canadian setting, we included an additional health state of liberation after the spontaneous breathing trial to formalize the weaning process and removal of the ventilator. For patients who fail the spontaneous breathing trial, mechanical ventilation continues until their next spontaneous breathing trial, whereas those who pass the spontaneous breathing trial are assessed for extubation. During the liberation phase, the ventilator is removed, and patients are then monitored closely to ascertain whether they can breathe unaided and clear secretions sufficiently without the need for an artificial airway (endotracheal tube). The outcomes of this state are remaining in the liberation phase (being in the ICU without mechanical ventilation), returning to mechanical ventilation in the ICU or progression to the general ward without mechanical ventilation.
Time horizon and discounting
The model base case considers a 1-year time horizon that aims to assess the immediate impact of a change in ventilation mode. Given that a stay in the ICU is often less than 1 month, all relevant clinical outcomes are expected to be realized within 1 year. Because the model adopts a year-long base-case time horizon, discounting was not required in the base case. We applied a half-cycle correction to both costs and quality of life.
Model outputs
The primary outcomes of interest in this analysis were total costs, quality-adjusted life years (QALYs) and the (incremental) cost per QALY gained, reported as an incremental cost-effectiveness ratio (ICER), for patients receiving mechanical ventilation with either PAV+ mode or PSV. We also estimated disaggregated costs and QALYs. The base-case model results reflect a 1-year time horizon.
Model inputs
To inform the model, we conducted a structured literature review (Appendix 1, available at www.cmajopen.ca/content/10/1/E126/suppl/DC1) to identify data relevant to adaptation of the model to the Canadian setting. Given that we identified multiple data sources on the efficacy of PAV+ mode versus PSV,15–17,23–26 to best summarize PAV+ mode efficacy, we performed a pragmatic meta-analysis to prevent introduction of bias from arbitrary selection of a single study (Appendix 1).
Cost inputs took a hospital-payer perspective and, where available, were identified through the targeted literature review. We sourced cost inputs that were not identified from the targeted search through reports from Canadian authorities27–30 and the Canadian Institute for Health Information Canadian Management Information System Database. All costs reflect the most recently available data at the time of writing, reported in 2017 Canadian dollars, with costs from earlier years inflated to 2017 with the use of the Canadian Consumer Price Index for health care.31 We assumed that PSV mechanical ventilation is already available in hospital, and so our analysis focused on the incremental cost-utility of introducing PAV+ mode.
As there appeared to be limited Canadian data on estimates of quality of life specific to patients who have been admitted to the ICU and require mechanical ventilation, we used the clinical expert opinion of 1 of the authors (K.J.B., ICU physician) to select relevant inputs for the quality of life utility (measured with the EuroQoL EQ-5D instrument32). We selected the utility derived from patients in critical care in the United States,22 as this population was well matched for age to the Canadian population evaluated in our analysis. We applied the utility for mechanical ventilation to health states of synchrony, asynchrony, spontaneous breathing trial and ventilator-associated pneumonia. A utility for ICU was applied to the ICU (no mechanical ventilation) health state, the general ward health state had a hospital utility, and patients in the home setting had a postdischarge utility applied for the first year. In analyses with a longer time horizon (see scenario analyses), the baseline utility with an annual decrement was applied in all subsequent years.
Additional inputs for mechanical ventilation that can affect costs and patient quality of life that we considered were the initial 1-time cost of PAV+ mode (included in the model as a cost per patient per day), duration of mechanical ventilation, time in the ICU, time in hospital, death in the ICU, death in hospital, occurrence of adverse events and death after discharge. We included in the model only the costs of specific adverse events; disutilities associated with these adverse events were excluded. This assumption was considered conservative because identified clinical data (Table 1) showed that PAV+ was associated with a lower rate of tracheostomy events and shorter duration of mechanical ventilation (resulting in a lower incidence of ventilator-associated pneumonia) than PSV. As such, adding a further disutility to adverse events that occur while patients are receiving mechanical ventilation would be expected to benefit only PAV+ and could potentially distort the findings of the analysis. Furthermore, receiving mechanical ventilation is already associated with low quality of life, possibly because adverse events common to mechanical ventilation are already considered in the estimate. We therefore considered that the quality of life disutility of adverse events while receiving mechanical ventilation was already reflected in the model.
Table 1:
Parameters of adapted Markov cohort cost-utility model*
| Parameter | Base case | Distribution† |
|---|---|---|
| Patient cohort demographic characteristics | ||
| Age, mean ± SD, yr | 67 ± 1215 | Normal |
| Female sex (SE), % | 39.7 (0.13)33 | β |
| Patient outcomes in acute phase of mechanical ventilation ‡ | ||
| Patients with clusters of ineffective efforts, mean (estimated 95% CI), % | 38 (29.1 to 47.3)33 | β |
| Patient asynchrony on entering model ‡ | ||
| Asynchrony > 10% at initiation of mechanical ventilation if clusters of ineffective efforts, % ± SD | 8.5 ± 1.833 | β |
| Asynchrony > 10% at initiation of mechanical ventilation if no clusters of ineffective efforts, % ± SD | 1.5 ± 1.033 | β |
| Reference efficacy standard of care (PSV), mean (95% CI) | ||
| Duration of mechanical ventilation, d | 8.1 (4.5 to 28.3)34 | Normal |
| Time in intensive care unit, d | 12.6 (7.4 to 33.3)34§ | Normal |
| Time in hospital, d | 43.5 (18.6 to 68.4)34§ | Normal |
| Spontaneous breathing trial success, % | 77.9 (73.8 to 82.1)23 | β |
| Liberation success, % (95% CI) | 85.3 (85.1 to 85.6)35 | β |
| Adverse event rates, mean (95% CI), % | ||
| Tracheostomy | 26.0 (8.1 to 44.0)15 | β |
| Ventilator-associated pneumonia | 8.8 (5.7 to 11.9)34 | β |
| Nosocomial infection | 0.85 (0.66 to 1.04)36 | β |
| Intensive care unit death | 25.4 (20.7 to 30.1)34 | β |
| Hospital death | 30.3 (25.3 to 35.3)34 | β |
| Postdischarge death | ||
| Year 1 | 12.5 (12.4 to 12.6)37 | β |
| Year 2 | 19.3 (19.2 to 19.5)37¶ | β |
| Year 3 | 27.5 (27.3 to 27.7)37¶ | β |
| Year 4 | onward Life tables¶ | β |
| Comparative effectiveness, PAV+ mode v. PSV ** | ||
| Total duration of mechanical ventilation, mean (95% CI), d | −1.53 (−2.24 to −0.83) | Normal |
| Intensive care unit length of stay, mean (95% CI), d | −1.54 (−2.19 to −0.90) | Normal |
| Hospital length of stay, mean (95% CI), d | −1.83 (−2.51 to −1.16) | Normal |
| Successful weaning/liberation, OR (95% CI) | 1.49 (0.59 to 3.79) | Log-normal |
| Intensive care unit death, OR (95% CI) | 0.70 (0.41 to 1.20) | Log-normal |
| Hospital death, OR (95% CI) | 0.70 (0.40 to 1.22) | Log-normal |
| Tracheostomy, OR (95% CI) | 0.76 (0.44 to 1.31) | Log-normal |
| Extubation failure/reintubation, OR (95% CI) | 0.52 (0.25 to 1.08) | Log-normal |
| Asynchrony index ≥ 10, OR (95% CI) | 0.13 (0.07 to 0.23) | Log-normal |
| Costs †† | ||
| Intensive care unit, cost per day, mean (range of reported means), $ | 2765 (2354–3690)38 | γ |
| General ward, cost per day, mean (range of reported means), $ | 019 (717–1400)38 | γ |
| Mechanical ventilation initiation, cost per event, mean (95% CI), $ | 139 (125 to 153)39 | γ |
| Mechanical ventilation maintenance, cost per day, mean (95% CI), $ | 851 (766 to 936)39 | γ |
| Tracheostomy, cost per event, mean (95% CI), $ | 4193 (3908 to 4477)40 | γ |
| Ventilator-associated pneumonia, cost per day, mean (95% CI), $ | 58 (30 to 73)41,42 | γ |
| Other nosocomial infection, cost per event, mean (± 10%), $ | 870 (783 to 956)43 | γ |
| PSV, purchase cost, $ | 0‡‡ | γ |
| PAV+ mode, 1-time purchase cost, $ | 27 00022§§ | γ |
| After discharge, annual cost, mean (95% CI), $ | ||
| Year 1–2 | 13 707 (6241 to 37 631)44¶ | γ |
| Year 3 onward | 10 032 (5835 to 17 169)44¶ | γ |
| Ventilator-associated pneumonia, additional length of stay, median (range), d | 9.5 (8.8–10.1)27 | Normal |
| Health state utility, mean (95% CI) | ||
| Baseline | 0.776 (0.677 to 0.899)22 | Normal |
| Mechanical ventilation | −0.390 (−0.590 to 0.090)22 | Normal |
| Intensive care unit | 0.402 (0.362 to 0.442)22 | Normal |
| Hospital | 0.520 (0.450 to 0.590)22 | Normal |
| After discharge to 1 yr | 0.550 (0.480 to 0.610)22 | Normal |
| Adverse event disutility | ||
| Tracheostomy | 0¶¶ | Normal |
| Ventilator-associated pneumonia | 0*** | Normal |
| Extubation failure | 0¶¶ | Normal |
Note: CI = confidence interval, OR = odds ratio, PAV+ mode = proportional-assist ventilation with load-adjustable gain factors, PSV = pressure-support ventilation, SD = standard deviation, SE = standard error.
Canadian data in italics.
We made the choice of distribution to reflect the uncertainty of each parameter from the perspective of population-level uncertainty as opposed to uncertainty at the individual patient level.
See Figure 1.
In the analysis by Sinuff and colleagues,34 no upper bound was presented owing to the patient’s remaining in hospital. For our calculations, we assumed that the upper bound is given by: mean + (mean – lower bound).
Used for scenario analyses only.
Seven clinical studies comparing PAV+ mode to PSV15–17,23–26 were identified by K.J.B. and in systematic reviews.45,46 As these systematic reviews did not report on all required outcomes, and no single study presented robust clinical data on the required model inputs, we determined the comparative efficacy of PAV+ mode versus PSV by means of a pragmatic meta-analysis (Appendix 1).
2017 Canadian dollars.
Conservative assumption.
Assumed to be $24.64 per day of use, assuming a 5-year life cycle and that the ventilator is in use on 60% of days. Probabilistic model inputs (used for the probabilistic sensitivity analysis) were based on input variance, calculated from reported CIs.
Assumed none in addition to mechanical ventilation.
Additional duration of mechanical ventilation is assumed to cover the disutility.
The full list of model parameters is provided in Table 1. Parameters for duration of mechanical ventilation, time in the ICU and time in hospital are not independent: for example, longer duration of mechanical ventilation leads to longer time in the ICU, and the average time in the ICU cannot be longer than the average time in hospital. For this reason, these inputs are connected via logic flows that prevent, for example, mechanical ventilation duration being longer than time in the ICU.
The input parameters used to calculate the transition probabilities between health states and the transition matrix for the PSV group can be found in Appendix 1, Table S3. This transition matrix is normalized for competing risk, such that all rows sum to 1. As data to inform the transition matrix come from multiple studies, the sum of all possible transitions may (initially) not be equal to 1. To account for this, a normalized transition matrix is created whereby each transition probability from health state X is divided by the sum of all transition probabilities from health state X.
Statistical analysis
We assessed the robustness of the base-case results through probabilistic sensitivity analysis, whereby the model is run repeatedly (2000 iterations) and, for each iteration, each input parameter is sampled randomly from a probabilistic distribution around the base case (default) value (the full list of distributions used is provided in Table 1). The distribution is defined by the uncertainty (standard deviation, interquartile range [IQR] or 95% confidence interval) provided in the source publications. The probabilistic sensitivity analysis explores uncertainty of outcomes at the population or cohort level as opposed to potential variation expected among individual patients. The results of these analyses are presented as median and 95% credible interval (CrI) and as a cost-effectiveness scatter plot. The willingness-to-pay threshold was set at $50 000 per QALY gained.
Scenario analyses
We conducted scenario analyses extensively to understand whether certain model parameters were driving the results and to explore how alternative modelling assumptions would affect model results. Specifically, we evaluated the impact of using alternative patient cohort characteristics through both a younger patient population and a patient population with a higher proportion of females. We also explored the impact of increasing the model time horizon to cover the assumed lifespan of a patient and including public-payer costs to provide a cost-effectiveness estimate relevant to health authorities and longer-term planning. In addition, where the targeted literature search identified alternative referenceable model inputs, we also explored the impact of using these inputs (comparative effectiveness estimates, asynchrony between PAV+ mode and PSV, PSV purchase cost, per-day hospital costs and utility values).
The meta-analysis showed that PAV+ mode was associated with significantly shorter duration of mechanical ventilation and time in hospital, but differences in most adverse events did not reach statistical significance (Table 1). Therefore, we evaluated the impact of running the model with only significant differences included.
Model validation
We assessed the face validity of the model using a variety of methods: by comparing inputs to model outputs on the assumption that these should be closely aligned; by looking at convergence of the model during sensitivity analysis and running tests for outliers; and through comparison of model estimates to published literature not used for a related input in our base case.
Ethics approval
The study is an economic model informed by aggregate data from published literature and, as such, did not require ethics approval.
Results
Base-case results
In the base case, PAV+ mode was dominant compared to PSV. Over 1 year, the cost of care per patient was $7642 less with PAV+ mode ($43 309) than with PSV ($50 951). Compared to PSV, PAV+ mode resulted in 0.04 more QALYs over the year (0.25 v. 0.29). Of the cost saving, $717 was accrued over the first 6 days (receiving mechanical ventilation), $2541 over the first 12 days (in the ICU) and $7159 over the first 45 days (in hospital). Differences in QALYs were negligible in the first 6 and 12 days, and reached 0.005 QALYs by day 45.
Probabilistic results
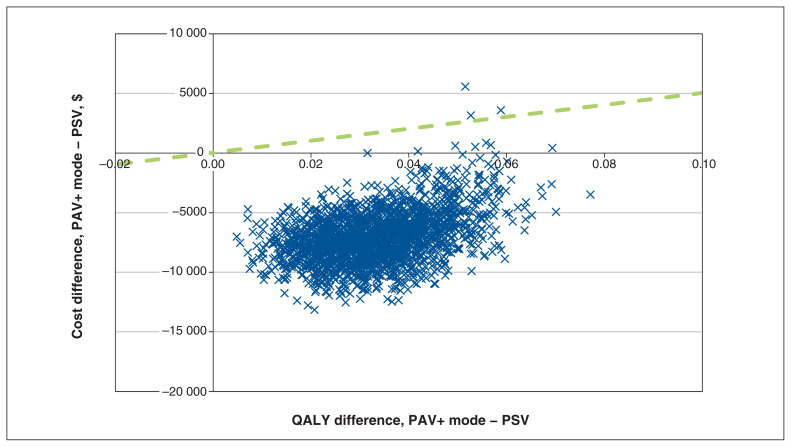
Based on the results of cost and QALY convergence tests (Appendix 1), we deemed a probabilistic sensitivity analysis with 2000 iterations appropriate given that total costs and QALYs converged toward their probabilistic values. The results of the probabilistic sensitivity analysis suggest that there is a 99.2% chance that PAV+ mode would be cost-saving in the population after 1 year (Figure 2), with a median probabilistic cost saving of $7147 (95% CrI $2072 to $10 719). The results suggest that there is a 100% chance of accumulating more QALYs with PAV+ than with PSV; use of PAV+ mode would result in 0.033 extra QALYs (95% CrI 0.014 to 0.055).
Figure 2:
Cost-effectiveness plane for proportional-assist ventilation with load-adjustable gain factors (PAV+ mode) versus pressure-support ventilation (PSV). The dashed green line (reference) indicates a willingness-to-pay threshold of $50 000 per quality-adjusted life year (QALY) gained. Points underneath this line are considered cost-effective. Simulations in the lower right quadrant (increase in QALY, decrease in cost) are considered dominant. Costs are in 2017 Canadian dollars.
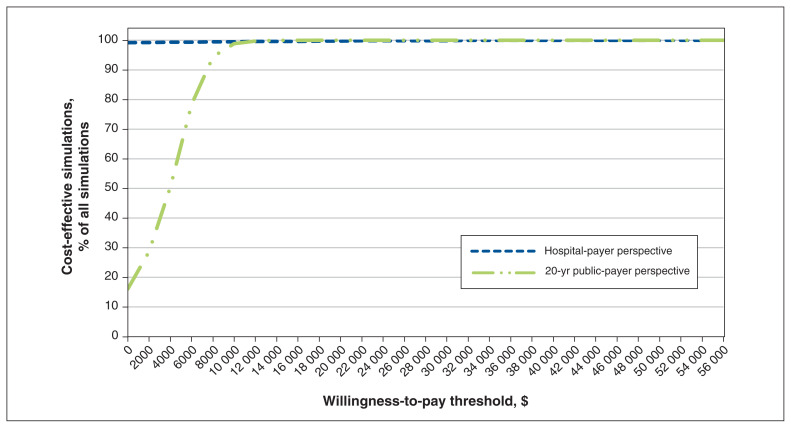
Given a willingness-to-pay threshold of $50 000 per QALY gained, the probabilistic sensitivity analysis results indicate that PAV+ mode was cost-effective in 99.2% of simulations. Varying the willingness-to-pay threshold influenced whether the use of PAV+ mode versus PSV mode would be considered cost-effective (Figure 3). We explored the impact of the specific seeded random number on this outcome and found that it had very limited impact on results (Appendix 1, Figure S5).
Figure 3:
Cost-effectiveness acceptability curve at 2 time horizons for proportional-assist ventilation with load-adjustable gain factors versus pressure-support ventilation. The proportion of simulations is shown according to varying thresholds of cost-effectiveness for a 1-year hospital-payer perspective and a 20-year public-payer perspective. Cases where the result was dominant (a decrease in costs accompanied by an increase in quality-adjusted life years) are counted among the cost-effective scenarios, hence the curves’ indicating nonzero proportions of simulations as cost-effective even at a willingness-to-pay threshold of $0. Costs are in 2017 Canadian dollars.
Scenario analyses
When we ran the model with only significant differences included from our meta-analysis (i.e., that PAV+ mode was associated with significantly shorter duration of mechanical ventilation and time in hospital [Table 1]), the cost and QALY benefit of PAV+ mode were reduced, but overall outcomes did not change (Table 2).
Table 2:
| Scenario | Cost difference, $ | QALY difference | ICER |
|---|---|---|---|
| Base case (default values) | −7643 | 0.04 | Dominant |
| Only significant differences in comparative effectiveness included | −7423 | 0.02 | Dominant |
| Long-term time horizon (20 yr, public-payer perspective)‡ | 6110 | 0.92 | $6624 |
| Younger patient population (50 yr) | 7643 | 0.04 | Dominant |
| Patient population 70% female | −7643 | 0.04 | Dominant |
| No difference in asynchrony between PAV+ mode and PSV | −6658 | 0.03 | Dominant |
| PSV also has purchase cost ($13 500) | −7761 | 0.04 | Dominant |
| Per-day total hospital costs: intensive care unit $3592, general ward $113528 | −9408 | 0.04 | Dominant |
| Per-day direct hospital costs: intensive care unit $1732.90, general ward $499.7029 | −5832 | 0.04 | Dominant |
| Canadian efficacy data only15 | −8080 | 0.00 | Cost saving |
| Alternative RR for successful spontaneous breathing trial, OR 1.1647 | −7123 | 0.03 | Dominant |
| Alternative utility value assumed for mechanical ventilation, 0.2922 | −7643 | 0.03 | Dominant |
Note: ICER = incremental cost-effectiveness ratio, OR = odds ratio, PAV+ mode = proportional-assist ventilation with load-adjustable gain factors, PSV = pressure-support ventilation, QALY = quality-adjusted life year, RR = relative risk.
Results are presented as PAV+ mode versus standard-care PSV, with difference in costs over difference in QALYs. The associated ICER is shown; in cases in which costs decrease and QALYs increase, the ICER is taken as dominant.
Costs in 2017 Canadian dollars.
A younger patient population or one with a higher proportion of females had little impact on absolute or relative base-case results, but extending the model time horizon to 20 years (to cover the assumed lifespan of patients and provide a public-payer perspective) was shown to lead to an ICER of $6624 per QALY gained (+$6110 and +0.92 QALYs) (Table 2).
Including costs per day for ICU and general wards from the Canadian Institute for Health Information Management Information System Database rather than from published literature increased the saving with PAV+ mode at 1 year by 23.1%, although when we considered only direct costs, the saving decreased by 23.7% (Table 2). The most notable change to the base case came from use of Canadian data instead of meta-analysis data: in that scenario, PAV+ mode was cost saving at 1 year (−$8080), with quality of life being equal.
Finally, we conducted scenario analyses using alternative referenceable inputs. Changing neither the odds ratio for a successful spontaneous breathing trial nor the utility value assumed for mechanical ventilation led to substantial changes in model outcomes.
Model validation
Face validity checks of our model indicated that the model was performing as expected, with an overall mortality rate of 29% at 68 days, compared to the input value of 30.3% at 68 days. Furthermore, with only 0.051% of the population alive after 20 years in the model, this was taken to represent the lifetime time horizon from the public-payer perspective.
Interpretation
Our health economic model showed that, relative to PSV, PAV+ mode reduced costs to payers in year 1 but increased costs over a longer time horizon, with a cost per QALY gained of $6624 over a 20-year time horizon. These results suggest that use of PAV+ mode has a high likelihood to be considered cost-effective in Canada.48
The observed increased costs with PAV+ mode align with previous analyses in the US and the United Kingdom, where higher patient survival with PAV+ mode resulted in higher costs of care (the survival paradox).22 When we used efficacy data from a pilot study specific to Canada15 as inputs for PAV+ mode, PAV+ mode was cost-saving but did not increase the QALYs accrued compared to PSV. Cost savings were driven mainly by shorter time in the ICU (−5 d), but a slightly higher ICU mortality rate (15% with PAV+ mode v. 13% with PSV) likely accounted for negation of the base-case QALY gains in this scenario analysis. Key drivers of cost in our model were duration of mechanical ventilation and time in the ICU.
The reasons why PAV+ mode results in shorter duration of mechanical ventilation and time in the ICU in this model can only be surmised and may be multifactorial. For example, the algorithms underlying PAV+ mode may help maintain the patient’s respiratory muscles, allowing for earlier success in spontaneous breathing trials or earlier readiness for liberation. Alternatively, or in addition, reduced incidence of tracheostomy may play a role, as time to tracheostomy has been associated with duration of mechanical ventilation and time in the ICU.49 Results of a large multicentre clinical trial (Proportional Assist Ventilation for Minimizing the Duration of Mechanical Ventilation [PROMIZING], ClinicalTrials.gov Identifier: NCT02447692) are pending and may help to answer these questions definitively.
To our knowledge, there are 3 published meta-analyses of PAV+ mode.45–47 Like us, the authors of 2 of these studies converted data reported as median and IQR to mean and standard deviation for analysis using the methods of Wan and colleagues50 (used by Kataoka and colleagues46) or Hozo and colleagues51 (used by Tirupakuzhi Vijayaraghavan and colleagues45 [Dr. Bharath Kumar Tirupakuzhi Vijayaraghavan, University of Toronto: personal communication, 2021]). Our results are more aligned to those of Kataoka and colleagues46 and Ou-Yang and colleagues,47 which appears to confirm the face validity of the pragmatic meta-analysis results that we conducted. (Our pragmatic meta-analysis, aimed at providing an objective assessment of the clinical effectiveness of PAV+ mode versus PSV for input into the cost-utility model, showed significant reductions in patient–ventilator asynchrony and duration of mechanical ventilation, time in the ICU and time in hospital.) Potential reasons for this may be the consensus use of the methodology presented by Wan and colleagues50 or the greater consistency in studies included in the meta-analyses (i.e., the number of studies included in both our meta-analysis and that of Kataoka and colleagues46 was greater than the number included in both our meta-analysis and that of Tirupakuzhi Vijayaraghavan and colleagues45).
Cost of care estimates in our model match up well to other published estimates. For example, looking at patients who received mechanical ventilation for less than 21 days, Hill and colleagues44 found that the median index admission cost was $32 994 (IQR $21 308–$54 736) (2013 Canadian dollars). Estimates from our model of $43 309 (PAV+ mode) to $50 951 (PSV) (2017 Canadian dollars) are within this IQR. Face validity checks of our model also indicated that the model was performing as expected. Furthermore, with only 0.051% of the population alive after 20 years in the model, this was taken to represent the lifetime time horizon from the public-payer perspective.
Limitations
As we did not conduct a systematic review, there remains the possibility that data relevant to the analysis were not captured. All data identified in previous meta-analyses45,46 were included, as well as data from a prospective observational case–control study.25 Cost data used in this analysis were not all from the same source, and their respective source publications often used different costing methodologies. In addition, costs were not all from the same year, some costs required adjustment via national health care inflation rates, and data for more recent years (2020 onward) were not available owing to delays in reporting on annual data and inflation rates. Furthermore, health state utilities and costs did not all come specifically from Canadian sources. Although we made efforts to source appropriate and comparable data, the lack of Canada-specific data in some areas is currently a limitation.
There is the potential for structural uncertainty in the model. This is difficult to assess and quantify, and our results should be treated as estimates and interpreted with respect to their uncertainty and the reader’s clinical judgment. Sensitivity and scenario analyses across a range of clinical parameters suggest that the results are robust, but confirmation in a real-world analysis in patients is recommended.
Conclusion
Based on available evidence to date, use of PAV+ mode is likely to be cost-effective in the Canadian setting, from the perspective of both hospital payers and public payers. Costs of care in the year of admission to the ICU are expected to be reduced. Over a 20-year time period, public health care costs would increase owing to longer patient life expectancy.
Supplementary Material
Footnotes
Competing interests: Rhodri Saunders is the owner and Jason Davis an employee of Coreva Scientific & Co, a consultancy for health economics and value-based health care. Coreva Scientific & Co received consultancy fees for its part in this research. Karen Bosma is co–principal investigator in the PROMIZING (Proportional Assist Ventilation for Minimizing the Duration of Mechanical Ventilation) clinical trial. She has previously consulted for Medtronic but received no remuneration for any part in this research project, the manuscript or any associated work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Rhodri Saunders and Karen Bosma contributed to the study conception and design. Rhodri Saunders and Jason Davis drafted the manuscript, and Karen Bosma revised it critically for important intellectual content. All of the authors contributed to data acquisition, analysis and interpretation, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by Medtronic.
Data sharing: Data relating to results presented will be made available on reasonable request to the corresponding author.
Disclaimer: Medtronic reviewed the manuscript for legal compliance but had no other involvement in any aspect of the study.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/1/E126/suppl/DC1.
References
- 1.Care in Canadian ICUs. Ottawa: Canadian Institute for Health Information; 2016. [accessed 2022 Jan. 26]. Available: https://secure.cihi.ca/free_products/ICU_Report_EN.pdf. [Google Scholar]
- 2.Long-term mechanical ventilation: toolkit for adult acute care providers. Toronto: Critical Care Services Ontario; 2013. [accessed 2021 Jan. 15]. Available: https://criticalcareontario.ca/wp-content/uploads/2020/10/Long-Term-Mechanical-Ventilation-Toolkit-for-Adult-Acute-Care-Providers.pdf. [Google Scholar]
- 3.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–87. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202:1–4. doi: 10.1164/rccm.202004-1385ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett K, Khan YA, Mac S, et al. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192:E640–6. doi: 10.1503/cmaj.200715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiseman SM, Crump RT, Sutherland JM. Surgical wait list management in Canada during a pandemic: many challenges ahead. Can J Surg. 2020;63:E226–8. doi: 10.1503/cjs.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein SK. How often does patient–ventilator asynchrony occur and what are the consequences? Respir Care. 2011;56:25–38. doi: 10.4187/respcare.01009. [DOI] [PubMed] [Google Scholar]
- 8.Thille AW, Rodriguez P, Cabello B, et al. Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–22. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 9.de Wit M, Miller KB, Green DA, et al. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med. 2009;37:2740–5. doi: 10.1097/ccm.0b013e3181a98a05. [DOI] [PubMed] [Google Scholar]
- 10.Blanch L, Villagra A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015;41:633–41. doi: 10.1007/s00134-015-3692-6. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M, Kindler F, Cecchini J, et al. Neurally adjusted ventilatory assist and proportional assist ventilation both improve patient–ventilator interaction. Crit Care. 2015;19:56. doi: 10.1186/s13054-015-0763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranieri VM, Grasso S, Mascia L, et al. Effects of proportional assist ventilation on inspiratory muscle effort in patients with chronic obstructive pulmonary disease and acute respiratory failure. Anesthesiology. 1997;86:79–91. doi: 10.1097/00000542-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Wysocki M, Meshaka P, Richard JC, et al. Proportional-assist ventilation compared with pressure-support ventilation during exercise in volunteers with external thoracic restriction. Crit Care Med. 2004;32:409–14. doi: 10.1097/01.CCM.0000108869.12426.51. [DOI] [PubMed] [Google Scholar]
- 14.Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis. 1992;145:114–20. doi: 10.1164/ajrccm/145.1.114. [DOI] [PubMed] [Google Scholar]
- 15.Bosma KJ, Read BA, Bahrgard Nikoo MJ, et al. A pilot randomized trial comparing weaning from mechanical ventilation on pressure support versus proportional assist ventilation. Crit Care Med. 2016;44:1098–108. doi: 10.1097/CCM.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 16.Botha J, Green C, Carney I, et al. Proportional assist ventilation versus pressure support ventilation in weaning ventilation: a pilot randomised controlled trial. Crit Care Resusc. 2018;20:33–40. [PubMed] [Google Scholar]
- 17.Elganady AA, Beshey BN, Abdelaziz AAH. Proportional assist ventilation versus pressure support ventilation in the weaning of patients with acute exacerbation of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2014;63:643–50. [Google Scholar]
- 18.Siebert U, Alagoz O, Bayoumi AM, et al. ISPOR-SMDM Modeling Good Research Practices Task Force. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health. 2012;15:812–20. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Briggs AH, Weinstein MC, Fenwick EAL, et al. ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15:835–42. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Eddy DM, Hollingworth W, Caro JJ, et al. ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32:733–43. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 21.Santos J, Palumbo F, Molsen-David E, et al. ISPOR Code of Ethics 2017 (4th edition) Value Health. 2017;20:1227–42. doi: 10.1016/j.jval.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Saunders R, Geogopoulos D. Evaluating the cost-effectiveness of proportional-assist ventilation plus vs. pressure support ventilation in the intensive care unit in two countries. Front Public Health. 2018;6:168. doi: 10.3389/fpubh.2018.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med. 2008;34:2026–34. doi: 10.1007/s00134-008-1209-2. [DOI] [PubMed] [Google Scholar]
- 24.Sasikumar S, Shanbhag V, Shenoy A, et al. Comparison of pressure support and proportional assist ventilation plus for weaning from mechanical ventilation in critically ill patients. Indian J Crit Care Med. 2013;7:34. [Google Scholar]
- 25.Aguirre-Bermeo H, Bottiroli M, Italiano S, et al. Pressure support ventilation and proportional assist ventilation during weaning from mechanical ventilation [article in Spanish] Med Intensiva. 2014;38:363–70. doi: 10.1016/j.medin.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira SN, Osaku EF, Lima de Macedo Costa CR, et al. Comparison of proportional assist ventilation plus, T-tube ventilation, and pressure support ventilation as spontaneous breathing trials for extubation: a randomized study. Respir Care. 2015;60:1527–35. doi: 10.4187/respcare.03915. [DOI] [PubMed] [Google Scholar]
- 27.Patient cost estimator. Ottawa: Canadian Institute for Health Information; [accessed 2022 Feb. 3]. Available: https://www.cihi.ca/en/patient-cost-estimator. [Google Scholar]
- 28.Care in Canadian ICUs: data tables. Table 13 Average daily cost for stay in ICU and general ward by hospital type, 2013–2014. Ottawa: Canadian Institute for Health Information; [accessed 2022 Jan. 26]. Available: https://www.cihi.ca›document›icu_datatables_en. [Google Scholar]
- 29.Trends in hospital spending, 2005–2006 to 2018–2019— data tables — Series C: average direct cost per patient by selected functional centre. Ottawa: Canadian Institute for Health Information; 2020. [accessed 2022 Jan. 26]. Available: https://www.cihi.ca/sites/default/files/document/hospital-spending-series-c-data-tables-2005-2006-to-2019-2020-en.xlsx. [Google Scholar]
- 30.CADTH methods and guidelines service line. Ottawa: CADTH; 2017. [accessed 2021 Jan. 15]. Guidelines for the economic evaluation of health technologies: Canada. Available: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. [Google Scholar]
- 31.Table 18-10-0005-01 (formerly CANSIM 326-0021) Ottawa: Statistics Canada; 2022. Jan 19, [accessed 2022 Jan. 26]. Available: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1810000501#timeframe. [Google Scholar]
- 32.EuroQol Group. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 33.Vaporidi K, Babalis D, Chytas A, et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med. 2017;43:184–91. doi: 10.1007/s00134-016-4593-z. [DOI] [PubMed] [Google Scholar]
- 34.Sinuff T, Muscedere J, Cook DJ, et al. Canadian Critical Care Trials Group. Implementation of clinical practice guidelines for ventilator-associated pneumonia: a multicenter prospective study. Crit Care Med. 2013;41:15–23. doi: 10.1097/CCM.0b013e318265e874. [DOI] [PubMed] [Google Scholar]
- 35.Miltiades AN, Gershengorn HB, Hua M, et al. Cumulative probability and time to reintubation in U.S. ICUs. Crit Care Med. 2017;45:835–42. doi: 10.1097/CCM.0000000000002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouadma L, Sonneville R, Garrouste-Orgeas M, et al. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43:1798–806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 37.Brinkman S, de Jonge E, Abu-Hanna A, et al. Mortality after hospital discharge in ICU patients. Crit Care Med. 2013;41:1229–36. doi: 10.1097/CCM.0b013e31827ca4e1. [DOI] [PubMed] [Google Scholar]
- 38.Evans J, Kobewka D, Thavorn K, et al. The impact of reducing intensive care unit length of stay on hospital costs: evidence from a tertiary care hospital in Canada. Can J Anaesth. 2018;65:627–35. doi: 10.1007/s12630-018-1087-1. [DOI] [PubMed] [Google Scholar]
- 39.Lachaine J, Beauchemin C. Economic evaluation of dexmedetomidine relative to midazolam for sedation in the intensive care unit. Can J Hosp Pharm. 2012;65:103–10. doi: 10.4212/cjhp.v65i2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu CC, Rudmik L. A cost-effectiveness analysis of early vs late tracheostomy. JAMA Otolaryngol Head Neck Surg. 2016;142:981–7. doi: 10.1001/jamaoto.2016.1829. [DOI] [PubMed] [Google Scholar]
- 41.Lee SJ. Cost-utility analysis of oral antiseptic chlorhexidine in decreasing ventilator-associated pneumonia in intensive care units [dissertation] Toronto: University of Toronto; 2016. [accessed 2021 Oct. 12]. Available: https://tspace.library.utoronto.ca/bitstream/1807/72749/1/Lee_So_J_201606_MSc_thesis.pdf. [Google Scholar]
- 42.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S120–5. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 43.Cooper K, Frampton G, Harris P, et al. Are educational interventions to prevent catheter-related bloodstream infections in intensive care unit cost-effective? J Hosp Infect. 2014;86:47–52. doi: 10.1016/j.jhin.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Hill AD, Fowler RA, Burns KEA, et al. Long-term outcomes and health care utilization after prolonged mechanical ventilation. Ann Am Thorac Soc. 2017;14:355–62. doi: 10.1513/AnnalsATS.201610-792OC. [DOI] [PubMed] [Google Scholar]
- 45.Tirupakuzhi Vijayaraghavan BK, Hamed S, Jain A, et al. Evidence supporting clinical use of proportional assist ventilation: a systematic review and meta-analysis of clinical trials. J Intensive Care Med. 2020;35:627–35. doi: 10.1177/0885066618769021. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka J, Kuriyama A, Norisue Y, et al. Proportional modes versus pressure support ventilation: a systematic review and meta-analysis. Ann Intensive Care. 2018;8:123. doi: 10.1186/s13613-018-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou-Yang LJ, Chen PH, Jhou HJ, et al. Proportional assist ventilation versus pressure support ventilation for weaning from mechanical ventilation in adults: a meta-analysis and trial sequential analysis. Crit Care. 2020;24:556. doi: 10.1186/s13054-020-03251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cape J, Beca JM, Hoch JS. Introduction to cost-effectiveness analysis for clinicians. Univ Toronto Med J. 2013;90:103–5. [Google Scholar]
- 49.Arabi YM, Alhashemi JA, Tamim HM, et al. The impact of time to tracheostomy on mechanical ventilation duration, length of stay, and mortality in intensive care unit patients. J Crit Care. 2009;24:435–40. doi: 10.1016/j.jcrc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.