KEY POINTS
Hypervirulent Klebsiella pneumoniae (hvKp) presents as a community-acquired pyogenic liver abscess with bacteremia and multiple sites of metastatic involvement including, but not limited to, endophthalmitis and meningitis.
This hypervirulent strain was first described in the Asian Pacific Rim but is increasingly emerging in countries outside of this region, most often affecting those with diabetes.
The key microbiologic features of hvKp are presence of a hypermucoviscous phenotype, shown by a positive string test, and numerous genetic virulence factors, including capsular serotypes K1 and K2, and rmpA, rmp2 and magA.
Management requires a combination of pathogen-directed antibiotic therapy and source control.
Antimicrobial resistance and nosocomial spread of hvKp are important emerging global health concerns.
A 51-year-old man presented to hospital with a 2-day history of pain, redness and vision loss in his right eye, and a 1-week history of fever, night sweats and 4.5 kg (10 lb) weight loss. Relevant medical history included type 2 diabetes and hypertension. He was taking no medications. He was born in Malaysia, had previously lived in Sri Lanka and moved to Canada 17 years before. He had not travelled recently.
On examination, he was febrile (38.7°C) and tachycardic (131 beats/min); his blood pressure and oxygen saturation were normal. He was alert and oriented. He had conjunctival injection in his right eye, a relative afferent pupillary defect and a 1.5 mm hypopyon, with his vision limited to perception of light only. This ophthalmologic examination was consistent with endogenous endophthalmitis. On chest auscultation, we heard crackles bilaterally. His abdominal examination was normal.
On investigation, he had elevated leukocyte (12.4 [normal 4–11] × 109/L), creatinine (111 [normal 44–106] μmol/L), alkaline phosphatase (218 [normal 40–120] IU/L), bilirubin (37 [normal < 20) μmol/L) and glycosylated hemoglobin (11.7% [target ≤ 7.0%]) levels. Gram staining of a vitreous aspirate showed Gram-negative bacilli. His chest radiograph showed bilateral nodular opacities.
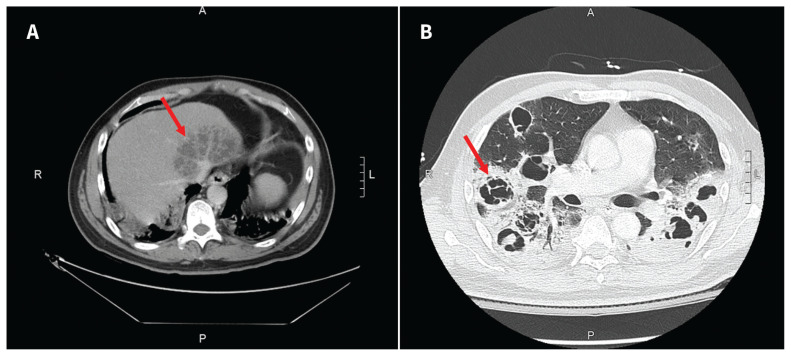
We admitted the patient and started him on intravenous meropenem after collecting blood cultures. Ophthalmology also provided intravitreal ceftazidime for the patient’s endophthalmitis. Computed tomography (CT) scans of his chest, abdomen and pelvis showed a 9.1 × 5.2 cm multiloculated liver abscess (Figure 1A), bilateral pulmonary nodules with central cavitation (Figure 1B) and bilateral renal hypodensities suggestive of pyelonephritis. Transthoracic echocardiogram showed no vegetations or substantial valvular abnormalities.
Figure 1:
Computed tomography (CT) scans of the abdomen and chest of a 51-year-old man who presented to hospital with visual symptoms and was found to have disseminated hypervirulent Klebsiella pneumoniae infection. (A) Multiloculated pyogenic liver abscess (arrow) measuring 9.1 × 5.2 cm, adjacent to the inferior vena cava confluence; (B) numerous large cavitary lesions (arrow indicates 1 example) representing multifocal pulmonary abscesses.
Within 24 hours of admission, the patient developed hypoxemic respiratory failure requiring intubation and transfer to the intensive care unit. Cultures of his blood, vitreous fluid, sputum, hepatic abscess aspirate and urine all showed growth of Klebsiella pneumoniae. Given the antibiotic susceptibilities, therapy was modified to intravenous ceftriaxone. The patient’s initial abdominal ultrasound suggested the hepatic abscess was not amenable to drainage, but repeat imaging 72 hours later showed liquification and the abscess was drained percutaneously. He later developed acute respiratory distress syndrome and required prolonged mechanical ventilation.
The patient’s clinical status gradually improved and he was extubated after 1 month. He was discharged to inpatient rehabilitation, 7 weeks after admission, on oral ciprofloxacin monotherapy. After a total of 3 months of antibiotic therapy, his hepatic and lung abscesses had resolved on repeat CT scans and antibiotics were discontinued. He was clinically stable 1 month after completion of antibiotic therapy. However, vision loss in his right eye persisted and the ophthalmology team deemed it irreversible.
K. pneumoniae isolates were consistent with a hypermucoviscous phenotype, based on the string test. Molecular testing, performed at the National Microbiology Laboratory in Winnipeg, Canada, found rmpA, iroB, iucA, ybtA, clbA and peg-344 genes, and the K1 capsular serotype, confirming disseminated infection due to hypervirulent K. pneumoniae.
Discussion
Hypervirulent K. (hvKp) is an emerging strain of the encapsulated Gram-negative, bacillary bacterium K. pneumoniae. The strain carries integrated virulence factors, conferring a hypermucoid phenotype, resulting in disseminated, community-acquired infections in immunocompetent hosts.1,2 This contrasts with classical K. pneumoniae, which is traditionally involved in nosocomial, single-organ infections, often in immunocompromised hosts.1,2 Disseminated infections due to hvKp typically occur in individuals with diabetes.1,2
Hypervirulent K. pneumoniae was first reported in China in 1986 in a case series of individuals with pyogenic liver abscess and endophthalmitis.1 Over the ensuing decades, cases were reported throughout the Asian Pacific Rim, with endemicity in several countries and high rates of colonic colonization with hvKp,3 which may contribute to community transmission. In recent years, cases have emerged in countries outside of this region.1,2 Retrospective surveillance-based cohort studies from Calgary and Quebec found that 10/134 (7.5%) and 1/110 (0.9%), respectively, of blood isolates of K. pneumoniae contained virulence factors suggestive of hvKp.4,5
Clinical manifestations
Hypervirulent K. pneumoniae typically causes communityacquired pyogenic liver abscess. Unlike with classical K. pneumoniae, liver abscesses due to hvKp are usually monomicrobial, without concomitant biliary disease, and associated with regional thrombophlebitis in up to one-third of cases (Table 1).1 Compared with pyogenic liver abscesses involving classical K. pneumoniae, monomicrobial liver abscesses due to hvKp are more likely to be single, unilobar, solid-appearing and multilocular in their radiographic appearance.6 The other distinguishing feature is metastatic involvement of other sites, notably endogenous endophthalmitis, meningitis, pneumonia and nonhepatic abscesses. Endophthalmitis was one of the first reported complications of hvKp, and occurs in about 5% of bacteremic patients.1 Visual symptoms progress rapidly despite systemic and intravitreal antibiotics, with vision reduced to light perception or worse in 89% of patients.1 Pulmonary manifestations range from lobar pneumonia to empyema or lung abscess.1,2 Other reported intra-abdominal manifestations include splenic abscess, pyelonephritis, perinephric abscess, epididymitis and prostatic abscess.1,2 Rarely, hvKp causes necrotizing fasciitis, osteomyelitis and deep neck infections.1,2
Table 1:
| Feature | Classical Klebsiella pneumoniae | Hypervirulent Klebsiella pneumonia |
|---|---|---|
| Predisposing factors |
|
|
| Distant spread (apart from hepatic abscess) | None | Multiple sites, including
|
| Hepatic abscess |
|
|
| Source of infection | Nosocomial | Community |
| Factors that increase virulence | Not applicable | K1 and K2 capsular serotypes rmpA, rmp2, magA, aerobactin (iuc), salmochelin (iro) |
| Treatment | Pathogen-directed antimicrobial therapy and abscess drainage | Pathogen-directed antimicrobial therapy and abscess drainage; surgical intervention should be considered if refractory |
| Antimicrobial resistance | Common | Emerging |
Diagnosis
There is no definitive diagnostic test for hvKp. Attributing infection to hvKp is based on the clinical presentation, laboratory demonstration of hypermucoviscous phenotype and confirmation of genetic markers. The hypermucoviscous phenotype is demonstrated by the “string test,” which is positive when a bacterial colony can be stretched more than 5 mm from the agar plate.1,2
Genetic markers associated with hvKp are most often encoded on plasmids, but in some cases are integrated in chromosomal elements. One driver of virulence is the enhanced polysaccharide capsule. Whereas classical K. pneumoniae produces capsules of serotypes ranging from K1 to K78, 93% of cases of hvKp have the K1 capsular serotype, and the remainder K2.2,7 K1 capsular serotypes increase the risk of metastatic infection in patients with liver abscess.8 Regulators of mucoid phenotype rmpA and rmpA2 are transcriptional regulators that lead to enhanced capsule production and hypermucoviscous phenotype; magA encodes an enzyme that is associated with the hypermucoviscous phenotype and confers resistance to phagocytosis.2,7 Hvpervirulence is also mediated by an increased ability to acquire iron through amplified siderophore production. The siderophores aerobactin (encoded by iuc) and salmochelin (encoded by iro) are rarely found in nonvirulent strains, but highly expressed in hvKp.2,7 Finally, hvKp harbours genes that enhance allantoin metabolism, improving its ability to obtain carbon and nitrogen.2,8 Molecular detection of these virulence factors is not routinely performed, but can be requested from reference laboratories.
Management
Treatment of hvKp infections requires source control and pathogendirected antimicrobial therapy. Much of the existing data about source control pertain to hepatic abscesses. No studies have examined percutaneous drainage versus surgery exclusively in cases of liver abscess due to hvKp. However, many studies likely included hypervirulent strains, given their geographical location and the cryptogenic nature of the abscesses.1 The largest of these studies suggested no difference in mortality between percutaneous drainage and surgical management (particularly if percutaneous drainage is followed by resection in refractory cases); however, surgical approaches may confer shorter length of stay and less treatment failure.9 If percutaneous drainage is necessary, a large-bore catheter with frequent flushing is often required,1 with surgery considered if conservative techniques fail.1
No randomized controlled trials have assessed optimal antibiotic therapy for hvKp, and treatment should be guided by sensitivity on culture. Tissue penetration must also be considered. For example, third-generation cephalosporins or carbapenems may be required for central nervous system infections; fluoroquinolones or trimethoprim-sulfamethoxazole may be considered for prostatic infections, and intravitreal therapy may be required for endophthalmitis.1,2 Therapies using passive immunization and bacteriophages have shown experimental efficacy but are still in developmental stages.1
Antimicrobial resistance
The development of antimicrobial resistance in hypervirulent strains of K. pneumoniae is an emerging concern. Extendedspectrum β-lactamase and AmpC-producing isolates are becoming increasingly prevalent. A prospective cohort study from China reported that 11/85 (13%) of hvKp isolates produced extendedspectrum β-lactamase.10 Notably, reports have also described carabapenemase production.11 One study reported a fatal outbreak caused by a carbapenem-resistant hypervirulent strain in eastern China; hvKp likely acquired this resistance genotype from a classical K. pneumoniae isolate.11 Despite in vitro susceptibility to colistin and tigecycline, all 5 cases were fatal regardless of appropriate therapy.11 Plasmid-mediated convergence of multidrug resistance and hypervirulence in K. pneumoniae has subsequently been reported in other Chinese provinces.11 Furthermore, hypervirulent strains are increasingly implicated in nosocomial infections such as ventilator-associated pneumonia.1 These evolutionary adaptations within K. pneumoniae represent a global challenge.
Conclusion
Hypervirulent K. pneumoniae causes bacteremia and pyogenic liver abscess, often with dissemination to multiple other sites, leading to endophthalmitis, meningitis or pulmonary involvement. This infection occurs more frequently in patients with diabetes. It was initially reported in the Asian Pacific Rim, but cases have now emerged outside of this geographic region. Clinicians should consider hvKp in patients with bacteremia and hepatic abscess due to K. pneumoniae and evaluate for possible metastatic foci of infection. Further investigation may show the hypermucoviscous phenotype and identify genetic markers of hypervirulence. Source control and antimicrobial therapy are the key to management but there is growing concern about antimicrobial resistance.
Acknowledgement
The authors acknowledge the Bacterial Pathogens Division at the National Microbiology Laboratory (Winnipeg, Manitoba) for its assistance with genetic sequencing.
Footnotes
Competing interests: Nisha Andany reports receiving research funds from Gilead Sciences, GlaxoSmithKline and Janssen, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Samik Doshi drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev 2019;32:e00001–00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choby JE, Howard-Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae — clinical and molecular perspectives. J Intern Med 2020;287:283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012. Jan19;12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peirano G, Pitout JD, Laupland KB, et al. Population-based surveillance for hypermucoviscosity Klebsiella pneumoniae causing community-acquired bacteremia in Calgary, Alberta. Can J Infect Dis Med Microbiol 2013;24:e61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol 2018;56:e00776–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsaif HS, Venkatesh SK, Chan DSG, et al. CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae. Radiology 2011;260:129–38. [DOI] [PubMed] [Google Scholar]
- 7.Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 2018;Jan 22;8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang CT, Lai S, Yi W, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007;45:284–93. [DOI] [PubMed] [Google Scholar]
- 9.Tan YM, Chang AY, Chow PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005;241:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother 2016;60:6115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Kreiswirth BN. Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect Dis 2018;18:2–3. [DOI] [PubMed] [Google Scholar]