Abstract
Background:
At present, the use of repetitive transcranial magnetic stimulation (rTMS) for generalized anxiety disorder (GAD) is limited to single-site interventions. We investigated whether dual-site frontoparietal stimulation delivered using cortical–cortical paired associative stimulation (ccPAS) had stronger clinical efficacy than single-site stimulation in patients with GAD.
Methods:
We randomized 50 patients with GAD to 1 Hz rTMS (10 sessions) using 1 of the following protocols: single-site stimulation over the right dorsolateral prefrontal cortex (dlPFC; 1500 pulses per session); single-site stimulation over the right posterior parietal cortex (PPC; 1500 pulses per session); repetitive dual-site ccPAS (rds-ccPAS) over the right dlPFC and right PPC with 1500 pulses per session (rd-ccPAS-1500); or rds-ccPAS over the right dlPFC and right PPC with 750 pulses per session (rd-ccPAS-750). Both rds-ccPAS treatments used a between-site interval of 100 ms.
Results:
Clinical scores for anxiety, depression and insomnia were reduced in all 4 groups after treatment. We found greater improvements in anxiety symptoms in the rds-ccPAS-1500 group compared to the rds-ccPAS-750 and single-site groups. We found greater improvements in depression symptoms and insomnia in the rds-PAS-1500 group compared to the single-site groups. The rds-ccPAS-1500 group also showed significant or trend-level improvements in anxiety symptoms and insomnia at 10-day and 1-month followup. More patients responded to treatment with rds-ccPAS-1500 than with single-site stimulation. The between-group differences in response rates persisted to the 3-month follow-up. Treatment using rds-ccPAS with a between-site interval of 100 ms induced a more significant improvement than the between-site interval of 50 ms we evaluated in a previous study.
Limitations:
These results need to be replicated in a larger sample using sham control and equal-pulse single-site stimulation.
Conclusion:
Frontoparietal rds-ccPAS may be a better treatment option for GAD.
Introduction
Generalized anxiety disorder (GAD) is characterized by excessive worry about everyday life matters.1 Although various treatments are available for GAD, a considerable proportion of patients cannot obtain relief.2 Among those who achieve remission, many experience adverse effects and addiction as a result of long-term medication use.1,2
Several studies have suggested that noninvasive repetitive transcranial magnetic stimulation (rTMS) may be beneficial for the treatment of anxiety disorders.3–8 Studies of the neural circuits of negative emotion and anxiety disorders provide a basis for us to choose stimulation sites for rTMS.9,10 It has been proposed that when someone receives negative stimuli, the limbic regions (such as the amygdala) are activated to perceive negative emotion. At the same time, the prefrontal cortex — especially the dorsolateral prefrontal cortex (dlPFC) — is activated; it transmits cognitive signals through the cortical–subcortical circuits to inhibit excessive response in the limbic system and ensure an appropriate emotional response.9,10 The classical neural hypothesis considers GAD to be an emotional dysregulation disorder associated with disturbance in the frontolimbic circuit, characterized by increased limbic activation to negative stimuli and dysregulation of the dlPFC with negative emotion.11–13 Both cortical and limbic abnormalities result in emotional and cognitive symptoms in patients with GAD.
Based on the core role of the dlPFC in emotion regulation and in the pathological mechanisms of anxiety disorders, studies of GAD have used the dlPFC as a stimulation site for rTMS.14 An open-label study3 applied 6 sessions of 1 Hz rTMS over the right prefrontal cortex (PFC) with a stimulating intensity of 90% of the resting motor threshold (RMT) and found that 6 of the 10 participants responded to treatment. Another open-label study6 applied 24 to 36 sessions of rTMS over the bilateral dlPFC in 13 patients with comorbid major depressive disorder and GAD. At the end of treatment, 11 patients (84.6%) showed remission. A randomized, sham-controlled pilot study4 of rTMS targeting the right dlPFC reported a higher response rate in the active (n = 13) versus sham (n = 12) conditions in participants with GAD.
Growing amounts of imaging evidence have allowed us to understand brain functional mechanisms from the perspective of large-scale neural networks.15 For complex brain functions, it is increasingly being recognized that multiple networks are involved and that several “hub” nodes exist in each functional network. Emotion regulation has been related to other cortical areas outside the PFC, such as the parietal lobe.16 The frontoparietal network is considered to be a “hub” network in the cortical cognitive system.17,18 The prefrontal and parietal lobes jointly regulate and control the limbic and sensorimotor systems.17,18 The posterior parietal cortex (PPC) disengages attention from emotional interference to reorient to task-relevant stimuli.15 Reducing the excitability of the intraparietal sulcus during shock threat could alleviate the physiologic arousal related to both fear and anxiety.19
In 36 patients with comorbid GAD and insomnia, a randomized, sham-controlled trial20 conducted by our group showed that 10 sessions of 1 Hz rTMS administered over the right PPC with an intensity of 90% RMT improved anxiety and insomnia symptoms in the active condition versus the sham condition. Although rTMS benefits a proportion of patients, its clinical efficacy has been inconsistent across studies and needs to be improved. Differences in stimulation sites, number of stimulating pulses, sample characteristics and more may explain such inconsistencies. For instance, the study6 that showed remission in most cases applied 24 to 36 sessions of rTMS — more than used in other studies. Our study described above20 applied only 10 sessions of rTMS. In addition to such inconsistencies in research methods, single-site stimulation is a critical technical issue that restricts the treatment efficacy and efficiency of rTMS.
It is increasingly being recognized that mental illnesses such as anxiety disorder are a product of dysfunction between interconnected networks.21 Based on this understanding, neuromodulation has been evolving from a single-site technique to a multisite stimulation technique.22 Double or multisite stimulation based on brain network characteristics is considered a promising pathway for optimizing the treatment of mental illness.22 Given that stimulating both the dlPFC and PPC is effective for patients with GAD,3,4,20 we speculated that associative stimulation of these 2 hubs would be more effective than single-site stimulation. The advantages of multisite over single-site rTMS has been confirmed in Parkinson disease and tinnitus.23,24 However, these studies stimulated a second brain site after sessions on the first site had been completed, making it twice as time-consuming as single-site stimulation.
Cortical–cortical paired associative stimulation (ccPAS), which delivers paired transcranial magnetic stimulation (TMS) pulses at predefined intervals over 2 brain sites, has been shown to modulate neural effects between the stimulating sites, purportedly via a mechanism similar to spike timing–dependent plasticity (STDP).25 According to the learning rule proposed by Hebbian in 1943, the synaptic efficacy of a synapse will increase if it can continuously elicit action potentials from the postsynaptic target neurons.26 Based on this rule, Henry Markram proposed the STDP learning principle, which says that the strength of connections between neurons is modulated according to the order of neuronal learning. If information from another neuron is generated before the activity of the neuron itself, the activity between the 2 neurons increases and causes long-term potentiation of the synapse. In contrast, if the neuron itself generates activity before receiving information from another neuron, the connection between the 2 neurons will be weakened and induce longterm depression.25 Based on the STDP principle, ccPAS delivered to 2 cortical areas at different time intervals may induce STDP-like inhibition or enhancement between the stimulating sites according to the dynamics of their activity.25,27–30
Therefore, ccPAS is considered to be a promising tool for modulating brain function, and translation to clinical applications has been encouraged. It has been reported that ccPAS over the PFC and PPC induces STDP-like changes in the PFC, based on the order of pulses.27 ccPAS over the supplementary motor area and inferior frontal gyrus affected inhibitory behaviours,28 and ccPAS that potentiated parietal-to-PFC activity (but not the reverse direction) increased goal-directed control.29
Our group investigated the effect of ccPAS with different stimulation intervals (i.e., 10, 20 and 50 ms) between 2 sites in 29 patients with GAD (no sham controls).31 We delivered 10 sessions of 1 Hz rds-ccPAS from the right dlPFC to the PPC at 1500 ccPAS pulses per session (a total of 3000 pulses). Although we used twice the number of single-site parietal stimulations used in another study,20 rds-ccPAS at stimulating intervals of 10 and 20 ms induced a reduction in Hamilton Rating Scale for Anxiety (HAM-A) scores of only 22% and 32%, substantially less than that of single-site stimulation.20 However, at an interval of 50 ms, the reduction in HAM-A scores reached 38%, second only to a reduction of 44% reported for single-site parietal stimulation.20 Combining these findings with the STDP principle, we speculate that at very short intervals, stimulating the right dlPFC may exert an STDP-like modulation in the PPC, which may antagonize the original TMS effect in the PPC. Such an antagonistic effect may be reduced when the stimulating interval is longer, resulting in an increase in treatment efficacy.
In the present study, we increased the stimulating interval to 100 ms, with the aim of improving the clinical efficacy of ccPAS even further. We performed a randomized, doubleblind clinical trial and recruited 4 groups of patients with GAD to compare rds-ccPAS and single-site stimulation: rds-ccPAS over the right dlPFC and right PPC at 1500 ccPAS pulses per session; rds-ccPAS over the right dlPFC and right PPC at 750 ccPAS pulses per session; single-site stimulation over the right dlPFC at 1500 pulses per session; and single-site stimulation over that right PPC at 1500 pulses per session.
Based on findings that the clinical efficacy of rds-ccPAS with a 50 ms between-site interval and 1500 pulses per session was similar to that of single-site stimulation at 1500 pulses per session, and that clinical efficacy increased with stimulation interval,31 we predicted that prolonging the interval to 100 ms would produce stronger clinical efficacy than single-site stimulation.17,18,20
We included a group with 750 rds-ccPAS pulses per session to clarify whether rds-ccPAS with a pulse number the same as single-site stimulation would have more clinical efficacy than single-site stimulation and explore whether frontal stimulation modulated the stimulatory effect in the parietal lobe. It was difficult to make exact assumptions about the clinical efficacy of rds-ccPAS with 750 pulses, given that large explanatory gaps still exist related to STDP in the context of neural networks instead of neurons.
Methods
Participants
Participants were patients with GAD aged 18 to 60 years, recruited from the psychosomatic disease clinic of the Neurology Department, Xuanwu Hospital, between September 2019 and February 2020. Patients were screened by a qualified psychiatrist for the following criteria: diagnosis of GAD according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition; Clinical Global Impression–Severity score of 4 or greater; a HAM-A score greater than 14; and a 17-item Hamilton Rating Scale for Depression (HAM-D) score of 22 or less. Participants who were taking medications had to have been stabilized for at least 4 weeks before study entry, but still have evident symptoms. Medications were maintained with the dose unchanged throughout the study.
Exclusion criteria included the presence of neurologic disorders, unstable medical conditions, other psychiatric diseases or substance use disorders within the last 6 months; pregnancy or breastfeeding; or contraindications for rTMS (e.g., metal implants in the body). All participants underwent resting-state electroencephalogram (EEG) recordings. Those who had epileptiform discharges, as determined by a qualified neurologist (Y.L.), were also excluded.
All procedures complied with the ethical standards of relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975 (revised 2008). All procedures were approved by the ethics committee of Xuanwu Hospital, and written informed consent was obtained for each participant.
rTMS protocol
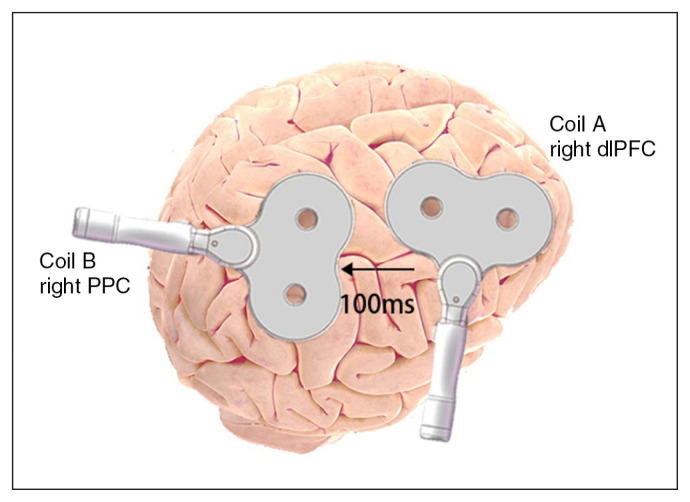
This trial adopted a randomized, double-blind design involving 10 sessions of rTMS plus clinical visits at 10 days, 1 month and 3 months (clinicaltrials.gov: ChiCTR1900028256). We performed treatment using a Magele-10 TMS stimulator (Tianjin Tams Medical Technology Co., Ltd.) and 2 figure-8 coils (width 191 mm, length 306 mm, thickness 19 mm). This size ensured that 2 coils could be placed on the head at the same time. One coil targeted the right dlPFC with the handle pointing backward and laterally, and oriented 45° from the sagittal plane. The other coil targeted the right PPC with the handle pointing backward and medially, and oriented approximately 45° from the sagittal plane. See Appendix 1, Figure S1 (available at www.jpn.ca/lookup/doi/10.1503/jpn.210201/tab-related-content), for the physical appearance of the coils and Figure S2 for their placement on the head.
Participants were randomized equally into groups for 1 of the 4 intervention types: single-site stimulation over the right dlPFC at 1500 pulses per session (ss-PFC); single-site stimulation over the right PPC at 1500 pulses per session (ss-PPC); rds-ccPAS over the right dlPFC and right PPC at 1500 pulses per session per site (rds-ccPAS-1500); or rds-ccPAS over the right dlPFC and right PPC at 750 pulses per session per site (rds-ccPAS-750). Because the goal of this study was to confirm the superiority of frontoparietal ccPAS to single-site stimulation, we chose single-site protocols as controls rather than sham stimulation.
All 4 intervention types used a low stimulation frequency of 1 Hz. In the rds-ccPAS groups, participants received stimulation first to the frontal lobe and then to the parietal lobe, with a between-site interval of 100 ms (Figure 1). The single-site protocol for each session consisted of 15 trains of 100 pulses, with inter-train intervals of 30 s. The rds-ccPAS-1500 protocol for each session consisted of 15 trains of 100 ccPAS pulses, with inter-train intervals of 30 s. The rds-ccPAS-750 protocol for each session consisted of 7 trains of 100 ccPAS pulses, with inter-train intervals of 30 s, plus 50 additional ccPAS pulses. The right dlPFC and right PPC sites corresponded to the F4 and P4 electrodes from the international 10–20 EEG system.32 We performed a total of 10 sessions of rTMS: 1 session per day within 14 days.
Fig. 1.
Frontoparietal paired associative stimulation. Coil A targeted the right dorsolateral prefrontal cortex (dlPFC), with the handle pointing backward and laterally, and oriented approximately 45o from the sagittal plane. Coil B targeted the right posterior parietal cortex (PPC), with the handle pointing backward and medially, and oriented approximately 45o from the sagittal plane.
Stimulation intensity was 90% of the RMT. We stimulated the right cerebral hemisphere and recorded motor evoked potentials on the left abductor pollicis brevis. We recorded the surface electromyogram using disc-shaped Ag-AgCl electrodes. The optimal stimulation site for the abductor pollicis brevis was usually 2 cm anterior and 2 cm lateral to the Cz (based on the international EEG 10–20 system). We defined RMT as the minimal magnetic stimulus intensity that could produce a TMS motor evoked potential in the relaxed abductor pollicis brevis with a peak-to-peak amplitude of greater than 50 μV in 5 of 10 trials.
An independent clinician (K.W.) developed the randomization schedule using a computerized random number generator. The double-blind design was mainly for clinical evaluators and participants; that is, the evaluators did not know which type of stimulation each participant received, and the participants did not know the pros and cons of the 4 stimulation protocols. We did not give participants any hints as to which paradigm might be better. We also asked participants not to discuss their feelings about treatments with each other.
Reasons for terminating the study included severe adverse events, missing 2 or more consecutive rTMS sessions, inability to attend a clinical evaluation and withdrawing consent.
Measures
We used the Clinical Global Impression–Severity scale to assess the global severity of the participant’s illness. Primary outcome measures were as follows: change in HAM-A score, number of treatment responders (defined as reduction of 50% or more on HAM-A) and number of patients who achieved remission (defined as HAM-A score less than 7). Secondary outcome measures were changes in the severity of depression symptoms (HAM-D) and insomnia symptoms (Pittsburgh Sleep Quality Index; PSQI). Clinical assessments were completed by the researchers (Q.Z., H.W., Y.L.), who were blinded to participants’ treatment conditions.
All participants were assessed at 4 time points: baseline, post-treatment, and 10 days and 1 month after the final treatment. Because patients who had not responded to rTMS treatment might have difficulty tolerating a long follow-up period, we performed 3-month follow-up visits only for those who were treatment responders at the 1-month visit. A longer follow-up time is clinically meaningful, but it is difficult to complete, and loss to follow-up would have been very common. As well, with a prolonged follow-up, patients would be more likely to seek and receive other treatments.
Statistical analysis
A professional statistician (S.H.) who was blinded to treatment conditions analyzed the data using SPSS version 22.0 (SPSS Inc.). For demographic and clinical data, we planned to use 1-way analysis of variance for normally distributed continuous variables (i.e., age, HAM-A, HAM-D, PSQI), the Kruskal–Wallis test for non-normally distributed continuous variables (i.e., education years, Clinical Global Impression–Severity) and the χ2 test for categorical variables (i.e., sex). However, we performed a normal distribution test and found that the data did not conform to normal distribution, so we could not use analysis of variance.
The generalized estimating equation (GEE),33 proposed by Liang and Zeger in 1986, is suitable for statistical models of data with different distributions, such as normal, binomial and Poisson. We had used the GEE model in a previous study to estimate the effect of transcranial direct current stimulation on epilepsy.34 Therefore, we used the GEE model for analysis of repeated measures based on Poisson log-linear distribution to compare clinical scores between the 4 time points and estimate group effects for each time period.
The GEE model included intercept, group effects, time and group × time interaction. If we found a significant group × time interaction, we used least significant difference to estimate the independent effects of group and time. To clarify the influence of individualized factors such as sex and age, we estimated the GEE model without covariates, and using sex or both sex and age as covariates. We used the Corrected Quasi Likelihood under Independence Model Criterion to evaluate goodness of fit (the smaller the value, the better the model).
We used the χ2 test to compare the number of responders and remitters between groups. We reported trend-level significance (p < 0.10) for hypothesis generation. We also performed a comparative analysis for reductions in HAM-A scores in the rds-ccPAS-1500 group using between-site intervals of 100 ms and 50 ms (results reported in our previous study31) to determine whether clinical efficacy was enhanced by prolonging the stimulation interval.
Results
Participants
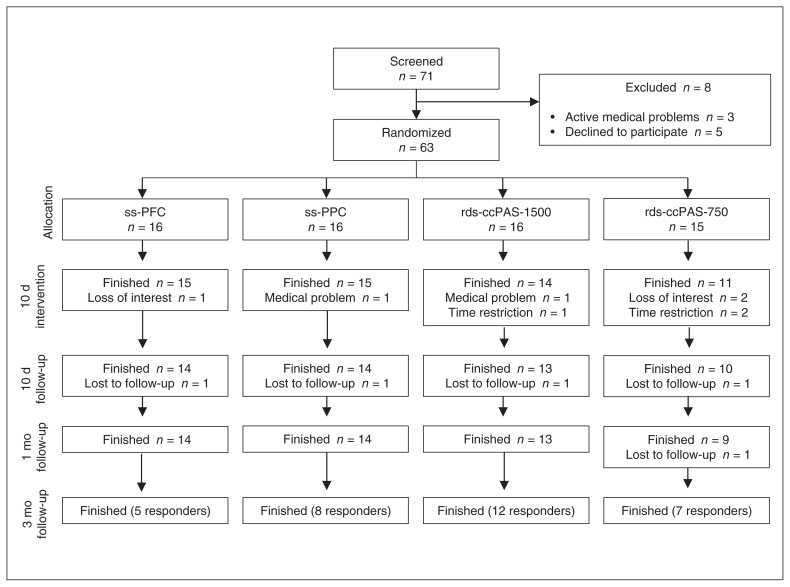
As shown in Figure 2, of the 71 patients we initially recruited, 8 passed the screening for enrolment criteria and signed an informed consent form but were excluded before they were assigned to treatment. During rTMS treatment, 1 patient in each of the ss-PFC and ss-PPC groups, 2 patients in the rds-ccPAS-1500 group and 4 patients in the rds-ccPAS-750 group discontinued the study because of time restrictions, medical problems or loss of interest. One patient in each of the 4 groups was lost to follow-up at the 10-day clinical visit. One patient in the rds-ccPAS-750 group was lost to follow-up at the 1-month visit. Retention rates were 83.3% (55/63) at post-treatment and 79.4% (50/63) at 1-month follow-up. We analyzed the data for the 50 patients who completed the treatment protocol and the 1-month follow-up. All patients who responded to rTMS treatment (including 9 in the rds-ccPAS-1500 group, 6 in the rds-ccPAS-750 group, 8 in the ss-PFC group and 6 in the ss-PPC group) completed the 3-month visit.
Fig. 2.
Trial profile. rds-ccPAS-750 = repetitive dual-site cortical–cortical paired associative stimulation, 750 pulses per session; rds-ccPAS-1500 = repetitive dual-site cortical–cortical paired associative stimulation, 1500 pulses per session; ss-PFC = single-site stimulation over the dorsolateral prefrontal cortex; ss-PPC = single-site stimulation over the right posterior parietal cortex.
Participant demographics (i.e., age, sex, education and employment status), clinical variables (i.e., medication status and scores on the Clinical Global Impression–Severity, HAM-A, HAM-D and PSQI scales) and RMT values at baseline did not differ between groups (Table 1). Group-level RMT values are shown in Table 1; participant-level RMT values can be found in Appendix 1, Table S1.
Table 1.
Participant demographic and clinical characteristics
| Characteristic | rds-ccPAS-1500 (n = 13) | rds-ccPAS-750 (n = 9) | ss-PFC (n = 14) | ss-PPC (n = 14) | F or χ2 | p value |
|---|---|---|---|---|---|---|
| Age, yr, mean ± SD | 40.15 ± 13.23 | 40.56 ± 9.30 | 40.71 ± 9.67 | 42.14 ± 6.98 | 0.129 | 0.94 |
| Male, n (%) | 3 (23.1) | 5 (55.6) | 6 (42.9) | 5 (35.7) | 2.577 | 0.46 |
| Education, yr, median (IQR) | 13.00 (11.00–13.00) | 13.00 (13.00–15.00) | 13.00 (10.25–15.00) | 15.00 (13.00–15.00) | 4.563 | 0.21 |
| Employed, n (%) | 12 (92.3) | 9 (100) | 14 (100) | 13 (92.9) | 1.780 | 0.62 |
| Taking medication, n (%) | 3 (23.1) | 1 (11.1) | 7 (50.0) | 2 (14.3) | 6.284 | 0.01 |
| CGI-S, median (IQR) | 5.00 (4.00–6.00) | 5.00 (4.50–5.50) | 5.00 (4.00–6.00) | 5.00 (4.00–5.00) | 1.164 | 0.76 |
| HAM-A score, mean ± SD | 23.2 ± 5.4 | 23.2 ± 3.9 | 22.1 ± 4.8 | 21.9 ± 4.9 | 0.272 | 0.85 |
| HAM-D score, mean ± SD | 15.1 ± 4.1 | 15.1 ± 3.8 | 14.2 ± 3.2 | 14.6 ± 4.6 | 0.145 | 0.93 |
| PSQI score, mean ± SD | 12.0 ± 3.9 | 12.0 ± 3.7 | 12.5 ± 3.3 | 11.9 ± 3.8 | 0.70 | 0.98 |
| RMT, mean ± SD | 41.5 ± 5.5 | 41.4 ± 6.6 | 45.4 ± 5.7 | 42.4 ± 4.8 | 1.52 | 0.22 |
CGI-S = Clinical Global Impression–Severity; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; IQR = interquartile range; PSQI = Pittsburgh Sleep Quality Index; rds-ccPAS-750 = repetitive dual-site cortical-cortical paired associative stimulation, 750 pulses per session; rds-ccPAS-1500 = repetitive dual-site cortical-cortical paired associative stimulation, 1500 pulses per session; RMT = resting motor threshold; SD = standard deviation; ss-PFC = single-site stimulation over the dorsolateral prefrontal cortex; ss-PPC = single-site stimulation over the right posterior parietal cortex.
Treatment efficacy
As shown in Table 2, the GEE models without covariates and with only sex as a covariate showed the best goodness of fit. Considering that the sex distribution between groups did not differ, we have presented the results of the GEE models without covariates here, and the other findings in Appendix 1, Table S2.
Table 2.
Between-group differences in clinical scores
| Adjusted difference | Mean difference (95% CI) p value, Cohen d | ||
|---|---|---|---|
|
| |||
| Post-treatment | 10-day follow-up | 1-month follow-up | |
| HAM-A | |||
| rds-ccPAS-1500 v. rds-ccPAS-750 | −5.26 (−9.30 to −1.21) | −2.37 (−7.66 to 2.93) | −2.86 (−8.96 to 3.32) |
| p = 0.011*, d = −1.147 | p = 0.38, d = −0.371 | p = 0.36, d = −0.394 | |
| rds-ccPAS-1500 v. ss-PFC | −6.14 (−9.06 to −3.22) | −3.42 (−7.94 to 1.09) | −4.66 (−9.85 to 0.52) |
| p < 0.001*, d = −1.577 | p = 0.14, d = −0.576 | p = 0.08†, d = −0.684 | |
| rds-ccPAS-1500 v. ss-PPC | −5.78* (−9.67 to −1.89) | −2.71 (−7.81 to 2.39) | −2.74 (−7.77 to 2.29) |
| p = 0.004*, d = −1.109 | p = 0.30, d = −0.402 | p = 0.29, d = −0.415 | |
| HAM-D | |||
| rds-ccPAS-1500 v. rds-ccPAS-750 | −2.79 (−6.47 to 0.88) | −0.62 (−4.82 to 3.59) | −1.39 (−7.01 to 1.09) |
| p = 0.14, d = −0.670 | p = 0.78, d = −0.127 | p = 0.63, d = −0.218 | |
| rds-ccPAS-1500 v. ss-PFC | −3.75 (−6.35 to −1.15) | −1.76 (−4.68 to 1.16) | −2.62 (−6.32 to 1.09) |
| p = 0.005*, d = −1.086 | p = 0.24, d = −0.458 | p = 0.17, d = −0.535 | |
| rds-ccPAS-1500 v. ss-PPC | −3.03 (−6.14 to 0.08) | −0.83 (−4.56 to 2.91) | −1.62 (−5.36 to 2.13) |
| p = 0.06†, d = −0.730 | p = 0.66, d = −0.167 | p = 0.40, d = −0.327 | |
| PSQI | |||
| rds-ccPAS-1500 v. rds-ccPAS-750 | −1.85 (−4.79 to 1.10) | −2.56 (−4.03 to 2.56) | −1.70 (−4.99 to 1.58) |
| p = 0.22, d = −0.547 | p = 0.66, d = −0.188 | p = 0.31, d = −0.436 | |
| rds-ccPAS-1500 v. ss-PFC | −3.20 (−6.56 to 0.16) | −3.06 (−5.76 to 0.64) | −2.71 (−5.83 to 0.41) |
| p = 0.06†, d = −0.712 | p = 0.12, d = −0.604 | p = 0.09†, d = −0.654 | |
| rds-ccPAS-1500 v. ss-PPC | −3.78 (−6.62 to −1.03) | −0.74 (−6.17 to 0.05) | −3.21 (−6.28 to −0.14) |
| p = 0.007*, d = −1.032 | p = 0.05†, d = −0.743 | p = 0.040*, d = −0.790 | |
CI = confidence interval; HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; PSQI = Pittsburgh Sleep Quality Index; rds-ccPAS-750 = repetitive dual-site cortical-cortical paired associative stimulation, 750 pulses per session; rds-ccPAS-1500 = repetitive dual-site cortical-cortical paired associative stimulation, 1500 pulses per session; ss-PFC = single-site stimulation over the dorsolateral prefrontal cortex; ss-PPC = single-site stimulation over the right posterior parietal cortex.
Significant difference as estimated by generalized estimating equation (p < 0.05).
Trend-level difference as estimated by generalized estimating equation (p < 0.1); degree of freedom was 1.
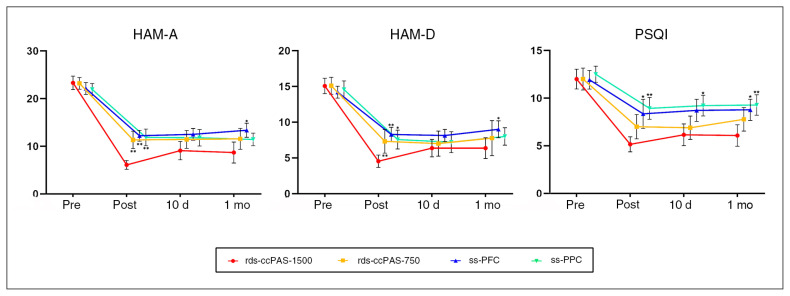
The GEE model without covariates showed significant group × time interactions for HAM-A score (χ2 = 26.367, p = 0.002), HAM-D score (χ2 = 18.445, p = 0.030) and PSQI score (χ2 = 24.295, p = 0.004). All 4 groups showed significant reductions in total scores on the HAM-A, HAM-D and PSQI at the post-treatment, 10-day and 1-month visits (p < 0.001). Model-estimated differences between groups in terms of treatment-related changes are shown in Table 2 and Figure 3.
Fig. 3.
Generalized estimating equation model for treatment-related differences between groups. Mean scores on the (A) HAM-A, (B) HAM-D and (C) PSQI estimated by the generalized estimating equation model for 4 groups at 4 time nodes. **Clinical scores of the rds-ccPAS-1500 group were significantly different from those of the other groups (p < 0.05). *Clinical scores of the rds-ccPAS-1500 group were marginally different from those of the single-site groups (p < 0.1). Bars represent standard error. HAM-A = Hamilton Anxiety Rating Scale; HAM-D = Hamilton Depression Rating Scale; PSQI = Pittsburgh Sleep Quality Index; rds-ccPAS-750 = repetitive dual-site cortical–cortical paired associative stimulation, 750 pulses per session; rds-ccPAS-1500 = repetitive dual-site cortical–cortical paired associative stimulation, 1500 pulses per session; ss-PFC = single-site stimulation over the dorsolateral prefrontal cortex; ss-PPC = single-site stimulation over the right posterior parietal cortex.
Post-treatment, the rds-ccPAS-1500 group showed significantly greater reductions in HAM-A scores than the rds-ccPAS-750 group (p = 0.011), the ss-PFC group (p < 0.001) and the ss-PPC group (p = 0.004). At the 1-month visit, we observed a trend-level significance in the reduction of HAM-A scores in the rds-ccPAS-1500 group compared to the ss-PFC group (p = 0.08).
Post-treatment, the rds-ccPAS-1500 group showed significantly greater reductions in HAM-D scores than the ss-PFC group (p = 0.005) and a trend-level greater reduction compared to the ss-PPC group (p = 0.06). Differences in HAM-D scores between the rds-ccPAS-1500 group and the single-site groups did not reach statistical significance at the 10-day or 1-month visits.
Post-treatment, the rds-ccPAS-1500 group showed significantly greater reductions in PSQI scores than the ss-PPC group (p = 0.007) and a trend-level greater reduction compared to the ss-PFC group (p = 0.06). We also found a trend-level greater reduction in the rds-ccPAS-1500 group compared to the ss-PPC group at the 10-day visit (p = 0.05). At the 1-month visit, the rds-ccPAS-1500 group showed significantly greater reductions in PSQI scores than the ss-PPC group (p = 0.040) and a trend-level greater reduction compared to the ss-PFC group (p = 0.09).
The results of the GEE model using sex or both sex and age were consistent with the results without covariates (Appendix 1, Tables S3 and S4).
Additional comparative analyses for rds-ccPAS-1500 using 100 ms and 50 ms between-site intervals showed that post-treatment, 100 ms was associated with a more significant reduction in HAM-A score than 50 ms (0.74 ± 0.16 v. 0.39 ± 0.23; t = −4.529, p < 0.001).
Responders and remitters
Post-treatment, significantly more patients achieved responder status in the rds-ccPAS-1500 group (92.3%, 12/13) than in the ss-PFC group (35.7%, 5/14; χ2 = 9.258, p = 0.002). We also found significantly more remitters in the rds-ccPAS-1500 group (53.8%, 7/13) than in the ss-PFC group (14.3%, 2/14; χ2 = 8.429, p = 0.004) or the ss-PPC group (14.3%, 2/14; χ2 = 8.429, p = 0.004). At the 1-month visit, we found significantly more remitters in the rds-ccPAS-1500 group (53.8%, 7/13) than in the ss-PFC group (7.1%, 1/14; χ2 = 7.052, p = 0.008). After Bonferroni correction (p < 0.05/6 = 0.0083), the differences in treatment response at the 3-month visit were still significant between the rds-ccPAS-1500 group (100%, 9/9) and the ss-PFC (50%, 4/8) and ss-PPC (50%, 3/6) groups.
Adverse events and safety
One patient in each the rds-ccPAS-1500 group and 1 patient in the ss-PPC group reported headaches and discontinued treatment. One patient in the ss-PFC group reported an aggravation of anxiety and insomnia symptoms within 2 days after starting treatment and withdrew from the study. No seizures, neurologic complications or subjective complaints about cognitive impairment were reported.
Discussion
This study compared the treatment efficacy of frontoparietal rds-ccPAS and single-site rTMS in patients with GAD. In the absence of a sham control, both rds-ccPAS and single-site stimulation induced significant reductions in clinical scores for anxiety, depression and insomnia. More importantly, compared to single-site stimulation, rds-ccPAS-1500 induced greater improvements in anxiety, depression and insomnia post-treatment, and in anxiety and insomnia at 10-day and 1-month follow-up. As well, the rds-ccPAS-1500 group had more treatment responders and remitters.
The results for single-site stimulation were consistent with the findings of previous studies.3,4,20 GAD is characterized by disturbances in emotional processing and regulation.11–13 As a critical region involved in emotional regulation, the dlPFC has been used as a stimulating site for rTMS treatment of GAD. Preliminary evidence with open-label3,6 and randomized, double-blind, sham-controlled4 designs suggests that low-frequency rTMS over the right dlPFC may improve symptoms in patients with GAD.
Although there is less research evidence for stimulation of the parietal lobe, some studies have suggested the potential of stimulating the right PPC to treat anxiety disorder. Abnormally increased cortical excitability has been observed in the right parietal cortex of patients with GAD as measured by somatosensory evoked potential,35 and inhibitory rTMS applied to the right PPC increased the reaction time cost for threatening distractors.36 Low-frequency rTMS over the right PPC has shown efficacy in the treatment of anxiety with insomnia.20
Our results showed that dual-site stimulation can lead to better therapeutic effects than single-site stimulation, by delivering twice as many pulses within approximately the same time. These findings may be explained by the aforementioned hypothesis of GAD10 and the “hub” role of the frontoparietal network in cognitive control.17,18 The coexisting functional abnormalities37–39 in the prefrontal and parietal cortices (i.e., functional connectivity and grey matter volume) in GAD also indicate that stimulating both the prefrontal and parietal cortices may lead to stronger treatment efficacy than single-site stimulation. A possible mechanism is that associative stimulation may improve the cognitive control ability of the frontoparietal network more strongly than single-site stimulation, subsequently inhibiting excessive emotional response generated by the limbic system. Further studies are needed to compare neural mechanisms between dual-site and single-site stimulation.
Our results indicate that a 100 ms between-site interval may be an optimized parameter for frontoparietal rds-ccPAS to treat GAD. The biological effect of ccPAS depends on both the stimulating interval and the direction of the 2 sites. By stimulating 2 interconnected areas with different intervals and directions, the physiologic effects can be very different. Using the opposite directions for ccPAS than we did in the present study produced distinct effects for prefrontal and parietal activation.26,28 Building on our previous finding31 of increased clinical efficacy with rds-ccPAS at 50 ms compared to shorter intervals, we prolonged the stimulating interval to 100 ms in the present study and found that at this interval, treatment efficacy was greater than with an interval of 50 ms. A possible explanation for this finding is that when the time interval is increased to 100 ms, the STDP-like antagonistic effect between the 2 sites may be eliminated.
Considering that the number of pulses may also be a reason for differences between dual-site and single-site stimulation, we created a dual-site group (rds-ccPAS-750) that received the same total number of pulses as the single-site stimulation groups. We found that the clinical efficacy of rds-ccPAS-1500 was stronger than that of rds-ccPAS-750, and that the rds-ccPAS-750 group did not show stronger clinical efficacy than single-site stimulation. It is possible that at a between-site interval of 100 ms, frontal stimulation did not produce a facilitation modulation on the stimulating effect of the parietal lobe; it is also possible that the stimulating effects for the 2 sites were independent.
We evaluated 3 clinical scales (HAM-A, HAM-D and PSQI) and found that the total scores for all scales decreased after treatment in all 4 groups. Between-group comparisons showed that scores on each of the 3 clinical scales decreased for at least 1 of the follow-up points. These results were consistent with the phenomenon of commonly coexisting symptoms of anxiety, depression and insomnia in patients with GAD.
We found significant or trend-level improvements in anxiety and insomnia symptoms at the 10-day and 1-month visits in several comparisons between rds-ccPAS-1500 group and the single-site groups (at 10 days for rds-ccPAS-1500 v. ss-PPC on the PSQI; at 1 month for rds-ccPAS-1500 v. ss-PFC on the HAM-A; at 1 month for rds-ccPAS-1500 v. ss-PFC on the HAM-D; at 1 month for rds-ccPAS-1500 v. ss-PFC and ss-PPC on the PSQI). Comparisons at other clinical visits did not show statistically significant differences, suggesting that the advantage of dual-site over single-site stimulation was only weakly maintained after treatment was terminated. This finding emphasizes the need for consolidation therapy to better maintain the advantage of dual-site rTMS. Evidence for GAD is lacking, but studies in treatment-resistant depression40,41 have confirmed a remarkable benefit of continuous treatment for patients who respond to rTMS.
The major contribution of this research is to indicate an appropriate stimulation interval for rds-ccPAS to produce better clinical efficacy than single-site stimulation. As indicated by our previous findings, frontoparietal associative stimulation does not necessarily produce a stronger effect than single-site stimulation or rds-ccPAS with very short intervals (i.e., 10 or 20 ms).31 Therefore, the appropriate between-site interval for dual-site stimulation is key to ensuring clinical efficacy. Another contribution is that we used a new technology — rds-ccPAS for rTMS treatment — which can deliver twice as many stimulation pulses than single-site stimulation in approximately the same time.
Limitations
The small n value for each treatment paradigm (n = 9–14) was one of the major limitations of this study, partly because the COVID-19 pandemic affected case collection. Recruiting a larger sample of patients with GAD may help to confirm our findings for rds-ccPAS and make it possible to follow responders for a longer period to identify the duration of treatment effect.
Another limitation may have been a lack of comparison between dual-site stimulation with 1500 ccPAS pulses and single-site stimulation with 3000 pulses, making it impossible to conclude whether it was the greater number of pulses or the dual-site approach that induced greater clinical improvement. It might be also useful to measure the effect on cortical dynamics of dual-site ccPAS with 10 ms versus 100 ms intervals, using electrophysiological technologies to determine whether ccPAS at 100 ms intervals enhanced inhibition of the PPC by increasing long-term depression, and whether the 10 ms interval has the opposite effect.
We used single-site stimulation as a control, but the most ideal control would have been dual-site sham stimulation, which could clarify whether clinical efficacy was because of TMS or a placebo effect.
Finally, although the EEG system has a reasonable correspondence with cortical areas, we could not rule out the possibility that the F4 and P4 electrodes included adjacent brain regions. The location of stimulating sites based on individualized brain activation may increase clinical efficacy.
Conclusion
Overall, our preliminary evidence suggests that a protocol of 10 sessions of 1 Hz frontoparietal rds-ccPAS with a between-site interval of 100 ms (rather than shorter intervals) provided better benefits to patients with GAD than single-site protocols, in almost the same treatment time. Future studies comparing dual-site and single-site stimulation with the same number of stimulating pulses will help to clarify whether the larger number of pulses (3000) or the dual-site approach led to improvements in clinical efficacy.
Supplementary Information
Acknowledgement
The authors are grateful to all participants for their cooperation.
Footnotes
Competing interests: None declared.
Contributors: Y. Yang and Y. Wang designed the study. L. Wang, Q. Zhou, K. Wang, H. Wang and Y. Yang acquired the data, which S. Hu and Y. Lin analyzed. L. Wang, Q. Zhou and S. Hu wrote the article, which K. Wang, H. Wang, Y. Yang, Y. Lin and Y. Wang reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: The work was supported by the National Key Research and Development Program of China (grant no. 2018YFC1314500); the National Natural Science Foundation of China (grant no. 81771398; 81801124); the China Postdoctoral Science Foundation (grant no. 2018M641413); and the Beijing Postdoctoral Funding Project (grant no. 2018-22-111).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth edition. Arlington (VA): American Psychiatric Association Publishing; 2013. [Google Scholar]
- 2.Stein MB, Sareen J. Clinical practice. Generalized anxiety disorder. N Engl J Med 2015;373:2059–68. [DOI] [PubMed] [Google Scholar]
- 3.Bystritsky A, Kaplan JT, Feusner JD, et al. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. J Clin Psychiatry 2008;69:1092–8. [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach GJ, Bragdon LB, Zertuche L, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry 2016; 209:222–8. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JM, Phan KL, Kennedy AE, et al. Prefrontal and amyg-dala engagement during emotional reactivity and regulation in generalized anxiety disorder. J Affect Disord 2017;218:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White D, Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann Clin Psychiatry 2015;27:192–6. [PubMed] [Google Scholar]
- 7.Rodrigues PA, Zaninotto AL, Neville IS, et al. Transcranial magnetic stimulation for the treatment of anxiety disorder. Neuropsychiatr Dis Treat 2019;15:2743–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Wang J, Li C, et al. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Rev 2014;2014:CD009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015;16:317–31. [DOI] [PubMed] [Google Scholar]
- 10.LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two system framework. Am J Psychiatry 2016;173:1083–93. [DOI] [PubMed] [Google Scholar]
- 11.Makovac E, Meeten F, Watson DR, et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol Psychiatry 2016;80:786–95. [DOI] [PubMed] [Google Scholar]
- 12.Porta-Casteràs D, Fullana MA, Tinoco D, et al. Prefrontal-amygdala connectivity in trait anxiety and generalized anxiety disorder: testing the boundaries between healthy and pathological worries. J Affect Disord 2020;267:211–9. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald JM, Phan KL, Kennedy AE, et al. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J Affect Disord 2017;218:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergallito A, Gallucci A, Pisoni A, et al. Effectiveness of noninvasive brain stimulation in the treatment of anxiety disorders: a meta-analysis of sham or behaviour-controlled studies. J Psychiatry Neurosci 2021;46:E592–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson PM, Jahanshad N, Ching CRK, et al. ENIGMA and global neuroscience: a decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry 2020;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petro NM, Gruss LF, Yin S, et al. Multimodal imaging evidence for a frontoparietal modulation of visual cortex during the selective processing of conditioned threat. J Cogn Neurosci 2017;29:953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole MW, Repovš G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist 2014;20:652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole MW, Reynolds JR, Power JD, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013;16:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balderston NL, Beydler EM, Goodwin M, et al. Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Transl Psychiatry 2020;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul 2018;11:1103–9. [DOI] [PubMed] [Google Scholar]
- 21.Braun U, Schaefer A, Betzel RF, et al. From maps to multi-dimensional network mechanisms of mental disorders. Neuron 2018;97:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng ZD, Luber B, Balderston NL, et al. Device-based modulation of neurocircuits as a therapeutic for psychiatric disorders. Annu Rev Pharmacol Toxicol 2020;60:591–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fricke C, Duesmann C, Woost TB, et al. Dual-site transcranial magnetic stimulation for the treatment of Parkinson’s disease. Front Neurol 2019;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehner A, Schecklmann M, Kreuzer PM, et al. Comparing single-site with multisite rTMS for the treatment of chronic tinnitus — clinical effects and neuroscientific insights: study protocol for a randomized controlled trial. Trials 2013;14:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo V, Siebner H, Morgante F, et al. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex 2009;19:907–15. [DOI] [PubMed] [Google Scholar]
- 26.Vignoud G, Venance L, Touboul JD. Interplay of multiple pathways and activity-dependent rules in STDP. PLOS Comput Biol 2018;14:e1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santarnecchi E, Momi D, Sprugnoli G, et al. Modulation of network-to-network connectivity via spike-timing-dependent noninvasive brain stimulation. Hum Brain Mapp 2018;39:4870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casula EP, Pellicciari MC, Picazio S, et al. Spike-timing-dependent plasticity in the human dorso-lateral prefrontal cortex. Neuroimage 2016;143:204–13. [DOI] [PubMed] [Google Scholar]
- 29.Kohl S, Hannah R, Rocchi L, et al. Cortical paired associative stimulation influences response inhibition: cortico-cortical and cortico-subcortical networks. Biol Psychiatry 2019;85:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nord CL, Popa T, Smith E, et al. The effect of frontoparietal paired associative stimulation on decision-making and working memory. Cortex 2019;117:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Chen P, Yang K, et al. Efficacy of repetitive dual-site paired associative transcranial magnetic stimulation in the treatment of generalized anxiety disorder. Brain Stimul 2020;13:1170–2. [DOI] [PubMed] [Google Scholar]
- 32.Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 2003;16:95–9. [DOI] [PubMed] [Google Scholar]
- 33.Hanley JA, Negassa A, Edwardes MD, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–75. [DOI] [PubMed] [Google Scholar]
- 34.Yang D, Wang Q, Xu C, et al. Transcranial direct current stimulation reduces seizure frequency in patients with refractory focal epilepsy: a randomized, double-blind, sham-controlled, and three-arm parallel multicenter study. Brain Stimul 2020;13:109–16. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z, Zhan S, Chen C, et al. The effect of insomnia on cortical excitability in patients with generalized anxiety disorder. Front Psychiatry 2019;9:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulckhuyse M, Engelmann JB, Schutter DJLG, et al. Right posterior parietal cortex is involved in disengaging from threat: a 1-Hz rTMS study. Soc Cogn Affect Neurosci 2017;12:1814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair KS, Geraci M, Smith B, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry 2012;72:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker JT, Dillon DG, Patrick LM, et al. Functional connectomics of affective and psychotic pathology. Proc Natl Acad Sci U S A 2019;116:9050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Z, Wang C, Hines CS, et al. Frontoparietal network abnormalities of gray matter volume and functional connectivity in patients with generalized anxiety disorder. Psychiatry Res Neuroimaging 2019;286:24–30. [DOI] [PubMed] [Google Scholar]
- 40.Benadhira R, Thomas F, Bouaziz N, et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res 2017;258:226–33. [DOI] [PubMed] [Google Scholar]
- 41.Haesebaert F, Moirand R, Schott-Pethelaz AM, et al. Usefulness of repetitive transcranial magnetic stimulation as a maintenance treatment in patients with major depression. World J Biol Psychiatry 2018;19:74–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.