Abstract
Background:
Patient management after bariatric surgery is important in controlling patients’ diabetes and recurrence prevention. This study aimed to meet the medical managements of patients with diabetes 6 months after the bariatric surgery.
Materials and Methods:
This cross-sectional study was performed on 77 type 2 diabetes patients’ candidates for bariatric surgery (Roux-en-Y [RYGP] and Omega). Postoperative implementation protocol was one-third of insulin for patients taking long-term insulin and the discontinuation of medications for patients of oral antidiabetic agents. Blood glucose (BG) level was checked regularly by the patients at home and the necessary medical management was applied. The weight, BG and HbA1C levels, and use of oral antidiabetic agents and insulin were assessed and recorded before 1, 3, and 6 months after the surgery.
Results:
BG levels and HbA1C percentage in the 1st, 3rd, and 6th months after the intervention in Omega group were significantly lower than RYGB group (P value < 0.05). At 1 and 3 months after surgery, the mean insulin dose received by the Omega and RYGB groups was reduced to <30 units/day and 10 units/day, respectively, following the management protocol in this study. Moreover, 23.1% and 7.7% of patients in RYGB group and 12.1% and 3% of patients in Omega group took oral antidiabetic agents 1and 3 months after surgery, respectively. Insulin and oral antidiabetic agents were completely discontinued 6 months after the surgery.
Conclusion:
The long-term management and support of the patients by the implementation of a standard protocol after surgery are of great significance in obtaining the optimal outcome after bariatric surgery.
Keywords: Antidiabetic Agents, Bariatric Surgery, Insulin, Patient Care Management, obesity, Roux-en-Y Anastomosis, Type 2 Diabetes
Introduction
The prevalence of obesity is increasing worldwide such that one-third and 20% of adults in some fully developed and developing countries such as Iran are obese, respectively.[1,2] Obese individuals are at the risk of type 2 diabetes (T2D), hypertension, osteoarthritis, and some cancers such as colorectal.[3,4] Severe obesity, as compared to the normal weight, is associated with a more than 8-fold increased relative risk of diabetes.[5] As more than 60% of individuals with diabetes are obese, the combined treatment of obesity and T2D is currently considered a public health priority.[6]
In this regard, the statement presented by the U. S. National Institutes of Health in 1991 expressed that all individuals with a BMI above 40 kg/m2 and those with a BMI between 40 and 35 kg/m2 with comorbidities and lifestyle interference were candidates for surgical treatment. The mentioned statement is the most accepted general guideline for identifying indications for the bariatric surgery for obese individuals with comorbidities. Among the mentioned categories of patients, those with T2D are the most common indications for surgery.[7,8]
Bariatric surgery is the surgical manipulation of part of the digestive system to limit food and/or calorie intake.[9] Over the last 6 years as compared to previous years, the number of bariatric surgeries has almost quadrupled.[10] Each type of this surgery can be significantly associated with the weight loss.[10,11,12] In addition, recent research has reported an improvement or reduction in diabetes, hypertension, and dyslipidemia following these surgeries. For instance, some studies have revealed that the use of antidiabetic agents was significantly reduced within 1 month after laparoscopic sleeve gastrectomy and was maintained or further reduced over longer periods.[13,14,15] In another study, a significant reduction was reported in terms of the use of antihypertensive agents, and nine patients discontinued their antihypertensive agents 6 months after the surgery.[16] In addition, Malone and Alger-Mayer examined the changes in the class of antidiabetic agents 1 year after the Roux-en-Y gastric bypass (RYGB) surgery and indicated a significant reduction in the use of antidiabetic and antihypertensive agents as well as lipid-lowering drugs.[17] In a retrospective study addressing more than 2000 patients with diabetes, 85% of patients, majority of whom received the RYGB surgery, did not take any more antidiabetic agents 2 years after surgery.[18]
Thus, the bariatric surgery seems to have been associated with promising results in reducing the use of antidiabetic agents;[13,14,19] however, fewer studies have paid attention to patient management after surgery. It appears that more acceptable results can be obtained if the status of patients’ blood glucose parameters is followed up after the surgery and antidiabetic medication is prescribed based on a specific protocol considering patients’ condition before surgery. Therefore, the present study aimed at examining whether the medical management of patients with diabetes 1, 3, and 6 months after the bariatric surgery (Omega and RYGB) can play an effective role in reducing the use of antidiabetic agents.
Materials and Methods
The population of the present, cross-sectional, prospective study included all patients with T2D that were candidates for the bariatric surgery and referred to Al-Zahra Hospital. The sample size of 87 patients was randomly selected from the mentioned population considering the confidence level of 95%, test power of 80%, the error level of 0.15, and the results of previous studies reporting the ratio of reducing the use of antidiabetic agents 1 month after the bariatric surgery to be equal to 50%.[20]
The inclusion criteria were patients that had T2D, aged over 19 years, were candidate for bariatric surgery (two classic procedures of Roux-en-Y and Omega), had the body mass index (BMI) of ≥35 kg/m2 and one or more comorbidities or had the BMI of >40 kg/m2 without any comorbidities, and consented to participate in the study. Moreover, the patients were excluded from the study in case of surgical complications, refusal to measure and deliver fasting blood glucose level after the surgery, and dissatisfaction with their cooperation in the study. In this study, ten patients were excluded from the study due to their lack of regular follow-ups and nonmeasurement of blood glucose level over 6 months so that the sample size was reduced to 77 patients.
It should be noted that the diagnosis was made by the surgeon, and typically patients that were candidates for the bariatric surgery had the same basic characteristics. In addition, before the surgery, all patients’ blood glucose level was adjusted for the surgery. Therefore, at the beginning of the study, the patients’ baseline characteristics and blood glucose levels could not be considered confounding factors.
The diagnosis of T2D was first defined and evaluated as the fasting glucose level equal to or >126 mg/dl or glycosylated hemoglobin (HbA1C) level ≥6.5%.[21] Other patients with medically-managed diabetes were identified through the medication profile as well as the medical history reported by the patients.
After obtaining the code of ethics from the Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.MED.REC.1399.990) and obtaining written consent from the eligible patients, their demographic and clinical characteristics such as age, sex, various diseases associated with obesity, systolic and diastolic blood pressure, HbA1C, fasting blood glucose, the use of oral antidiabetic agents and insulin before and after the surgery, and anthropometric measurements before and after the surgery such as weight, height, and BMI were recorded.
It should be taken into consideration that after surgery, the drug dose and its administration procedure before surgery were specified based on the duration of diabetes and according to the standard protocol of oral antidiabetic agents or insulin. In details, after surgery, one-third of insulin was prescribed for patients that had been taking insulin for a long time, and their blood glucose level was regularly checked and reported by the patient. For patients taking oral antidiabetic agents, the medications were discontinued completely. Moreover, the patients were regularly supposed to check their blood glucose level at home and were requested to consult with a physician if their blood glucose level reached above 180 mg/dl.[20]
It should be noted that a diet protocol including food abstinence and food consumption instructions was provided for patients from 1 month before the surgery to prevent the potential effect of the patients’ diet. Moreover, patients were encouraged to continue following this protocol after the surgery. To ensure the patients’ due attention in this respect, the patients were called twice a month and requested to follow the instructions. Our assumption was based on patients’ honesty.
The reduction or the nonuse of oral antidiabetic agents or insulin[20] was questioned and recorded in person at the center or on telephone 1, 3, and 6 months after the surgery. Moreover, the percentage of change in total body weight was calculated and recorded using the following formula: ([W0-W1]/W0) ×100%.[22]
Finally, the collected data were entered into the SPSS software version 26. Data were presented as means ± standard deviation or n (%). At the level of inferential statistics, the Chi-squared test, independent samples t-test, and univariate analysis were used by adjusting the confounding factors, including age, anthropometric indices, blood pressure, and comorbidities to compare the mean of the variable between the two groups in each of the studied times. In addition, repeated measures ANOVA were also used to compare the mean changes of the variable over 6 months in each of the two groups. The significance level of <0.05 was considered in all analyses.
Results
In the present study, out of 77 patients that underwent the bariatric surgery, 43 and 34 patients underwent the Omega and RYGB surgery, respectively. In the Omega group with the mean age of 40.2 ± 11.23 years, 11 (14.3%) and 66 (85.7%) patients were male and female, respectively. In the RYGB group with a mean age of 41.37 ± 12.44 years, 7 (16.3%) and 36 (83.7%) patients were male and female, respectively (P > 0.05). In addition, the two groups did not differ significantly in terms of other basic and clinical characteristics including height, weight, BMI, comorbidities, and blood pressure (P > 0.05) [Table 1].
Table 1.
Basic and clinical characteristics of patients in the two groups
| Characteristics | Total (n=77) | Omega (n=43) | RYBP (n=34) | P |
|---|---|---|---|---|
| Age (years) | 40.61±12.03 | 40.02±11.23 | 41.37±12.44 | 0.618 |
| Sex, n (%) | ||||
| Male | 11 (14.3) | 7 (16.3) | 4 (11.8) | 0.574 |
| Female | 66 (85.7) | 36 (83.7) | 30 (88.2) | |
| Weight (kg) | 117.77±19.90 | 118.65±22.19 | 116.65±16.83 | 0.664 |
| Height (m) | 1.59±0.06 | 1.58±0.08 | 1.61±0.04 | 0.085 |
| BMI (kg/m2) | 46.59±8.01 | 47.61±8.74 | 45.30±6.90 | 0.212 |
| Comorbidity, n (%) | ||||
| T2D | 77 (100) | 43 (100) | 34 (100) | - |
| Hypertension | 31 (40.2) | 18 (41.9) | 13 (38.2) | 0.083 |
| Blood pressure (mm Hg) | ||||
| Systolic | 132.80±9.54 | 131.34±11.38 | 134.64±6.21 | 0.132 |
| Diastolic | 81.64±12.92 | 81.93±12.56 | 81.27±13.53 | 0.657 |
T2D: Type 2 diabetes, BMI: Body mass index, RYBP: Roux-en-Y gastric bypass
Furthermore, the patients’ fasting glucose and HbA1C levels did not differ significantly between the two groups before the surgery (P > 0.05). However, there was a significant decrease in the fasting glucose and HbA1C levels in both groups from the 1st month after the surgery. Fasting glucose levels in the 1st, 3rd, and 6th months after the intervention in the Omega group with the mean of 116.23 ± 29.93 mg/dl, 110.77 ± 31.65 mg/dl, and 107.42 ± 31.74 mg/dl, respectively, were significantly lower than those of the RYGB group with the mean of 150.12 ± 40.37 mg/dl, 126.09 ± 30.79 mg/dl, and 122.91 ± 36.26 mg/dl (P value < 0.05). Moreover, 1, 3, and 6 months after the surgery, the HbA1C percentages in the Omega group with the mean of 6.01 ± 1.35, 5.81 ± 1.22, and 5.72 ± 1.31 were significantly lower than those of the RYGB group with the mean of 6.66 ± 1.32, 6.42 ± 1.25, and 6.45 ± 1.32, respectively (P < 0.05) [Table 2].
Table 2.
Comparison of the patients’ mean fasting glucose and hemoglobin A1C levels between the two groups
| Variables | Total (n=77) | Omega (n=43) | RYBP (n=34) | P a |
|---|---|---|---|---|
| Fasting glucose (mg/dl) | ||||
| Baseline | 171.44±45.91 | 171.86±44.80 | 170.91±47.94 | 0.929 |
| 1 month after surgery | 131.19±38.59 | 116.23±29.93 | 150.12±40.37 | <0.001 |
| 3 months after surgery | 117.53±32.00 | 110.77±31.65 | 126.09±30.79 | 0.036 |
| 6 months after surgery | 114.26±34.46 | 107.42±31.74 | 122.91±36.26 | 0.048 |
| P b | <0.001 | <0.001 | <0.001 | |
| HbA1C (%) | ||||
| Baseline | 7.94±1.57 | 8.08±1.32 | 7.76±1.84 | 0.384 |
| 1 month after surgery | 6.33±1.36 | 6.01±1.35 | 6.66±1.32 | 0.038 |
| 3 months after surgery | 6.08±1.26 | 5.81±1.22 | 6.42±1.25 | 0.034 |
| 6 months after surgery | 6.04±1.35 | 5.72±1.31 | 6.45±1.32 | 0.018 |
| P b | <0.001 | <0.001 | 0.003 |
aSignificance level obtained from the univariate analysis by adjusting for the age, anthropometric indices, blood pressure, and comorbidities to compare the mean of the variable between the two groups in each of the studied times, bSignificance level obtained from the repeated measures ANOVA comparing the changes in the mean of the variable over 6 months in each of the two groups. HbA1C: Hemoglobin A1C, RYBP: Roux-en-Y gastric bypass
Insulin was used before the surgery for 10 (23.3%) patients with an average dose of 74.00 ± 17.76 units/day in the Omega group and for 8 (23.5%) patients with an average dose of 77.50 ± 19.09 units/day in the RYGB group (P > 0.05). According to the postoperative diabetes management protocol in this study, each patient's insulin intake was reduced to one-third of daily unit. Thus, 1 month after the surgery, the average insulin dose received by Omega and RYGB groups was reduced to 29.10 ± 12.07 and 29.50 ± 14.99 units/day, respectively (P > 0.05). In the 3rd month after the surgery, the insulin dose in the Omega and RYGB groups was 7.70 ± 7.77 and 10.25 ± 9.04 units/day, respectively (P = 0.529). In the 6th month after the surgery, insulin intake was completely discontinued and no patient received insulin [Table 3]. Moreover, before the surgery, 33 (76.7%) patients in the Omega group and 26 (76.5%) patients in the RYGB group had taken oral antidiabetic agents. Moreover, oral antidiabetic agents were prescribed for 4 (12.1%) and 1 (3%) patients in the Omega group and 6 (23.1%) and 2 (7.7%) patients in the RYGB group 1 and 3 months after the surgery, respectively. However, no patient required oral antidiabetic agents 6 months after the surgery [Figure 1].
Table 3.
Patients’ insulin intake before and after the surgery in the two groups
| Insulin | Omega (n=43) | RYBP (n=34) | P |
|---|---|---|---|
| Baseline, n (%) | 10 (23.3) | 8 (23.5) | |
| 74.00±17.76 | 77.50±19.09 | 0.693 | |
| 1 month after surgery | 29.10±12.07 | 29.50±14.99 | 0.951 |
| 3 months after surgery | 7.70±7.77 | 10.25±9.04 | 0.529 |
| 6 months after surgery | - | - | - |
RYBP: Roux-en-Y gastric bypass
Figure 1.
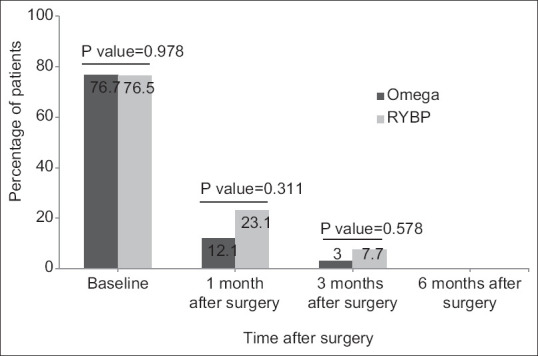
Determination and comparison of the percentage of patients taking oral antidiabetic agents after the surgery between the two groups
Finally, the percentage of weight changes in patients was not significantly different between the two groups 1, 3, and 6 months after the surgery (P > 0.05), and both groups had a significant weight loss (P < 0.001) [Table 4].
Table 4.
Comparison of the percentage of patients’ mean weight loss in the two groups
| Variables | Total (n=77) | Omega (n=43) | RYBP (n=34) | P a |
|---|---|---|---|---|
| Percentage of excess weight lost | ||||
| 1 month after surgery | 18.06±9.64 | 17.24±8.82 | 19.09±10.63 | 0.407 |
| 3 months after surgery | 27.18±6.07 | 28.21±6.98 | 25.88±4.45 | 0.094 |
| 6 months after surgery | 33.38±9.74 | 32.84±10.59 | 34.06±8.65 | 0.588 |
| P b | <0.001 | <0.001 | <0.001 |
aSignificance level obtained from an independent samples t-test to compare the percentage of the patients’ mean weight loss between the two groups in each of the studied times, bSignificance level obtained from the repeated measures ANOVA analysis comparing the percentage of the patients’ mean weight loss over 6 months in each of the two groups. RYBP: Roux-en-Y gastric bypass
Discussion
The results of the present study revealed that the effect of these two types of surgery on patients’ weight loss was not significantly different, although generally patients’ blood glucose and HbA1C reduction in the Omega procedure was much higher than that of the RYGB procedure.
Similarly, similar evidence suggests that the bariatric surgery is associated with 45%–95% cure of diabetes depending on the type of the surgery.[23,22,25] The results of the largest meta-analysis study have indicated that in addition to the effectiveness of the bariatric surgery in patients’ weight loss, it was also associated with a significant reduction in the blood glucose and HbA1C levels[23] so that diabetes was completely eliminated or improved in 70%–80% of cases. Weight loss and diabetes recovery had the highest rates for biliopancreatic diversion, followed by RYGB and then adjustable gastric banding (AGB). Vertical sleeve gastrectomy (VSG) and mini or Omega loop gastric bypass have not been included in the mentioned meta-analysis. In contrast, our study has addressed the Omega procedure and revealed that diabetes recovery and blood glucose reduction in the Omega group were much better than the RYGB group, although the weight loss was the same in both groups.
In addition, a retrospective study of patients with T2D undergoing the bariatric surgery showed that patients with preoperative HbA1C level of more than 6.5% had a poorer postoperative blood glucose control and a lower weight loss as compared to patients with the HbA1C level of <6.5%.[26]
Furthermore, as mentioned, 23.3% of the patients in the Omega group and 23.5% of the patients in the RYGB group received insulin before the surgery, and the rest of the patients took oral antidiabetic agents. According to the protocol implemented in this study, after surgery, one-third of insulin was prescribed for patients that had been taking insulin for a long time, and the blood glucose level was regularly checked and reported by the patient. The results of this study indicated that the insulin dose in these patients gradually decreased significantly after the surgery so that the insulin intake was completely stopped for all patients 6 months after the surgery, and oral antidiabetic agents were prescribed only for two of the patients 6 months after the surgery.
In addition, regarding the patients with a complete discontinuation of oral antidiabetic agents, the researchers of the current study had to prescribe oral antidiabetic agents for 12.1% and 23.1% of patients in the Omega and RYGB groups, respectively 1 month after the surgery. Moreover, oral antidiabetic agents were prescribed for 3% and 7.7% of patients in the Omega and RYGB groups, respectively, 3 months after the surgery. It should be noted that the medication prescription was reduced in the 3rd month, and no oral medication was administered to any of the patients in the 6th month. Therefore, the most imperative point in the management of patients’ diabetes after surgery from the present researchers’ perspective is to train the patients with respect to monitoring along with periodic screenings of their diet as well as their co-operation in self-management according to the physician's instructions. However, the availability of a physician or diabetes specialist is also very important in the long-term follow-up of these patients after the surgery.
In this regard, Pournaras et al. examined the rate of diabetes recovery in the 10-year follow-up of patients with T2D undergoing three surgeries of RYGB, VSG, and AGB in the UK and revealed that totally 34.4% of patients had a complete cure of diabetes. The recovery rate after RYGB, VSG, and AGB was 40.6%, 26%, and 7%, respectively. Therefore, the recovery rate for RYGB was significantly lower than that of the other two procedures. In the present study, the effectiveness of this method was less than that of the Omega procedure.[27]
Similarly, Karim et al. stated that the role of different metabolic surgery procedures should be attended to by the clinician while guiding care. For instance, substantial effects of RYGB on the reduction of diabetes have been reported with no concomitant requirements for the oral therapy. Therefore, pharmacotherapy regimen of patients following the RYGB may require being more considerably fine-tuned. Similarly, the degree of control and diabetes history may provide implications for both postoperative and inpatient care. Further intensive insulin dosing regimens may be required preoperatively for patients with a poor control in their preoperative period, which predicts a lower reduction of blood glucose level after the surgery. Thus, it is less probable to stop their dependence on the oral therapy.[28]
It should be noted that the patients were not controlled for a long time before the surgery in the current study, and their blood glucose level was controlled for a short time before the surgery, and although they were not comparable in this regard, the consideration of the preoperative diabetes management protocol can also be of great importance before the surgery.
Consistent with the findings of the present study, the results of some previous studies have also shown the blood glucose control and diabetes recovery in approximately 80% of patients undergoing RYGB.[26,29,30] Insulin can also be eliminated in most patients with T2D according to another review study.[31] However, diabetes is a chronic disease, and an increase in glucose levels may recur in some patients when the weight stabilization is achieved (usually 12–18 months after surgery). It is ambiguous whether metformin or lifestyle interventions such as exercise can prevent the recurrence of diabetes. However, as metformin is not associated with the risk of hypoglycemia or weight gain, it may be retained or re-prescribed in patients unless gastrointestinal side effects or contraindications such as renal failure are followed by at least annual HbA1c. In our study, we had to maintain or re-prescribe oral antidiabetic agents, most of which were metformin, for <20% of patients.
It is important to note that some studies have suggested the necessity of controlling the blood glucose level before the surgery through diet control, exercise, and anti-diabetic agents. In fact, the need for a careful monitoring of glucose control and adjustment of treatment along with hypoglycemia in patients with diabetes before surgery is recommended.[28,30,32] However, the current study has not addressed the mentioned point, which can be one of the limitations of the study. We controlled the patients’ blood glucose level few days before the surgery and could observe the effective role of the postoperative diabetes medical management in the discontinuation of antidiabetic agents by checking the patients’ blood glucose level daily, the exact implementation of the mentioned protocol, and the support and advice of physicians in this regard. The mentioned point can be regarded as one of the strengths of the present study. Therefore, considering the increasing prevalence of obesity, changes in the lifestyle, and unhealthy eating habits, it can be stated that this surgery alone cannot be effective in reducing weight, improving patients’ diabetes, and reducing their need for antidiabetic agents so the patients should be followed-up, managed, and treated for a long time after the surgery. Improving the lifestyle and diets of these patients can also be influential in increasing effectiveness of medical management of these patients. Therefore, it is recommended that more studies with larger sample sizes and longer follow-ups before and after the surgery be performed so that the results can be generalized to the target population with more confidence.
Conclusion
According to the results of the present study, generally bariatric surgery has had an effective role in patients’ weight loss and T2D recovery. Although the effectiveness of the Omega procedure in controlling and reducing patients’ blood glucose and HbA1C levels was greater than that of the RYGB procedure, both procedures were significantly effective in reducing patients’ weight. In addition, the main purpose of the present study was to manage the patients’ diabetes up to 6 months after the surgery. According to the protocol implemented in this study, reducing patients’ insulin to one-third of the dose taken before the surgery, along with frequent daily blood glucose monitoring and physician-patient consultation could reduce the need for insulin in these patients and terminate its use 6 months after the surgery. Moreover, oral antidiabetic agents were prescribed for <25% of patients 1 and 3 months after the surgery and were gradually decreased with the daily control of the patients’ blood glucose level such that the use of oral antidiabetic agents also terminated 6 months after the surgery. Therefore, it seems that the management and support of these patients after the surgery are of great importance in the long-term feedback of bariatric surgeries, regardless of its type. In fact, the patients’ need for oral antidiabetic agents and insulin can be terminated with patients’ long-term control and support.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P. Obesity and diabetes–not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018;34:e3042. doi: 10.1002/dmrr.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Drummen M, Tischmann L, Gatta-Cherifi B, Adam T, Westerterp-Plantenga M. Dietary protein and energy balance in relation to obesity and co-morbidities. Front Endocrinol (Lausanne) 2018;9:443. doi: 10.3389/fendo.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscot MJ, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimäki T, et al. BMI Trajectories associated with resolution of elevated youth BMI and incident adult obesity. Pediatrics. 2018;141:e20172003. doi: 10.1542/peds.2017-2003. [DOI] [PubMed] [Google Scholar]
- 5.Tarride JE, Haq M, Taylor VH, Sharma AM, Nakhai-Pour HR, O’Reilly D, et al. Health status, hospitalizations, day procedures, and physician costs associated with body mass index (BMI) levels in Ontario, Canada. Clinicoecon Outcomes Res. 2012;4:21–30. doi: 10.2147/CEOR.S24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovascular diabetology. 2018;17:1–9. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review) J Diabetes Complications. 2013;27:508–18. doi: 10.1016/j.jdiacomp.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan LM. What bariatric surgery can teach us about endoluminal treatment of obesity and metabolic disorders. Gastrointestl Endosc Clin. 2017;27:213–31. doi: 10.1016/j.giec.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8:464. doi: 10.4239/wjd.v8.i11.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English WJ, DeMaria EJ, Hutter MM, Kothari SN, Mattar SG, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16:457–63. doi: 10.1016/j.soard.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Angrisani L, Formisano G, Santonicola A, Hasani A, Vitiello A. Bariatric and Metabolic Surgery. Milano: Springer; 2017. Bariatric surgery worldwide; pp. 19–24. [Google Scholar]
- 13.Leonetti F, Capoccia D, Coccia F, Casella G, Baglio G, Paradiso F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: A prospective cohort study of laparoscopic sleeve gastrectomy vs.medical treatment. Arch Surg. 2012;147:694–700. doi: 10.1001/archsurg.2012.222. [DOI] [PubMed] [Google Scholar]
- 14.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med. 2017;376:641–51. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater BJ, Bellatorre N, Eisenberg D. Early postoperative outcomes and medication cost savings after laparoscopic sleeve gastrectomy in morbidly obese patients with type 2 diabetes. J Obes. 2011;2011:350523. doi: 10.1155/2011/350523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Tovar J, Oller I, Tomas A, Llavero C, Arrovo A, Calero A, et al. Midterm impact of gleeve gastrectomy, calibrated with a 50-Fr bougie, on weight loss, glucose homeostatsis, lipid profiles, and comorbidities in morbidly obese patients. Am Surg. 2012;78:969–74. [PubMed] [Google Scholar]
- 17.Mottalib A, Kasetty M, Mar JY, Elseaidy T, Ashrafzadeh S, Hamdy O. Weight management in patients with type 1 diabetes and obesity. Curr Diab Rep. 2017;17:92. doi: 10.1007/s11892-017-0918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makary MA, Clark JM, Shore AD, Magnuson TH, Richards T, Bass EB, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg. 2010;145:726–31. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 19.Yip S, Plank LD, Murphy R. Gastric bypass and sleeve gastrectomy for type 2 diabetes: A systematic review and meta-analysis of outcomes. Obes Surg. 2013;23:1994–2003. doi: 10.1007/s11695-013-1030-z. [DOI] [PubMed] [Google Scholar]
- 20.Dillon C, Peddle J, Twells L, Lester K, Midodzi W, Manning K, et al. Rapid reduction in use of antidiabetic medication after laparoscopic sleeve gastrectomy: The newfoundland and Labrador bariatric surgery cohort (BaSCo) study. Can J Hosp Pharm. 2015;68:113–20. doi: 10.4212/cjhp.v68i2.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenberg R, Punthakee Z. Canadian diabetes association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2013;37(Suppl 1):S8–11. doi: 10.1016/j.jcjd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Powers KA, Rehrig ST, Jones DB. Financial impact of obesity and bariatric surgery. Med Clin North Am. 2007;91:321–38, ix. doi: 10.1016/j.mcna.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Laferrère B. Diabetes after bariatric surgery. Can J Diabetes. 2017;41:401–6. doi: 10.1016/j.jcjd.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Khoury L, Chouillard E, Chahine E, Saikaly E, Debs T, Kassir R. Metabolic surgery and diabesity: A systematic review. Obes Surg. 2018;28:2069–77. doi: 10.1007/s11695-018-3252-6. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Pevida B, Escalada J, Miras AD, Frühbeck G. Mechanisms underlying type 2 diabetes remission after metabolic surgery. Front Endocrinol. 2019;10:641. doi: 10.3389/fendo.2019.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perna M, Romagnuolo J, Morgan K, Byrne TK, Baker M. Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis. 2012;8:685–90. doi: 10.1016/j.soard.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Angrisani L, Cutolo PP, Formisano G, Nosso G, Vitolo G. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 10-year results of a prospective, randomized trial. Surg Obes Relat Dis. 2013;9:405–13. doi: 10.1016/j.soard.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Kheniser KG, Kashyap SR. Diabetes management before, during, and after bariatric and metabolic surgery. J Diabetes Complications. 2018;32:870–5. doi: 10.1016/j.jdiacomp.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Seridi L, Leo GC, Dohm GL, Pories WJ, Lenhard J. Time course metabolome of Roux-en-Y gastric bypass confirms correlation between leptin, body weight and the microbiome. PLoS One. 2018;13:e0198156. doi: 10.1371/journal.pone.0198156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorell A, Hagström-Toft E. Treatment of diabetes prior to and after bariatric surgery. J Diabetes Sci Technol. 2012;6:1226–32. doi: 10.1177/193229681200600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: A narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11071644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the obesity management task force of the European Association for the Study of obesity for the post-bariatric surgery medical management. Obesity Facts. 2017;10:597–632. doi: 10.1159/000481825. [DOI] [PMC free article] [PubMed] [Google Scholar]


