Abstract
Purpose
We report a case of a 10-year-old with Moring glory disc anomaly (MGDA) associated with Moyamoya disease and pituitary stalk duplication.
Observations
A 10-year-old Asian child presented with decreased vision in the right eye and bilateral nystagmus. Both dilated fundus exam and magnetic resonance imaging (MRI) of the orbit confirmed MGDA of the right eye. MRI of the brain demonstrated duplication of the pituitary stalk. Magnetic resonance angiography (MRA) of the brain revealed bilateral severe narrowing (greater on the right side) of the distal supraclinoid internal carotid arteries with bilateral reconstitution at the carotid terminus and prominent collaterals, suggestive of Moyamoya disease.
Conclusions
Patients with MGDA should undergo neuroimaging due to the associated central nervous system (CNS) anomalies.
Keywords: Morning glory disc anomaly, Moyamoya disease
1. Introduction
Morning glory disc anomaly (MGDA) is an uncommon excavated congenital optic nerve anomaly. Some MGDA cases have systemic anomalies including midline CNS defects and cerebrovascular anomalies. Moyamoya disease (MMD) is a progressive narrowing of the distal part of the internal carotid artery (ICA) and proximal middle and anterior cerebral arteries. It can be complicated by hemorrhagic or ischemic symptoms. Here, we present a case of MGDA that is associated with MMD and pituitary stalk duplication, demonstrating the importance of neuroimaging studies in these patients.
2. Case presentation
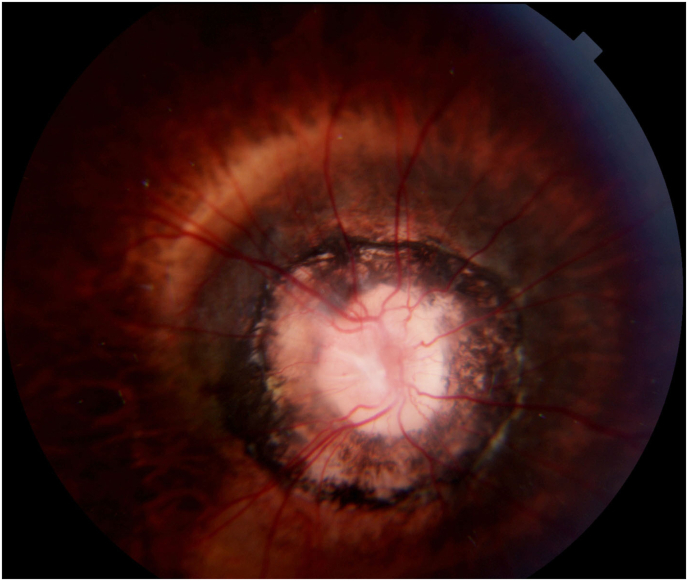
A 10-year-old Asian child presented with gradual diminution of vision and nystagmus. Upon initial ophthalmic examination, best-corrected visual acuity (BCVA) was 20/800 and 20/30 for right and left eyes, respectively. Intraocular pressure (IOP) was 14 mmHg in the right eye and 17 mmHg in the left eye. A relative afferent pupillary defect (RAPD) in the right eye along with bilateral jerk nystagmus with high frequency, low amplitude was observed. The patient had a right exotropia of 20 prism diopters. Color vision was normal in both eyes. On slit-lamp examination, faint lens opacifications in both eyes were present. Fundoscopic examination of the right eye showed an enlarged funnel-shaped optic nerve head and circumferential anomalous radiating retinal blood vessels. The peripapillary retina showed chorioretinal pigmentary changes (Fig. 1). In addition, the retinal blood vessels were attenuated. The fundus of the left eye was normal. However, our patient did not have the typical central glial tissue. These findings were consistent with the possible diagnosis of MGDA in the right eye. The atypical appearance of the optic nerve head suggests, that in addition to the MGDA, that the patient also has posterior staphyloma.
Fig. 1.
Fundus photograph of the right optic nerve head.
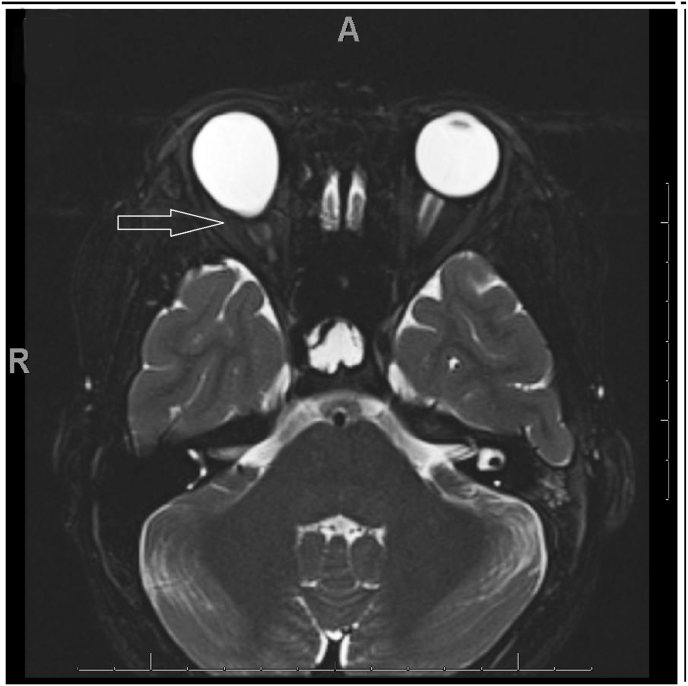
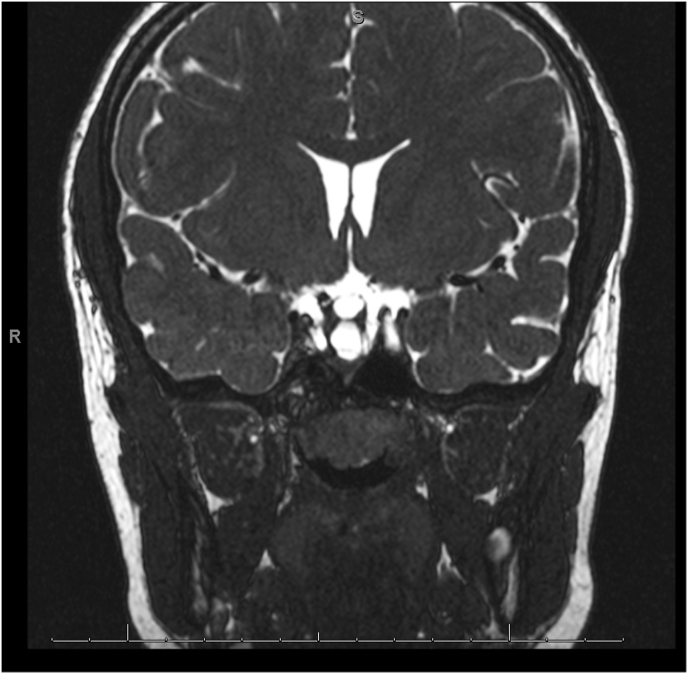
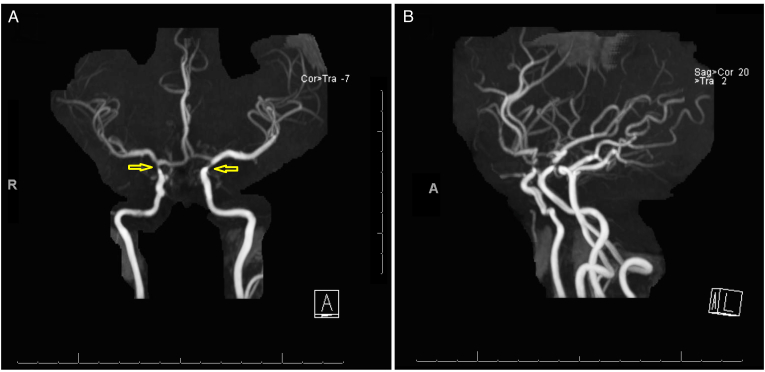
The patient underwent MRI brain and orbits and MRA of the brain. MRI of the orbits revealed an excavation of the posterior pole of the right eye with choroidal discontinuity with abnormal tissue associated with the distal intraorbital segment of the right optic nerve optic nerve and effacement of the subarachnoid space at that level (Fig. 2). MRI of the brain showed hypophyseal stalk duplication (Fig. 3). On MRA of the brain, there was severe narrowing of the distal supraclinoid ICA bilaterally, greater on the right side, with prominent ophthalmic arteries. Flow reconstitution was noted at the bilateral carotid terminus. Additionally, there were prominent lenticulostriate vessels that arose from the bilateral M1, P1, and posterior communicating arteries (Fig. 4A and B). Posterior cerebral circulation was normal. Cerebral angiography also revealed right ICA stenosis >75% and left ICA stenosis >70% with patent posterior circulation collaterals.
Fig. 2.
Outpouching of the posterior right globe noted in axial T2 scan.
Fig. 3.
Coronal T2-weighted images demonstrated duplication of the pituitary stalk.
Fig. 4.
A. MRA of the head which shows severe narrowing with near occlusion of the bilateral distal supraclinoid internal carotid arteries (yellow arrows). There is reconstitution noted at the carotid terminus bilaterally. B. There are additionally prominent collateral vessels arising from the bilateral M1, A1 and posterior communicating arteries. These findings are keeping with Moyamoya disease. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The diagnosis of Moyamoya disease (MMD) was made followed by an assessment with a multidisciplinary medical team consisting of pediatrics, endocrinology, neuro-ophthalmology, and neurosurgery specialists. On further evaluation, the growth hormone level was low in the patient, prompting hormonal replacement therapy initiation. Follow-up every 6 months with repeat MRI and MRA given absence of any signs or symptoms of ischemia, likely as a result of patent collaterals from the posterior circulation.
3. Discussion
MGDA is a rare congenital anomaly involving the optic nerve head and surrounding peripapillary retina. MGDA is most commonly unilateral, and predominant in females. Although the majority of MGDA cases are solitary (not associated with other systemic manifestations), some have systemic associations. Transsphenoidal basal encephalocele is reported to be a common association of MGDA. Patients with basal encephalocele often have dysmorphic facial features, such as flat nose, hypertelorism and cleft lip in addition to agenesis of the corpus callosum.1,2 Malformations of the cereberal vasculature, including Moyamoya disease, have been noted in MGDA patients. Other reported associations include renal malformations and PHACE syndrome (posterior fossa malformation, large facial hemangioma, arterial anomalies, cardiac anomalies, eye anomalies).2 MGDA may develop due to non-closure of the optic fissure, dysgenesis of the terminal optic stalk, or as a primary mesenchymal disorder.2
Clinically, MGDA is characterized by a funnel-shaped excavation of the optic nerve head and peripapillary retina. The optic disc has a central glial tuft with dense the retinal vessels emerging in a radial pattern. The peripapillary area is elevated with a ring of pigmentation, which may be indicative of a previous detachment. Patients usually present with decreased vision, strabismus, or nystagmus.3
Moyamoya disease is an idiopathic cerebrovascular disease characterized by progressive stenotic changes at the distal portion of the ICA, proximal middle cerebral artery (MCA), and anterior cerebral artery (ACA). On angiography, the collateral arterial network resembles a puff of smoke which means “moyamoya” in Japanese.4 The incidence of Moyamoya disease in the United States is 0.293 per 100,000 individuals with female-to-male ratio around 2.15. Patients of Asian origin have a higher incidence of Moyamoya disease, and this could be explained by the higher association of RNF213 gene mutation that has a possible role in vascular development. Moyamoya disease has bimodal age distribution with two peaks; 5 to 9 and 55–59 years.5,6
Moyamoya disease is referred to as Moyamoya syndrome when associated with systemic disorders such as Hirschsprung disease, coarctation of the aorta, renal artery stenosis, hyperthyroidism, and NF1.7 These associations support the theory that Moyamoya disease could be due to defective development of neural crest cells.8 Histopathological changes in Moyamoya include thinning of the media, tortuosity of the internal elastic lamina, and fibrous intimal hyperplasia caused by proliferation of the vascular smooth muscle cells, which cause intravascular lumen narrowing and thrombosis.9
The most common presenting symptoms of Moyamoya disease are ischemic strokes, transient ischemic attacks (TIA), and hemorrhage in the territories of ICA and MCA. Less commonly, Moyamoya disease patients can present with headache, seizures, visual complaints, or chorea. Hyperventilation, exertion, or dehydration can precipitate ischemic stroke and TIA, especially in children. Hemorrhage is more common in adults and can be interventricular, intraparenchymal, or subarachnoid.7
MGDA is considered the most common ophthalmic manifestation of MMD. In a retrospective study evaluating the associated neurological anomalies of 20 patients with MGDA and control group of 40 patients with other neurological complaints, Lenhart et al. reported that 45% of the MGDA group had cerebrovascular anomalies compared to 15% in the control group. The reported intracranial vascular anomalies in this study ranged from mild congenital variants to progressive severe stenosis or occlusion, as in Moyamoya disease. Moyamoya disease was present in 15% of patients with MGDA but was not reported in any patient of the control group.10
Conventional cerebral angiography historically was the gold standard for the diagnosis of MDD. However, recently, MRA has replaced conventional angiography as the preferred imaging modality due to high specificity and being non-invasive.11 The intravascular stenosis noticed in MMD is irreversible and currently, no treatment can prevent or reverse the disease progression. The treatment aims in MMD patients are to prevent ischemic and hemorrhagic complications and improve symptoms of the disease. The benefits of medical treatment are still controversial. Vasodilators, anti-platelet aggregation drugs, and anticoagulants may be useful in chronic ischemic moyamoya disease. However, long-term usage of antiplatelet drugs can increase the risk of bleeding.12
Surgical revascularization is indicated in patients with ischemic attacks and has proven to decrease the risk of infarction, frequency of TIAs, and improve quality of life after surgery but the role of surgery is still controversial in hemorrhagic patients. Surgical revascularization options include direct, indirect or combined revascularization surgeries.11
Pituitary stalk duplication is rarely reported in association with MGDA.13 The exact causes or mechanisms of pituitary gland or stalk duplication are unknown. Various mechanisms have been suggested, including incomplete splitting of the developing pituitary gland and stalk, duplication of the prochordal plate cells and anterior notochordal process or splitting of the notochordal tissue as a primary event or due to mechanical changes.14 The pituitary stalk duplication noted in our patient is the possible cause of decreased growth hormone levels and subsequently his short stature.
4. Conclusions
Although MGDA is not a common optic nerve head pathology, the exclusion of associated vascular and structural CNS anomalies is crucial in proper management of those cases. This underscores the importance of neuroimaging studies in patient with MGDA.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The authors have no relevant conflicts of interest to disclose with this manuscript.
Acknowledgments
None.
References
- 1.Quah B.L., Hamilton J., Blaser S., Héon E., Tehrani N.N. Morning glory disc anomaly, midline cranial defects and abnormal carotid circulation: an association worth looking for. Pediatr Radiol. 2005;35(5):525–528. doi: 10.1007/s00247-004-1345-y. [DOI] [PubMed] [Google Scholar]
- 2.Harasymowycz P., Chevrette L., Décarie J.C., et al. Morning glory syndrome: clinical, computerized tomographic, and ultrasonographic findings. J Pediatr Ophthalmol Strabismus. 2005;42(5):290–295. doi: 10.3928/0191-3913-20050901-11. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M. Retinal complications associated with congenital optic disc anomalies determined by swept source optical coherence tomography. Taiwan J Ophthalmol. 2016;6(1):8–14. doi: 10.1016/j.tjo.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Zheng L., Feng L. Epidemiology, diagnosis and treatment of moyamoya disease. Exp Ther Med. 2019;17(3):1977–1984. doi: 10.3892/etm.2019.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaffari-Rafi A., Ghaffari-Rafi S., Leon-Rojas J. Socioeconomic and demographic disparities of moyamoya disease in the United States. Clin Neurol Neurosurg. 2020;192(105719) doi: 10.1016/j.clineuro.2020.105719. [DOI] [PubMed] [Google Scholar]
- 6.Uchino K., Johnston S.C., Becker K.J., Tirschwell D.L. Moyamoya disease in Washington state and California. Neurology. 2005;65(6):956–958. doi: 10.1212/01.wnl.0000176066.33797.82. [DOI] [PubMed] [Google Scholar]
- 7.Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 8.Komiyama M. Moyamoya disease is a vascular form of neurocristopathy: disease of the embryologic cephalic neural crest. Childs Nerv Syst. 2017;33(4):567–568. doi: 10.1007/s00381-017-3369-2. [DOI] [PubMed] [Google Scholar]
- 9.Lin R., Xie Z., Zhang J., et al. Clinical and immunopathological features of Moyamoya disease. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenhart P.D., Lambert S.R., Newman N.J., et al. Intracranial vascular anomalies in patients with morning glory disk anomaly. Am J Ophthalmol. 2006;142(4):644–650. doi: 10.1016/j.ajo.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura M., Tominaga T. Diagnosis of moyamoya disease: international standard and regional differences. Neurol Med -Chir. 2015;55(3):189–193. doi: 10.2176/nmc.ra.2014-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang S., Zhou D., Ya J., et al. Progress in moyamoya disease. Neurosurg Rev. 2020;43(2):371–382. doi: 10.1007/s10143-018-0994-5. [DOI] [PubMed] [Google Scholar]
- 13.Taşkintuna I., Oz O., Teke M.Y., Koçak H., Firat E. Morning glory syndrome: association with moyamoya disease, midline cranial defects, central nervous system anomalies, and persistent hyaloid artery remnant. Retina. 2003;23(3):400–402. doi: 10.1097/00006982-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Burke M., Zinkovsky S., Abrantes M.A., Riley W. Duplication of the hypophysis. Pediatr Neurosurg. 2000;33(2):95–99. doi: 10.1159/000028983. [DOI] [PubMed] [Google Scholar]