Abstract
Despite advances in immunosuppressive prophylaxis and overall supportive care, gastrointestinal (GI) graft-versus-host disease (GVHD) remains a major, lethal side effect after allogeneic hematopoietic stem cell transplantation (allo-HSCT). It has become increasingly clear that the intestinal epithelium, in addition to being a target of transplant-related toxicity and GVHD, plays an important role in the onset of GVHD. Over the last two decades, increased understanding of the epithelial constituents and their microenvironment has led to the development of novel prophylactic and therapeutic interventions, with the potential to protect the intestinal epithelium from GVHD-associated damage and promote its recovery following insult. In this review, we will discuss intestinal epithelial injury and the role of the intestinal epithelium in GVHD pathogenesis. In addition, we will highlight possible approaches to protect the GI tract from damage posttransplant and to stimulate epithelial regeneration, in order to promote intestinal recovery. Combined treatment modalities integrating immunomodulation, epithelial protection, and induction of regeneration may hold the key to unlocking mucosal recovery and optimizing therapy for acute intestinal GVHD.
Introduction
Damage to the gastrointestinal (GI) tract is a common occurrence following allogeneic hematopoietic stem cell transplantation (allo-HSCT)1,2. Several factors are thought to contribute to this damage, including pretransplant conditioning, posttransplant activation of alloreactive T cells, and both tissue-targeted and immunomodulatory effects of the intestinal microbiota (Figs. 1, 2). Before transplantation of a donor allograft, the recipient receives chemotherapy ± irradiation conditioning to kill residual malignant cells, weaken the recipient’s immune system, and create space for donor hematopoietic engraftment. However, the required pretransplant conditioning can also cause significant damage to cycling cells in the epithelial gut lining, resulting in mucositis and disruption of the mucosal barrier. Impaired barrier function leads to exposure of the basolateral intestinal epithelial cell (IEC) membranes and lamina propria leukocytes to luminal contents. Activation of the immune system in this context may cause the development of acute Graft-versus-Host Disease (aGVHD). In aGVHD, transplanted donor T cells recognize antigens in the recipient and launch an inflammatory attack against the recipients’ tissues such as the skin, intestines, and liver1,3,4. Despite prophylactic immunosuppression and careful HLA-matching, ~30–50% of allo-HSCT patients develop GVHD symptoms, half of which include significant GI tract involvement manifesting in nausea, anorexia, and diarrhea. In addition, poor barrier function contributes to potentially life-threatening bloodstream infections in these immunocompromised patients. The immunosuppression and high dose corticosteroids necessary for treatment of GI-GVHD provide additional potential complications, even when GVHD can be successfully treated. As such, GI-GVHD remains an important cause of transplant-related morbidity and mortality1,2.
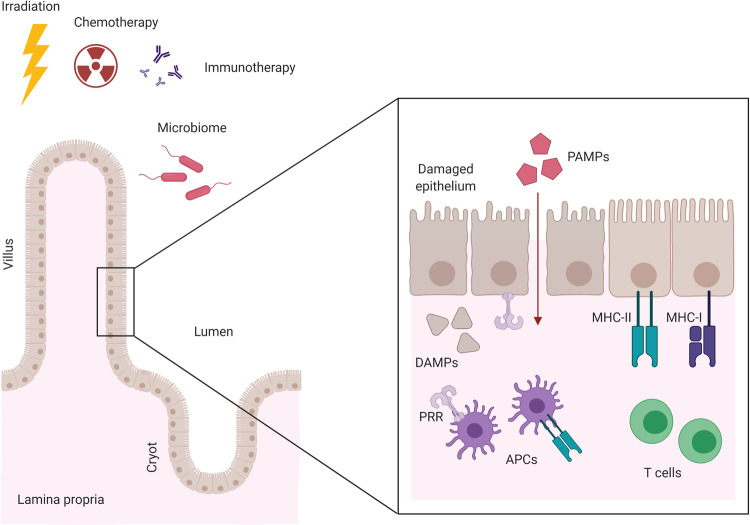
Fig. 1. The pivotal role of the intestinal epithelium and epithelial damage in GVHD onset.
Irradiation, chemotherapy, and/or immunotherapy used in the conditioning regimen before HSCT damages the intestinal epithelial cells and disrupts barrier protecting the recipient from luminal pathogens. Translocating pathogen-associated molecular patterns (PAMPs) and released damage-associated molecular patterns (DAMPs) bind to their corresponding pattern recognition receptors (PRRs) and activate the innate immune system, including professional antigen presenting cells (APCs). Antigen presentation by APCs, including the intestinal epithelium, lead to the propagation and activation of alloreactive T cells, which cause further damage through cytokine- and cell–cell-mediated toxicity in the then developed GVHD. Created with BioRender.com.
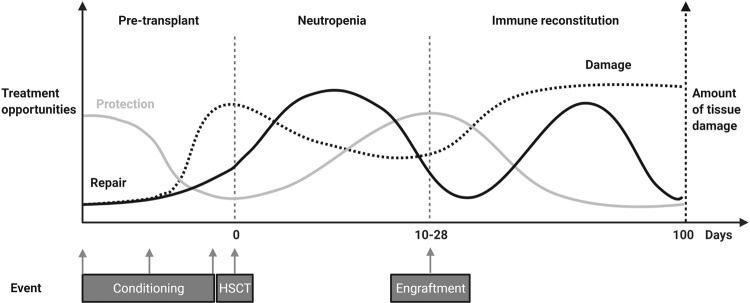
Fig. 2. Opportunities for intestinal protection and repair over the course of HSCT.
Damage to the intestinal epithelium over the course of HSCT occurs in different phases. As such, opportunities for protection against the insult and repair of the injury occur in parallel, rather than after the fact. The timing of these different approaches will be crucial, since certain treatment opportunities may have pleiotropic effects on other cell types at different time points during the posttransplant period. Created with BioRender.com.
Current GI-GVHD treatment focuses mainly on suppressing posttransplant aberrant immune responses with corticosteroids3,5, but this approach is often ineffective. Up to 50% of patients develop steroid-refractory (SR)-GVHD and require additional treatment6. The Jak1/2-inhibitor Ruxolitinib (Rux) is currently the only FDA-approved treatment for SR-GVHD7. Other second and third-line therapies lack consistent demonstrated benefit, and are mostly based on providing additional immunosuppression. This causes most GVHD therapeutic approaches to be accompanied by an increased risk of infection and a potentially reduced graft-versus-leukemia (GVL) effect.
An evolving understanding of intestinal homeostasis and its related epithelial constituents has led to new treatment opportunities that aim to protect the intestines peri-transplant, without impairing the recovery of physiologic immune function posttransplant. It has also become increasingly clear that the intestinal epithelium is not only a direct target of GVHD-associated damage, but in addition may take part in the development and propagation of the disease, and possibly in its resolution as well8. In this review we focus specifically on the role of the intestinal epithelium and epithelial injury in GVHD initiation, and how it can be protected from transplant-associated insult. Furthermore, strategies to promote epithelial restoration by improving regeneration and augmenting posttransplant epithelial recovery are discussed, both through regulating epithelial-intrinsic constituents as well as factors supplied by the microenvironment.
Preventing intestinal epithelial damage
Minimizing conditioning-induced injury
The intestinal epithelial barrier forms the first line of defense between the lumen and the underlying immune system in the gut9. As such it protects the recipient from harmful gut contents, including pathogens. The barrier is formed by the plasma membranes of a single layer of IECs that are tightly connected with tight junctions (TJ). The IEC lining is covered with a protective, extracellular layer of mucus produced by epithelial Goblet cells, which inhibits direct contact between the IEC and gut luminal particles and bacteria. The mucosal immune cells are present within the epithelial compartment, as well as in the lamina propria below, and in designated lymphoid regions called Peyer’s patches. In addition to providing a physical barrier, the epithelial cell layer is essential for the absorption and transport of nutrients and water. Both integrity and functionality of the epithelial barrier are crucial elements in the course of transplant, as loss is associated with systemic infections, severe GI symptoms like anorexia/diarrhea and poor outcome in general2.
Pretransplant conditioning is recognized as an early insult to this barrier integrity10. In both preclinical models and human studies overall aGVHD severity is associated with the intensity of the pretransplant conditioning regimen11,12. In mice, high intensity regimens were associated with reduced mucus layer thickness and the presence of bacterial RNA in the colon lamina propria13. Indeed, total body irradiation (TBI) and chemotherapy treatment led to significant leakage of orally administered FITC-labeled dextran into the bloodstream, indicating consequential compromise of the epithelial barrier14. In a study by Nalle et al. preconditioning-induced damage to the epithelium was even required to induce aGVHD in a MHC-matched minor-histocompatibility-antigen (miHA)-mismatched transplantation10,15. In humans, compromised epithelial integrity, as measured by 51Cr-EDTA absorption, has been documented in association with myeloablative regimens even 14 days after stem cell infusion16,17. Conditioning toxicity is also associated with the release of pro-inflammatory cytokines in the GI tract, which can contribute to GVHD development11,18,19. In particular, TBI appears to be associated with both a higher aGVHD incidence20 and treatment-related mortality21.
The implementation of reduced intensity conditioning (RIC) regimens has decreased conditioning-associated tissue toxicity and enabled older and more fragile patients to undergo allo-HSCT. Regimens typically include an alkylating agent like busulfan (Bu), melphalan or cyclophosphamide (Cy) and a purine analog such as fludarabine (Flu), with or without low-dose TBI. Clinical GI toxicity of RIC transplants is reported to be moderate22,23, and the associated intestinal epithelial damage16,17 and mucositis24 have been found to be less severe. The occurrence of aGVHD after RIC is reduced as well25. Additionally, more favorable combinations of agents have been applied to this approach. For example, in reducing the number of combined alkylators, Bu/Flu has a more favorable toxicity profile than Bu/Cy in patients across HSCT indications, while still providing a myeloablative regimen26–28.
More recent developments in conditioning regimen tolerability have focused on tailored dosing based on chemotherapy plasma levels of the individual patient to reduce exposure and consequent epithelial damage. This concept is known as reduced toxicity conditioning (RTC). The superiority of pharmacokinetic (PK)-directed dosing for intravenous Bu in adults has been established for over a decade with both enhanced safety29 and efficacy30. Similar results were found in children31,32. Additionally, Flu exposure, as calculated by a PK-model, was recently retrospectively demonstrated to be a strong predictor of HSCT survival in adults33. In conclusion, PK-directed dosing of favorable chemotherapeutic combinations will hopefully further reduce conditioning-related toxicity and development of acute GVHD in the near future.
Preventing deleterious responses to PAMPS and DAMPS
Upon intestinal barrier breach, translocating pathogen-associated molecular patterns (PAMPs) and tissue-released damage-associated molecular patterns (DAMPs) are recognized by pattern recognition receptors (PRRs), which activate the innate immune system. Concurrently, the development of alloreactive responses can be initiated34–36. As such, many DAMPs, PAMPs and corresponding PRRs have been implicated in the development of GVHD, and multiple approaches have been taken to dampen the response at this level (Table 1). Firstly, scavenging or breaking down the PAMPs and DAMPs would ascertain they do not reach the target immune cell. Examples are treatment with anti-LPS37, HSP90-inhibitor 17AAG38, locked nucleic acid anti-miRNA-29a39, uricase for uric acid40, apyrase for ATP41, NecroX-7 for HMGB1 blockade42 and alpha-1-antitrypsin (AAT) targeting heparin sulfate43, all of which have been shown to reduce GVHD in mouse models and AAT in addition SR-GVHD in humans44. Secondly, binding of DAMPs to target immune cells can be blocked, e.g., by P2X7R antagonists, blocking ATP binding41,45, or anti-TIM-1 monoclonal antibodies, inhibiting binding to phosphatidylserine on apoptotic cell debris46. Thirdly, responsiveness of immune cells to DAMPS could be modulated. MicroRNA-155 deficiency in host DCs protected against GVHD through reduced purinergic receptor and inflammasome-associated gene expression, and concurrent reduced IL-1β release47. Inhibiting microRNA-155 with antagomir could be a promising new approach. Contrarily, Siglecs play a crucial role in mitigating specifically DAMP-induced immune responses48,49. Following conditioning-mediated tissue damage, the interaction of Siglec-G on host APCs with the glycoprotein CD24 on T cells was essential for GVHD protection in both a MHC-matched and mismatched mouse model50. Enhancing the Siglec-CD24 interaction with a CD24-Fc fusion protein mitigated GVHD in experimental GVHD50,51. The results of a phase II trial testing the safety of CD24-Fc for the prevention of aGVHD following myeloablative allo-HSCT are expected shortly.
Table 1.
All DAMPS/PAMPS implicated in GI-GVHD and targeted therapy options.
| Receptor | DAMP/PAMP | Signaling pathway | Effect on GVHD | Therapeutic options | Ref. |
|---|---|---|---|---|---|
| TLR3 | dsRNA | TRIF | = | – | 215 |
| TLR2/4 | HMGB1 | MyD88 | − | NecroX-7 | 42 |
| TLR4 | LPS | MyD88/TRIF | − | Anti-LPS | 37 |
| TLR4 | Heparan sulphate | MyD88 | − | AAT | 43,44 |
| TLR4 | S100 proteins | MyD88 | − | – | 216 |
| TLR4/CD14 | HSP90 | MyD88 | − | 17AAG | 38 |
| TLR5 | Flagellin | MyD88 | + | Flagellin treatment | 217 |
| TLR7/8 | ssRNA MiR29a | MyD88 | − | locked nucleic acid anti-miRNA-29a | 39,218 |
| TLR9 | Bacterial DNA | MyD88 | − | – | 54,184 |
| cGAS | Bacterial DNA | STING | + | DNA treatment | 184 |
| RIG-I | dsRNA | MAVS | + | 3pRNA treatment | 184 |
| Caspase-11 | LPS | Pyroptosis/NLRP3 | − | – | 56 |
| ? | Uric acid | NLRP3 | − | Uricase | 40 |
| P2X7 | ATP | NLRP3 | − | Apyrase P2X7R antagonists | 41,45 |
| NOD2 | Eg MDP | NLRC | + | – | 190 |
| ? | ? | NLRP6 | − | – | 52 |
| TIM | Phosphatidylserine | ? | − | Anti-TIM | 46 |
| ST2 | IL-33 | MyD88 | − | ST2-Fc treatment | 219 |
+ Alleviating, = No effect, − Worsening.
Additionally, targeting innate signaling pathways downstream of PRRs might be a future therapeutic approach52–56. Interestingly, host TLR deficiency was found to be protective against GVHD in murine studies53,54, but inhibition of TLR and inflammasome pathway signaling (via MyD88 and TRIF) only in host hematopoietic cells did not reduce GVHD55. This suggests a GVHD-promoting role of non-hematopoietic tissue signaling. More importantly, deficiency of TLR954 and NLRP6 inflammasome52 was protective only when it was restricted to the non-hematopoietic compartment, indicating that TLR signaling at the tissue level, which includes the intestinal epithelial compartment, may be a future target for GVHD reduction. Despite the numerous possibilities of molecules and pathways to block, only very few damage-modulating agents have led to successful clinical trial results. Probably, the concurrent involvement of many different damage molecules as well as redundant downstream signaling pathways, make achieving significant clinical improvements by targeting just one molecule unlikely. Alternatively, involved molecules and signaling pathways may have concurrent roles in the resolution of GVHD, and targeting them would abrogate this, giving no net improvement.
Preventing cell death within the epithelial compartment
Under homeostatic conditions, the intestinal epithelium is continuously regenerated by stem and progenitor cells that are present within the crypt region (Fig. 3). While a point of longstanding debate, work from the last 15 years has identified Lgr5-expressing crypt base columnar (CBC) cells as intestinal stem cells (ISCs) capable of giving rise to all other cell types of mouse and human intestinal epithelium in vivo and ex vivo57–59. Olfm4 is another marker identifying ISCs60 in humans and in mouse small intestine. ISCs are maintained by both secreted and membrane-bound molecules of surrounding cells, together constituting the ISC niche61. These niche cells include Paneth cells (PCs), which lie interspersed between CBC ISCs at the bottom of the crypt and promote stemness through the release of Wnt3 and EGF, which bind to their respective receptors Frizzled–LRP5–LRP6 complex and ERBB1 on CBCs62,63. PCs also express Notch ligands DLL1 and DLL4 on the cell-surface that directly interact with ISC Notch receptors such as NOTCH1 to maintain stemness and inhibit differentiation into secretory-cell lineages62. Crypt-adjacent stromal cells also promote ISC maintenance through the secretion of Wnts64 and R-spondins (Rspo)65,66. Rspo-binding of LGR5 on the ISC potentiates Wnt signaling by phosphorylation and stabilization of β-catenin in the cytoplasm, thus promoting subsequent translocation to the nucleus67. To protect ISCs from mesenchymal-derived epithelium-maturating BMP2 and BMP4 signals68,69, myofibroblasts and smooth muscle cells around the crypt bottom secrete BMP-inhibitor proteins such as Gremlin 1 and Gremlin 2 that sequester BMPs before they can bind the BMP receptors70,71. As epithelial precursors continue to proliferate and move up the crypt, they give rise to the highly proliferative transit amplifying (TA) cell compartment. Finally, differentiation into the destined cell type of for instance the absorptive or secretory lineage occurs under the influence of both environmental and intrinsically programmed factor dynamics63.
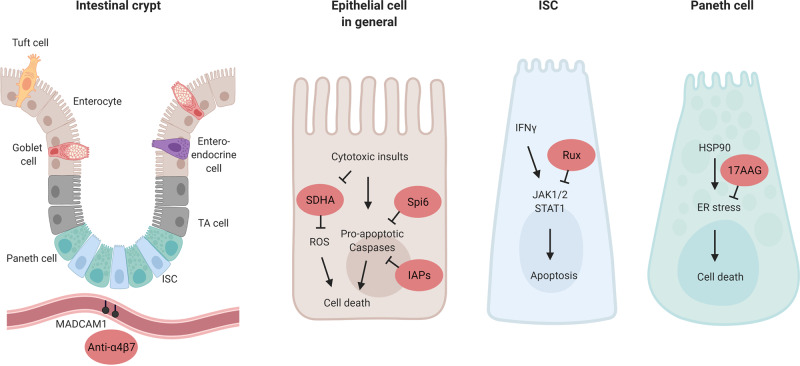
Fig. 3. The intestinal crypt as a GVHD target, and mechanisms of protection.
The intestinal epithelium is maintained by intestinal stem cells (ISCs) which reside at the base of intestinal crypts, interspersed between their supportive Paneth cells (PCs) in the small intestine. Along the crypt-villus axis the ISCs differentiate into transit amplifying (TA) cells and their destined lineage, including absorptive (e.g., enterocyte), secretory (e.g., PC, Goblet cell, Tuft cells) and enteroendocrine cells. In the vasculature near the intestinal crypt the addressin MAdCAM-1 is expressed, which binds α4β7-integrin expressed on gut-directed immune cells. Several approaches of protection at the level of the intestinal epithelial cell in general, or in addition at the ISC and PC level specifically, are indicated in red. A4β7 blockade inhibits the influx of T cells into the lower crypt regions of the small intestine; the serine protease inhibitor Spi6 present in the epithelium protects against GVHD-induced damage, possibly through inhibition of caspase 3/7; intestinal epithelial Inhibitor of Apoptosis Proteins (IAPS) inhibits the function of pro-apoptotic caspases; the SDHA enzyme is reduced in IECs after allo-T cell insult, increasing reactive oxygen species (ROS) levels; Ruxolitinib (Rux) inhibits JAK1/2-STAT1 signaling, relieving interferon (IFN)-y induced epithelial apoptosis; and 17AAG was reported to suppress ER stress and thereby cell death in a.o. Paneth cells. Created with BioRender.com.
When aGVHD of the gut develops, the histopathology characteristically demonstrates epithelial apoptosis within intestinal crypts72,73. In addition to crypt loss, the number of CBC ISCs per crypt is reduced in experimental GVHD74–77. Severe colonic crypt loss at the time of GVHD has been associated with delayed recovery, persistence of symptoms, and the development of SR-GVHD78, suggesting an impaired capacity to recover beyond the initial insult. In addition, PCs are reduced in GVHD76,77,79–83, which may hamper their ability to provide the indispensable niche factors Wnt3, EGF and membrane-bound Notch ligands for the maintenance of ISC integrity. Besides their role as regulators of ISC proliferation, PCs play an important physiologic role in the production of antimicrobial (AMP) and the release of immunomodulatory proteins (e.g., IgA, IL-1β), both key components of host defense in the gut84. Α-defensins are a major class of AMPs produced by PCs, and their production is markedly reduced in experimental GVHD80,81, as well as in GVHD patients83. Loss of PC α-defensins has been associated with decreased bacterial diversity and domination of bacterial species such as Proteobacteria at the phylum level, Enterobacteriales at the order level, and Escherichia and Bacteroides at the genus level, some of which pathogenic80,81. Interestingly, increased plasma levels of the AMP REG3α act as a biomarker of GI-GVHD, predictive of response to therapy, non-relapse mortality and survival85. Murine transplant recipients deficient in Reg3γ, the claimed mouse ortholog of REG3α, developed more severe GI-GVHD with increased crypt apoptosis86. As such, PC deficiency may contribute to GVHD pathology due to the impairment of ISC niche functions as well as through a reduction in bacterial containment.
Up until recently it was uncertain whether crypt loss in GVHD was directly caused by the effector mechanisms of allo-T cells. Using 3D microscopy, donor T cells were shown to primarily invade the intestinal crypt region early after allo-BMT77,87. The potential of allo-T cells to damage the intestinal crypt compartment was studied using so-called intestinal organoid cultures; self-organizing, 3D mini-guts, that form from isolated crypts or purified ISCs when cultured in the presence of defined ISC niche growth factors EGF, Rspo-1 and BMP-inhibitor Noggin58. Intestinal organoids contain multiple epithelial cell types, including Lgr5+ ISCs, and ex vivo culture models with allogeneic T cells recapitulated in vivo ISC damage77. Interferon (IFN)-γ secreted by allo-T cells directly caused ISC apoptosis through signaling via the IFNγ receptor (IFNγR) expressed by ISCs77. Interestingly, blocking IFNγR signaling with the JAK1/2-specific inhibitor Ruxolinitib early after allo-BMT protected ISCs from IFNγ-induced damage77. Ruxolitinib has recently been approved by the FDA for treatment of SR-GVHD7. The rationale for its use in GVHD is based on the suppression of allo-T cell activation, proliferation, cytokine production, and promoting a more favorable regulatory T cell to conventional T cell ratio. The findings of this study indicate that earlier posttransplant use might have a beneficial, target-tissue-protective effect in patients developing GI-GVHD88.
Finally, other protective mechanisms downstream of T cell-induced cytotoxicity have recently been described, that could protect epithelial cells in preclinical models. For example, inhibition of HSP90 by 17AAG after allo-HSCT protected the ISC niche38. HSP90 is released during tissue damage and induces the intracellular response to ER stress, which PCs are particularly sensitive to89. Administration of 17AAG was found to decrease the ER stress actor expression and increase the level of spliced XBP1 important for the regulation of the unfolded-protein response, and preserved both PCs and ISCs in two MHC-mismatched models38. A second example concerns serine protease inhibitor 6 (Spi6), the only known endogenous inhibitor of the cytolytic serine protease Granzyme B (GzmB) which protects immune cells from GzmB-mediated damage. In a GVHD model, host Spi6 expression in the non-hematopoietic compartment played a prominent role in GVHD protection, independently of donor-derived GzmB, and Spi6 was upregulated in the intestinal epithelium upon irradiation and subsequent GVHD induction90. A third example is found in the inhibitors of apoptosis proteins (IAPs), which are classically involved in the inhibition of cell death proteases such as caspase 3. IAP inhibition was found to exacerbate GVHD, but not when IAP1/XIAP deficiency was limited to the immune system. This suggests intact tissue IAPs are relevant to tissue protection in GVHD91. Patients with XIAP deficiency undergoing allo-HSCT after myeloablative conditioning appear to have a poor overall outcome and extra protection against GVHD may be crucial for successful transplantation of these recipients92. A fourth example suggests manipulating epithelial integrity regulators to protect the epithelium in GVHD93. The expression of MLCK210, an established factor in epithelial tight junction regulation, was found to be increased in the intestinal epithelium of GVHD patients and mice93. MLCK210 deficiency in the host led to decreased barrier dysfunction, lower clinical GVHD sores and increased survival in multiple mouse GVHD models. Target-tissue allo-T cell numbers were lower in MLCK210-deficient hosts, and the GzmB-expressing CD8 T cell fraction in mesenteric lymph nodes (MLNs) was reduced93. Multiple mechanisms can attribute to this however, including activation, proliferation and homing of the allo-T cells, as well as epithelial–T cell interaction specific factors. Lastly, a recent study describes the disruption of oxidative phosphorylation in IECs exposed to allo-T cells, caused by a reduction in succinate dehydrogenase A (SDHA), a component of mitochondrial complex II94. In colonic biopsies from confirmed GI-GVHD patients the amount of SDHA was reduced in comparison to patients suspected of GI-GVHD that could not be histopathological confirmed. Genetically increasing SDHA levels in an experimental GVHD model reduced clinical and histopathological intestinal GVHD severity. Further study of all four approaches is required to establish usefulness in the clinical setting.
Preventing T cell trafficking to the gut
Given the epithelial damage caused by allo-T cells, blocking their entry into the intestines provides a promising strategy for tissue protection without increasing global immunosuppression and associated risks of relapse or infection elsewhere. Expression of α4β7 integrin is an important contributor to T cell homing to the GI tract, and plays a major role in the homing of allo-T cells to the GI tract as well95,96. Blocking α4β7 binding to its constitutively expressed receptor MAdCAM-1 on intestinal endothelium with anti-MAdCAM-1 antibody after GVHD induction96, or using α4β7-deficient donor T cells95, selectively reduced CD8 T cell infiltration in the gut96 and led to less GI-GVHD95. Interestingly, it was recently discovered that MAdCAM-1 expression in the small intestine vasculature localizes predominantly to vessels located in the lower crypt region, offering a possible explanation for the observed pattern of allo-T cells invading the crypt region early posttransplant. As such, inhibition of the α4β7-integrin/MAdCAM-1 axis reduced T cell infiltrate into the crypt base region of the mucosa and protected the ISC compartment from GVHD87. In a clinical setting, patients with GI-GVHD had a significantly higher percentage of α4β7-expressing memory T cell subsets than patients with skin-only GVHD or patients with no evidence of GVHD97. Retrospective studies indicated potential efficacy of vedolizumab, an anti-α4β7 antibody, for reduction of GI-GVHD severity98,99. Results of a prospective, dose-finding trial of vedolizumab starting 1 day prior to transplant are promising; the treatment was well tolerated, and the incidence of subsequent GI-GVHD development was low100. Blocking the α4β7 integrin pathway with monoclonal antibodies is therefore a promising strategy for protecting the crypt compartment following allo-HSCT. Antibodies that block only the β7 subunit may hold promise as well. These, in addition to antagonizing the α4β7-MAdCAM-1 mediated T cell influx, also target the αEβ7-E-cadherin interaction, believed to be important for T cell retention in the intraepithelial compartment101. A phase II clinical trial of the recombinant human anti-β7 etrolizumab for inflammatory bowel disease had promising results102. Future study will have to show if it can be useful in the treatment of GVHD as well.
In addition to protecting the intestinal epithelium from the effector phase of aGVHD, prophylactic inhibition of T cell entry to the gut may also protect against epithelium-dependent contributions to GVHD development. It was recently reported that intestinal epithelial antigen presentation can propagate alloreactive T cell responses. This is contrary the notion that host and donor professional antigen presenting cells (APCs) are the principle APC populations contributing to the activation of alloreactive donor T cells103. Despite the dominant role of hematopoietic APCs in propagating MHC-I-restricted/CD8 T cell-dependent GVHD104, radio-resistant non-hematopoietic APCs could contribute to the initiation of MHC-I-dependent GVHD as well105. Furthermore, profound deletion of professional host APCs did not decrease CD4-dependent GVHD in both a MHC-matched miHA-mismatched and a MHC-II-mismatched mouse model106. The action of recipient non-hematopoietic, non-professional APCs was sufficient to induce lethal GVHD107. MHC-II expression on IECs specifically could thus initiate lethal GVH immune responses, even in the presence of other types of APCs108. As such, approaches to reduce intestinal epithelial MHC-II expression, for instance through the initiation of a high fat diet in mice109, may reduce the development of experimental GVHD. Nonetheless, preventing donor T cells from reaching the intestinal epithelium with agents such as vedolizumab may be the most promising approach for reducing intestinal epithelial antigen presentation at the present time.
Preventing acute GVHD to prevent chronic GVHD
In some cases acute GVHD can progress or contribute to the development of chronic GVHD (cGVHD)110,111, and aGVHD is a well-defined risk factor for cGVHD12. While certain aspects of acute GVHD pathophysiology may be shared with cGVHD, such as the involvement of Th17/Tc17112,113, there is a paucity in research data studying the links between intestinal epithelial injury and the development of consequent cGVHD of the gut. Most recent insight in the pathobiology include an allogeneic ‘auto-immune’-like course of events, with defective thymic deletion of self-reactive T cells and aberrant B cell activation and production of antibodies114. Therefore, new targets of therapy include T and B cell-signaling pathways that are operational during cGVHD115. As aberrant tissue repair mechanisms, an inflammatory local milieu and continuous antigen exposure are also contributors in the development of cGVHD116, approaches discussed above to prevent intestinal epithelial injury and the development of acute GVHD are in essence applicable in the prevention of cGVHD as well.
Stimulating epithelial cell restoration
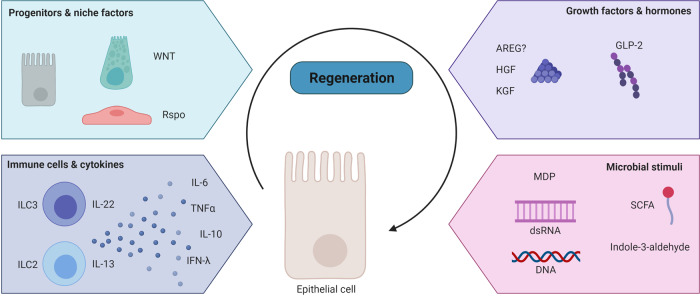
GVHD treatments have traditionally emphasized immunosuppression, and advances have focused on novel ways to accomplish this. In order to make continued meaningful progress, it is necessary to approach GVHD from additional perspectives. A promising and complimentary approach may be to focus on stimulating epithelial repair. Several processes are involved in the maintenance and recovery of the epithelium, including proliferation and differentiation of intestinal cells, as well as cell migration. Major pathways involved in these processes are multifactorial, and some examples are listed in Table 2. While it has been postulated that GVHD is mainly a disease of the inability to regenerate8, evidence exists that regeneration does take place, but may not be enough to overcome the continuous insult. This is for instance illustrated by the fact that intestinal epithelial crypts that survive allo-T cell insult in fact proliferate more than crypts of matched controls77. In addition, enterocytes of patients suffering from refractory GI-GVHD showed significant telomere shortening, which is associated with compensatory proliferation117. Below we will discuss regenerative approaches that hold promise to support the epithelium in the context of GVHD, either by stimulating epithelial constituents to promote recovery of the lining itself or by influencing the mucosal microenvironment (Fig. 4).
Table 2.
Major pathways involved in intestinal epithelial regeneration and repair.
| Signaling pathway | (S)timulation/(I)nhibition | Effect | Example of eliciting factor and/or mechanism | Ref. |
|---|---|---|---|---|
| mTORC1/SIRT1 | S | ISC expansion | Caloric restriction | 220 |
| PI3K/AKT | S | IEC proliferation, G1 cell cycle progression | Binding of EGF, TGF-α | 221 |
| WNT/R-spondin/β-catenin | S | ISC proliferation, suppressed IEC differentiation | Arachidonic acid presence | 67,222 |
| STAT5/NFκβ | STAT5 S | ISC proliferation, crypt regeneration | Cytokine receptor activation | 223 |
| NFκβ I | Mucosal wound healing | Decreased MLCK phosphorylation and TJ permeability | 224 | |
| Hippo/YAP-TAZ | Hippo I, YAP S | Intestinal regeneration in DSS colitis | Binding of stroma-derived Immunoglobulin superfamily containing leucin-rich repeat protein (ISLR) | 225,226 |
| Low Wnt signaling, wound-healing response | ||||
| Excessive PC differentiation, crypt regeneration | ||||
| Increased organoid growth | Binding of bile acids to TGR5 | 227 | ||
| Hippo S, YAP I | Maintenance Wnt signaling, canonical stem cell function | – | 228 | |
| SMAD | S | Increased barrier function through TJ protein upregulation | Binding of TGF-β | 229 |
| BMP/SMAD | I | ISC maintenance, expansion | Relief of direct, HDAC1-mediated transcriptional repression of stem cell signature genes | 230 |
| ERK/MAPK | S | ISC expansion, crypt formation, IEC proliferation | Binding of HGF to MET | 175 |
| Increased barrier function through TJ protein upregulation | Binding of TGF-β | 229 | ||
| Enhanced IEC migration | Binding of Flagellin | 231 | ||
| STAT3 | S | Intestinal mucosa regeneration, organoid formation | Downstream FAK activation and integrin signaling | 148 |
| ISC expansion, crypt formation, organoid proliferation | Binding of IL-22 to IL-22R | 76 | ||
| Myd88/NFκβ | S/I | Regulation of intestinal epithelial integrity and inflammatory responses | NFκβ inhibition leads to severe chronic inflammation and epithelial apoptosis | 232 |
| Epithelial MyD88 required for survival in multiple colitis models | 233 | |||
| c-Jun/AP-1 | S | Promotion of epithelial restitution after wounding through cell migration | Upregulation of PLCγ1-induced Ca2+ signaling | 234 |
| JNK2 | S | Epithelial barrier maintenance, enhanced Goblet cell and EEC differentiation and mucus production | Protection from DSS colitis, reduced barrier dysfunction and enterocyte apoptosis, increased Atoh1 expression | 235 |
Fig. 4. Regenerative treatment options in GI-GVHD.
Restoration of the epithelial barrier during the course of GVHD may occur at several levels. The epithelium reconstitutes from within, deriving from progenitors under the influence of supportive niche factors. It can also be supported in its regeneration from its immediate surroundings, for instance through the action of immune cells or particular excreted cytokines, growth factors and hormones. Finally microbial components can contribute to intestinal epithelial healing. Created with BioRender.com.
Restoration from within the epithelial compartment
Despite growing insights into ISC maintenance under homeostatic conditions, the principles underlying epithelial regeneration for maintenance of barrier function after tissue damage remain incompletely understood. Although radiation injury can cause a significant loss of ISCs, the Lgr5+ CBC cell pool is relatively resistant to radiation injury, reportedly due to their ability to repair DNA damage118. Crypt repopulation originated from surviving CBC cells118, which are essential, as Lgr5 genetic deletion and subsequent irradiation severely hinders the regenerative response119. There appears to be considerable plasticity in intestinal progenitor cells in response to damage. Upon CBC ablation, progenitors were able to dedifferentiate and regain stemness, thereby replenishing the ISC pool and subsequently the mature enterocytes at the epithelial surface120. Both secretory121,122 and enterocyte123 progenitors are capable of this reversion, and even fully differentiated enterocytes can contribute to crypt repopulation under specific circumstances of extreme damage124. In both instances, the expression of Lgr5 reappeared at the base of the crypt121,123, preceded by the re-expression of the ISC-restricted transcription factor Ascl2125. Even a subset of Paneth cells acquired multipotency upon irradiation through Notch activation126. The reprogramming of adult differentiated cells appears to have a developmental link122, as fetal mouse IECs can give rise to the adult ISC pool irrespective of their location or Lgr5 status127. Additionally, during infectious insult fetal-type gene expression programs play a role in epithelial recovery, as the murine ISC niche can revert to a fetal-like state upon parasitic helminth infection128. The importance of these complex crypt stem cell and progenitor dynamics in regeneration during GVHD-induced damage is currently unknown. The prolonged damage to the GI tract present in GVHD likely includes substantial insult to the cells with regenerative potential that are responsible for overall epithelial reconstitution.
Restoration through replenishment of ISC niche factors
Several niche factors secreted by cells in the microenvironment of the crypt compartment could contribute to restoration of crypt damage in allo-HSCT. Wnt signaling is essential for ISC maintenance, with cytoplasmic β-catenin translocating to the nucleus, interacting with transcription factors of the TCF/LEF family, and subsequently activating expression of target proteins involved in proliferation, such as Myc67. Wnt is required for crypt regeneration after damage129,130 and during inflammation, as seen in a DSS colitis model131. Short term Wnt agonism has been proposed as a therapeutic countermeasure against irradiation-induced gastrointestinal damage in mice132. GSK3β is an essential kinase of the Wnt/β-catenin pathway involved in the control of the cytoplasmic levels of β-catenin and its inhibition increases β-catenin availability and downstream Myc expression133. In an observational pilot study the known GSK3β-inhibitor lithium was used to salvage SR-GVHD, with promising results134.
Another approach to potentiate the Wnt pathway in the experimental transplant setting is through R-spondin-dependent modulation of Lgr5 signaling. It has recently been proposed that the most abundant R-spondin in the intestines, Rspo-3, is predominantly produced by lymphatic endothelial cells (LECS) in the lamina propria. LECS were found to be reduced in number and their Rspo-3 production impaired in experimental GVHD135. Exogenous administration of Rspo-3 promoted stem cell recovery and epithelial regeneration in the colon in a murine DSS-induced colitis model124. Another source of R-spondins are the recently described MAP3K2-regulated intestinal stromal cells at the bottom of colon crypts, which release Rspo-1 to maintain Lgr5+ ISCs during DSS colitis66. A prophylactic strategy of enhancing Wnt signaling with administration of Rspo-1 reduced murine colon pathology resulting from radiation136 and chemotherapy injury136,137. Furthermore, in a MHC-mismatched allogeneic BMT model, pretransplant treatment with Rspo-1 was associated with increased Olfm4+ ISCs and reduced GVHD mortality75. In addition, Rspo1 administration stimulated differentiation of ISCs towards PCs, increasing their numbers, with a positive impact on the secretion of luminal α-defensins and microbiome diversity81. Rspo administration might therefore be a promising approach, but future clinical studies are necessary to investigate efficacy and safety.
Also other stem cell niche factors are important for crypt regeneration and intestinal epithelial integrity after damaging or inflammatory insults. For instance, mice with impaired EGF-receptor signaling in the gut were more susceptible to inflammation in DSS colitis, due to impaired IEC regeneration and consequent barrier compromise138. In a phase I study, patients receiving urinary-derived human chorionic gonadotropin (uhCG) that could supplement EGF as supportive care in aGVHD had a promising biochemical response correlating with day 28 clinical response139. Also Notch ligands like Jag1 and DLL3 have been implicated in intestinal epithelial reconstitution and proliferation during inflammation, downstream of YAP pathway activation140, but have thus far not been studied in the context of GVHD.
Restoration through the regulation of immune cells & cytokines
In addition to epithelial and stromal contributions to the ISC niche, there is a growing appreciation that the local immune system can regulate the ISC compartment and its regeneration141. The IL-10-type cytokine IL-22142 is produced by a variety of immune cells and is involved in antimicrobial immunity and in both induction and resolution of inflammation in the intestine143–145. In addition, IL-22 has been implicated in the maintenance of the intestinal barrier and epithelial repair, due to its influence on mucus production145 and IEC proliferation76,146. IL-22 derived from group 3 ILCs (ILC3s) was shown to be protective in the GI tracts of transplant recipients in experimental GVHD74. However, the pathophysiological process of gut GVHD leads to loss of intestinal ILC3s and their protective IL-22 production74. Furthermore, patients with low numbers of ILCs in circulation prior to transplant had an increased risk of developing GVHD147. Interestingly, in vivo treatment of transplanted mice with the recombinant human IL-22 dimer/Fc fusion molecule F-652 (Generon Corp., Shanghai) reduced GVHD-related clinical scoring and mortality in a MHC-matched GVHD model76. IL-22 activated STAT3 phosphorylation in small intestine ISCs and organoids, promoting ISC survival and expansion as well as overall epithelial regeneration and recovery76. Findings of the role of the IL-22-STAT3 axis in crypt regeneration have been validated by the fact that STAT3 was required for damage-induced crypt regeneration after radiation injury148. In addition, IL-22 was required for an effective DNA damage response in protecting ISCs from genotoxic stress149. The influence of IL-22 on crypt regeneration under homeostatic conditions in vivo has not yet been studied. A Phase II trial for treatment of newly diagnosed GI-GVHD with a combination of corticosteroids and a recombinant human IL-22 dimer has recently been performed to investigate the safety potential of this novel tissue-regenerative approach to GVHD treatment (NCT02406651).
While therapy with IL-22 appears promising, additional immune-mediated pathways of regeneration may hold translational potential as well. IL-22-independent effects of ILC3s on epithelial regeneration involving the Hippo-YAP1 pathway have been described after methotrexate-induced GI damage150. The authors proposed a dichotomy between stem cell maintenance, which could be ILC3/IL-22/STAT3 dependent, and crypt proliferation, which they found to occur in a ILC3-dependent but IL-22 independent manner150. As such, the application of an ILC3-based cell therapy, instead of only administering IL-22, may have additional benefits for GVHD patients.
Also another type of innate lymphocyte cells, ILC2s residing in MLNs and Peyer’s patches, are known to support the intestinal barrier function, by inducing Goblet cell expansion through IL-13 secretion in response to Tuft-cell-derived IL-25151. Goblet cells are important for barrier function by secreting mucus that shields the intestinal epithelium from gut contents and microbes, and were found to be reduced in GVHD in mice and patients13. As mentioned earlier, ILCs are lost in GVHD, but pretransplant administration of IL-25 led to protective Goblet cell induction, decreased bacterial translocation, and ameliorated GVHD, increasing survival in a haploidentical and MHC-mismatched model13. In addition, ILC2-derived IL-13 may have a direct regenerative effect through binding the IL-13R expressed on ISCs. IL-13 increased ISC self-renewal and β-catenin signaling152.
Recently, it was reported that type III IFNs (IFN-λ), known for their role in epithelial viral defense, have an epithelial protective effect in experimental GVHD153. In vivo treatment of naïve mice with recombinant IFN-λ in the form of PEGylated (PEG-)IL-29, which has been tested in phase I-III clinical trials as an adjunctive treatment for hepatitis C virus, increased ISC numbers and led to more efficient ISC-derived organoid growth ex vivo. In experimental GVHD, prophylactic PEG-IL-29 administration prolonged survival, reduced GVHD severity and increased epithelial proliferation153.
Some other, classic pro-inflammatory cytokines have been shown to also play a role in maintaining epithelial integrity. Many of these cytokines may be inhibited by immunosuppressive GVHD therapies. For instance, TNFα has long been implicated in GI-GVHD pathogenesis154, but also has epithelial-supportive effects in vitro. Low-dose TNFα increased the number of human fetal intestinal organoids, while higher doses impaired organoid formation155. Mechanistically, TNF treatment directly promoted Wnt/B-catenin signaling156 and increased the expression of several stem cell markers in murine intestinal156 and human fetal intestinal organoids155, including Acl2. This epithelial-supportive mechanism provides insights as to why TNFα-blockade has had inconsistent results in the treatment of GI-GVHD157. A similar paradox can be found with the pro-inflammatory cytokine IL-6. IL-6 inhibition through blockade of IL6R-signaling with tocilizumab has had some promising results in both experimental and clinical GVHD, and has been associated with induction of allo-T cell suppressive Tregs158,159. However, IL-6 administration in healthy mice has been associated with STAT3-induced epithelial regenerative effects such as increased intestinal villus height, elongated enterocyte lifespan and a concurrent decrease in pro-apoptotic caspase activity160. Accordingly, in a GI damage model of mechanical wound injury, IL-6 inhibition resulted in impaired healing due to decreased proliferation161. As such, care should be taken with IL-6-blocking therapeutic approaches in GI-GVHD.
An additional example of cytokine-mediated restoration can be found in the regulatory cytokine IL-10. IL-10- and IL10R deficiency are known to cause severe intestinal disease in both mice and humans162, and disruption of the IL-10 signaling pathway resulted in exacerbation of experimental GVHD163. Nonetheless, treatment of IL-10 or co-culture with peripherally induced Tregs led to the expansion of ISC numbers in murine organoids, and increased clonogenicity after passage164. In addition, human recombinant IL-10 was shown to promote intestinal epithelial proliferation by activation of CREB signaling165. Despite the fact that treatment of GVHD by exogenous IL-10 does not seem to be clinically feasible due to its pleiotropic and divergent effects163, there may be a crypt-protective effect if epithelial-targeted administration would be possible.
Restoration through use of growth factors and hormones
In addition to previously discussed niche factors, stromal cells surrounding the epithelial crypts are important sources of EGF-like growth factors for the intestinal epithelium. Keratinocyte growth factor (KGF) is one of the most well-studied for its protective role during conditioning-induced damage and oral mucositis166. While initially described as a growth factor for skin epithelium, KGF can enhance intestinal epithelial proliferation167, and crypt cell survival after irradiation168. In experimental GVHD, KGF administration started prior to and continued after the transplant reduced GVHD mortality and severity in the GI tract169,170. However, administration of palifermin, a recombinant human KGF, did not reduce GVHD incidence or improve overall survival in allo-transplant patients in two randomized controlled trials171,172, although it did reduce mucositis incidence and severity in a subgroup of patients171. Another EGF-like growth factor in preclinical development is the potent liver mitogen Hepatocyte Growth Factor (HGF) produced by intestinal fibroblasts and macrophages173. Using a human HGF expression vector injected into muscle at the time of transplant, stable expression of hHGF in HSCT recipient mice reduced GVHD histopathology and crypt apoptosis174. Interestingly, HGF was found to be a possible substitute for EGF in intestinal organoid cultures. Mice lacking the receptor for HGF in their epithelium had reduced numbers of proliferating crypts and ISCs after irradiation175. Perhaps the protective effect of HGF in experimental GVHD is a result of directly targeting the intestinal epithelium. Lastly, it has been postulated that amphiregulin (AREG), a weak EGF-receptor agonist produced by a multitude of immune, stromal and epithelial cells, may have intestinal epithelial regenerative effects in the GVHD setting. It has been implicated as a possible plasma biomarker for risk stratification and steroid response in aGVHD176. Genetic disruption of Areg significantly impaired intestinal regeneration after radiation injury in full knockout mice177. Nonetheless, the beneficial effects of AREG on experimental GVHD incidence and mortality observed thus far do not directly implicate epithelial regeneration as the main mechanism and could still be ascribed to allo-immune suppression, such as through Treg function enhancement178–180. Taken together, the intestinal regenerative effect of growth factor substituents may be promising, but seems to have limited application in the clinic thus far.
In addition to growth factors, enteroendocrine hormones may have intestinal epithelial protective effects181 in the context of GI-GVHD. Glucagon-like peptide (GLP)-2 is produced by intestinal L-cells, which are a subset of enteroendocrine cells. L-cells are reduced in mice and patients that develop GVHD182. In vivo, GLP-2 agonism acutely increased the proportion of Lgr5+ ISCs in S-phase and prolonged treatment increased numbers of Olfm4+ ISCs per crypt183. GLP-2 stimulation of intestinal organoids led to increased organoid size182. Prophylactic treatment with a GLP-2 agonist injected subcutaneously in a MHC-mismatched mouse model, improved survival, decreased gut GVHD histopathology scores, and restored ISC loss, even when applied as an additive to steroids182. Future clinical studies will have to investigate its utility in clinical GVHD patients.
Restoration through the supply of protective microbial stimuli
Despite the pro-inflammatory effects of some innate immune signaling pathways, it was demonstrated that specific innate pattern recognition pathways can exert a protective effect on the intestinal epithelium during GVHD in mice. The RIG-I/MAVS pathway is involved in the sensing of dsRNA during infection, while the cGAS/STING signaling pathway is involved in the recognition of DNA. Perturbation of these innate pathways with genetic STING knockouts, changed the sensitivity to GVHD with contrasting effects on outcomes depending on the donor/recipient disparity and the specifics of the transplant models utilized184,185. In a MHC-mismatched model, treatment with 3pRNA or DNA prior to allo-HSCT protected mice from conditioning-induced intestinal damage and GVHD without diminishing the GVL activity of allo-T cells. Mechanistically, activation of the pathways led to the expression of protective type I IFNs (IFN-Is), which were indispensable for the maintenance of gut epithelial barrier integrity, but only when they were induced prior to the TBI insult. Treatment of intestinal organoids with 3pRNA and DNA confirmed the direct epithelial effects with increased IFN-I-dependent proliferation184. Intestinal epithelial IFN-I signaling was recently implicated in the regulation of stemness and differentiation into secretory-cell lineages. Mice lacking IEC Interferon regulatory factor 2, which downregulates IFN-signaling, had fewer ISCs, accumulation of immature PCs and impaired regeneration after damage186. In clinical studies, treatment with IFN-α before HSCT187 or after relapse post-HSCT188 was associated with a higher incidence of overall acute GVHD. Tight regulation of IFN-signaling induction during injury thus appears to be crucial.
Another cytosolic innate immune pathway implicated in the protection against intestinal epithelial injury is NOD2. It binds to the peptidoglycan muramyl dipeptide (MDP), which is produced by most bacteria. In a T cell-induced enteropathy model, NOD2 deficiency outside the intestinal epithelial compartment led to more severe crypt damage, apoptosis and delayed epithelial regeneration189. Similarly, in mouse BMT models, host NOD2 expression in the hematopoietic compartment is protective against the development of GVHD190. Nevertheless, MDP was shown to directly increase organoid-forming potential of intestinal crypts and to protect ISCs from oxidative-stress-induced cell death191. NOD2 also supported intestinal crypt survival and regeneration after irradiation, both in organoid cultures of NOD2 knockout mice and in vivo192. Given these findings, a non-hematopoietic protective role of NOD2 signaling in GVHD protection may also be possible. Nonetheless, more study is required to appreciate whether these mechanisms can also be exploited in GVHD patients to promote regeneration.
Over the past decade, several bacterial metabolic products have been associated with gut barrier integrity, including in the context of GVHD. Many studies have focused on short chain fatty acids (SCFAs), such as butyrate and propionate. Butyrate contributes to intestinal health in multiple ways193–195. It was found to directly increase epithelial regeneration in 3D organoid cultures196 and improve wound healing through tight junction protein upregulation196,197. In the setting of GVHD, intragastric administration of butyrate to allogeneic recipients improved IEC junctional integrity, decreased expression of apoptotic proteins in IECs, and led to decreased GVHD, independent of the induction of Tregs196. Clostridia commensals are known butyrate producers, and administration of a microbial cocktail including 17 strains of Clostridiales elevated intraluminal butyrate concentrations, decreased GVHD clinical scores, and increased survival in a mouse GVHD model196. This could explain why the presence of Clostridiales198 and its protection in the microbiome by selective antibiotic use was found to be associated with reduced GVHD-related mortality in clinical studies199,200. It also provides a rationale for the use of fecal microbiota transplants for the treatment of GVHD201–203. Recently, it was demonstrated that signaling through non-hematopoietic GPR43, a metabolite sensor, is critical for the GVHD treatment effects of SCFAs, independent of baseline microbiota constitution204. Given the clear associations of microbial constituents and GVHD outcomes, manipulation of the enteric flora or associated metabolites represent promising approaches for clinical prevention and treatment of GVHD.
The tryptophan catabolite indole and its derivatives are other product of commensal bacteria with gut immunomodulatory effects. Lactobacillus-derived indole-3-aldhehyde, for instance, engages the aryl hydrocarbon receptor (AHR), an environmental sensor and crucial transcription factor for ILC3s in the gut. As such, it can expand ILC3s and their IL-22 production in the intestinal mucosa205, as well as influence the immune response via many other immune cell types206. AHR ligation however also has a direct epithelial effect, as it was shown to regulate ISC differentiation and thereby maintain barrier integrity207. In a GVHD mouse model, administration of indole-3-aldehyde reduced GVHD severity, intestinal epithelial damage, and gut bacterial translocation. The effects were mediated through an IFN-I response observed at the transcriptional level in whole gut samples208. In allo-HSCT patients, higher levels of urine 3‐indoxyl sulfate, an indole-derived metabolite, correlated with lower treatment-related mortality and higher overall survival209. Therefore, indole-3-aldehyde administration represents another potential interventional approach of interest for treatment of GVHD.
Concluding remarks and future prospects
The intestinal epithelium experiences substantial toxicity during the course of allogeneic transplantation. Given the pivotal role of alloreactive T cells in GVHD pathogenesis, effective immunosuppression is the cornerstone of GVHD treatment strategies. However, in addition to control of the alloreactive immune response, development of target-organ-focused strategies that can protect the epithelium and stimulate its regeneration is important for further progress in improving clinical outcomes for transplant patients. Given advancements in both experimental and clinical research, it is possible that this hope may be realized in the near future.
Most epithelial-targeted factors and pathways discussed here have pleiotropic effects with complex feedback mechanisms in multiple tissues and different cell types. The mere stimulation or inhibition on the systemic level therefore may not result in the intended outcome. New ways to specifically target the intestine, for example through intestine-directed genetically engineered cells210 or carriers such as nanoparticles211–213, might make additional GI-targeted approaches more feasible in the future, similar to the wide use of oral budesonide for more targeted administration of corticosteroids to the GI tract. On an even smaller scale, a better understanding of the structural design of factors involved may enable the decoupling of protective functions from pro-inflammatory effects. In a recent study authors were able to design a STAT3-biased IL-22 receptor agonist, which elicited tissue selective STAT3 activation in vivo214.
A combinatory approach of factors that hold promise in preliminary trials at different levels of epithelial support could be considered (Table 3). To have an effect, adequate timing of the different strategies will be essential (Fig. 2). Wider use of drug-exposure-targeted pretransplant conditioning to limit the initial damage29–31,33 in combination with early initiation of Jak1/2 inhibition to shield the epithelium from allo-T cell-derived IFNy and protect ISCs77 may represent a currently attainable approach to improve GVHD prophylaxis. Should GVHD develop, pro-regenerative therapies such as IL-22 could be administered in the front-line setting along with corticosteroids to promote epithelial recovery76. Attention must also be paid to maintaining a supportive enteric microbial environment, including preservation of healthy anaerobic commensals such as butyrate producers199,200. A comprehensive approach involving these strategies as well as the implementation of additional tissue-targeted modalities currently in development will be necessary to fully incorporate epithelial biology into GVHD treatment strategies and optimize outcomes for HSCT patients.
Table 3.
Ongoing trials aimed at protecting or regenerating the intestinal epithelium in GI-GVHD treatment or prevention (per February 1st, 2022).
| Trial agent | (Proposed) mechanism of action | Phase | Trial number |
|---|---|---|---|
| Reducing DAMPs or response to DAMPS | |||
| Alpha-1 Antitrypsin (AAT) | Serine protease inhibitor degrading heparan sulfate | III | NCT04167514 |
| II/III | NCT03805789 | ||
| Blocking alloreactive T cell influx to the gut | |||
| Vedolizumab | α4β7-integrin inhibitor | III | NCT03657160 |
| Natalizumab | Selective α4 subunit adhesion molecule inhibitor | II | NCT02133924 |
| Blocking cytokine-mediated killing | |||
| Ruxolitinib | JAK1/2 inhibitor | II | NCT04384692 |
| II | NCT04061876 | ||
| II | NCT03701698 | ||
| I/II | NCT03491215 | ||
| IV | NCT02386800 | ||
| I | NCT05121142 | ||
| Baricitinib | JAK1/2 inhibitor | I | NCT04131738 |
| Pacritinib | JAK2 inhibitor | I/II | NCT02891603 |
| Itacitinib | JAK1 inhibitor | I | NCT04070781 |
| II | NCT03846479 | ||
| I | NCT03755414 | ||
| Tocilizumab | IL-6 inhibitor | II | NCT04395222 |
| I | NCT04070781 | ||
| II | NCT03434730 | ||
| II | NCT04688021 | ||
| Jaktinib | JAK1/2/3 inhibitor | II | NCT04971551 |
| TQ05105 | JAK2 inhibitor | I/II | NCT04941404 |
| Regeneration of the epithelium | |||
| Pregnyl | human Chorionic Gonadotrophin (hCG)/EGF | I/II | NCT02525029 |
| I/II | NCT05123040 | ||
| IL-22Fc | IL-22R binding | Ib | NCT04539470 |
| Lactobacillus Plantarum | Producers of indole-3-aldhehyde | III | NCT03057054 |
| Galacto-oligosaccharide | Prebiotic sustaining butyrate-producing bacteria | I/II | NCT04373057 |
Acknowledgements
This research was supported by NIH award numbers R01-HL125571, R01-HL146338, and R01-HL145631 (A.M.H., C.A.L.), the WKZ Fund of the UMC Utrecht (C.A.L.), and Alexandre Suerman Stipend of the UMC Utrecht (S.A.J.).
Author contributions
S.A.J. drafted the paper. E.E.N. and A.M.H. provided expert advice and edited the text. C.A.L. supervised the work. All authors read and approved the paper.
Competing interests
C.A.L. and A.M.H. hold IP related to use of IL-22 in GVHD.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naymagon S, et al. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017;14:711–726. doi: 10.1038/nrgastro.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeiser R, Blazar BR. Acute graft-versus-host disease. N. Engl. J. Med. 2018;378:586. doi: 10.1056/NEJMc1716969. [DOI] [PubMed] [Google Scholar]
- 5.Penack O, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167. doi: 10.1016/S2352-3026(19)30256-X. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiser R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 2020;382:1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 8.Chakraverty R, Teshima T. Graft-versus-host disease: a disorder of tissue regeneration and repair. Blood. 2021;138:1657–1665. doi: 10.1182/blood.2021011867. [DOI] [PubMed] [Google Scholar]
- 9.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalle SC, et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci. Transl. Med. 2014;6:243ra87. doi: 10.1126/scitranslmed.3008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill GR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. doi: 10.1182/blood.V90.8.3204. [DOI] [PubMed] [Google Scholar]
- 12.Flowers MED, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ara, T. et al. Intestinal goblet cells protect against GVHD after allogeneic stem cell transplantation via Lypd8. Sci. Transl. Med. 12, eaaw0720 (2020). [DOI] [PubMed]
- 14.Fischer JC, Wintges A, Haas T, Poeck H. Assessment of mucosal integrity by quantifying neutrophil granulocyte influx in murine models of acute intestinal injury. Cell. Immunol. 2017;316:70–76. doi: 10.1016/j.cellimm.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Zuo L, Kuo W-T, Turner JR. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020;10:327–340. doi: 10.1016/j.jcmgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson JE, Brune M, Ekman T. The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell transplantation with reduced intensity conditioning. Bone Marrow Transplant. 2001;28:737–742. doi: 10.1038/sj.bmt.1703230. [DOI] [PubMed] [Google Scholar]
- 17.Johansson J-E, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig. Dis. Sci. 2007;52:2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- 18.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. doi: 10.1182/blood.V95.9.2754.009k25_2754_2759. [DOI] [PubMed] [Google Scholar]
- 19.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. doi: 10.1182/blood.V83.8.2360.2360. [DOI] [PubMed] [Google Scholar]
- 20.Nakasone H, et al. Impact of conditioning intensity and TBI on acute GVHD after hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:559–565. doi: 10.1038/bmt.2014.293. [DOI] [PubMed] [Google Scholar]
- 21.Kebriaei P, et al. Intravenous busulfan compared with total body irradiation pretransplant conditioning for adults with acute lymphoblastic leukemia. Biol. Blood Marrow Transplant. 2018;24:726–733. doi: 10.1016/j.bbmt.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slavin S, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. doi: 10.1182/blood.V91.3.756. [DOI] [PubMed] [Google Scholar]
- 23.Blum W, et al. Low-dose (550 cGy), single-exposure total body irradiation and cyclophosphamide: consistent, durable engraftment of related-donor peripheral blood stem cells with low treatment-related mortality and fatal organ toxicity. Biol. Blood Marrow Transplant. 2002;8:608–618. doi: 10.1053/bbmt.2002.v8.abbmt080608. [DOI] [PubMed] [Google Scholar]
- 24.Eduardo FP, et al. Retrospective study of the digestive tract mucositis derived from myeloablative and non-myeloablative/reduced-intensity conditionings with busulfan in hematopoietic cell transplantation patient. Support. Care Cancer. 2019;27:839–848. doi: 10.1007/s00520-018-4362-3. [DOI] [PubMed] [Google Scholar]
- 25.Abdul Wahid SF, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells Dev. 2014;23:2535–2552. doi: 10.1089/scd.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rambaldi A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–1536. doi: 10.1016/S1470-2045(15)00200-4. [DOI] [PubMed] [Google Scholar]
- 27.Bartelink IH, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol. Blood Marrow Transplant. 2014;20:345–353. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Harris AC, et al. Comparison of pediatric allogeneic transplant outcomes using myeloablative busulfan with cyclophosphamide or fludarabine. Blood Adv. 2018;2:1198–1206. doi: 10.1182/bloodadvances.2018016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson BS, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol. Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 30.Kharfan-Dabaja MA, et al. Higher busulfan dose intensity appears to improve leukemia-free and overall survival in AML allografted in CR2: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Leuk. Res. 2015;39:933–937. doi: 10.1016/j.leukres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Bartelink IH, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3:e526–e536. doi: 10.1016/S2352-3026(16)30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartelink IH, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. J. Am. Soc. Blood Marrow Transplant. 2009;15:231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Langenhorst JB, et al. Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes. Blood Adv. 2019;3:2179–2187. doi: 10.1182/bloodadvances.2018029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toubai T, Mathewson ND, Magenau J, Reddy P. Danger signals and graft-versus-host disease: current understanding and future perspectives. Front. Immunol. 2016;7:539. doi: 10.3389/fimmu.2016.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara JL, Smith CM, Sheets J, Reddy P, Serody JS. Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J. Clin. Investig. 2017;127:2441–2451. doi: 10.1172/JCI90592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penack O, Holler E, van den Brink MRM. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 37.Cooke KR, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J. Clin. Investig. 2001;107:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joly A-L, et al. The HSP90 inhibitor, 17AAG, protects the intestinal stem cell niche and inhibits graft versus host disease development. Oncogene. 2016;35:2842–2851. doi: 10.1038/onc.2015.242. [DOI] [PubMed] [Google Scholar]
- 39.Zitzer NC, Garzon R, Ranganathan P. Toll-like receptor stimulation by microRNAs in acute graft-vs.-host disease. Front. Immunol. 2018;9:2561. doi: 10.3389/fimmu.2018.02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankovic D, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J. Exp. Med. 2013;210:1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilhelm K, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 42.Im K-I, et al. The free radical scavenger necroX-7 attenuates acute graft-versus-host disease via reciprocal regulation of Th1/regulatory T cells and inhibition of HMGB1 release. J. Immunol. 2015;194:5223–5232. doi: 10.4049/jimmunol.1402609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcondes AM, et al. Response of steroid-refractory acute GVHD to alpha1-antitrypsin. Biol. Blood Marrow Transplant. 2016;22:1596–1601. doi: 10.1016/j.bbmt.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Magenau JM, et al. alpha1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood. 2018;131:1372–1379. doi: 10.1182/blood-2017-11-815746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong X, et al. The impact of P2X7 receptor antagonist, brilliant blue G on graft-versus-host disease in mice after allogeneic hematopoietic stem cell transplantation. Cell. Immunol. 2016;310:71–77. doi: 10.1016/j.cellimm.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Iliopoulou BP, et al. Blockade of TIM-1 on the donor graft ameliorates graft-versus-host disease following hematopoietic cell transplantation. Blood Adv. 2019;3:3419–3431. doi: 10.1182/bloodadvances.2019000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S, et al. MicroRNA-155-deficient dendritic cells cause less severe GVHD through reduced migration and defective inflammasome activation. Blood. 2015;126:103–112. doi: 10.1182/blood-2014-12-617258. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Chen G-Y, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G-Y, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toubai T, et al. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014;123:3512–3523. doi: 10.1182/blood-2013-12-545335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toubai, T. et al. Siglec-G represses DAMP-mediated effects on T cells. JCI insight2, e92293 (2017). [DOI] [PMC free article] [PubMed]
- 52.Toubai T, et al. Host NLRP6 exacerbates graft-versus-host disease independent of gut microbial composition. Nat. Microbiol. 2019;4:800–812. doi: 10.1038/s41564-019-0373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, et al. TLR4 inactivation protects from graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Cell. Mol. Immunol. 2013;10:165–175. doi: 10.1038/cmi.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calcaterra C, et al. Critical role of TLR9 in acute graft-versus-host disease. J. Immunol. 2008;181:6132–6139. doi: 10.4049/jimmunol.181.9.6132. [DOI] [PubMed] [Google Scholar]
- 55.Li H, et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J. Immunol. 2011;186:230–241. doi: 10.4049/jimmunol.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, et al. Caspase-11 signaling enhances graft-versus-host disease. Nat. Commun. 2019;10:4044. doi: 10.1038/s41467-019-11895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 58.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 59.Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 60.Schuijers J, van der Flier LG, van Es J, Clevers H. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Rep. 2014;3:234–241. doi: 10.1016/j.stemcr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- 64.Kabiri Z, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 65.Kang E, Yousefi M, Gruenheid S. R-Spondins are expressed by the intestinal stroma and are differentially regulated during citrobacter rodentium- and DSS-induced colitis in mice. PLoS ONE. 2016;11:e0152859. doi: 10.1371/journal.pone.0152859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu N, et al. MAP3K2-regulated intestinal stromal cells define a distinct stem cell niche. Nature. 2021;592:606–610. doi: 10.1038/s41586-021-03283-y. [DOI] [PubMed] [Google Scholar]
- 67.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haramis A-PG, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 69.Hardwick JCH, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 70.Martín-Alonso M, et al. Smooth muscle-specific MMP17 (MT4-MMP) regulates the intestinal stem cell niche and regeneration after damage. Nat. Commun. 2021;12:6741. doi: 10.1038/s41467-021-26904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosinski C, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl Acad. Sci. U.S.A. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980;78:764–771. doi: 10.1016/0016-5085(80)90681-2. [DOI] [PubMed] [Google Scholar]
- 73.Bombi JA, et al. Assessment of histopathologic changes in the colonic biopsy in acute graft-versus-host disease. Am. J. Clin. Pathol. 1995;103:690–695. doi: 10.1093/ajcp/103.6.690. [DOI] [PubMed] [Google Scholar]
- 74.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takashima S, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J. Exp. Med. 2011;208:285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takashima, S. et al. T cell-derived interferon-gamma programs stem cell death in immune-mediated intestinal damage. Sci. Immunol. 4, eaay8556 (2019). [DOI] [PMC free article] [PubMed]
- 78.Melson J, Jakate S, Fung H, Arai S, Keshavarzian A. Crypt loss is a marker of clinical severity of acute gastrointestinal graft-versus-host disease. Am. J. Hematol. 2007;82:881–886. doi: 10.1002/ajh.20976. [DOI] [PubMed] [Google Scholar]
- 79.Jenq RR, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eriguchi Y, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120:223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 81.Hayase E, et al. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J. Exp. Med. 2017;214:3507–3518. doi: 10.1084/jem.20170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levine JE, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122:1505–1509. doi: 10.1182/blood-2013-02-485813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber D, et al. The association between acute graft-versus-host disease and antimicrobial peptide expression in the gastrointestinal tract after allogeneic stem cell transplantation. PLoS ONE. 2017;12:e0185265. doi: 10.1371/journal.pone.0185265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lueschow SR, McElroy SJ. The paneth cell: the curator and defender of the immature small intestine. Front. Immunol. 2020;11:587. doi: 10.3389/fimmu.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferrara JLM, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao D, et al. Survival signal REG3alpha prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J. Clin. Investig. 2018;128:4970–4979. doi: 10.1172/JCI99261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu Y-Y, et al. T cell recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity. 2019;51:90–103.e3. doi: 10.1016/j.immuni.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang B, et al. Ruxolitinib early administration reduces acute GVHD after alternative donor hematopoietic stem cell transplantation in acute leukemia. Sci. Rep. 2021;11:8501. doi: 10.1038/s41598-021-88080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaser A, Martinez-Naves E, Blumberg RS. Endoplasmic reticulum stress: implications for inflammatory bowel disease pathogenesis. Curr. Opin. Gastroenterol. 2010;26:318–326. doi: 10.1097/MOG.0b013e32833a9ff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammadpour H, et al. Host-derived serine protease inhibitor 6 provides granzyme B-independent protection of intestinal epithelial cells in murine graft-versus-host disease. Biol. Blood Marrow Transplant. 2018;24:2397–2408. doi: 10.1016/j.bbmt.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toubai T, et al. IAPs protect host target tissues from graft-versus-host disease in mice. Blood Adv. 2017;1:1517–1532. doi: 10.1182/bloodadvances.2017004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marsh RA, et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood. 2013;121:877–883. doi: 10.1182/blood-2012-06-432500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nalle SC, et al. Graft-versus-host disease propagation depends on increased intestinal epithelial tight junction permeability. J. Clin. Investig. 2019;129:902–914. doi: 10.1172/JCI98554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujiwara H, et al. Mitochondrial complex II in intestinal epithelial cells regulates T cell-mediated immunopathology. Nat. Immunol. 2021;22:1440–1451. doi: 10.1038/s41590-021-01048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrovic A, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 96.Ueha S, et al. Intervention of MAdCAM-1 or fractalkine alleviates graft-versus-host reaction associated intestinal injury while preserving graft-versus-tumor effects. J. Leukoc. Biol. 2007;81:176–185. doi: 10.1189/jlb.0306231. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y-B, et al. Up-Regulation of alpha4beta7 integrin on peripheral T cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2009;15:1066–1076. doi: 10.1016/j.bbmt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Floisand Y, et al. Safety and effectiveness of vedolizumab in patients with steroid-refractory gastrointestinal acute graft-versus-host disease: a retrospective record review. Biol. Blood Marrow Transplant. 2019;25:720–727. doi: 10.1016/j.bbmt.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Coltoff A, Lancman G, Kim S, Steinberg A. Vedolizumab for treatment of steroid-refractory lower gastrointestinal acute graft-versus-host disease. Bone Marrow Transplant. 2018;53:900–904. doi: 10.1038/s41409-018-0094-8. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y-B, et al. Vedolizumab for prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:4136–4146. doi: 10.1182/bloodadvances.2019000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J. Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- 102.Vermeire S, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–318. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 103.Koyama M, Hill GR. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood. 2019;134:2139–2148. doi: 10.1182/blood.2019000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 105.Toubai T, et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radiosensitive host hematopoietic-derived antigen-presenting cells. Blood. 2012;119:3844–3853. doi: 10.1182/blood-2011-10-384057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H, et al. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J. Immunol. 2012;188:3804–3811. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koyama M, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat. Med. 2011;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 108.Koyama M, et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity. 2019;51:885–898.e7. doi: 10.1016/j.immuni.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beyaz S, et al. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell. 2021;28:1922–1935.e5. doi: 10.1016/j.stem.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jagasia MH, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. blood marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoemans HM, et al. EBMT-NIH-CIBMTR task force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53:1401–1415. doi: 10.1038/s41409-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yi T, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlson MJ, et al. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MacDonald KPA, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129:13–21. doi: 10.1182/blood-2016-06-686618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129:22–29. doi: 10.1182/blood-2016-08-686659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N. Engl. J. Med. 2017;377:2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 117.Hummel S, et al. Telomere shortening in enterocytes of patients with uncontrolled acute intestinal graft-versus-host disease. Blood. 2015;126:2518–2521. doi: 10.1182/blood-2015-03-633289. [DOI] [PubMed] [Google Scholar]
- 118.Hua G, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 120.Buczacki SJA, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 121.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Higa T, et al. Spatiotemporal reprogramming of differentiated cells underlies regeneration and neoplasia in the intestinal epithelium. Nat. Commun. 2022;13:1500. doi: 10.1038/s41467-022-29165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tetteh PW, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 124.Harnack C, et al. R-spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat. Commun. 2019;10:4368. doi: 10.1038/s41467-019-12349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murata K, et al. Ascl2-Dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell. 2020;26:377–390.e6. doi: 10.1016/j.stem.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu S, et al. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell. 2018;23:46–59.e5. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guiu J, et al. Tracing the origin of adult intestinal stem cells. Nature. 2019;570:107–111. doi: 10.1038/s41586-019-1212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nusse YM, et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature. 2018;559:109–113. doi: 10.1038/s41586-018-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ashton GH, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu S, et al. Lgr4 gene deficiency increases susceptibility and severity of dextran sodium sulfate-induced inflammatory bowel disease in mice. J. Biol. Chem. 2013;288:8794–8803. doi: 10.1074/jbc.M112.436204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Romesser PB, et al. Preclinical murine platform to evaluate therapeutic countermeasures against radiation-induced gastrointestinal syndrome. Proc. Natl Acad. Sci. U.S.A. 2019;116:20672–20678. doi: 10.1073/pnas.1906611116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raup-Konsavage WM, Cooper TK, Yochum GS. A role for MYC in lithium-stimulated repair of the colonic epithelium after DSS-induced damage in mice. Dig. Dis. Sci. 2016;61:410–422. doi: 10.1007/s10620-015-3852-0. [DOI] [PubMed] [Google Scholar]
- 134.Steinbach G, et al. Pilot study of lithium to restore intestinal barrier function in severe graft-versus-host disease. PLoS ONE. 2017;12:e0183284. doi: 10.1371/journal.pone.0183284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogasawara R, et al. Intestinal lymphatic endothelial cells produce R-Spondin3. Sci. Rep. 2018;8:10719. doi: 10.1038/s41598-018-29100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao J, et al. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc. Natl Acad. Sci. U.S.A. 2009;106:2331–2336. doi: 10.1073/pnas.0805159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim K-A, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 138.Chalaris A, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 2010;207:1617–1624. doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holtan SG, et al. Facilitating resolution of life-threatening acute GVHD with human chorionic gonadotropin and epidermal growth factor. Blood Adv. 2020;4:1284–1295. doi: 10.1182/bloodadvances.2019001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pestka S, et al. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 143.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 146.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Munneke JM, et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood. 2014;124:812–821. doi: 10.1182/blood-2013-11-536888. [DOI] [PubMed] [Google Scholar]
- 148.Oshima H, et al. Stat3 is indispensable for damage-induced crypt regeneration but not for Wnt-driven intestinal tumorigenesis. FASEB J. 2019;33:1873–1886. doi: 10.1096/fj.201801176R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gronke K, et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019;566:249–253. doi: 10.1038/s41586-019-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Romera-Hernandez M, et al. Yap1-driven intestinal repair is controlled by group 3 innate lymphoid cells. Cell Rep. 2020;30:37–45.e3. doi: 10.1016/j.celrep.2019.11.115. [DOI] [PubMed] [Google Scholar]
- 151.von Moltke J, Ji M, Liang H-E, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu P, et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat. Immunol. 2019;20:183–194. doi: 10.1038/s41590-018-0297-6. [DOI] [PubMed] [Google Scholar]
- 153.Henden AS, et al. IFN-λ therapy prevents severe gastrointestinal graft-versus-host disease. Blood. 2021;138:722–737. doi: 10.1182/blood.2020006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Levine JE. Implications of TNF-α in the pathogenesis and management of GVHD. Int. J. Hematol. 2011;93:571–577. doi: 10.1007/s12185-011-0803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schreurs RRCE, et al. Human fetal TNF-α-Cytokine-Producing CD4(+) effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity. 2019;50:462–476.e8. doi: 10.1016/j.immuni.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 156.Bradford EM, et al. Epithelial TNF receptor signaling promotes mucosal repair in inflammatory bowel disease. J. Immunol. 2017;199:1886–1897. doi: 10.4049/jimmunol.1601066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Couriel DR, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2009;15:1555–1562. doi: 10.1016/j.bbmt.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Drobyski WR, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol. Blood Marrow Transplant. 2011;17:1862–1868. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kennedy GA, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15:1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 160.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 161.Kuhn KA, Manieri NA, Liu T-C, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE. 2014;9:e114195. doi: 10.1371/journal.pone.0114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shouval DS, et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang P, Hill GR. Interleukin-10 mediated immune regulation after stem cell transplantation: Mechanisms and implications for therapeutic intervention. Semin. Immunol. 2019;44:101322. doi: 10.1016/j.smim.2019.101322. [DOI] [PubMed] [Google Scholar]
- 164.Biton M, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 2018;175:1307–1320.e22. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Quiros M, et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J. Clin. Investig. 2017;127:3510–3520. doi: 10.1172/JCI90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. 2007;18:817–826. doi: 10.1093/annonc/mdl332. [DOI] [PubMed] [Google Scholar]
- 167.Housley RM, et al. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J. Clin. Investig. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khan WB, Shui C, Ning S, Knox SJ. Enhancement of murine intestinal stem cell survival after irradiation by keratinocyte growth factor. Radiat. Res. 1997;148:248–253. doi: 10.2307/3579609. [DOI] [PubMed] [Google Scholar]
- 169.Krijanovski OI, et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94:825–831. doi: 10.1182/blood.V94.2.825. [DOI] [PubMed] [Google Scholar]
- 170.Clouthier SG, et al. Repifermin (keratinocyte growth factor-2) reduces the severity of graft-versus-host disease while preserving a graft-versus-leukemia effect. Biol. Blood Marrow Transplant. 2003;9:592–603. doi: 10.1016/S1083-8791(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 171.Blazar BR, et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) Blood. 2006;108:3216–3222. doi: 10.1182/blood-2006-04-017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jagasia MH, et al. Palifermin for the reduction of acute GVHD: a randomized, double-blind, placebo-controlled trial. Bone Marrow Transplant. 2012;47:1350–1355. doi: 10.1038/bmt.2011.261. [DOI] [PubMed] [Google Scholar]
- 173.D’Angelo F, et al. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin. Exp. Immunol. 2013;174:60–72. doi: 10.1111/cei.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuroiwa T, et al. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J. Clin. Investig. 2001;107:1365–1373. doi: 10.1172/JCI11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Joosten SPJ, et al. MET signaling mediates intestinal crypt-villus development, regeneration, and adenoma formation and is promoted by stem cell CD44 isoforms. Gastroenterology. 2017;153:1040–1053.e4. doi: 10.1053/j.gastro.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 176.Holtan SG, et al. Amphiregulin modifies the minnesota acute graft-versus-host disease risk score: results from BMT CTN 0302/0802. Blood Adv. 2018;2:1882–1888. doi: 10.1182/bloodadvances.2018017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shao J, Sheng H. Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology. 2010;151:3728–3737. doi: 10.1210/en.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zaiss DMW, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bruce DW, et al. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J. Clin. Investig. 2017;127:1813–1825. doi: 10.1172/JCI91816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bruce DW, et al. Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD. Blood Adv. 2021;5:4578–4589. doi: 10.1182/bloodadvances.2020001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rowland KJ, Brubaker PL. The ‘cryptic’ mechanism of action of glucagon-like peptide-2. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G1–G8. doi: 10.1152/ajpgi.00039.2011. [DOI] [PubMed] [Google Scholar]
- 182.Norona, J. et al. Glucagon like peptide-2 for Intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood10.1182/blood.2020005957 (2020). [DOI] [PMC free article] [PubMed]
- 183.Chen, M. E. et al. Glucagon-like peptide-2 stimulates S-phase entry of intestinal Lgr5+ stem cells. Cell. Mol. Gastroenterol. Hepatol. 10.1016/j.jcmgh.2022.02.011 (2022). [DOI] [PMC free article] [PubMed]
- 184.Fischer, J. C. et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 9, eaag2513 (2017). [DOI] [PMC free article] [PubMed]
- 185.Bader, C. S. et al. STING differentially regulates experimental GVHD mediated by CD8 versus CD4 T cell subsets. Sci. Transl. Med. 12, eaay5006 (2020). [DOI] [PMC free article] [PubMed]
- 186.Sato T, et al. Regulated IFN signalling preserves the stemness of intestinal stem cells by restricting differentiation into secretory-cell lineages. Nat. Cell Biol. 2020;22:919–926. doi: 10.1038/s41556-020-0545-5. [DOI] [PubMed] [Google Scholar]
- 187.Pigneux A, et al. Prior treatment with alpha interferon does not adversely affect the outcome of allogeneic transplantation for chronic myeloid leukaemia. Br. J. Haematol. 2002;116:193–201. doi: 10.1046/j.1365-2141.2002.03235.x. [DOI] [PubMed] [Google Scholar]
- 188.Henden AS, et al. Pegylated interferon-2α invokes graft-versus-leukemia effects in patients relapsing after allogeneic stem cell transplantation. Blood Adv. 2019;3:3013–3019. doi: 10.1182/bloodadvances.2019000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zanello G, et al. The cytosolic microbial receptor Nod2 regulates small intestinal crypt damage and epithelial regeneration following T cell-induced enteropathy. J. Immunol. 2016;197:345–355. doi: 10.4049/jimmunol.1600185. [DOI] [PubMed] [Google Scholar]
- 190.Penack O, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J. Exp. Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nigro G, Rossi R, Commere P-H, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 192.Lee, C. et al. NOD2 Supports crypt survival and epithelial regeneration after radiation-induced injury. Int. J. Mol. Sci. 20, 4297 (2019). [DOI] [PMC free article] [PubMed]
- 193.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Willemsen LEM, Koetsier MA, van Deventer SJH, van Tol EAF. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Donohoe DR, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mathewson ND, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016;17:505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma X, et al. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012;90:266–268. doi: 10.2527/jas.50965. [DOI] [PubMed] [Google Scholar]
- 198.Romick-Rosendale LE, et al. Antibiotic exposure and reduced short chain fatty acid production after hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 2018;24:2418–2424. doi: 10.1016/j.bbmt.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 199.Jenq RR, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weber D, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:1087–1092. doi: 10.1038/bmt.2016.66. [DOI] [PubMed] [Google Scholar]
- 201.van Lier, Y. F. et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 12, eaaz8926 (2020). [DOI] [PubMed]
- 202.Kakihana K, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.DeFilipp Z, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2:745–753. doi: 10.1182/bloodadvances.2018017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fujiwara H, et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018;9:3674. doi: 10.1038/s41467-018-06048-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zelante T, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 206.Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Metidji A, et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2018;49:353–362.e5. doi: 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Swimm A, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132:2506–2519. doi: 10.1182/blood-2018-03-838193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weber D, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015;126:1723–1728. doi: 10.1182/blood-2015-04-638858. [DOI] [PubMed] [Google Scholar]
- 210.Trivett, M. T. et al. Preferential small intestine homing and persistence of CD8 T cells in rhesus macaques achieved by molecularly engineered expression of CCR9 and reduced ex vivo manipulation. J. Virol. 93, e00896–19 (2019). [DOI] [PMC free article] [PubMed]
- 211.Lee Y, et al. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020;19:118–126. doi: 10.1038/s41563-019-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Praveschotinunt P, et al. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019;10:5580. doi: 10.1038/s41467-019-13336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gou S, et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54. doi: 10.1016/j.biomaterials.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 214.Saxton RA, et al. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity. 2021;54:660–672.e9. doi: 10.1016/j.immuni.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Toubai T, et al. Host-derived CD8+ dendritic cells are required for induction of optimal graft-versus-tumor responses after experimental allogeneic bone marrow transplantation. Blood. 2013;121:4231–4241. doi: 10.1182/blood-2012-05-432872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reinhardt K, et al. Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J. Immunol. 2014;193:3355–3365. doi: 10.4049/jimmunol.1400983. [DOI] [PubMed] [Google Scholar]
- 217.Hossain MS, et al. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. J. Immunol. 2011;187:5130–5140. doi: 10.4049/jimmunol.1101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Taylor PA, et al. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;112:3508–3516. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reichenbach DK, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015;125:3183–3192. doi: 10.1182/blood-2014-10-606830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Igarashi M, Guarente L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell. 2016;166:436–450. doi: 10.1016/j.cell.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 221.Sheng H, Shao J, Townsend CMJ, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Q, et al. Arachidonic acid promotes intestinal regeneration by activating WNT signaling. Stem Cell Rep. 2020;15:374–388. doi: 10.1016/j.stemcr.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gilbert S, et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015;4:209–225. doi: 10.1016/j.stemcr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gilbert S, et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol. Med. 2012;4:109–124. doi: 10.1002/emmm.201100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xu J, et al. Secreted stromal protein ISLR promotes intestinal regeneration by suppressing epithelial Hippo signaling. EMBO J. 2020;39:e103255. doi: 10.15252/embj.2019103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 227.Sorrentino G, et al. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology. 2020;159:956–968.e8. doi: 10.1053/j.gastro.2020.05.067. [DOI] [PubMed] [Google Scholar]
- 228.Cheung P, et al. Regenerative reprogramming of the intestinal stem cell state via hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell. 2020;27:590–604.e9. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005;167:1587–1597. doi: 10.1016/S0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Qi Z, et al. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat. Commun. 2017;8:13824. doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kondo Y, et al. Stimulation of cell migration by flagellin through the p38 MAP kinase pathway in cultured intestinal epithelial cells. J. Cell. Biochem. 2016;117:247–258. doi: 10.1002/jcb.25272. [DOI] [PubMed] [Google Scholar]
- 232.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 233.Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529.e2. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang P-Y, et al. c-Jun enhances intestinal epithelial restitution after wounding by increasing phospholipase C-gamma1 transcription. Am. J. Physiol. Cell Physiol. 2017;312:C367–C375. doi: 10.1152/ajpcell.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mandic AD, et al. c-Jun N-terminal kinase 2 promotes enterocyte survival and goblet cell differentiation in the inflamed intestine. Mucosal Immunol. 2017;10:1211–1223. doi: 10.1038/mi.2016.125. [DOI] [PubMed] [Google Scholar]