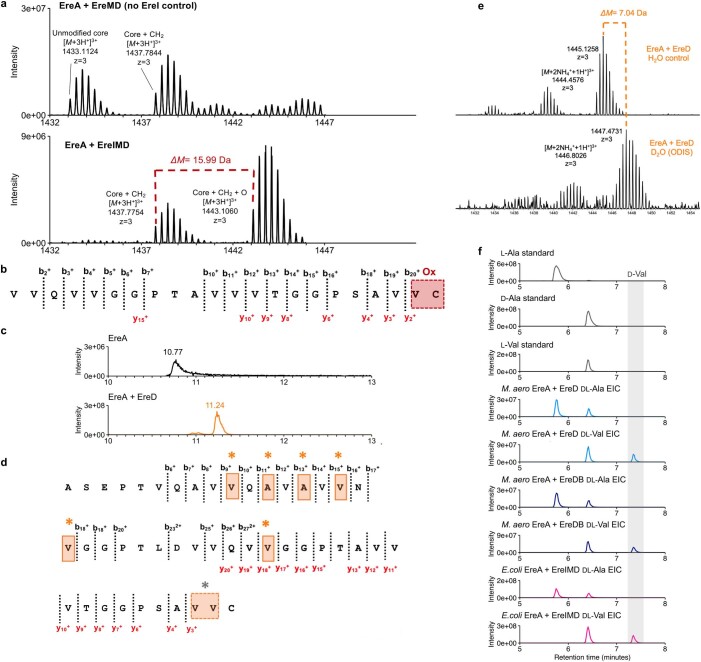
Extended Data Fig. 11. EreI mass shift and high resolution tandem mass spectroscopy (MS2); EreD retention time shift and ODIS and advanced Marfey's analysis for d-Val/d-Ala.
(a) Mass spectra and MS2-fragmentation of the LahT-digested Nhis-EreA modified by EreI, EreM, and EreD. Bottom: A mass shift of +15.99 Da corresponding to incorporation of one oxygen into the mono-methylated core (EreA + EreIMD, [M+3H+]3+ = 1443.1060 Da) was observed after co-expressions of ereAIMD. Top: No oxygen incorporation was observed in Nhis-EreA modified by EreM and EreD (EreA + EreMD) controls lacking the aspartinyl-asparaginyl β-hydroxylase protein family protein, EreI. Notably, the +15.99 Da modification was only observed on methylated, LahT-released EreA cores ([M+3H+]3+ = 1437.7754 Da) and not on the non-methylated core ([M+3H+]3+ = 1433.1124 Da) as observed by in vivo ereAI or ereAID co-expressions and in vitro assays with purified NHis-EreI and Nhis-EreA or Nhis-EreA modified by EreD. (b) MS2-fragmentation of [M+3H+]3+ = 1443.1060 Da. The data localize oxygen (Ox) incorporation to the C-terminus of the peptide but cannot distinguish between terminal cysteine (C46) or valine (V45). MS2-fragmentation data including calculated and observed masses for all b- and y- ions are available in Supplementary Table 5. (c) Extracted ion chromatograms (EICs) at 1433.1123 Da of LahT-digested precursors Nhis-EreA (black trace, top) and epimerized Nhis-EreA from co-productions with radical SAM epimerase EreD (orange trace, bottom), which show a retention time shift of 0.47 min. No mass shift was observed, since l- to d- amino acid epimerization is a mass-neutral modification, requiring the use of the orthogonal D2O induction systems (ODIS) to localize epimerization sites. (d) MS2-fragmentation of m/z = 1447.47308 Da patterns enabled localization of epimerized residues to V10, A12, A14, V16, V18, V29 (orange asterisks) and either V44 or V45 (grey asterisk). MS2-fragmentation data are available in Supplementary Table 5. (e) Expressions using ODIS results in a shift of +7.04 Da corresponding to the incorporation of 7 deuterium atoms. A mixture of products is observed due to slowdown of epimerization in the presence of deuterium. (f) EICs from advanced Marfey’s analysis of epimerized and modified cores from two heterologous hosts: E. coli (pink) and M. aerodenitrificans (light and dark blue) consistently yielded d-Val and d-Ala (grey shading) as the only d-amino acids detected in EreA cores, as compared to d-Thr, d-Asp, and d-Ser standards (not shown), for which no corresponding peaks were detected. Both d-Val and d-Ala were measured in ratios of 1:3 to their l-amino acid counterparts. Based on EreA core amino acid composition, these ratios correspond to approximately 2 d-Ala and 5 d-Val per core consistent with ODIS results.