Abstract
Background
The gold standard for COVID-19 diagnosis–reverse-transcriptase polymerase chain reaction (RT-PCR)– is expensive and often slow to yield results whereas lateral flow tests can lack sensitivity.
Methods
We tested a rapid, lateral flow antigen (LFA) assay with artificial intelligence read (LFAIR) in subjects from COVID-19 treatment trials (N = 37; daily tests for 5 days) and from a population-based study (N = 88; single test). LFAIR was compared to RT-PCR from same-day samples.
Results
Using each participant's first sample, LFAIR showed 86.2% sensitivity (95% CI 73.6%−98.8) and 94.3% specificity (88.8%−99.7%) compared to RT-PCR. Adjusting for days since symptom onset and repeat testing, sensitivity was 97.8% (89.9%−99.5%) on the first symptomatic day and decreased with each additional day. Sensitivity improved with artificial intelligence (AI) read (86.2%) compared to the human eye (71.4%).
Conclusion
LFAIR showed improved accuracy compared to LFA alone. particularly early in infection.
Keywords: COVID-19, SARS-CoV-2, Diagnostic accuracy, Validity, Rapid antigen test, Artificial intelligence
1. Introduction
The COVID-19 pandemic is a global public health crisis with over 500 million cases and 6 million deaths globally to date [1]. To contain infectious outbreaks like COVID-19, rapid and accurate diagnostic testing is critical. Reverse transcriptase polymerase chain reaction (RT-PCR) is the current reference standard [2]. Although it is the reference standard, RT-PCR testing falls short of being a perfect test for several reasons. Even where widely available, RT-PCR can take hours to complete and days to get results. In the United States (US), turnaround time for RT-PCR is 1 to 2 days during which time, infection is likely to be transmitted [2,3]. RT-PCR is also comparatively expensive, requiring trained laboratory technicians. The average cost of one RT-PCR test ranges from $100 to $300 in the US [4]. In low- and middle-income countries (LMICs), cost and personnel constraints can make RT-PCR screening for COVID prohibitive [[5], [7], [6]]. Thus, despite high analytic sensitivity, RT-PCR tests are ineffective for surveillance because of slow turnaround and limited availability [8].
Antigen tests have been touted as a better screening tool to detect infectious agents like SARS-CoV-2 [9]. Antigen tests are less expensive than RT-PCR; the average cost of antigen tests in the US is $25 per test kit with the global cost around $5 [6]. The World Health Organization (WHO) also predicts the price of these antigen tests will decrease [6]. Additionally, antigen tests do not require trained health care professionals and can provide results in 15 to 20 minutes. This rapid time to results facilitates quicker isolation of infected individuals. A recent study assessing various surveillance approaches through simulation models found that the speed of case identification was far more important than test sensitivity [10]. With this in mind, the CDC recently published interim guidelines for the use of antigen tests instead of PCR as an effective testing solution [11].
To date, 4 antigen tests have earned Emergency Use Approval (EUA) from the U.S. Federal and Drug Administration (FDA) for at-home use [9,12]. Although sensitivity and specificity of these tests in symptomatic adults has been published (range 64%–90% and 85.7%–99%, respectively), little is known about their performance throughout the course of illness in comparison to RT-PCR [[14], [13], [15]]. This comparison is of particular interest since RT-PCR often detects non-viable or non-transmissible virus late in the course of illness [16].
Two biotechnology companies, Cellex and Exa Health, have recently partnered to create a twenty-minute, lateral flow antigen test that is read by an artificial intelligence algorithm accessed through a smartphone application (Lateral Flow with AI Read; LFAIR). This rapid test can provide results at home, but also, if desired, simultaneously transmit results to health care providers and public health authorities. Therefore, the LFAIR has the potential to make SARS-CoV-2 testing available to anyone with a smartphone and a cellular connection, whether in the US or in resource limited settings.
We assessed the diagnostic accuracy of the Cellex and Exa Health, LFAIR to detect SARS-CoV-2 compared to the reference standard, RT-PCR. We then conducted analyses to assess how test results relate to days since symptom onset, test the sensitivity of AI compared to users’ interpretations, and SARS-CoV-2 viral load as approximated by RT-PCR cycle threshold (CT) values.
2. Material and methods
2.1. Participants
Participants, 125 in total, were recruited from 2 study populations that were obtaining nasal swabs for RT-PCR. These participants included:
-
1)
Thirty-seven from COVID treatment trials [NCT04524663 and NCT04346628] that included outpatients with mild to moderate COVID-19 symptoms who had tested positive for SARS-CoV-2 via RT-PCR within 72 hours of enrollment. These subjects underwent anterior nares swab by study personnel on days 1, 5, and 10 of study participation and swabbed themselves on days 2 to 4 and 6 to 9. Accumulated samples were refrigerated in viral transport medium before being sent for RT PCR on days 1, 5 and 10. Subjects in this group were provided with 5 kits and asked to conduct them on 5 days on which nasal swabs were obtained for RT-PCR.
-
2)
88 from a longitudinal surveillance study of COVID-19 incidence (TrackCOVID study). These subjects were not known to be infected with SARS-CoV-2. The subjects were provided with a single LFAIR kit to be performed at home on the same day that study personnel swabbed the subjects’ anterior nares and sent the swabs for RT-PCR.
Inclusion criteria included fluency in English, age over 18 years, and reported comfort using iPhone applications. Exclusion criteria included receiving treatment with convalescent plasma or other antibody therapy related to SARS-CoV-2 infections, participating in a SARS-CoV-2 vaccine study, and testing positive for SARS-CoV-2 more than 2 weeks prior to enrollment without an interim negative RT-PCR test. Eligible participants were recruited from December 2020 to March 2021.
All subjects provided informed consent and the study was approved by Stanford's Institutional Review Board (protocol #58444).
2.2. Data collection
Participants completed a survey with demographic information, symptom status, and if symptomatic, date of symptom onset and of prior positive RT-PCR. If a participant did not own an iPhone, one was loaned to them.
Each home test kit had a unique quick response (QR) code that was recorded before participants took the kit home. To simulate individuals purchasing kits at a store, researchers did not provide verbal instructions for the rapid antigen test. A brief instruction sheet directed subjects to scan the QR code to download the Exa Health app, which was only compatible with iPhones at the time of the study. The app then provided detailed video instructions to complete the test. A timer within the application indicated when 15 minutes had transpired and an image of the LFAIR should be uploaded to Exa Health with the smartphone camera. Only the image, no PHI or clinical information, was transferred. Participants were not provided with their results.
Results for RT-PCR assays, including CT values, were provided to this study by the PIs of the parent trials using study IDs connected to the QR codes. Subjects in NCT04346628 had their RT-PCR assays performed by Quest which amplified 2 sequences of the nucleocapsid gene (N1 and N3). All other RT-PCR assays were performed at the Stanford clinical lab using an RT-PCR of the E gene. Deidentified antigen test results, including AI binary results, the intensity score for the LFA antigen test line, and user read, were received from Exa Health with their attached QR codes.
2.3. LFAIR assay
At-home antigen test kits include a SARS-CoV-2 antigen test device, a buffer solution, a sterile swab, an extraction tube, and an instruction sheet. The plastic antigen test device includes the LFA test strip, consisting of a sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad is made of colloidal-gold conjugated with monoclonal antibodies that bind to SARS-CoV-2 nucleocapsid protein (N protein); the N protein is detectable in upper respiratory specimens during the acute phase of SARS-CoV-2 infection [9].
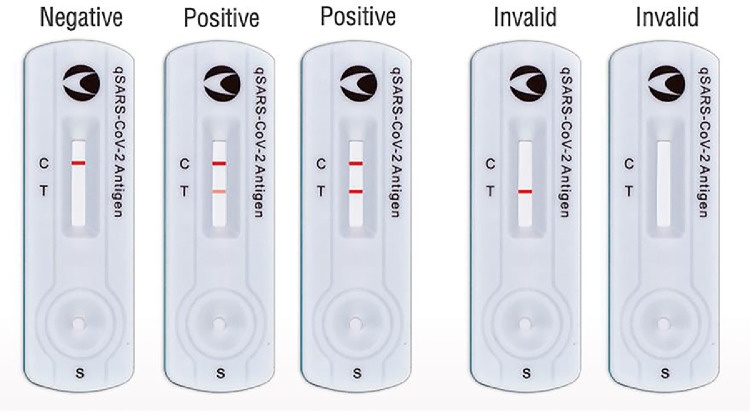
After swabbing their noses, subjects place the nasal swab into the buffer solution. After 1 minute, 3 drops of the buffer are added to the sample well on the test device. When SARS-CoV-2 antigen is present in the sample, the antibody conjugates and the N protein will form an antibody-antigen complex, which is captured by the monoclonal antibodies on the test line (T) region. Presence of the T line indicates a positive test result. All valid test strips must also have a control line (C) to indicate that the sample had sufficient volume of buffer and that membrane wicking was successful (Fig. 1 ) [17].
Fig. 1.
Example antigen test device results after sample has developed for 15 minutes. C indicates control line and T indicates test line. Invalid test strips do not have a C line, meaning there was insufficient sample volume or unsuccessful membrane wicking [18].
The artificial intelligence algorithm (Exa Health, Inc., Los Altos, CA) determines if a test is positive, negative, or invalid by determining the intensity of both the C and T lines. Test results are stored as either positive, negative, or invalid. The algorithm also stores the test line intensity value, which reflects the amount of SARS-CoV-2 antigen bound to the antibody in the LFA device. This test line intensity value ranges from 0 (no line visible) to 1.0 (most intense line). We also collected the test users’ interpretations of their results. Participants were prompted through the smartphone application to indicate if they could see a line next to the control (C) and test (T) markers.
2.4. Statistical analysis
We report distribution and mean (standard deviation) of participant demographic and symptom characteristics, infection status determined by RT-PCR results, RT-PCR CT values, and antigen test line intensity.
RT-PCR results were used as the reference test to determine the sensitivity and specificity with 95% confidence intervals for each participant's first antigen test regardless of symptom status.
Since SARS-CoV-2 positive participants underwent multiple reference and index tests, ranging from 1 to 5 pairs of tests, we also estimated sensitivity using a repeated-measures analysis including each index-reference pair completed on the same day. To understand how the LFAIR sensitivity varies over time, we stratified test sensitivity over days since symptom onset based on subject self-report. We also used the generalized estimating equation (GEE) approach to estimate sensitivity with robust standard errors for correlated observations [[20], [18], [19]]. We implemented a GEE model with a binary distribution and logit link function.
In subjects from the treatment cohort, we compared the sensitivity of LFAIR with that of the test user's reading of the C and T lines using McNemar's Exact test. We also used a linear regression to investigate the relationship between antigen test line intensity and RT-PCR CT values. Finally, we determined the LFAIR diagnostic accuracy compared to RT-PCR results stratified by participant age and education level. We created dichotomous variables for participant age (<40 and ≥40 years) and educational attainment (less than a 4 years college degree or college graduate). Data was analyzed using statistical programs R (Version 1.2.5019) and Stata (Version 16.1, StataCorp, College Station, TX).
3. Results
Of 37 participants recruited from the COVID treatment trials, 33 (89%) completed at least 1 antigen test successfully with a total of 119 assays performed (mean 3.6 per person). Paired RT-PCR results were available for 110 of these 119 LFAIR results (mean 3.3 per person). Of the 88 individuals from the longitudinal TrackCOVID study, 66 (75%) completed their LFAIR successfully and all 66 had paired PCR results. The mean age of participants was 47.7 years (SD = 14.8) and 55.1% were female (Table 1 ). Of the 185 rapid LFA antigen tests completed, 1 yielded an invalid result and was removed from subsequent analysis.
Table 1.
Descriptive statistics of participants’ demographic information including age, race, ethnicity, sex, and education level.
| Characteristics, n (%) | Participants (N = 98) |
|---|---|
| Age | |
| 18−24 | 1 (1.0%) |
| 25−44 | 42 (42.9%) |
| 45−64 | 41 (41.8%) |
| 65+ | 14 (14.3%) |
| Race | |
| Asian | 19 (19.4%) |
| Black | 4 (4.1%) |
| White | 58 (59.2%) |
| Other | 17 (10.2%) |
| Ethnicity | |
| Hispanic/Latino | 17 (17.3%) |
| Not Hispanic/Latino | 81 (82.7%) |
| Sex | |
| Female | 54 (55.1%) |
| Male | 44 (44.9%) |
| Education | |
| High school degree | 4 (4.1%) |
| Associates degree | 10 (10.2%) |
| Four-year college degree | 41 (41.8%) |
| Graduate degree | 43 (43.9%) |
Overall, 29 participants—28 from the COVID treatment trials–were positive by RT-PCR on at least 1 swab. Among the 70 SARS-CoV-2 negative participants, 65 (92.9%) were recruited from the TrackCOVID population-based study. Only 1 SARS-CoV-2 positive individual, recruited from the TrackCOVID trials, was asymptomatic.
The mean (SD) CT values for the N1 gene, N3 gene, E gene, and RNaseP control sequence were 23 (4.9), 24 (4.9), 24.7 (6.8), and 21.3 (3.2), respectively.
Stanford University and Quest report any CT values of 40 or less as positive for SARS-CoV2. Using this RT-PCR cutoff and only the first test from each person in study, the sensitivity and specificity of the LFAIR were 86.2% (95% CI 73.6%–98.8%) and 94.3% (95% CI 88.8%–99.7%), respectively (Table 2 ).
Table 2.
Test characteristics of index test (LFAIR) compared to reference test (RT-PCR) for all participants’ first test.
| RT-PCR Results |
|||
|---|---|---|---|
| Positive | Negative | Total | |
| LFAIR Positive | 25 | 4 | 29 |
| LFAIR Negative | 4 | 66 | 70 |
| 29 | 70 | 99 | |
Sensitivity = 86.2% (95% CI 73.6%−98.8%) Specificity = 94.3% (95% CI 88.8%−99.7%).
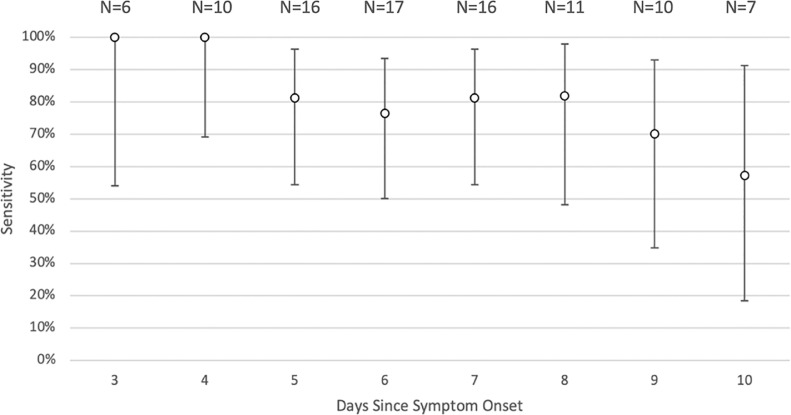
Since almost all (96.4%) SARS-CoV-2 positive individuals performed multiple tests over several days, we assessed sensitivity with RT-PCR results as the reference test stratified over days since symptom onset. The at-home rapid LFA antigen test had a high sensitivity over the first few days after symptom onset, and the sensitivity declined as the days progress (Fig. 2 and Supplementary Appendix, Table S1).
Fig. 2.
At-home LFA rapid antigen test sensitivity by days since symptom onset. Sensitivities are illustrated with open circles with confidence intervals included. Data for this figure is in the Supplementary Appendix (Table S1). LFA = lateral flow antigen.
The GEE model used all 97 tests from 28 individuals in the treatment trial population. The model, adjusted for repeated measures, predicted a sensitivity of 97.8% (95% CI 89.9%–99.5%) on the first symptomatic day decreasing with each additional day by 0.35 on a log odds scale. For example, the model predicts a sensitivity of 94.0% 3 days after symptom onset (Model, coefficient estimates, and predicted sensitivities are available in Supplementary Appendix, Figure S1 and Table S2).
Comparing the sensitivity of LFAIR to human user interpretation, the sensitivity of the test using artificial intelligence was 86.2% (95% CI 73.6%–98.8%) and the sensitivity of the test with user interpretation was 71.4% (95% CI 54.7%–88.2%; P = 0.0625) (Supplementary Appendix, Table S3).
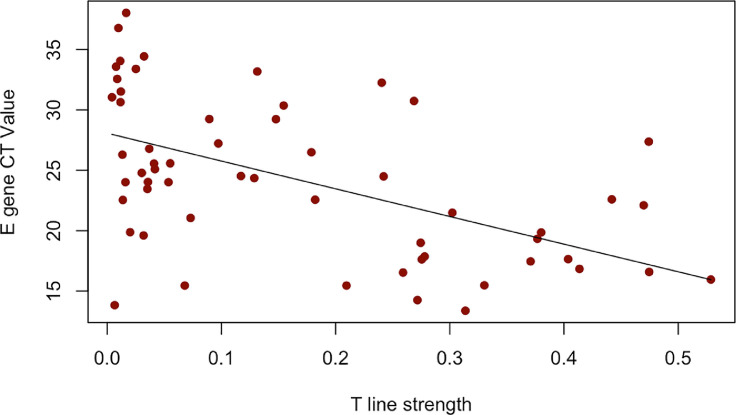
Because we only had sufficient samples with E gene CT values (58, compared to 14 for the N1 and N3 genes), we assessed the relationship of the E gene CT value with the LFAIR test line intensity. We detected a linear association between the E gene CT value and LFAIR test line intensity (r = -0.53, P < 0.001) (Fig. 3 ). A decrease in LFAIR test line was associated with an increase in CT value, a proxy for viral load.
Fig. 3.
E gene CT value plotted by LFAIR test (T) line strength. E gene CT value associated with test line strength (r = - 0.53; P < 0.001). LFAIR = Lateral Flow with AI Read.
We did not observe any significant differences in sensitivity and specificity of the LFAIR by age group or level of educational attainment (Supplementary Appendix, Tables S4 and S5).
4. Discussion
In our study cohort we found the LFAIR test had a sensitivity of 86.2% and specificity of 94.3% for the first LFAIR test completed, ignoring days since first symptom onset. These values are similar to the reported sensitivities and specificities of extant EUA-approved tests that range from 64% to 95% and 96% to 100%, respectively. Sensitivity was better using AI interpretation than when the assay was judged visually by the test user.
When accounting for days since symptom onset, we found that the at-home rapid antigen test sensitivity was much higher over the first few days after symptoms began. We saw this trend not only when we stratified the first test per participants but also when we accounted for all data collected, including all repeat LFAIR and RT-PCR tests. When accounting for the consecutive testing, the GEE model predicted a test sensitivity of 97.78% on day one of symptoms.
Both the crude stratification and GEE model indicate that LFAIR is more sensitive in early stages of disease onset when SARS-CoV-2 viral loads are likely higher in nasopharyngeal and salivary samples and, therefore, individuals are more likely to transmit the virus to others. False negatives in later stages of disease (higher CT values) when individuals are less likely to transmit SARS-CoV-2 to others may be less problematic from a public health perspective. A study assessing virus cultures of respiratory tract secretions as a proxy for virus shedding found that shedding was undetectable at RNA load thresholds above 7 log10 RNA copies per milliliter [16]. These findings of high sensitivity in early stages of SARS-CoV-2 infection, when viral load is high, suggest that the LFAIR can serve as an effective early diagnostic tool to mitigate the spread of SARS-CoV-2. However, in high-risk populations and areas, the impact of false negative results should not be underestimated.
The association between the LFAIR test line intensity and the E gene CT value suggests that the antigen test may provide more than a binary answer. However, the variance in the intensity was considerable, and at least for now, LFAIR should be treated as a binary test. Although our study was not adequately powered to assess the relationship of symptom type with LFAIR sensitivity or intensity, our findings suggest additional research into the relationship between symptoms and LFA accuracy is warranted.
A key strength of our study is the real-life conditions of our testing. Participants completed all of their LFAIR tests at-home, which is the setting for which these tests were developed.
Although these conclusions point towards the LFAIR being a useful public health tool for COVID-19, we note that the generalizability of our findings is limited by spectrum bias, iPhone application use, and low racial and ethnic diversity of our sample. For spectrum bias, since we only had one asymptomatic SARS-CoV-2-positive individual, we could not estimate sensitivity in asymptomatic individuals. Moreover, most participants were tested 3 or more days after symptom onset, when viral load peaks. Therefore, it is unclear how sensitive the at-home LFA rapid antigen test is in the first 2 days after symptom onset. This spectrum bias may mean that LFAIR's sensitivity will be lower in practice [21].
Additionally, the diversity of our cohort does not adequately reflect the general public, limiting the generalizability of our findings. The similar LFAIR test diagnostic accuracy by age and education, however, suggest that the test can be used by a wide variety of individuals. That most positive patients had already received a confirmed diagnosis of SARS-CoV-2 also introduces the possibility of bias and subsequent user interpretation bias could have underestimated the discrepancy between the LFAIR and human eye sensitivity.
We should note how the persistence of RT-PCR positivity after the infectious period might affect our results. Since RT-PCR tests can be positive even when an individual is no longer contagious, some LFAIR false negatives that occurred in patients with non-viable or non-transmissible virus might actually be considered true negatives. We tried to assess the importance of this consideration by looking at the test sensitivity stratified by days since symptom onset.
Results from this study indicate that this LFAIR test could be integral to improving SARS-CoV-2 screening and slowing the spread of and reducing the deaths caused by SARS-CoV-2. Moreover, this rapid testing has the potential to improve access to diagnostic testing because compared to the current reference standard, RT-PCR, it is less expensive and can produce test results in the absence of laboratories and medical personnel. This LFAIR test could be particularly useful in resource limited settings. For example, in developing countries with high smartphone use, the LFAIR test would enable diagnostic results to be sent from individuals’ phones directly to public health officials and physicians.
5. Conclusions
In summary, this lateral flow assay, when performed at home, has good sensitivity and specificity, particularly when read with artificial intelligence rather than with the human eye. The test appears most sensitive early in the course of illness when viral load is highest and transmission most likely. Because the artificial intelligence algorithm will improve as more testing is completed, and because results can be automatically transmitted to health department and health care providers, we expect this and similar assays will become useful tools for controlling SARS-CoV-2 in areas with high smartphone coverage.
Funding
This work was funded by Exa Health, Inc. (IC-2020 SPO 205666, Los Altos, CA ). The company was not involved in the data analysis or in manuscript preparation or writing. The artificial intelligence algorithm that Exa Health produced for the smartphone application was set at the start of the study; none of the study's data was used to develop the algorithm.
Authors statement
The authors contributed to the paper in the following manner: Shannon Richardson: Data curation, formal analysis, investigation, visualization, Roles/writing – original draft. Michael Kohn : Formal analysis, Methodology, Visualization, Writing—review and editing. Jenna Bollyky : Methodology, Project administration, Supervision, Writing – review & editing. Julie Parsonnet: Formal analysis, Funding Acquisition, Investigation, Methodology, Project administration, Writing – Reviewing and Editing
Authors’ contributions
Shannon Richardson: study design, data collection, data analysis and interpretation, drafted manuscript, and revised manuscript
Michael Kohn: data analysis and revised manuscript
Jenna Bollyky: data collection and revised manuscript
Julie Parsonnet: study design, data collection, and revised manuscript
Declaration of competing interest
The authors report no conflicts of interest relevant to this article
Acknowledgments
We thank Yvonne A Maldonado, MD and Upinder Singh, MD for their collaboration with the TrackCOVID study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.diagmicrobio.2022.115763.
Appendix. Supplementary materials
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context. Sci Transl Med. 2020;12(546):1–7. doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- 3.Sallam M. Covid-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):1–15. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krouse S, Abbott B. What kind of covid test should i get? Answers on cost, accuracy and more. Wall Str J. 2020:1–7. https://www.wsj.com/articles/covid-19-tests-answers-on-cost-accuracy-and-turnaround-time-11599134378 Accessed August 30, 2022. [Google Scholar]
- 5.Khoury P, Azar E, Hitti E. COVID-19 response in lebanon: current experience and challenges in a low-resource setting. JAMA - J Am Med Assoc. 2020;324(6):548–549. doi: 10.1001/jama.2020.12695. [DOI] [PubMed] [Google Scholar]
- 6.Mahase E. Covid-19: 120 million rapid tests pledged to low and middle income countries. BMJ. 2020;371:m3857. doi: 10.1136/bmj.m3857. [DOI] [PubMed] [Google Scholar]
- 7.Kobia F, Gitaka J. COVID-19: are Africa's diagnostic challenges blunting response effectiveness? AAS Open Res. 2020;3(4):1–11. doi: 10.12688/aasopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity — A strategy for containment. N Engl J Med. 2020;383(22):e120. doi: 10.1056/nejmp2025631. [DOI] [PubMed] [Google Scholar]
- 9.Ravi N, Cortade DL, Ng E, Wang SX. Diagnostics for SARS-CoV-2 detection: a comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1) doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Interim guidance for antigen testing for SARS-CoV-2. Centers Dis Control Prevent. 2020 https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html [Google Scholar]
- 12.FDA. In vitro diagnostics EUAs - antigen diagnostic tests for SARS-CoV-2 FDA. 2021. p. 1-5. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2. Accessed August 30, 2022.
- 13.Prince-Guerra JL, Almendares O, Nolen LD, Gunn JKL, Dale AP, Buono SA, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites — Pima County, Arizona, November 3–17, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(3):100–105. doi: 10.15585/mmwr.mm7003e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollock NR, Tran K, Jacobs JR, Cranston AE, Smith S, O’Kane CY, et al. Performance and operational evaluation of the access bio carestart rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. Open Forum Infect Dis. 2021;8(7):1–7. doi: 10.1093/ofid/ofab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BD. BD Veritor SARS-CoV-2 System : easy-to-use, portable rapid point-of-care antigen diagnostic test in just 15 minutes.https://www.bd.com/en-za/about-bd/news-and-media/press-releases/jun-30-2021-bd-veritor-sars-cov-2-system. Published 2021.
- 16.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12(1):267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spartan Medical Cellex QSARS-CoV-2 Antigen Rapid Test. 2022 [Google Scholar]
- 18.Liang KY, Zeger SL. Longitudinal data analysis using GLM. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 19.Zeger L, Liang S. Longitudinal data analysis for discrete and continuous outcomes author (s): Scott L. zeger and kung-yee liang published by : international biometric society stable. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 20.Genders TSS, Spronk S, Stijnen T, Steyerberg EW, Lesaffre E, Hunink MGM. Methods for calculating sensitivity and specificity of clustered data: a tutorial. Radiology. 2012;265(3):910–916. doi: 10.1148/radiol.12120509. [DOI] [PubMed] [Google Scholar]
- 21.Kohn MA, Carpenter CR, Newman TB. Understanding the direction of bias in studies of diagnostic test accuracy. Acad Emerg Med. 2013;20(11):1194–1206. doi: 10.1111/acem.12255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.