Abstract
Objectives
This meta-analysis of randomized controlled trials (RCTs) investigated the usefulness of mesenchymal stromal cells (MSCs) to treat patients with COVID-19. Methods: PubMed, Embase, Ovid MEDLINE, the Cochrane Library, and Clinicaltrials.gov were searched for RCTs published before November 7, 2021. Only RCTs that compared the clinical efficacy and safety of MSCs with other alternative treatments or placebos in the treatment of patients with COVID-19 were included.
Results
Six RCTs were included, in which the MSC and control groups consisted of 158 and 135 patients, respectively. The patients who received MSCs had a significantly lower 28-day mortality rate (7.6% vs 21.5%; OR, 0.18; 95% CI, 0.06–0.52; I2 = 0%) and significantly higher clinical improvement rate (OR, 6.05; 95% CI, 2.31–15.83; I2 = 0%) than the controls. The patients who received MSCs were associated with a similar risk of adverse events (AEs) and serious AEs to the control group (AEs: OR, 33; 95% CI, 0.09–1.18; I2 = 59%; serious AEs: OR, 0.30; 95% CI, 0.02–4.41; I2 = 53%).
Conclusions
MSC treatment may help to improve the clinical outcomes of patients with COVID-19. In addition, MSC treatment appears to be a safe therapeutic option for patients with COVID-19.
Abbreviations: AE, adverse event; COVID-19, coronavirus disease 2019; MSC, mesenchymal stem cell; MV, mechanical ventilation; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: COVID-19, Mesenchymal stromal cell, Mortality, SARS-CoV-2
Introduction
The COVID-19 pandemic has spread around the world since its first appearance at the end of 2019 [1], with more than 535 million cumulative cases and 6 million deaths globally [2]. The clinical manifestations of SARS-CoV-2 infection range from asymptomatic, acute respiratory disease, pneumonia, and acute respiratory distress syndrome (ARDS) [3], [4], [5]. Severe or critical COVID-19 can be associated with cytokine storm and cause a high morbidity and mortality [4]. Therefore, anti-inflammatory agents are an important treatment modality in addition to initial anti-viral agents [6], [7], [8]. At present, only corticosteroids and anti-interleukin-6 (IL-6) agents have demonstrated clinical efficacy in reducing the mortality of patients with COVID-19 [9], [10]. Mesenchymal stromal cells (MSCs), which can be collected from a variety of adult and neonatal tissues and mediated partly by conditioned media or through secreted extracellular vesicles, have been shown to exhibit significant immunomodulatory and tissue repair capabilities [11], [12], [13], [14]. SARS-CoV-2 infection can cause life-threatening illness due to an activated immune response. Due to the potent anti-inflammatory activity of MSCs and their secretomes, MSCs have been repurposed to treat COVID-19. Many clinical studies of MSCs have demonstrated their potential efficacy and safety in the treatment of SARS-CoV-2 infection during this global pandemic, however, only a small number of studies with limited information have been completed [15], [16], [17], [18], [19], [20], [21]. A systematic search and meta-analysis is therefore urgently needed to comprehensively review the results of these studies and provide solid evidence on the potential benefit of MSCs. Therefore, we conducted this systematic review and meta-analysis using updated data to provide robust and timely evidence.
Methods
Study search and selection
We searched PubMed, Embase, Ovid MEDLINE, the Cochrane Library, and Clinicaltrials.gov were searched for RCTs published before November 7, 2021. The following search terms were used: “mesenchymal stromal cells,’ “mesenchymal stem cells,” “MSCs,” “mesenchymal stromal cell conditioned medium,” “exosomes,” “mesenchymal stromal cell extracellular vesicles,” “microvesicles,” “Covid-19,” “SARS-CoV-2,” “coronavirus,” “2019-nCoV,” and ”corona-virus.” Only RCTs that compared the clinical efficacy and safety of MSCs with other alternative agents or placebos in the treatment of patients with COVID-19 were included. The inclusion criteria were: (1) studies with a RCT design; (2) study subjects were patients with COVID-19; (3) intervention was MSCs or their secretomes derived from any tissue source (e.g., bone marrow, adipose, umbilical cord, dental pulp, placenta, etc.), which could be syngeneic, allogeneic, or xenogeneic; and (4) comparator was a conventional therapy for COVID-19 (e.g., antivirals, immunomodulatory drugs, anticytokine drugs, combination therapies, etc.) or placebo. The exclusion criteria were: (1) not a RCT design (case reports, case series, observational studies, and retrospective cohort studies); (2) in vitro studies; (3) pharmacodynamic or pharmacokinetic studies; and (4) no available data regarding the outcome of interest. Two authors (CYC and WCC) independently reviewed the identified abstracts and selected articles for full review. Disagreements were resolved by the third author (CKH). For each included study, we extracted the following data: year of publication, study design, interventions, patient populations, methods of MSC isolation and characterization, primary and secondary clinical outcomes, and adverse event (AEs). This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [22] and the study protocol is registered in PROSPERO (CRD42021288552).
Outcome measurements
The primary outcome of this meta-analysis was 28-day overall mortality, and the secondary outcomes were the rate of clinical improvement, time to recovery, length of hospital stay, the need for mechanical ventilation (MV), and the risk of AEs.
Data analysis
The Cochrane risk of bias tool [23] was used by two authors (CYC and WCC) to assess the quality of the included RCTs and their associated risk of bias. Any discrepancies between these two authors were resolved by a third author (CCL). All statistical analyses were performed using Review Manager (version 5.3; Nordic Cochrane Center, Copenhagen, Denmark). Heterogeneity was evaluated using Q statistics generated by the χ2 test, and the I 2 statistic was used to assess statistical heterogeneity. Heterogeneity was significant when the P value was< 0.10 or I 2> 50%. We used a fixed-effects model when the data were homogeneous and a random-effects model when the data were heterogeneous. We calculated pooled odds ratios (ORs) and mean differences (MDs) along with 95% confidence intervals (CIs) for outcome analyses.
Results
Study selection
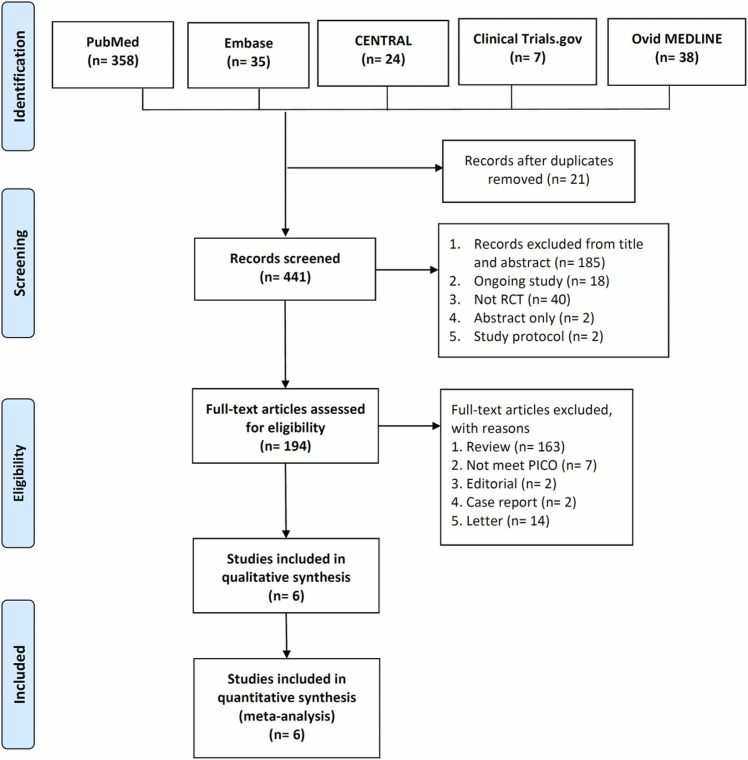
The search results yielded a total of 441 studies (n = 358 from PubMed; n = 35 from Embase; n = 38 from Ovid MEDLINE; n = 24 from the Cochrane Library; and n = 7 from Clinicaltrials.gov), of which 21 were excluded as duplicates. In addition, we determined that 247 studies were not relevant to this review after screening their titles and abstracts. Finally, we included 6 RCTs [15], [16], [18], [20], [21], [24] in this meta-analysis ( Fig. 1).
Fig. 1.
Flow diagram of study identification and assessment for eligibility.
Study characteristics
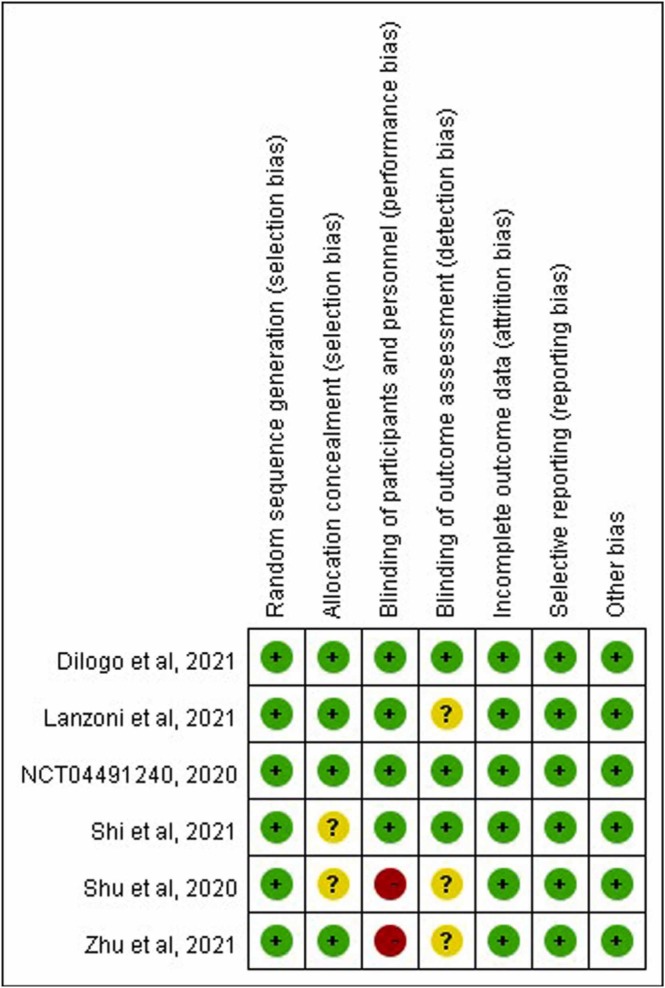
Of the six RCTs, four were single center studies [16], [20], [21], [24] ( Table 1). Three RCTs were conducted in China [18], [20], [21], and one each in the US, [16] Indonesia [15], and Russia [24]. The inclusion criteria varied; three included patients with severe COVID-19 [18], [21], [24] and two included critical patients with ARDS. [15], [16] The regimens of MSCs also varied. Overall, the number of the patients in study group treated with MSCs in addition to standard of care was 158, and the control group consisted of 135 patients ( Table 2). The risk of bias in each study is shown in Fig. 2. Two studies [20], [21] had high risk of performance bias. Unknown risk of detection and selection bias was found for three [16], [20], [21] and two studies [18], [21], respectively.
Table 1.
Characteristics of the included studies.
| Study | Study design | Study site | Study period | Subjects | Regimen of MSCs | Comparator |
|---|---|---|---|---|---|---|
| Dilogo et al., 2021[15] | double-blind, randomized clinical trial | 4 centers in Indonesia | May 1, 2020 to October 10, 2020 | Patients with critical COVID-19 | a single intravenous infusion of 1 × 106/kg UC-MSCs on day 8 | Placebo |
| Lanzoni et al., 2021[16] | double-blind, randomized, controlled, early phase clinical trial | 1 center in the US | April 25, 2020 to July 21, 2020 | Patients with COVID-19 and ARDS | 2 intravenous infusions of 100 ± 20 × 106 cells/kg UC-MSCs on days 0 and 3 | Placebo |
| Shi et al., 2021[18] | randomized, placebo-controlled, double-blind trial | 3 centers in China | March 5, 2020 and March 28, 2020 | Patients with severe COVID-19 | 3 intravenous infusions of 4.0 × 107 cells UC-MSCs on days 0, 3, and 6 | Placebo |
| Shu et al., 2020[21] | open-label, individually randomized, standard treatment-controlled trial | 1 center in China | Feb 12 to March 25, 2020 | Patients with severe COVID-19 | Intravenous infusion of UC-MSCs at 2 × 106 cells | Standard of care |
| Zhu et al., 2021[20] | randomized, single-blind, placebo-controlled | 1 center in China | January 30, 2020 to March 30, 2020 | Patients with COVID-19 | Intravenous infusion of UC-MSCs at 1 × 106 cells/kg | Placebo |
| NCT04491240, 2020[24] | double-blind, randomized clinical trial | 1 center in Russia | July 20, 2020 to October 20, 2020 | Patients with severe COVID-19 | Inhaled MSC exosomes twice daily for 10 days | Placebo |
ARDS, acute respiratory distress syndrome; UC, umbilical cord; MSC, mesenchymal stem cell.
Table 2.
The inclusion criteria and the demographic features of included patients in each study.
| Study | Inclusion criteria | No of patients |
Mean or median age of patients |
Male (%) |
|||
|---|---|---|---|---|---|---|---|
| MSC group | Control group | MSC group | Control group | MSC group | Control group | ||
| Dilogo et al., 2021[15] | Respiratory failure with ARDS, shock, multiorgan failure and monitored in ICU | 20 | 20 | NA | NA | 75 | 75 |
| Lanzoni et al., 2021[16] | Peripheral oxygen saturation ≤ 94% on room air or requiring supplemental oxygen; PaO2/FiO2 ratio ≤ 300 mg; bilateral infiltrations on chest radiograph or bilateral ground glass opacities on CT | 12 | 12 | 58.6 | 58.8 | 41.7 | 66.7 |
| Shi et al., 2021[18] | CT confirmed pneumonia combed with lung damage and any of the following: (1) respiratory rate ≥ 30; (2) oxygen saturation of 93% or lower on room air; (3) PaO2/FiO2 ratio ≤ 300 mg; (4) pulmonary imaging showing the foci progressed by > 50% in 24–48 h | 65 | 35 | 60.7 | 59.9 | 56.9 | 54.3 |
| Shu et al., 2020[21] | CT indicated pneumonia and any of the following: (1) respiratory rate ≥ 30; (2) oxygen saturation ≤ 93% in the resting state; (3) PaO2/FiO2 ratio ≤ 300 mg | 12 | 29 | 61.0 | 57.9 | 58.5 | 66.7 |
| Zhu et al., 2021[20] | Severe or critically severe COVID-19 | 29 | 29 | 64 | 66 | 41.4 | 34.5 |
| NCT04491240, 2020[24] | Pneumonia requiring hospitalization, oxygen saturation of < 94% indoors or a need for auxiliary oxygen, the confirmed volume of lung damage of 30–80% by CT | 20 | 10 | 50.2 | 53.3 | 30 | 50 |
ARDS, acute respiratory distress syndrome; CT, computed tomography; ICU, intensive care unit; PaO2, arterial oxygen partial pressure; FiO2, fraction of inspired oxygen.
Fig. 2.
Summary of risk of bias assessment.
Primary outcome
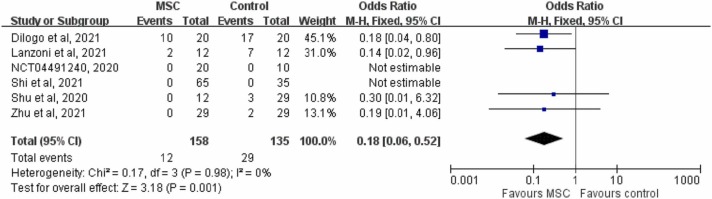
Pooled analysis of the six RCTs [15], [16], [18], [20], [21], [24] showed that the 28-day mortality rates were 7.6% (12/158) and 21.5% (29/135) in the study and control groups, respectively. Overall, the patients who received MSCs had a significant lower 28-day mortality rate than the controls (OR, 0.18; 95% CI, 0.06–0.52; I 2 = 0%, Fig. 3). The results remained unchanged when the random-effects model was used (OR, 0.18; 95% CI, 0.06–0.50; I 2 = 0%). The results also remained consistent in the leave-one-out sensitivity test by excluding one study from each analysis. Furthermore, the MSC group was associated with a lower mortality rate than the control group in the subgroup analysis of patients with critical COVID-19 (OR, 0.16; 95% CI, 0.05–0.53; I 2 = 0%). The subgroup analysis of patients with severe COVID-19 also showed that the study group had a lower mortality rate than the control group, however the difference did not reach statistical significance (OR, 0.24; 95% CI, 0.03–2.09; I 2 = 0%). In pooled analysis of five RCTs [15], [16], [18], [21], [24] that reported 14-day mortality, the study group had a lower 14-day mortality rate than the control group (8.5% [11/129] vs 21.7% [23/106]; OR, 0.24; 95% CI, 0.08–0.74; I 2 = 0%).
Fig. 3.
Forest plot of 28-day mortality.
Secondary outcomes
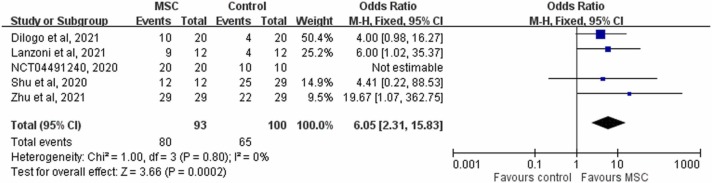
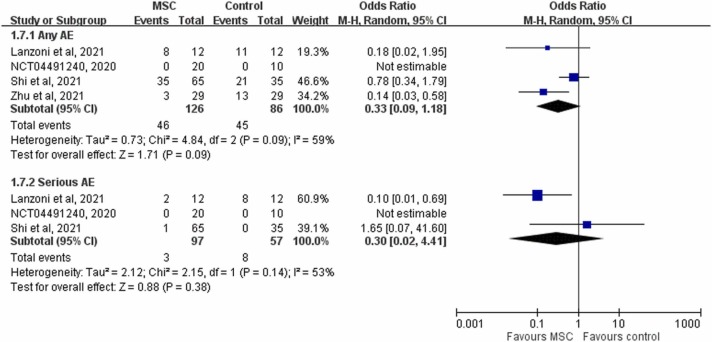
In analysis of the five RCTs [15], [16], [20], [21], [24] that reported clinical improvement, the study group was associated with a significantly higher clinical improvement rate than the control group (86.0% [80/93] vs 65.0% [65/100], 6.05; 95% CI, 2.31–15.83; I 2 = 0%, Fig. 4). In pooled analysis of the two RCTs [20], [21] that had available data on the time to clinical improvement, the study group was associated with a faster time to recovery than the control group (MD, −4.02 days; 95% CI, −6.42 to −1.63; I 2 = 0). In addition, the study group was associated with a shorter length of hospital stay than the control group (MD, −3.84 days; 95% CI, −6.19 to −1.50; I 2 = 0). In analysis of the three RCTs [18], [20], [21] that had available data on the need for MV, none of 106 patients in the study group needed MV, but 6.5% (6/93) of the patients in the control group needed MV. However, the difference did not reach statistical significance (OR, 0.21; 95% CI, 0.12–1.79; I 2 = 0%). Finally, the study group was associated with a similar risk of AEs and serious AEs to the control group (AEs: OR, 0.33; 95% CI, 0.09–1.18; I 2 = 59%; serious AEs: OR, 0.30; 95% CI, 0.02–4.41; I 2 = 53%, Fig. 5).
Fig. 4.
Forest plot of the rate of clinical improvement.
Fig. 5.
Forest plot of the risk of adverse events (AEs).
Discussion
In this meta-analysis, we reviewed six RCTs [15], [16], [18], [20], [21], [24] including 293 patients to investigate the additional use of MSCs for the treatment of patients with COVID-19. Overall, we found that treatment with MSCs was associated with improved clinical outcomes of patients with COVID-19, which was supported by the following evidence. First, we observed that the patients who received MSCs were associated with a lower mortality rate than the control group, and that this result remained consistent in the subgroup analyses and sensitivity test. In addition, this finding is consistent with an observational study [25] in which critical patients who received MSCs had a lower mortality rate than controls (20% vs. 55.6%). Second, the patients who received MSCs had a higher clinical improvement rate and a faster time to clinical improvement. Third, the patients who received MSCs were associated with a shorter length of hospital stay than the controls. Fourth, the patients who received MSCs had numerically less need of MV than the controls, although no statistically significant difference was observed between the two groups. These findings regarding the benefits of MSC treatment for patients with COVID-19 are consistent with those of recent observational studies [25], [26]. One small series of five patients showed that MSC infusion could help improve the pulmonary function and overall outcome of patients with COVID-19 and ARDS [25]. In addition, another series of seven patients with COVID-19 pneumonia showed that MSC treatment could cure or significantly improve lung function without observed AEs [26]. Moreover, our findings were consistent with previous meta-analyses, which based on the analyses of RCT and non-RCTs [27], [28], [29]. In contrast, our findings were based on the analyses of only RCTs, and could provide more solid evidence than previous meta-analyses [27], [28], [29]. Finally, we found that MSCs was associated with the similar risk of any AE or serious AE to the comparator, and these findings were consistent with previous studies [25], [26], [27], [28], [29]. Thus, MSC treatment is a safe therapeutic option for patients with COVID-19. Taken together, these findings indicate that MSC treatment may positively impact the clinical outcomes of patients with COVID-19, and suggest a potential clinical role for MSC treatment.
Although this study cannot provide plausible mechanisms, several studies have demonstrated the anti-inflammatory activity of MSCs when treating patients with COVID-19 [15], [16], [20], [21]. Shu et al. showed that C-reactive protein (CRP) and IL-6 levels were significantly lower after infusion of MSCs, and that the time for the lymphocyte count to return to normal range was significantly faster in the MSC group than in the control group [21]. Zhu et al. also demonstrated that MSC infusion could reduce the levels of CRP, proinflammatory cytokines, and neutrophil extracellular traps, and promote the maintenance of SARS-CoV-2-specific antibodies [20]. Moreover, Lanzoni et al. observed significant differences between MSC and control groups in the concentrations of GM-CSF, IFNg, IL-5, IL-6, IL-7, TNFa, TNFb, PDGF-BB, and RANTES after treatment [16]. Taken together, these anti-inflammatory activities of MSCs may explain the improved clinical outcomes of patients with COVID-19.
This study has several limitations. First, the number of study patients was small; hence, some differences between the study group and control group did not reach statistical significance. Additional large-scale RCTs are required to validate our findings. Second, we observed some differences in the study subjects and MSC regimens among the included RCTs. However, most of the outcome analyses in this study demonstrated low heterogeneity, which may minimize the confounding effect of heterogeneity. Finally, two [20], [21] of included five RCTs carried the high risk of performance bias, so our findings should be interpreted with caution.
Conclusions
This meta-analysis found that MSCs may help improve the clinical outcomes of patients with COVID-19, and suggest its potential clinical role. However, further large-scale research is required to validate our findings.
Ethical approval
Not required.
CRediT authorship contribution statement
Ching-Yi Chen, Chih-Cheng Lai: Conception, Drafting and writing. Ching-Yi Chen, Wang-Chun Chen, Chih-Cheng Lai: Study design. Ching-Yi Chen, Wang-Chun Chen, Chi-Kuei Hsu, Chih-Cheng Lai: Analysis and interpretation. Chih-Cheng Lai: Substantially revised and critically reviewed.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
This paper did not receive any funding. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Acknowledgments
None.
Consent for publication
Not Applicable.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 〈https://covid19.who.int/〉 Accessed on June 17, 2022.
- 3.Lai C.C., Liu Y.H., Wang C.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi R.T., Lynch J.B., Del Rio C. Mild or Moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 6.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Lessons learned from COVID-19 therapies: Critical perspectives from the IDSA COVID-19 treatment guideline panel. Clin Infect Dis., 2021. [DOI] [PMC free article] [PubMed]
- 7.National Institutes of Health. 〈https://www.covid19treatmentguidelines.nih.gov/〉 Accessed on January 18, 2022.
- 8.Alhazzani W., Evans L., Alshamsi F., et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med. 2021;49(3):e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 9.Shankar-Hari M., Vale C.L., Godolphin P.J., et al. Association between administration of il-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. Jama. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. Jama. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13(6):419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Németh K., Leelahavanichkul A., Yuen P.S., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S., Lu G., Wu Y., et al. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci. 2012;66(1):29–36. doi: 10.1016/j.jdermsci.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Mousaei Ghasroldasht M., Matin M.M., Kazemi Mehrjerdi H., et al. Application of mesenchymal stem cells to enhance non-union bone fracture healing. J Biomed Mater Res A. 2019;107(2):301–311. doi: 10.1002/jbm.a.36441. [DOI] [PubMed] [Google Scholar]
- 15.Dilogo I.H., Aditianingsih D., Sugiarto A., et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl Med. 2021;10(9):1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanzoni G., Linetsky E., Correa D., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L., Huang H., Lu X., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei F., Kong D., Li T., et al. Efficacy and safety of umbilical cord mesenchymal stem cells for the treatment of patients with COVID-19. Clin (Sao Paulo) 2021;76 doi: 10.6061/clinics/2021/e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu R., Yan T., Feng Y., et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021:1–19. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu L., Niu C., Li R., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ClinicalTrials.gov. 〈https://clinicaltrials.gov/ct2/show/study/NCT04491240〉 Accessed on June 17, 2022.
- 25.Häberle H., Magunia H., Lang P., et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. 2021;36(6):681–688. doi: 10.1177/0885066621997365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arabpour E., Khoshdel S., Tabatabaie N., Akhgarzad A., Zangiabadian M., Nasiri M.J. Stem cells therapy for COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021:8. doi: 10.3389/fmed.2021.737590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Wei Z., Ma X., et al. Efficacy and safety of mesenchymal stromal cells therapy for COVID-19 infection: a systematic review and meta-analysis. Curr Stem Cell Res Ther. 2021 doi: 10.2174/1574888X16666211206145839. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Shi P., Chen D., et al. Research status of the safety and efficacy of mesenchymal stem cells in the treatment of COVID-19-related pneumonia: a systematic review and meta-analysis. Stem Cells Dev. 2021;30(19):947–969. doi: 10.1089/scd.2021.0179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.