Abstract
The development of new natural drugs for Helicobacter pylori (H. pylori) management has recently received significant attention. Iris confusa (I. confusa) was long used for the treatment of bacterial infections and gastritis. This study aimed at evaluating its effect on management of H. pylori infection and exploring its bioactive metabolites. The inhibitory potential of the polar (PF), non-polar (NPF) fractions and the isolated compounds against H. pylori using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay in addition to their cyclooxygenases (COX-1 and COX-2), and nitric oxide (NO) inhibitory activities were assessed. The most biologically active compound was tested for its selective H. pylori inosine-5′-monophosphate dehydrogenase (HpIMPDH) inhibitory potential. Chromatographic purification of PF and NPF allowed isolation of tectoridin, orientin, irigenin, tectorigenin, isoarborinol and stigmasterol. The PF exhibited significant anti-H. pylori (MIC 62.50 µg/mL), COX-1, COX-2 (IC50 of 112.08 ± 0.60 and 47.90 ± 1.50 µg/mL respectively, selectivity index SI of 2.34), and NO (IC50 47.80 ± 0.89 µg/mL) inhibitory activities, while irigenin was the most potent isolated compound. Irigenin was found to have a promising activity against HpIMPDH enzyme (IC50 of 2.07 ± 1.90 μM) with low activity against human hIMPDH2 (IC50 > 10 μM) than clarithromycin, assuring its selectivity. Overall, I. confusa and its isolated compounds may serve as a potential source of plant-based drugs for H. pylori control. This study scientifically validated the claimed anti-bacterial activity of I. confusa and revealed irigenin potential as a novel lead exhibiting anti H. pylori activity in a first record.
Subject terms: Target validation, Peptic ulcers
Introduction
Helicobacter pylori (H. pylori) is a Gram negative bacterium that colonizes 50% of the world population stomach1. It is considered as a main factor of chronic gastritis and gastric/duodenal ulcers2. A marked reduction in gastric or duodenal ulcers recurrence rates associated with H. pylori infection was achieved by curing the infection3. H. pylori infection is the major risk factor associated with gastric cancer development4. H. pylori infection triggers the transition from normal mucosa to non-atrophic gastritis and initiates precancerous lesions which after that progress to intestinal metaplasia and atrophic gastritis5. H. pylori is classified by the International Agency for Research on Cancer6 and World Health Organization (WHO) as group one carcinogen.
Noticeable infiltration of inflammatory cells along with release of the inflammatory mediators occur due to H. pylori colonization in the gastric mucosa7. Also, H. pylori infection activates the mucosal defense mechanisms2. Prostaglandins (PGs) play an important role as mucosal defense factors protecting the gastric mucosa against injury by inducing gastric mucus and bicarbonate secretion, inhibiting acid secretion and decreasing gastric motility8. PGs are synthesized through cyclooxygenase (COX) enzymes.
COX-1 is constitutively expressed in the stomach, while COX-2 expression is induced at inflammation sites9. COX-1 participates in PGs production under normal physiological conditions. Thus, it is regarded as the isoform generating PGs associated with housekeeping functions, as protective action on the gastric mucosa against ulceration. On the other hand, COX-2 is involved in the PGs production associated with the development of several diseases10 such as autoimmune diseases including rheumatoid arthritis11,12, allergic sign and symptoms13, Alzheimer’s disease14, and the development and prognoses of numerous cancers15. Moreover, COX-2 was proved to be involved in gastrointestinal cancer16. H. pylori increases COX-2 expression consequently stimulating the release of PGs in H. Pylori associated premalignant and malignant gastric lesions17.
Thus, selective COX-2 inhibitors decrease PGs levels in inflammatory sites only and they have no effect on gastric mucosal PGs levels or integrity . On the other hand, specific COX-2 inhibitors, which cause no significant inhibition of COX-1, have been reported to delay healing of ulcers18,19.
Inflammation predisposes to the development of cancer and promotes all stages of tumorigenesis. inflammation drives tumor initiation, growth, progression, and metastasis20. Therefore, NSAIDs can aid in cancer prevention21 and a dual anti-H. pylori/anti-inflammatory compound could both help in eradicating the microorganism and avoid the development of H. pylori-related cancers.
Safe NSAID COX-2-inhibitors must have selectivity indices (IC50 COX-2/IC50 COX-1) ranging from 5 to 5022. Since super selective COX-2-inhibitors like Rofecoxib were withdrawn from the market because of the increased risk of heart attack and stroke. Thus, it becomes essential to find alternative safe NSAID COX-2-inhibitors. Most of the natural products tend to be more selective towards COX-1 than COX-2 but they can be further modified to increase COX-2 selectivity23.
Antibiotics such as amoxicillin, tetracycline, metronidazole or clarithromycin in addition to proton pump inhibitors or bismuth salts are considered as the only known current therapy for H. pylori infection24. This triple therapy is not always successful in eradication of the infection. Reduced treatment efficacy can occur due to emergence of antibiotic resistant H. pylori. Moreover, non-steroidal anti-inflammatory drugs (NSAIDs), which have been commonly used for COX-2 inhibition, resulted in serious adverse effects as gastrointestinal bleeding and gastric mucosa damage after prolonged intake25. H. pylori infection and NSAIDs are the main causative risk factors for gastroduodenal ulcers26. Therefore, discovering alternative therapy for management of H. pylori infection and the associated inflammation is crucial to overcome the undesirable effects of the currently used therapy.
Since ancient times, the use of medicinal plants has been adopted and considered the origin of modern medicine27. Many plants are not deeply investigated, although they have been reported to contain various secondary metabolite classes of well known anti-microbial and anti-inflammatory potentials.
Iris is the largest genus in the family Iridaceae with the greatest taxonomic diversity28. Numerous interesting secondary metabolites such as triterpenoids and phenolics; flavonoids and xanthones were detected in genus Iris. Extracts and pure compounds of the irises were reported to have several biological activities such as antimicrobial, anti-oxidant, anti-inflammatory, phytoestrogenic, anti-diabetic, anti-tumor, and anti-cholinesterase activities29,30.
Long time ago irises were used for the treatment of bacterial infections in the Mongolian folk medicine31. Irises have been found in Karnak temple engraved on a marble panel of the ancient Egyptians32. In traditional Chinese medicine, the rhizomes of Iris confusa, one of the irises native to western China, were used extensively to treat tonsillitis, acute bronchitis, colitis, and chronic atrophic gastritis33.
Reviewing the current literature, very few reports were traced concerning I. confusa Sealy (bamboo iris) phytochemical constituents29,34. In addition, nothing was traced about the biological activity of its isolated compounds except the study conducted by Chen et al. 2018 who studied their anti- hepatitis B potential34. In a continuation of our interest in exploring irises metabolites and their possible activities35, I. confusa was selected for this study. Its polar and non-polar fractions were subjected to chromatographic fractionation and purification with the aim of isolation of its major constituents and testing their anti H. pylori and selective anti-inflammatory potencies.
Material and methods
Plant material, extraction and fractionation
Iris confusa Sealy was collected from Al-Mansouria, Giza, Egypt in the flowering stage after permission from Agricultural Research Center, Giza, Egypt in compliance with the national guidelines. The plant material identity was kindly authenticated and verified by Dr Nina Davies, the curator of the African Iridaceae, Royal Botanic Gardens, Kew, London, UK. Specimen was deposited in Pharmacognosy Department Herbarium, Cairo University (registration no. 15.1. 2019I). The air-dried powdered underground parts of I. confusa were extracted and fractionated according to Salem et al. yielding polar fraction (PF) and non-polar fraction (NPF)36. Collection of plant material, complied with the institutional, national, and international guidelines and legislation.
Major constituents isolation
The PF and NPF were screened on pre-coated silica gel 60 F254 using solvent system S1 (methylene chloride:methanol:formic acid 85:15:0.2 v/v/v) and S2 (methylene chloride:methanol 97:3 v/v), respectively. The spots were examined in UV light before and after ammonia vapour exposure and AlCl3 spraying and after being sprayed with p-anisaldehyde/H2SO4 followed by heating at 110 °C. PF and NPF purification using a vacuum liquid chromatography column (VLC) packed with silica gel H 60, ion exchange resin (diaion HP-20), sephadex LH 20 and silica gel 60 were illustrated in detail in Fig. S1.
Similar fractions were pooled together and evaporated to dryness under reduced pressure yielding compounds P1 (yellow powder, Rf = 0.28, S1), P2 (yellow powder, Rf = 0.18, S1), P3 (yellow crystals, Rf = 0.69, S1) and P4 (yellow crystals, Rf = 0.55, S1) from the PF and N1 (white crystals, Rf = 0.62, S2) and N2 (white crystals, Rf = 0.43, S2) from the NPF.
Determination of anti-Helicobacter pylori activity
The anti-Helicobacter pylori activity of the PF and NPF as well as the isolated compounds was evaluated against H. pylori ATCC 700392 (type strain, obtained from the American Type Culture Collection unit (ATCC), using microwell dilution method and clarithromycin as standard drug37. The detailed procedures were described in the supplementary file.
Determination of COX-1 and COX-2 inhibitory activity
The tested samples and the standard drugs (ibuprofen and celecoxib) were prepared in dimethyl sulfoxide and subsequent eight twofold dilutions (125–0.98 µg) were carried out in a 96-well plate. The inhibitory COX activity was assayed colourimetrically by examining the inhibition of the ovine COX-1 and human recombinant COX-2 enzymes as described by George et al.38, using a COX inhibitor screening assay kit. The detailed procedures were described in the supplementary file.
Determination of nitric oxide (NO) inhibitory activity
The macrophage cell line, RAW 264.7 was obtained from Vaccines, Sera and Drugs Egyptian Company (VACSERA). It was cultured in (RPMI, 1640) medium supplemented with 10% fetal bovine serum and 1% gentamicin39. The detailed procedures were described in the supplementary file.
In vitro HpIMPDH inhibition assay
The most potent isolated compound, as revealed from the previous assays, was screened at different concentrations (10–0.078 µM) in triplicates. The assay was carried out according to Galal40 and was described in detail in the supplementary file.
In vitro hIMPDH2 inhibition assay
Human inosine-5′-monophosphate dehydrogenase (hIMPDH2) was purchased from NovoCIB SAS (Lyon, France). It was used for the in vitro screening of the most potent isolated compound, as revealed from the previous assays, against H. pylori and the standard drug at the concentrations (10–0.078 µM) in triplicates40. The detailed procedures were described in the supplementary file.
Quantitative determination of the main phytochemical classes and antioxidant assay
Total phenolic content (TPC)
Determination of TPC was carried out according to the European Pharmacopeia procedure41, using the Folin–Ciocalteu colourimetric method. It depends on measuring the intensity of the blue colour produced in correlation to the reducing power of existing phenolics. Determinations were carried out in triplicates; results are the mean values ± standard deviations and expressed as μg gallic acid equivalent (GAE) per mg dry fraction (μg GAE/mg).
Total flavonoid content (TFC)
TFC was determined based on measuring the intensity of the yellow colour developed upon reaction of flavonoids with aluminium chloride reagent42. Quercetin was used to compute the standard calibration curve. Determinations were carried out in triplicates; results were the mean values ± standard deviation and expressed as μg quercetin equivalent (QE) per mg dry fraction (μg QE/mg).
Total triterpene content (TTC)
TTC was determined based on measuring the intensity of the red–purple colour developed upon reaction of perchloric acid-oxidized triterpenes in glacial acetic acid with vanillin43. Ursolic acid was used to compute the standard calibration curve. Determinations were carried out in triplicates; results were the mean values ± standard deviations and expressed as μg ursolic acid equivalent (UAE) per mg dry fraction (μg UAE/mg).
DPPH assay
The DPPH anti-oxidant assay was carried out as described by Romano et al.44 with some modifications. The PF and NPF of I. confusa underground parts were separately dissolved in methanol by the aid of sonication to give a set of serial dilutions for each sample. Experiments were performed in triplicates using a 96-wells plate. The reaction mixture in each case consisted of 22 μL of the tested sample and 200 μL of 0.004% DPPH in methanol. The non-quenched DPPH radicals were assessed spectrophotometrically at λmax = 492 nm using a micro-plate reader.
Ethical statement
Collection of the plant material, complied with relevant institutional, national, and international guidelines and legislation.
Results
Purification of PF and NPF
Chromatographic purification of I. confusa underground parts PF allowed the isolation of three isoflavonoids; tectoridin (P1), irigenin (P3), and tectorigenin (P4), and one flavone, orientin (P2). On the other hand, purification of the NPF led to the isolation of one triterpene, isoarborinol (N1), and one sterol, stigmasterol (N2).
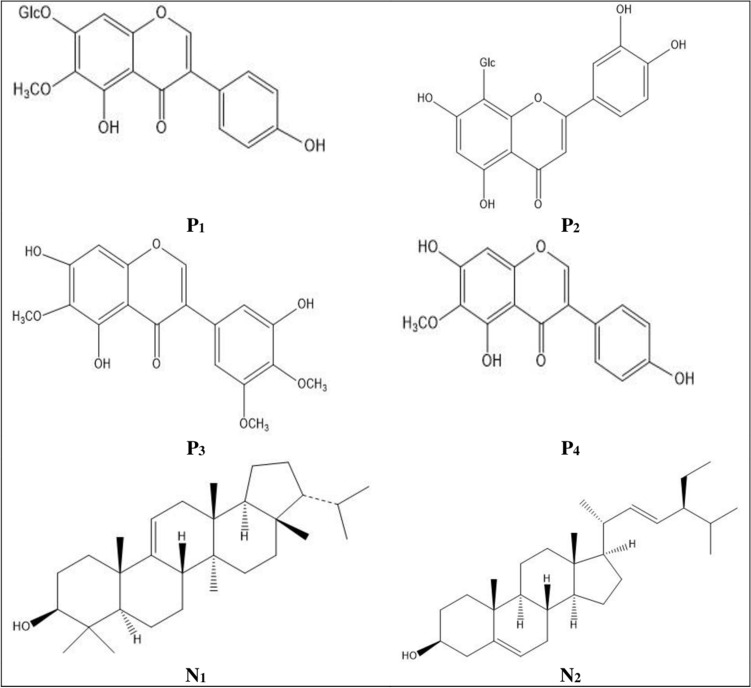
The isolated compounds (P1–P4 and N1–N2) were identified through their physicochemical characters, spectroscopic analysis, UV spectral data and via comparing their 1H and 13C NMR data with the published data (Tables S1–S4 in the supplementary file). Their structures are shown in Fig. 1.
Figure 1.
Structure of the isolated compounds. P1 tectoridin, P2 orientin, P3 irigenin, P4 tectorigenin, N1 isoarborinol, N2 stigmasterol.
Helicobacter pylori inhibitory activity
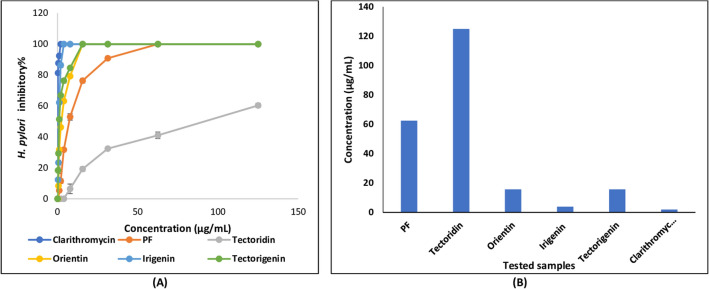
I. confusa underground parts PF inhibited the growth of H. pylori with MIC value of 62.50 μg/mL (Table 1 and Fig. 2). Compounds isolated from PF showed more potent activity than their crude fraction (irigenin: MIC 3.90 μg/mL; orientin: MIC 15.53 μg/mL and tectorigenin: MIC 15.63 μg/mL) except tectoridin which showed MIC > 125 μg/mL. The most potent isolated compound was irigenin (MIC 10.82 μM) followed by orientin (MIC 34.64 μM) and tectorigenin (MIC 52.05 μM). I. confusa underground parts NPF as well as its isolated compounds (isoarborinol and stigmasterol) exhibited no H. pylori inhibitory potential.
Table 1.
Minimum inhibitory concentration of I. confusa underground parts PF, NPF and isolated compounds against Helicobacter pylori and their IC50 on COX-1, COX-2, and nitric oxide (NO).
| I. confusa | Compounds isolated from PF | Compounds isolated from NPF | Positive control* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PF | NPF | Tectoridin (P1) | Orientin (P2) | Irigenin (P3) | Tectorigenin (P4) | Isoarborinol (N1) | Stigmasterol (N2) | ||
| MIC (H. pylori) | 62.50a | ND | > 125a | 15.53a | 3.90a | 15.63a | ND | ND | 1.95a |
| > 270.33b | 34.64b | 10.82b | 52.05b | ND | ND | 2.61b | |||
| IC50 (COX-1) | 112.08 ± 0.60a | ND | ND | 73.30a ± 0.60 | 12.70a ± 1.40 | 122.30a ± 0.90 | 31.30a ± 1.00 | 33.60a ± 1.10 | 8.07a ± 1.40 |
| 163.48a ± 0.60 | 35.25b ± 1.40 | 407.31b ± 0.90 | 73.35b ± 1.00 | 81.42b ± 1.10 | 39.12b ± 1.40 | ||||
| IC50 (COX-2) | 47.90 ± 1.50a | > 125a | ND | 46.50a ± 1.70 | 3.90a ± 0.86 | 56.70a ± 1.50 | 22.10a ± 2.10 | 25.20a ± 2.10 | 0.28a ± 0.10 |
| 103.70b ± 1.70 | 10.83b ± 0.86 | 188.84b ± 1.50 | 51.79b ± 2.10 | 61.06b ± 2.10 | 0.73b ± 0.10 | ||||
| SI (COX-1/COX-2) | 2.34 | ND | ND | 1.58 | 3.26 | 2.16 | 1.42 | 1.33 | |
| IC50 (NO) | 47.80 ± 0.89a | ND | ND | 55.60a ± 0.86 | 15.30a ± 1.80 | > 125a | 18.90a ± 1.70 | 53.80a ± 1.30 | 2.80a ± 0.69 |
| 124b ± 0.86 | 42.46b ± 1.80 | > 416.31b | 44.29b ± 1.70 | 130.36b ± 1.30 | 12.62b ± 0.69 | ||||
aµg/mL; bµM; IC50 concentration at which the tested sample produces 50% inhibition, MIC minimum inhibitory concentration, ND not detected, NPF non-polar fraction, PF polar fraction, SI selectivity index; *Positive control clarithromycin for MIC, and L-N6-(1-iminoethyl) lysine hydrochloride for nitric oxide.
Figure 2.
(A) Percentage inhibition and (B) MIC values of I. confusa underground PF and isolated compounds against H. pylori. All determinations were carried out in a triplicate manner and values are expressed as the mean ± SD; PF polar fraction.
COX-1 and COX-2 inhibitory activity
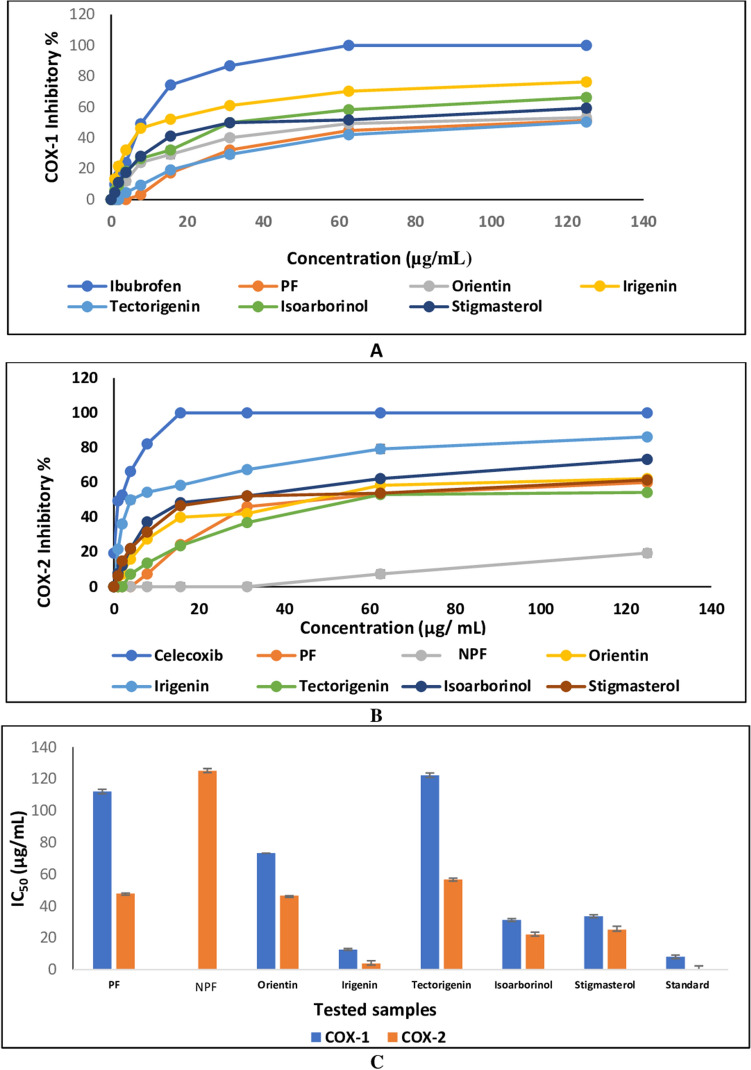
I. confusa underground parts PF inhibited COX-1 and COX-2 by an IC50 of 112.08 ± 0.60 and 47.9 ± 1.50 µg/mL, respectively (Table 1 and Fig. 3). On the other hand, the NPF did not show any inhibition to COX-1 and IC50 > 125 µg/mL against COX-2.
Figure 3.
Effect of I. confusa underground parts PF, NPF and isolated compounds on (A) COX-1 and (B) COX-2 inhibition, (C) IC50 compared to reference drugs. All determinations were carried out in triplicate and values are expressed as the mean ± SD; NPF non-polar fraction, PF polar fraction, IC50 concentration at which the tested sample produces 50% inhibition, Standard ibuprofen for COX-1, celecoxib for COX-2.
The isolated compounds were more potent than the corresponding crude fractions except for tectorigenin (COX-1 IC50 of 122.30 ± 0.90 and COX-2 IC50 of 56.70 ± 1.50 µg/mL) and its glycoside tectoridin which showed no inhibition against both COX-1 and COX-2. Among the isolated compounds, irigenin was the most potent COX-1 (IC50 of 35.25 ± 1.40 μM) and COX-2 (IC50 of 10.83 ± 0.86 μM) inhibitor followed by isoarborinol (COX-1 IC50 of 73.35 ± 1.00 μM and COX-2 IC50 of 51.79 ± 2.10 μM).
The isolated compounds showed higher inhibitory potential against COX-2 than COX-1 enzyme. Among all tested samples, irigenin showed the highest selectivity for COX-2 (SI = 3.26).
Nitric oxide assay
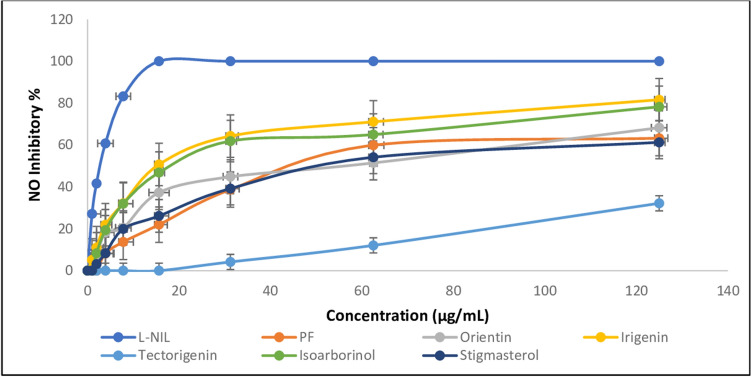
In the same manner, I. confusa underground parts PF exhibited nitric oxide inhibition by an IC50 of 47.80 ± 0.89 µg/mL while the NPF was inactive (Table 1 and Fig. 4). Irigenin was the most active tested compound. It showed nitric oxide inhibition by an IC50 of 42.46 ± 1.80 μM followed by isoarborinol (IC50 of 44.29 ± 1.70 μM).
Figure 4.
Effect of I. confusa fractions and isolated compounds on NO inhibition. All determinations were carried out in triplicate and values are expressed as the mean ± SD; L-NIL L-N6-(1-iminoethyl) lysine hydrochloride, PF polar fraction.
IMPDH inhibition assays
On the basis of the previous in vitro assays on I. confusa underground parts PF and NPF as well as their isolated compounds (Table 1), the most active compound was irigenin. Thus, irigenin was selected to test its HpIMPDH inhibitory activity by monitoring NADH production at concentration range of 10–0.078 µM compared to clarithromycin as standard drug. Also, to examine irigenin selectivity toward the bacterial IMPDH, human hIMPDH2 inhibition potential was assayed.
Results showed that irigenin (P3) exhibited potent inhibitory potential against HpIMPDH enzyme with IC50 of 2.07 ± 1.90 μM (Table 2). Whereas the IC50 value of clarithromycin (standard drug) was 0.51 ± 0.58 μM. In addition, irigenin was less active and safer against hIMPDH2 with IC50 > 10 μM, compared with clarithromycin (IC50 3.10 ± 2.10 μM), which make sure that its selectivity is toward HpIMPDH in contrast of clarithromycin. I. confusa underground parts PF exhibited high DPPH scavenging activity with EC50 of 41.68 ± 6.67 μg/mL. The polar fraction TPC amounted 99.05 ± 0.02 μg GAE/mg dried fraction and TFC amounted 98.31 ± 0.04 μg QE/mg dried fraction while, the non-polar fraction TTC amounted 126.30 ± 0.04 μg UAE/mg dried (Table 3).
Table 2.
HpIMPDH and hIMPDH2 inhibitory activity of irigenin (P3) and the standard drug clarithromycin.
| Sample conc. | Irigenin (P3) | Clarithromycin | ||
|---|---|---|---|---|
| HpIMPDH | hIMPDH2 | HpIMPDH | hIMPDH2 | |
| 10 | 83.25 ± 0.58 | 22.68 ± 1.00 | 100 ± 0.00 | 76.32 ± 0.50 |
| 5 | 71.08 ± 1.30 | 10.36 ± 0.72 | 100 ± 0.00 | 60.24 ± 1.50 |
| 2.5 | 59.31 ± 1.40 | 4.81 ± 0.91 | 81.22 ± 1.60 | 46.32 ± 2.20 |
| 1.25 | 24.19 ± 1.70 | 0.00 | 69.31 ± 2.10 | 27.32 ± 1.70 |
| 0.625 | 10.25 ± 2.80 | 0.00 | 56.06 ± 0.58 | 19.35 ± 1.50 |
| 0.313 | 6.14 ± 1.90 | 0.00 | 38.25 ± 1.50 | 9.74 ± 0.67 |
| 0.156 | 0.00 | 0.00 | 21.82 ± 2.10 | 0.00 |
| 0.078 | 0.00 | 0.00 | 9.14 ± 1.70 | 0.00 |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| IC50 (µM) | 2.07 ± 1.90 | > 10 | 0.51 ± 0.58 | 3.10 ± 2.10 |
Table 3.
Total phenolic, flavonoid, and triterpene content of the polar and non-polar fractions of I. confusa underground parts and their DPPH scavenging activity.
| TPC μg GAE/mg dried fraction ± SD |
TFC μg QE/mg dried fraction ± SD |
TTC μg UAE/mg dried fraction ± SD |
EC50 ± SD (μg/mL) | |
|---|---|---|---|---|
| PF | 99.05 ± 0.02 | 98.31 ± 0.04 | 25.38 ± 0.04 | 41.68 ± 6.67 |
| NPF | 6.55 ± 0.01 | 13.03 ± 0.01 | 126.30 ± 0.04 | 430.61 ± 2.78 |
*Average of three determinations; GAE gallic acid equivalent, NPF non-polar fraction, PF polar fraction, SD standard deviation, TPC total phenolic content, QE quercetin equivalent, TFC total flavonoid content, TTC total triterpene content, UAE ursolic acid equivalent. EC50 = effective concentration of the sample required to scavenge 50% of the DPPH free radical.
Discussion
The eradication of H. pylori infection has been proven to prevent gastric or duodenal relapses. Direct correlation was observed between the development of gastric adenocarcinoma and the long-term infection with H. Pylori. Therefore, antibiotics such as clarithromycin have been used for treatment. The declining eradication rates provoked the search for alternative therapeutic options which should be more effective and safer.
Exploring medicinal plants along with their phytoconstituents for H. pylori infection control is not just a way to discover safer pharmaceutical alternatives, but also is a trial to discover a natural affordable effective drug especially in developing countries.
The selection of Iris in this study was based on our previous experience with this genus diverse metabolites content and antibacterial potential29,35. As well as, I. confusa has been used a long time ago in the treatment of bacterial infections and gastritis in traditional Chinese medicine33. Hence, the in vitro H. pylori, COX-1, COX-2, and NO inhibition potency of I. confusa underground parts PF, NPF, and their isolated compounds were assessed. Additionally, the selective IMPDH inhibition activity of the most potent isolated compound, irigenin, was examined.
Purification of the PF led to the isolation of four metabolites (P1–P4) while two compounds (N1–N2) were purified from the NPF. Compounds P1–P4 spectral data were in agreement with the reported data of tectoridin45, orientin46, irigenin47, and tectorigenin45. Compounds N1–N2 spectral data were in agreement with the reported data of isoarborinol48 and stigmasterol49. This is the first report for the presence of isoarborinol (N1) in genus Iris. P1–P4 and N2 are herein isolated from I. confusa for the first time.
I. confusa underground parts PF and its isolated compounds showed significant inhibitory effects on H. pylori. Irigenin was superior over tectorigenin as anti-H. pylori. This was attributed to the presence of methoxy group at C-4′ in irigenin, which increases the H. pylori inhibitory effect than the hydroxyl group in C-4′ of tectorigenin as previously reported by Park et al.50.
Although tectorigenin (P4) exhibited certain inhibitory activity against H. pylori, its corresponding O-glycoside; tectoridine (P1) exhibited no activity. This was attributed to the effect of glycosylation at 7-OH of tectoridin which caused dramatic decrease in the H. pylori inhibitory activity compared to its aglycone tectorigenin. This dramatic decrease in the activity of the isoflavone glycosides was well documented before50. The observed anti-H. pylori activity of orientin matched that previously reported by Król-Kogus et al.51.
The inflammation usually associated with H. pylori infection drained the authors interest to explore the COX-1 and COX-2 inhibitory potential. The need to explore drugs with selective COX-2 inhibitory potential that are able to decrease PGs dependent inflammation while maintaining protective gastric mucosal PGs synthesis intact has increased in recent decades. Finding COX-2 inhibitors, but not specific and still having a degree for COX-1 inhibition18,52 was the main target.
Irigenin was found to exhibit COX-2 selective inhibitory activity three times more than COX-1 (SI = 3.26) followed by tectorigenin (SI of 2.16) and orientin (SI of 1.58).
Nitric oxide is free oxygen radical and has cytotoxic effect in pathological processes, particularly in inflammatory disorders. Nitric oxide is a potent proinflammatory molecule secreted during inflammation and causes vasodilation and cellular migration. At higher concentrations, it downregulates adhesion molecules and induces apoptosis of inflammatory cells. Inhibition of NOS (inducible nitric oxide synthase) is beneficial for the treatment of inflammatory disease53–56. Irigenin exhibited significant potential in inhibiting nitric oxide followed by isoarborinol.
Many microbial infections are characterized by rapid proliferation that is supported by guanine nucleotide pool expansion in the rapidly dividing cells. Inosine 5ʹ‐monophosphate dehydrogenase (IMPDH), an important enzyme required for the new synthesis of guanine nucleotides, is an interesting target for antimicrobial drug development57. This enzyme, IMPDH, catalyzes the oxidation of inosine 5ʹ‐monophosphate (IMP) to xanthosine 5ʹ‐monophosphate (XMP) leading to reduction of nicotinamide adenine dinucleotide (NAD+), which is an important step in guanine nucleotides de novo synthesis. IMPDH inhibition surely leads to major fall in guanine nucleotide pool which consequently blocks proliferation57.
The most active anti H. pylori and anti-inflammatory tested compound; irigenin (P3) was chosen to test its inhibitory potential against the bacterial HpIMPDH enzyme and to evaluate its selectivity relative to the host enzyme (hIMPDH2). It was found to have promising activity against the HpIMPDH enzyme, and to be safer and less active against the hIMPDH2, which reflected its selectivity.
Therefore, I. confusa underground parts and its isolated compounds can be used as excellent therapy to control gastric ulcers via eradication of H. pylori and exerting its anti-inflammatory potentials.
The PF of I. confusa underground parts exhibited high DPPH scavenging activity which was in accordance with its high TPC and TFC. The DPPH scavenging activity could be attributed to its total phenolic and flavonoid contents58. The observed anti-oxidant potential of the NPF could be attributed to the recently detected I. confusa xanthones and triterpenoids29. Xanthones and triterpenoids metabolites are well documented anti-oxidants59,60.
In recent years, irigenin, isolated from Belamcanda chinensis (Iridaceae) has been a hot research topic due to its important bioactivities. Irigenin controlled the metastatic progression in lung carcinoma cells61. It was recently proved to exhibit beneficial potentials in management of cardiac injuries. It alleviated doxorubicin (DOX)-induced cardiotoxicity62 and protected HUVECs (human umbilical vein endothelial cell lines) from angiotensin II-induced oxidative stress and apoptosis injury63. Irigenin exhibited inhibitory effects on prostaglandin E2 and nitric oxide production in murine macrophage RAW 264.7 cells64.
Our results presented irigenin (P3) as a new anti‐H. pylori, COX, NO and HpIMPDH inhibitor beside being previously reported to significantly enhance TRAIL-regulated apoptosis in Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistant gastric cancer cells65. This highlights the promising expected results upon future testing of its in vivo potential in gastric ailments.
It is noteworthy to mention that, this is the first time to isolate irigenin from I. confusa by such classical chromatographic techniques. Thus, we herein present I. confusa as another natural source of irigenin other than Belamcanda chinensis (Iridaceae).
Besides irigenin, isolated in this study, the anti-H. pylori activity of I. confusa underground parts is attributed to its content of flavonoids, isoflavonoids, and xanthones which were recently reported in the PF29. Flavonoids and isoflavonoids react with superoxide anion, lipid peroxy, and hydroxyl radicals thus can protect the gastric mucosa against reactive O2 species formed during the infection50. In addition, several natural flavonoids were reported to exhibit potent bactericidal potential against antibiotics resistant H. pylori strains66. Xanthones also are well documented to exhibit anti H. pylori activity67,68. Mangiferin xanthone reported in I. confusa PF29 is a very potent gastroprotective69,70 and anti inflammatory71 compound.
It is worthy to note that, the H. pylori, COX-1, COX-2, and NO inhibitory potentials of irigenin (P3), were much higher than that of PF from which it was isolated. Thus, fractionation and purification of I. confusa crude fractions caused significant increase in the aforementioned activities which may indicate a chance for isolation of more active constituents.
To the best of our knowledge, this is the first study that demonstrates the anti-H. pylori activity as well as COX-2 and IMPDH selective inhibitory activity of I. confusa underground parts and its isolated compounds.
Novel strategies are urgently required to control H. pylori infection. Our findings recommend further studies on I. confusa bioactive metabolites and also suggest irigenin as an important lead for management of H. pylori infection after detailed in vivo and clinical studies.
Conclusion
Irigenin exhibited promising activity against HpIMPDH enzyme with low activity against human hIMPDH2 than clarithromycin, assuring its selectivity in addition to it’s selective COX-2 inhibitory potential. This study scientifically validated the claimed anti-bacterial activity of I. confusa and has put strong focus on exploring traditional Chinese medicine for novel anti- H. pylori infections controlling drugs.
Supplementary Information
Abbreviations
- COX-1 and COX-2
Cyclooxygenases
- H. pylori
Helicobacter pylori
- hIMPDH2
Human inosine-5′-monophosphate dehydrogenase
- HpIMPDH
Helicobacter pylori inosine-5′-monophosphate dehydrogenase
- IMPDH
Inosine-5′-monophosphate dehydrogenase
- MTT
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- NAD+
Nicotinamide adenine dinucleotide
- NO
Nitric oxide
- NPF
Non-polar fraction
- PF
Polar fraction
- WHO
World Health Organization
- XMP
Xanthosine 5ʹ‐monophosphate
Author contributions
P.M.A.B. Writing—original draft, Data curation, Formal analysis, Funding acquisition, Investigation, Resources, Methodology. M.M.E.S.: Conceptualization, Supervision, Writing—review and editing. A.E.K.: Conceptualization, Supervision, Writing—review and editing. M.M.A.A.: Investigation, Methodology, Resources. M.M.O.: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing—original draft. All authors read and approved the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-15361-w.
References
- 1.Stege P, et al. Antimicrobial activity of aqueous extracts of Larrea divaricata Cav (jarilla) against Helicobacter pylori. Phytomedicine. 2006;13:724–727. doi: 10.1016/j.phymed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S, Fujita T, Yamamoto A. Role of cyclooxygenase-2 in Helicobacter pylori-induced gastritis in Mongolian gerbils. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G791–G798. doi: 10.1152/ajpgi.2000.279.4.G791. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman, J. in Seminars in Gastrointestinal Disease 156–163. [PubMed]
- 4.Matthews GM, Butler RN. Cellular mucosal defense during Helicobacter pylori infection: A review of the role of glutathione and the oxidative pentose pathway. Helicobacter. 2005;10:298–306. doi: 10.1111/j.1523-5378.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 5.Díaz P, Valenzuela Valderrama M, Bravo J, Quest AF. Helicobacter pylori and gastric cancer: Adaptive cellular mechanisms involved in disease progression. Front. Microbiol. 2018;9:5. doi: 10.3389/fmicb.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC. in Schistosomes, Liver Flukes and Helicobacter pylori 218–220 (1994). [PMC free article] [PubMed]
- 7.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL, Bell CJ. Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol. 1996;12:503–511. doi: 10.1097/00001574-199611000-00003. [DOI] [Google Scholar]
- 9.Herschman HR. Prostaglandin synthase 2. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 10.Langenbach R. COX-2 Blockade in Cancer Prevention and Therapy. Springer; 2003. pp. 147–155. [Google Scholar]
- 11.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 12.Lucas S, Rothwell N, Gibson R. The role of inflammation in CNS disease and injury. Br. J. Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harizi H, Corcuff J-B, Gualde N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krysan K, Reckamp KL, Sharma S, Dubinett SM. The potential and rationale for COX-2 inhibitors in lung cancer. Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem. Anti-Cancer Agents) 2006;6:209–220. doi: 10.2174/187152006776930882. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann KC, et al. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 17.Saukkonen K, et al. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin. Cancer Res. 2001;7:1923–1931. [PubMed] [Google Scholar]
- 18.Schmassmann A, et al. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br. J. Pharmacol. 1998;123:795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everts B, Währborg P, Hedner T. COX-2-Specific inhibitors—The emergence of a new class of analgesic and anti-inflammatory drugs. Clin. Rheumatol. 2000;19:331–343. doi: 10.1007/s100670070024. [DOI] [PubMed] [Google Scholar]
- 20.Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong RS. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv. Pharmacol. Sci. 2019;2019:3418975. doi: 10.1155/2019/3418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner TD, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attiq A, Jalil J, Husain K, Ahmad W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018;9:976. doi: 10.3389/fphar.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman JS, Cave DR. Treatment of Helicobacter pylori. Curr. Opin. Gastroenterol. 2001;17:30–34. doi: 10.1097/00001574-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Mellemkjaer L, et al. Upper gastrointestinal bleeding among users of NSAIDs: A population-based cohort study in Denmark. Br. J. Clin. Pharmacol. 2002;53:173–181. doi: 10.1046/j.0306-5251.2001.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langman MJ, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:1075–1078. doi: 10.1016/s0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 27.Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health. 2020;17:3376. doi: 10.3390/ijerph17103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niketić M, Tomović G, Siljak-Yakovlev S. A new spontaneous hybrid between the cultivated and wild Iris species from Serbia. Bull. Nat. His. Museum. 2018;11:189–210. doi: 10.5937/bnhmb1811189N. [DOI] [Google Scholar]
- 29.Okba MM, et al. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J. Ethnopharmacol. 2022;282:114658. doi: 10.1016/j.jep.2021.114658. [DOI] [PubMed] [Google Scholar]
- 30.Hoang L, et al. Phytochemical composition and in vitro biological activity of Iris spp. (Iridaceae): A new source of bioactive constituents for the inhibition of oral bacterial biofilms. Antibiotics. 2020;9:403. doi: 10.3390/antibiotics9070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhary MI, et al. Five new peltogynoids from underground parts of Iris bungei: A Mongolian medicinal plant. Chem. Pharm. Bull. 2001;49:1295–1298. doi: 10.1248/cpb.49.1295. [DOI] [PubMed] [Google Scholar]
- 32.Crisan I, Cantor M. New perspectives on medicinal properties and uses of Iris sp. Hop. Med. Plants. 2016;24:24–36. [Google Scholar]
- 33.Ma J, Clemants S. A history and overview of the Flora Reipublicae Popularis Sinicae (FRPS, Flora of China, Chinese edition, 1959–2004) Taxon. 2006;55:451–460. doi: 10.2307/25065592. [DOI] [Google Scholar]
- 34.Chen X, et al. Iridal-type triterpenoids with anti-HBV activity from Iris confusa. Fitoterapia. 2018;129:126–132. doi: 10.1016/j.fitote.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Okba MM, Abdel Baki PM, Khaleel AE, El-Sherei MM, Salem MA. Discrimination of common Iris species from Egypt based on their genetic and metabolic profiling. Phytochem. Anal. 2021;32:172–182. doi: 10.1002/pca.2945. [DOI] [PubMed] [Google Scholar]
- 36.Salem MA, Jüppner J, Bajdzienko K, Giavalisco P. Protocol: A fast, comprehensive and reproducible one-step extraction method for the rapid preparation of polar and semi-polar metabolites, lipids, proteins, starch and cell wall polymers from a single sample. Plant Methods. 2016;12:1–15. doi: 10.1186/s13007-016-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonacorsi C, Raddi MSG, Carlos IZ, Sannomiya M, Vilegas W. Anti-Helicobacter pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied. (Malpighiaceae) BMC Complement. Altern. Med. 2009;9:1–7. doi: 10.1186/1472-6882-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George A, et al. Anti-inflammatory effects of Polygonum minus (Huds) extract (Lineminus™) in in-vitro enzyme assays and carrageenan induced paw edema. BMC Complement. Altern. Med. 2014;14:355. doi: 10.1186/1472-6882-14-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chantaranothai C, Palaga T, Karnchanatat A, Sangvanich P. Inhibition of nitric oxide production in the macrophage-like RAW 264.7 cell line by protein from the rhizomes of Zingiberaceae plants. Prep. Biochem. Biotechnol. 2013;43:60–78. doi: 10.1080/10826068.2012.697958. [DOI] [PubMed] [Google Scholar]
- 40.Galal AM, Mohamed HS, Abdel-Aziz MM, Hanna AG. Development, synthesis, and biological evaluation of sulfonyl-α-L-amino acids as potential anti-Helicobacter pylori and IMPDH inhibitors. Arch. Pharm. 2021;354:e2000385. doi: 10.1002/ardp.202000385. [DOI] [PubMed] [Google Scholar]
- 41.Druckerei, C. European Pharmacopoeia 4th edn (2002).
- 42.Kiranmai M, Kumar CM, Mohammed I. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. J. Pharm. Biol. Chem. Sci. 2011;2:254–261. [Google Scholar]
- 43.Chang, C. L., Lin, C. S. & Lai, G. H. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid. Based Complement. Alternat. Med. 2012 (2012). [DOI] [PMC free article] [PubMed]
- 44.Romano CS, Abadi K, Repetto V, Vojnov AA, Moreno S. Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 2009;115:456–461. doi: 10.1016/j.foodchem.2008.12.029. [DOI] [Google Scholar]
- 45.Singab ANB. Flavonoids from Iris spuria (Zeal) cultivated in Egypt. Arch. Pharm. Res. 2004;27:1023–1028. doi: 10.1007/BF02975425. [DOI] [PubMed] [Google Scholar]
- 46.de Oliveira DM, Siqueira EP, Nunes YR, Cota BB. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. Farmacogn. 2013;23:614–620. doi: 10.1590/S0102-695X2013005000061. [DOI] [Google Scholar]
- 47.Roger B, Jeannot V, Fernandez X, Cerantola S, Chahboun J. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochem. Anal. 2012;23:450–455. doi: 10.1002/pca.1379. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen PQD, et al. In vitro cytotoxic activity of constituents of the aerial parts of Glycosmis parviflora. Trop. J. Nat. Prod. Res. 2020;4:703–707. doi: 10.26538/tjnpr/v4i10.8. [DOI] [Google Scholar]
- 49.Koay YC, Wong KC, Osman H, Eldeen I, Asmawi MZ. Chemical constituents and biological activities of Strobilanthes crispus L. Rec. Nat. Prod. 2013;7:59–64. [Google Scholar]
- 50.Park W, et al. Anti-Helicobacter pylori compounds from Maackia amurensis. Nat. Prod. Sci. 2015;21:49–53. doi: 10.20307/nps.2015.21.4.282. [DOI] [Google Scholar]
- 51.Król-Kogus B, Głód D, Hałasa R, Krauze-Baranowska M, Pobłocka-Olech L. 2D LC as a tool for standardization of Foenugraeci semen extracts containing compounds with anti-Helicobacter pylori activity. Food Funct. 2021;12:2686–2692. doi: 10.1039/D1FO00226K. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno H, et al. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 53.Aktan F, Henness S, Roufogalis BD, Ammit AJ. Gypenosides derived from Gynostemma pentaphyllum suppress NO synthesis in murine macrophages by inhibiting iNOS enzymatic activity and attenuating NF-κB-mediated iNOS protein expression. Nitric Oxide. 2003;8:235–242. doi: 10.1016/S1089-8603(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 54.Kröncke K, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rees D, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minhas R, Bansal Y, Bansal G. Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 2020;40:823–855. doi: 10.1002/med.21636. [DOI] [PubMed] [Google Scholar]
- 57.Shah CP, Kharkar PS. Inosine 5′-monophosphate dehydrogenase inhibitors as antimicrobial agents: Recent progress and future perspectives. Future Med. Chem. 2015;7:1415–1429. doi: 10.4155/fmc.15.72. [DOI] [PubMed] [Google Scholar]
- 58.Ullah F, et al. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var. florentina. Nat. Prod. Res. 2015;30:1440–1444. doi: 10.1080/14786419.2015.1057585. [DOI] [PubMed] [Google Scholar]
- 59.Montilla MP, et al. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003;69:472–474. doi: 10.1055/s-2003-39698. [DOI] [PubMed] [Google Scholar]
- 60.Fouotsa H, et al. Antibacterial and antioxidant xanthones and benzophenone from Garcinia smeathmannii. Am. Assoc. Adv. Sci. 2015;81:594–599. doi: 10.1055/s-0035-1545841. [DOI] [PubMed] [Google Scholar]
- 61.Amin A, et al. Irigenin, a novel lead from Western Himalayan chemiome inhibits fibronectin-extra domain A induced metastasis in lung cancer cells. Sci. Rep. 2016;6:1–13. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo L, et al. Irigenin treatment alleviates doxorubicin (DOX)-induced cardiotoxicity by suppressing apoptosis, inflammation and oxidative stress via the increase of miR-425. Biomed. Pharmacother. 2020;125:109784. doi: 10.1016/j.biopha.2019.109784. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, et al. Irigenin alleviates angiotensin II-induced oxidative stress and apoptosis in HUVEC cells by activating Nrf2 pathway. Drug Dev. Res. 2021;82:999–1007. doi: 10.1002/ddr.21802. [DOI] [PubMed] [Google Scholar]
- 64.Ahn KS, et al. Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E2 production in murine macrophage RAW 264.7 cells. Life Sci. 2006;78:2336–2342. doi: 10.1016/j.lfs.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y, Gao C-C, Pan Z-G, Zhou C-W. Irigenin sensitizes TRAIL-induced apoptosis via enhancing pro-apoptotic molecules in gastric cancer cells. Biochem. Biophys. Res. Commun. 2018;496:998–1005. doi: 10.1016/j.bbrc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 66.González A, et al. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klesiewicz K, Karczewska E, Budak A, Marona H, Szkaradek N. Anti-Helicobacter pylori activity of some newly synthesized derivatives of xanthone. J. Antibiot. 2016;69:825–834. doi: 10.1038/ja.2016.36. [DOI] [PubMed] [Google Scholar]
- 68.Chin Y-W, Kinghorn AD. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-Rev. Org. Chem. 2008;5:355–364. doi: 10.2174/157019308786242223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q-J, Yue L. Inhibitory activity of mangiferin on Helicobacter pylori-induced inflammation in human gastric carcinoma AGS cells. Afr. J. Tradit. Complement. Altern. Med. 2017;14:263–271. doi: 10.21010/ajtcam.v14i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stohs S, et al. A review on antioxidant, anti-inflammatory and gastroprotective abilities of mango (Mangifera indica) leaf extract and mangiferin. J. Nutr. Health Sci. 2018;5:303. [Google Scholar]
- 71.Mei S, Ma H, Chen X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021;149:111997. doi: 10.1016/j.fct.2021.111997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).