Abstract
BN‐heteroarenes, which employ both boron and nitrogen in aromatic hydrocarbons, have gained great attention in the fields of organic chemistry and materials science. Nevertheless, the extensive studies on BN‐heteroarenes are largely limited to 1,2‐azaborine‐based compounds with B–N covalent bonds, whereas 1,3‐ and 1,4‐BN‐heteroarenes are relatively rare due to their greater challenge in the synthesis. Recently, significant progresses have been achieved in the synthesis and applications of BN‐heteroarenes featuring 1,4‐azaborines, especially driven by their significant potential as multiresonant thermally activated delayed fluorescence (MR‐TADF) materials. Therefore, it is timely to review these advances from the chemistry perspective. This review summarizes the synthetic methods and recent achievements of 1,4‐azaborine‐based BN‐heteroarenes and discusses their unique properties and potential applications of this emerging class of materials, highlighting the value of 1,4‐BN‐heteroarenes beyond MR‐TADF materials. It is hoped that this review would stimulate the conversation and cooperation between chemists who are interested in azaborine chemistry and materials scientists working in the fields of organic optoelectronics, metal catalysis, and carbon‐based nanoscience etc.
Keywords: azaborines, boron, conjugated materials, heteroarenes, heteroatom, organic semiconductors, polycyclic aromatic hydrocarbons
Boron and nitrogen (BN)‐heteroarenes have gained great attention in organic chemistry and materials science. Recently, significant progresses have been achieved in the synthesis and applications of BN‐heteroarenes featuring 1,4‐azaborine moieties. This review summarizes the synthetic methods and recent achievements of 1,4‐azaborine‐based BN‐heteroarenes, and discusses their unique properties and potential applications, in order to stimulate the synergistic development of azaborine chemistry and materials research.
1. Introduction
Since the discovery of benzene by Faraday in 1825, monocyclic and polycyclic aromatic hydrocarbons (PAHs) have been attracting wide attention in the fields of organic chemistry and materials science.[ 1 ] During the past decades, there has been increasing interest in introducing BN units into the backbone of benzene and PAHs to modulate their chemical and physical properties.[ 2 ] The diatomic polar BN units and nonpolar CC units are isoelectronic moieties while showing great discrepancy in π‐electron distribution. The resulting BN‐heteroarenes display unique properties compared with all‐carbon analogs, demonstrating great potential for applications in biological systems, coordinate chemistry, and organic electronics.[ 2 , 3 ]
In 1926, Stock and Pohland reported the synthesis of borazine,[ 4 ] which is known as inorganic benzene (Figure 1a). The discovery of borazine has promoted the development of partially BN‐substituted benzene derivatives, namely azaborines. According to the relative positions of embedded boron and nitrogen atoms, azaborines are classified into three isomers: 1,2‐azaborine, 1,3‐azaborine, and 1,4‐azaborine. Theory calculations have revealed that the thermodynamic stability of these three isomers follows: 1,2‐azaborine > 1,4‐azaborine > 1,3‐azaborine (Figure 1b).[ 5 ] The synthesis of 1,2‐azaborine‐embedded PAHs was pioneered by Dewar and co‐workers in 1958,[ 6 ] whereas the monocyclic 1,2‐dihydro‐1,2‐azaborine was only successfully synthesized by the Liu group in 2008.[ 7 ] Up to date, 1,2‐azaborine and its π‐extended conjugated molecules have been widely studied and thoroughly reviewed.[ 3 , 8 ] By comparison, 1,3‐azaborine displays the lowest thermodynamic stability and possesses the greatest challenge in the synthesis. Only the Liu group reported the synthesis of monocyclic 1,3‐azaborine in 2011 (note that the parent 1,3‐dihydro‐1,3‐azaborine remains elusive),[ 9 ] whereas π‐extended conjugated molecules featuring 1,3‐azaborine have still been an unexplored area.
Figure 1.
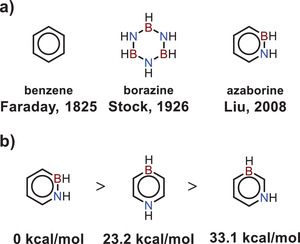
a) Chemical structures of benzene, borazine, and 1,2‐dihydro‐1,2‐azaborine. b) Thermodynamic stability of three azaborine isomers.
Despite higher stability of 1,4‐azaborine compared with its 1,3‐isomer, only a few examples were reported before 2006. The first 1,4‐azaborine‐embedded anthracene derivatives were reported by Maitlis and co‐workers in 1961.[ 10 ] The same backbone with a different substituent was synthesized by Clark and co‐workers in 1992, and the structure was confirmed by single‐crystal X‐ray analysis (Figure 2 ).[ 11 ] From 2006 to 2010, Kawashima and co‐workers explored a series of 1,4‐azaborine‐embedded acenes, demonstrating their potential applications in organic light‐emitting diodes (OLEDs) and optical anion sensors.[ 12 ] Monocyclic 1,4‐azaborine was first reported by Braunschweig and co‐workers in 2012 via rhodium‐catalyzed cycloaddition reaction,[ 13 ] whereas the parent 1,4‐dihydro‐1,4‐azaborine has remained elusive. In 2014, Liu and co‐workers successfully synthesized 1,4‐azaborine‐embedded naphthalene,[ 14 ] and disclosed its unique coordination behavior with platinum(II) metal centers. In 2016, Hatakeyama and co‐workers reported 1,4‐azaborine‐based multiresonant thermally activated delayed fluorescence (MR‐TADF) materials and demonstrated their advantages in OLEDs with high external quantum efficiencies (EQEs) as well as excellent color purity.[ 15 ] Since then, the diversity of 1,4‐BN‐heteroarenes have been greatly enriched and the interest in this class of compounds has been growing rapidly.
Figure 2.
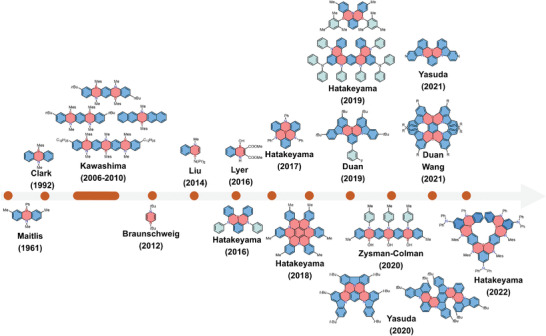
The development history of 1,4‐BN‐heteroarenes showing representative chemical structures.
There have been more than 60 years since the first synthesis of 1,4‐azaborines, but only recently have they drawn broad attention, especially driven by the development of MR‐TADF materials. Although several reviews partly covering 1,4‐azaborines have been reported in recent years, they are mainly focused on the molecular structures or the specific OLED applications.[ 3c ‐ 3 , 16 ] Up to date, a chemistry‐oriented review to address the synthetic progresses and challenges, the fundamental structure‐property relationships, and the wide applications (especially beyond MR‐TADF OLEDs) of 1,4‐BN‐heteroarenes has been elusive. Therefore, in this review, we aim to provide a chemistry perspective of BN‐heteroarenes featuring 1,4‐azaborine by summarizing the synthetic strategies and recent advances, as well as their intriguing properties and potential applications in a variety of research fields.
2. Synthetic Strategies
According to how the 1,4‐azaborine ring is formed, synthetic strategies toward 1,4‐azaborine derivatives can be generally classified into the following three types: i) transition‐metal‐catalyzed reactions, ii) nucleophilic substitution reactions, and iii) electrophilic C—H borylation reactions. In the following, the three strategies will be discussed in detail together with the synthetic advances.
2.1. Transition‐Metal‐Catalyzed Reactions
[2+2+2] cycloaddition is a powerful strategy to construct aromatic rings, such as substituted benzene and pyridine derivatives.[ 17 ] In 2012, Braunschweig and co‐workers tried to prepare 1,2‐azaborines from iminoborane 1 with excess alkyne in the presence of [{(iPr3P)2RhCl}2].[ 13 ] Surprisingly, they found that the reaction selectively afforded 1,4‐azaborine 2 in moderate yields. The reaction was demonstrated to proceed via intermediate 3 through a tandem [2+2]/[2+4] cycloaddition process, which was different from common alkyne cyclotrimerization reaction (Scheme 1 ). To further extend the scope of this reaction, in 2016, they synthesized a variety of functionalized 1,4‐azaborines (4) with different substituents at C(2) position, including the molecules with two and three discrete 1,4‐azaborine moieties (5–7).[ 18 ]
Scheme 1.
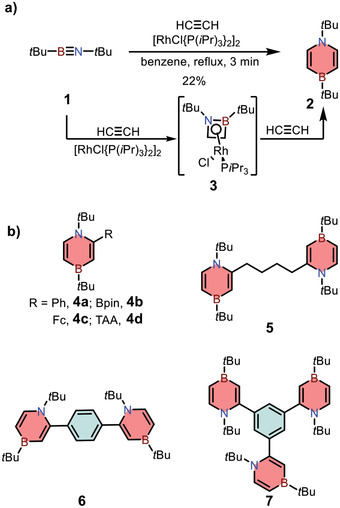
a) Synthetic route to monocyclic 1,4‐azaborine 2 via rhodium‐catalyzed cycloaddition reaction. b) Other 1,4‐azaborine derivatives.
By comparison, in 2016, Liu and co‐workers reported the synthesis of monocyclic 1,4‐azaborines via a different method.[ 19 ] As illustrated in Scheme 2 , various substituted bis(2‐bromoallyl)amines 9 were used as building blocks for dual Li/Br exchange and the subsequent nucleophilic substitution reactions with iPr2NBCl2, affording precursor 10 with exocyclic alkenes. Then the corresponding 1,4‐azaborines 11 were obtained efficiently via a Ru‐catalyzed isomerization. Direct replacement of the N,N‐diisopropylamino group in 11a with OMe or Cl group was achieved, and the resulting 1,4‐azaborines with B‐Cl (12a) or B‐OMe (12b) group could further react with various anionic nucleophiles, leading to functionalized 1,4‐azaborines 13 (Scheme 2). It is worth noting that compounds 102 and 103 were obtained by this approach and exhibited distinct photophysical properties compared with their all‐carbon analogs, which will be discussed in detail in Section 3.1.
Scheme 2.
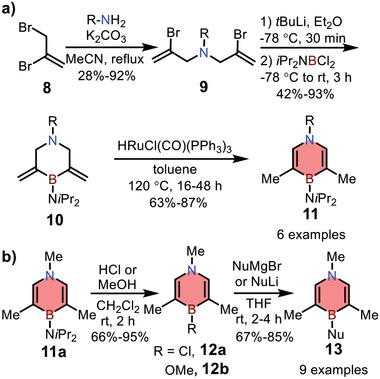
a) Synthetic routes to monocyclic 1,4‐azaborines via Ru‐catalyzed olefin isomerization reaction. b) Nucleophilic substitution towards B‐substituted 1,4‐azaborines.
Apart from monocyclic 1,4‐azaborines, Liu and co‐workers also reported the synthesis of 1,4‐azaborine‐embedded naphthalene 15 in 2014.[ 14 ] The key precursor 14 was achieved from commercially available 2‐bromoaniline in four steps, and then was converted into compound 15 in the presence of Ru‐based Grubbs second‐generation catalyst (Scheme 3a). Similarly, the diisopropylamino substituent in 15 could be quantitatively replaced by methoxy group in the presence of methanol. Then the afforded 16 was further transformed into various functionalized 1,4‐azaborines 17 by nucleophilic substitutions. Interestingly, a unique k 2‐N‐η2‐BC mode coordination was found by treating 17a with [{PtMe2(μ‐SMe2)}2] or trans‐[{PtCl(μ‐Cl)(PEt3)}2], resulting in charge‐neutral Pt complex 18a and cationic Pt complex 19, respectively (Scheme 3b). Notably, the similar complex could not be obtained from the corresponding carbonaceous ligand under the same reaction conditions. Furthermore, the utility of this novel skeleton in organic synthesis was carried out by using 1,4‐azaborine‐based phosphine ligand 17b for Pd‐catalyzed 1,3‐enyne hydroboration reaction, leading to preferable trans addition products (Scheme 3c).
Scheme 3.
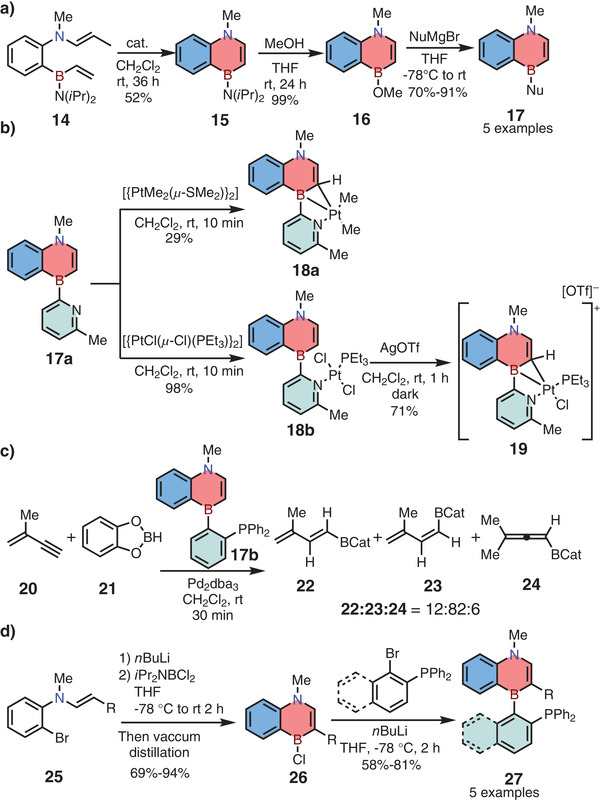
a) Synthetic route to 1,4‐azaborine‐embedded naphthalene via ring‐closing metathesis (RCM) reaction. b) Synthesis of k 2‐N‐ɳ 2‐BC Pt complex. c) Unique selectivity in Pd‐catalyzed hydroboration reaction. d) Synthesis of 1,4‐azaborine‐embedded SenPhos ligands.
In 2016, the same group developed a new strategy to access 1,4‐azaborine‐based SenPhos ligands 27 (Scheme 3d).[ 20 ] The key enamine 25 was treated with nBuLi firstly, and then the formed lithium reagents were trapped by iPr2NBCl2, followed by intramolecular electrophilic borylation to give air‐sensitive 26 in one pot. Finally, the target ligands 27 were obtained successfully by the nucleophilic substitution reaction using phosphine‐containing organolithium nucleophiles. Indeed, this strategy superseded the original method in terms of the scale of preparation. Meanwhile, the use of 27 as ligands in 1,3‐enyne hydroboration reactions showed higher trans selectivity (see Section 3.3).
In 2017, Chatani and co‐workers reported the preparation of 1,4‐azaborine‐embedded anthracene by palladium‐catalyzed borylation (Scheme 4 ).[ 21 ] Dihalide 28 was selected as the key building block and reacted with diisopropylaminoborane 29 in the presence of Pd(OAc)2, forming 1,4‐azaborine 30 with air‐sensitive character in one pot. The intermediate 30 could be further hydrolyzed by MeOH or aqueous NH4Cl, affording product 31 in 42% yield. Although the same skeleton of compound 31 was already reported in 1961 by nucleophilic substitution, this is the first example of constructing 1,4‐azaborine‐embedded anthracene without using organolithium reagents. In addition, this palladium‐catalyzed borylation could be explored for the preparation of various dibenzo‐1,4‐heteroborines containing O or S atoms in moderate yields.
Scheme 4.
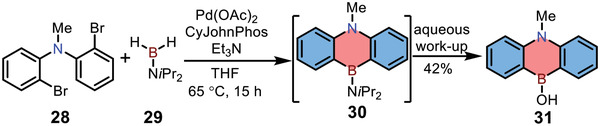
Synthetic route to 1,4‐azaborine‐embedded anthracene by Pd‐catalyzed borylation.
2.2. Nucleophilic Substitution Reactions
The first 1,4‐azaborine‐embedded anthracene was synthesized by Maitlis in 1961 via nucleophilic substitution reaction, and the single‐crystal X‐ray structure of the same skeleton was further confirmed by Clark and co‐workers in 1992.[ 10 , 11 ] From 2006 to 2010, Kawashima and co‐workers developed a series of 1,4‐azaborine‐based linear acenes by the same synthetic strategy (Scheme 5 ).[ 12a – d ] Taking 1,4‐azaborine‐embedded anthracene 34 for example, the synthesis started from Li/Br exchange of 2,4‐dibromo‐N‐(2,4‐dibromophenyl)‐N‐hexylaniline 32 to form intermediate 33, followed by nucleophilic substitution reaction with MesB(OMe)2 or TipB(OMe)2 to give target compounds in one pot. Furthermore, 1,4‐azaborine‐based acenes 35–38 were obtained successfully by the same method. Nearly coplanar geometry of compound 36 was determined by X‐ray single‐crystal analysis, indicating good π‐extension across the conjugated framework. This character was in accordance with the bathochromic shift of both the absorption and emission maxima of 38 (the maximum λ abs at 608 nm and λ em at 625 nm) compared with 36 (λ abs at 523 nm, λ em at 534 nm). Meanwhile, π–π stacking interaction of compound 36 was hampered by the large steric group (mesityl group, Mes), which was beneficial to the high photoluminescence quantum yield (PLQY) of up to 69%.
Scheme 5.
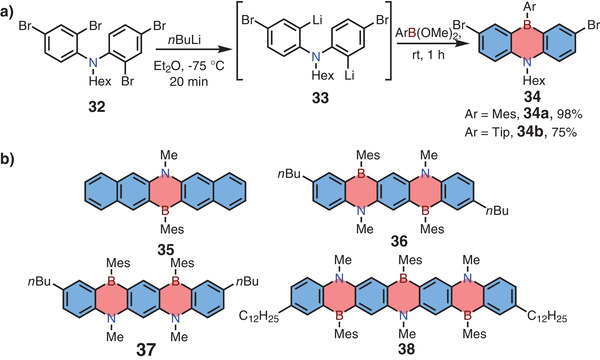
a) Nucleophilic substitution reaction to afford 1,4‐azaborine‐embedded anthracenes and b) 1,4‐azaborine‐embedded pentacene and heptacene.
Apart from novel skeletons, Kawashima and co‐workers also explored various peripherally functionalized 1,4‐azaborine‐embedded anthracene derivatives from 34 via the Buchwald‐Hartwig amination, Li/Br exchange in conjunction with nucleophilic substitution, or Sonogashira cross‐coupling reactions, giving products 39, 40, and 41, respectively (Scheme 6 ).[ 12b ] Notably, although compound 39c with carbazolyl moiety shared the similar low‐energy absorption maximum with the parent 1,4‐azaborine‐embedded anthracene (around 400 nm), the former exhibited an ultrahigh PLQY up to 100%. In 2019, the potential of 39c as emitting materials in OLEDs was examined by Cheng and co‐workers by replacing hexyl group at nitrogen with phenyl moiety, and the resulting device exhibited a narrow emission band (full‐width at half‐maximum, FWHM = 51 nm) with TADF character.[ 22 ] Kawashima and co‐workers also reported the synthesis of water‐soluble dicationic 1,4‐azaborines 43 by treating precursor 42 with iodomethane and further demonstrated their potential as optical anion sensors (Scheme 6b).[ 12e ] In 2021, Agou and co‐workers reported the synthesis of π‐extended 1,4‐azaborines 45 via palladium‐catalyzed C—H activation reaction. Through fusing carbazole moieties, compounds 45 showed improved PLQY and higher electrochemical stability.[ 23 ] On the other hand, Kawashima and co‐workers further studied the functionalization of 1,4‐azaborine‐embedded anthracenes at the nitrogen position and synthesized donor–acceptor type π‐conjugated dendrimers 46 successfully.[ 12c ] The emission of dendrimers 46a and 46c both revealed obviously bathochromic shift (λ em > 600 nm) because of the intramolecular charge transfer (ICT) from the azaborine dendrons to the benzothiadiazole core. By contrast, the non‐emissive character of ICT state in 46b was responsible for the absence of emission around 600 nm.
Scheme 6.
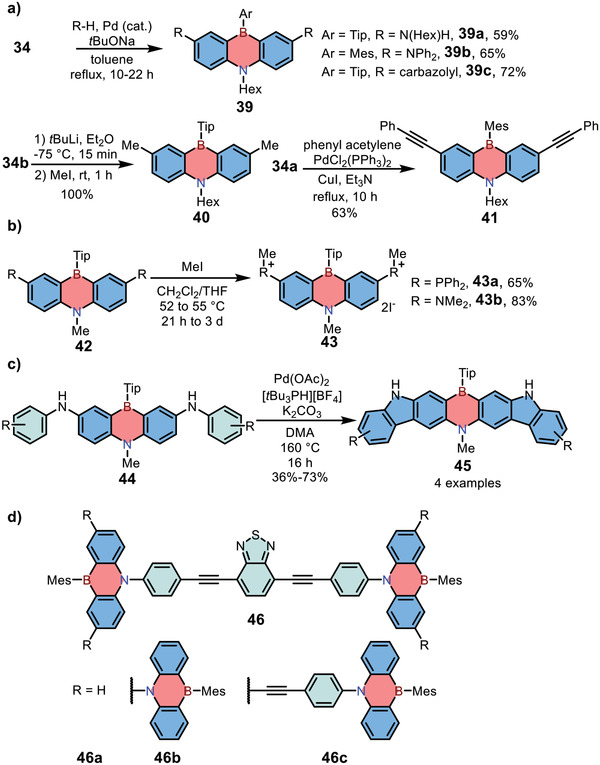
a–d) Synthesis of 1,4‐azaborine‐embedded anthracene derivatives.
In 2019, Yamashita and co‐workers further explored the scope of functionalized 1,4‐azaborine‐embedded anthracenes, achieving compounds 52–54 with various substituents at nitrogen or boron positions (Scheme 7 ).[ 24 ] Bis(2‐bromophenyl)amine 47 was used as the starting material and reacted with nBuLi, then the reaction was trapped by BCl3, giving air‐sensitive intermediate 49 with the 1,4‐azaborine backbone. Then compound 49 was further quenched by aqueous K2CO3, affording corresponding boronic acid 50 in 21% yield for two steps. Notably, compound 50 could also be prepared in 70% yield from 47 via intermediate 48. Functionalization of 50 on the boron atom was achieved by the activation of hydroxyl group in conjunction with the nucleophilic substitution, giving products 52 in 43–95% yields. Finally, functionalization of 52a was carried out via nucleophilic alkylation or Buchwald–Hartwig amination, affording target compounds 53 and 54, respectively.
Scheme 7.
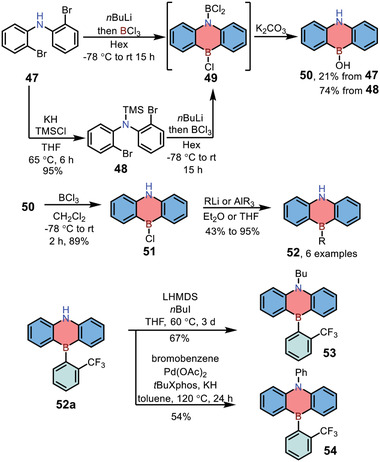
Synthesis of 1,4‐azaborine‐embedded anthracenes via orthogonal functionalization.
In 2015, Iyer and co‐workers reported the synthesis of 1,4‐azaborine‐embedded naphthalenes 56 by the nucleophilic [4+2] annulation of 2‐aminophenylboronic acids/boronates 55 with alkynes (Scheme 8 ).[ 25 ] The scope of this reaction was broad, and various substituted 1,4‐azaborines 56 were afforded in good yields (65–88%). Furthermore, compounds 56a could be further transformed to corresponding indoles 57 in the presence of palladium acetate.
Scheme 8.
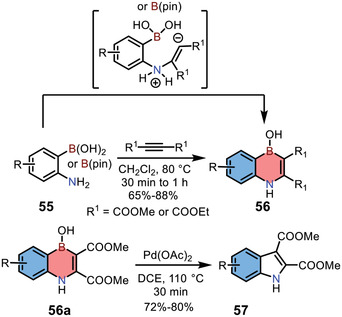
Synthesis and transformation of 1,4‐azaborine‐embedded naphthalenes.
2.3. Electrophilic C—H Borylation Reactions
Electrophilic direct C—H borylation reaction was first reported in the 1950s, and a great number of conjugated molecules incorporating boron atoms have been explored via this method, including 1,2‐azaborine‐based PAHs.[ 3 , 26 ] In 2016, Hatakeyama and co‐workers reported the synthesis of N,B,N‐type 1,4‐azaborines 59 by the electrophilic C—H borylation.[ 15 ] The obtained compounds revealed separated HOMO–LUMO distribution and exhibited narrow emission bands (FWHM = 28 nm for both two compounds) as the emitting layer in OLED devices. As shown in Scheme 9a, the key precursor 58 were prepared from commercially available 1‐bromo‐2,3‐dichlorobenzene via Buchwald–Hartwig coupling reactions. Then 58 was treated with tBuLi for Li/Br exchange, and the intermediate was trapped with BBr3 to form one C—B bond. The final two C—B bonds were constructed by intramolecular direct C—H borylation in tert‐butylbenzene at high temperature, forming 59 in one pot. To tune the emission properties of 1,4‐azaborines 59, in 2019, Zhang and co‐workers reported the synthesis of carbazole‐fused 1,4‐azaborines 61 by the similar synthetic strategy (Scheme 9b).[ 27 ] Precursor 60 was achieved by the nucleophilic substitution reaction of 2‐bromo‐1,3,5‐trifluorobenzene or the nucleophilic substitution in conjunction with Suzuki coupling reactions from 5‐bromo‐2‐chloro‐1,3‐difluorobenzene. Compounds 61a with electron‐deficient unit at the para‐position to boron exhibited a red‐shifted green emission without compromising the color purity due to the enhanced ICT effect.
Scheme 9.
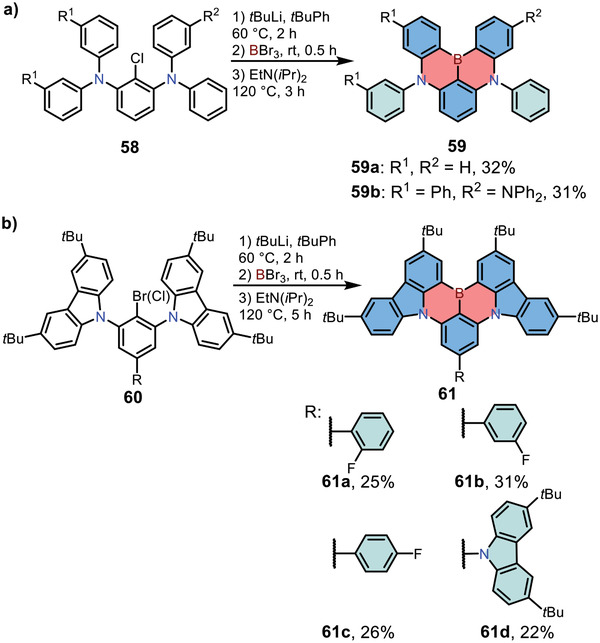
a,b) Preparation of N,B,N‐type 1,4‐azaborine‐based PAHs via electrophilic direct C—H borylation reaction.
Varying the substituents is effective to systematically modulate the emission properties of these N,B,N‐type 1,4‐azaborine‐based MR‐TADF materials, which will be discussed in Section 3.6 (Figure 6) and will not be detailed here. We would, however, like to highlight a series of novel N,B,N‐type 1,4‐azaborine skeletons that were synthesized via the C—H borylation method in recent years (Scheme 10 ). For example, in 2020, Yasuda and co‐workers reported the synthesis of N,B,N‐type 1,4‐azaborines 63, 65, 137, and 139 from the halogenated precursors that were prepared by the nucleophilic substitution (Scheme 10a and Figure 6).[ 28 ] The emission maxima of these compounds were well tunable covering nearly the whole visible region (from 466 to 615 nm) by increasing (or decreasing) the electronic strength of boron (as acceptor) and nitrogen (as donor) atoms. In 2021, Yasuda and co‐workers further developed a new family of N,B,N‐type 1,4‐azaborine derivatives 67, 134, and 135 by the similar strategy, demonstrating their potential as color‐tunable MR‐TADF materials (Scheme 10b and Figure 6).[ 29 ]
Figure 6.
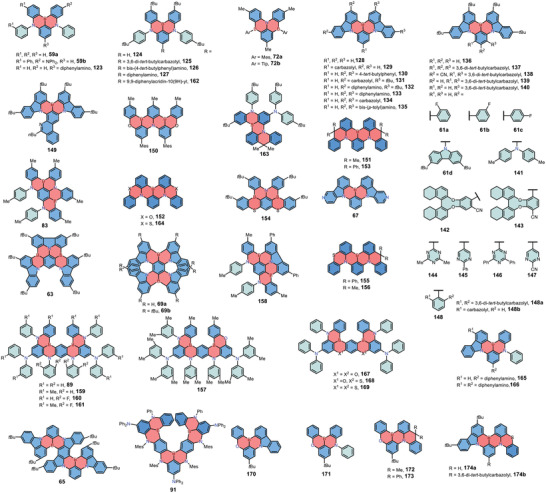
Chemical structures of recently reported MR‐TADF emitters employed for OLED devices.
Scheme 10.
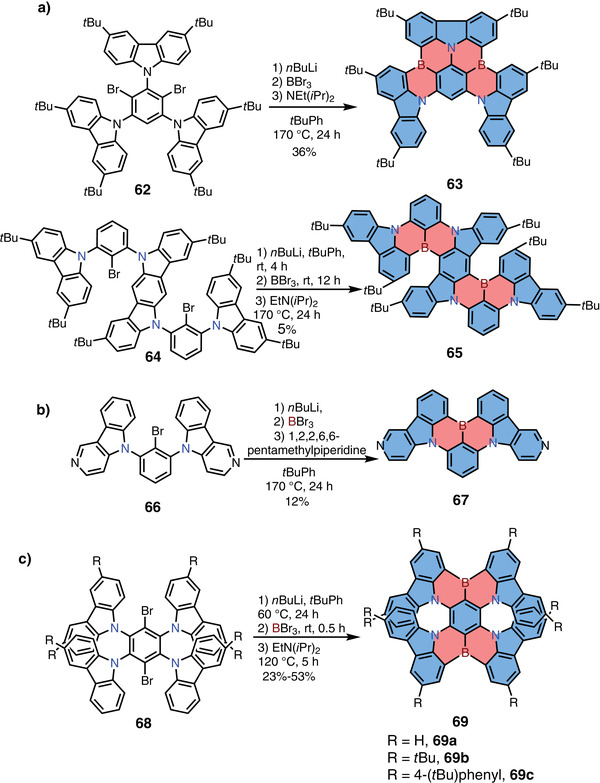
a–c) Synthetic routes to π‐extended 1,4‐azaborine derivatives.
In 2021, Zhang and co‐workers reported the synthesis of N,B,N‐type 1,4‐azaborine‐embedded PAHs 69a,b (Scheme 10c).[ 30 ] Precursors 68 were prepared by nucleophilic substitution reaction from 1,4‐dibromo‐2,3,5,6‐tetrafluorobenzene in excellent yields. Then compounds 68 were treated with nBuLi for Li/Br exchange, and the lithium reagent was reacted with BBr3, followed by intramolecular C—H borylation at high temperatures to form 69a,b in one pot. The OLED devices employing compounds 69a,b as the emitters exhibited deep‐red emission with EQEs up to 28%. Notably, at the same time, Wang and co‐workers independently reported compounds 69a,b as well as compound 69c, and disclosed their excellent chiroptical properties as a new type of B,N‐embedded double hetero[7]helicenes, such as high absorption dissymmetry factors (g abs) and efficient circularly polarized luminescence (see Section 3.2).[ 31 ]
In addition to the well‐developed N,B,N‐type 1,4‐BN‐heteroarenes, in 2019, Hatakeyama and co‐workers also reported the synthesis of B,N,B‐type 1,4‐azaborine‐based PAHs 72, which shared the similar backbone with compounds 59 (Scheme 11a).[ 32 ] In their synthesis, the key precursor 70 was treated with tBuLi first, and then the resulting lithium reagent was trapped by two equivalents of BBr3, forming intermediate 71 with one 1,4‐azaborine ring. The second 1,4‐azaborine ring was built up via direct electrophilic borylation reaction. Finally, the bromine at boron atoms was replaced by steric groups to improve the stability. Compounds 72 were successfully used as MR‐TADF materials and displayed sky‐blue emission (around 480 nm). Besides, in 2020, Wang and co‐workers reported the same skeleton (74 and 75) via a different strategy (Scheme 11b).[ 33 ] In their synthesis, the same precursor 70 was treated with tBuLi firstly, and then reacted with MesB(OMe)2, forming air‐stable 1,4‐azaborine 73. Compound 73 was found to display a unique B‐Mes bond cleavage reactivity under different equivalents of BBr3, affording 1,4‐azaborines with various steric groups.
Scheme 11.
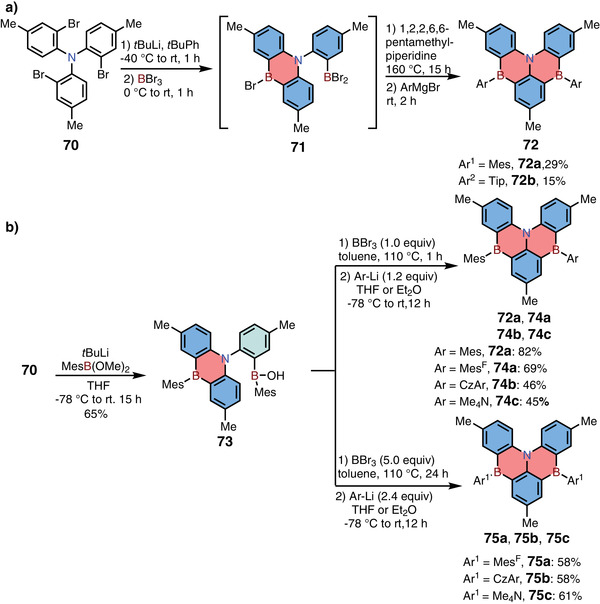
a,b) Syntheses of B,N,B‐type 1,4‐azaborine‐based PAHs.
Electrophilic C—H borylation can also be used for constructing 1,4‐azaborine‐embedded triangulenes. In 2017, Hatakeyama and co‐workers reported the synthesis of 1,4‐azaborine‐embedded [3]triangulenes 77 (Scheme 12 ).[ 34 ] The key precursor triphenylcyclotrianiline 76 was synthesized from 2‐chloro‐diphenylbenzene‐1,3‐diamine via two intermolecular and one intramolecular Buchwald‐Hartwig amination reactions. Then the boron atom was introduced into the central core via nucleophilic substitution in conjunction with electrophilic borylation reaction, forming 1,4‐azaborine 77 with a fully constrained structure. Note that this strategy could be further expanded to construct various hetero[3]triangulenes with different central heteroatoms/groups (e.g., P, P═S, P═O, and SiMe). In 2021, the same group synthesized cationic 1,4‐azaborine 79 with tetrabromoborate (BBr4 −) as a counter ion through the same method (Scheme 12b).[ 35 ] Due to the electron‐accepting character of boron atom, compound 79 was sensitive towards nucleophilic reagents, which could lead to air‐stable derivatives 80. Interestingly, treatment of compound 79 with AgOTf afforded another cationic derivative 81, which showed high air stability in the solid state. Salt 81 exhibited reversible two‐electron reduction waves in electrochemistry and could be further transformed into 2D brickwork‐type π‐electronic ion pair by treating it with 1,2,3,4,5‐pentacyanocyclopentadienyl anion, demonstrating its potential as electronic materials.
Scheme 12.
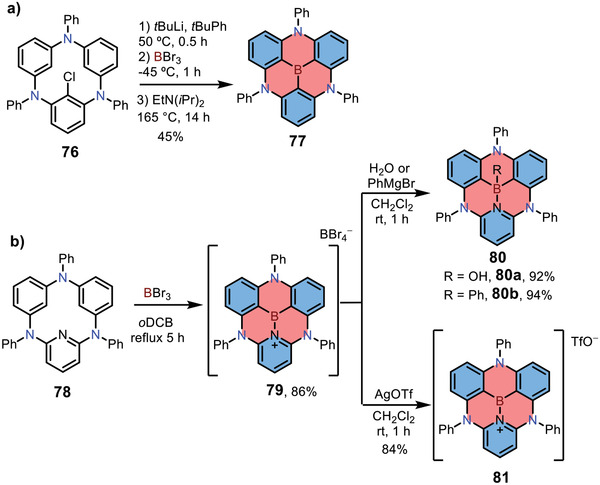
a,b) Synthesis of 1,4‐azaborine‐embedded [3]triangulenes.
The above examples generally involve two steps for forming new C—B bonds: nucleophilic substitution of corresponding lithium reagent with BBr3 and subsequent direct C—H borylation reaction. The procedure is somewhat complicated, so it would be simpler if 1,4‐azaborine‐based PAHs could be obtained by direct C—H borylation on electron‐rich substances. In 2018, Hatakeyama and co‐workers reported the synthesis of multiple 1,4‐azaborine‐embedded PAHs via one‐pot direct borylation method (Scheme 13a).[ 36 ] The p‐phenyl‐1,3,5‐triamine 82 was synthesized from 1,3,5‐tribromobenzene via Buchwald‐Hartwig coupling reaction. The products of direct electrophilic borylation highly depended on the reaction temperature as well as the equivalents of triiodoborane (BI3). Doubly boron‐fused 1,4‐azaborine 83 was achieved by treating precursor 82 with five equivalents of BI3 in the presence of triphenylborane (BPh3) at 190°C, whereas threefold boron‐embedded 1,4‐azaborine 84 was obtained as the main product when the reaction temperature was increased to 200 °C in 1,2,4‐trichlorobenzene. Besides, in the presence of excess BI3 (without BPh3), multi‐borylation of triamine 82 occurred, affording main product 85 with four boron atoms, whereas other boron sources (i.e., BCl3 or BBr3) could not give 85 at all. In 2021, Hatakeyama and co‐workers reported carbazole‐fused 1,4‐azaborines 87 via the same strategy (Scheme 13b).[ 37 ] Two boron atoms were selectively introduced at the ortho‐position of the carbazolyl group because of the higher electron‐donating abilities of carbazolyl. The resulting carbazole‐fused compound 87 exhibited slightly bathochromic emission (λ max: 491 nm) compared with 83 (λ max: 455 nm).
Scheme 13.
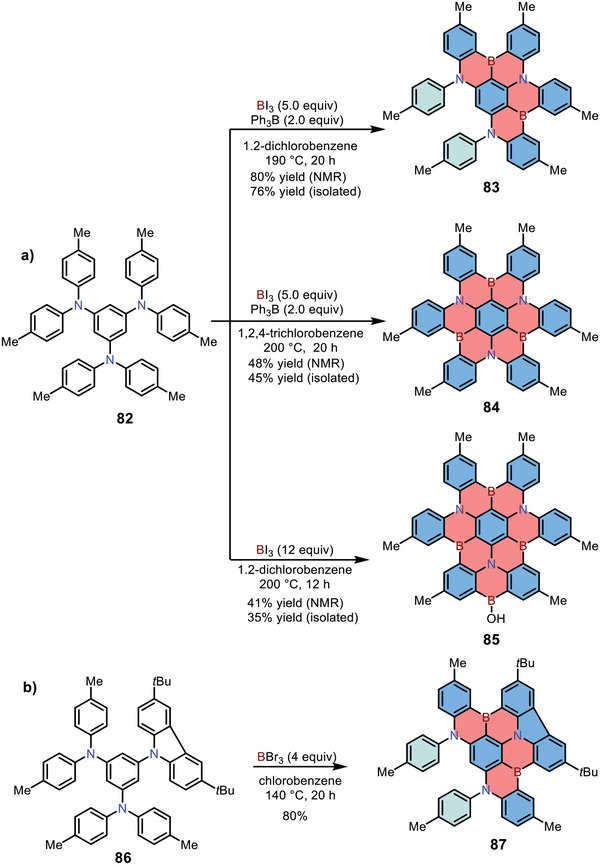
a,b) Syntheses of 1,4‐BN‐heteroarenes via one‐pot multiple electrophilic borylation reactions.
Multiborylation reaction can also be used to build 1,4‐azaborine‐embedded PAHs with other topologies. In 2019, Hatakeyama and co‐workers developed a π‐extended 1,4‐azaborine 89 (Scheme 14a).[ 38 ] The key precursor 88 was synthesized from commercially available 1,3‐dibromo‐5‐chlorobenzene via two sequential Buchwald‐Hartwig coupling reactions. Then 88 was treated with BBr3 directly in 1,2‐dichlorobenzene at 180 °C, forming six C—B bonds in one pot to provide 89 in 73% yield. The OLED device employing 89 as the emitter exhibited a narrow emitting band (λ max: 469 nm, FWHM: 18 nm), demonstrating its advantage as an ultrapure blue‐emitting material. In 2022, the same group reported the synthesis of an expanded 1,4‐azaborine‐based PAH 91 with a twisted structure.[ 39 ] The product 91 was prepared by treating diphenylamine derivatives 90 with excess BBr3 at a high temperature (180 °C), forming nine C—B bonds in one pot (Scheme 14b). The π‐extended framework of 91 exhibited narrowband sky‐blue emission with a small FWHM of 16 nm and a high k RISC of 4.4 × 105 s−1.
Scheme 14.
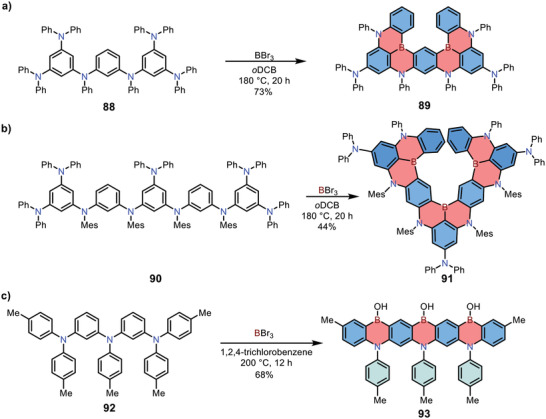
a–c) Synthesis of ladder‐type 1,4‐azaborine‐embedded PAHs via one‐pot multiple electrophilic borylation reactions.
In 2020, Zysman‐Colman and co‐workers reported the synthesis of 1,4‐azaborine‐embedded heptacene 93 (Scheme 14c).[ 40 ] Precursor 92 was obtained through a sequence of Ullman and Buchwald–Hartwig coupling reactions with a moderate yield for two steps. Interestingly, although excess BBr3 (18 equivalents) was added, the reaction mainly afforded a structurally rigid linear backbone. Then the reaction was quenched by aqueous sodium acetate and 1,4‐azaborine 93 was achieved successfully. Compound 93 showed a dramatic combination of both MR‐TADF and triplet‐triplet annihilation (TTA) characters and was considered as a highly efficient deep‐blue emitting material for OLEDs.
In addition to dibenzo‐1,4‐azaborines, Mitsudo and co‐workers also reported the synthesis of dithieno‐fused 1,4‐azaborines 95 (Scheme 15 ).[ 41 ] The precursors 94 were prepared from 3‐bromothiophene derivatives via the Buchwald–Hartwig amination, and then were treated with PhBCl2 for the direct electrophilic borylations to form two C—B bonds in the presence of triethylamine, giving products 95 in good yields.
Scheme 15.
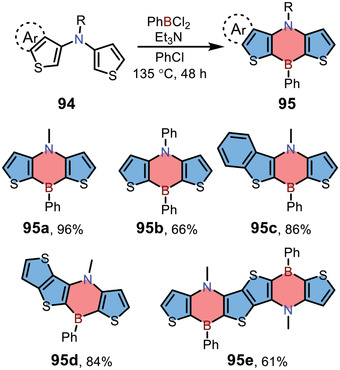
Synthesis of dithieno‐fused 1,4‐azaborines.
In 2021, Feng and co‐workers developed a series of boron‐embedded PAHs, including 1,4‐azaborines, by a one‐pot cascade reaction (Scheme 16 ).[ 42 ] Compounds 96 was selected as the key precursor for electrophilic borylation, and was treated with BBr3 in the presence of 2,4,6‐tri‐tert‐butylpyridine, forming one benzene ring with a BBr2 substituent. Then the obtained intermediate 97 underwent 1,4‐boron migration, affording intermediate 98. The subsequent intramolecular twofold electrophilic borylations generated a variety of boron‐embedded PAHs, including 1,4‐azaborines 99. This strategy featured simplicity and a broad substrate scope and could be used for building various expanded B‐doped graphene nanostructures.
Scheme 16.
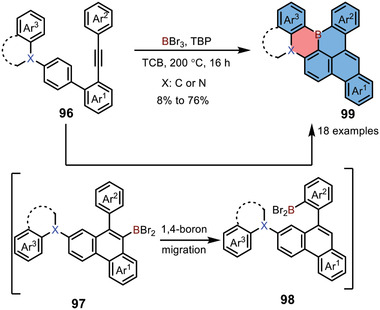
Synthesis of 1,4‐azaborine‐based PAHs via a key 1,4‐boron migration step.
3. The Value of Incorporating 1,4‐Azaborines
During the past two decades, the chemistry of 1,4‐azaborines has achieved significant development, and numerous compounds have revealed unique properties compared with their carbonaceous counterparts. In this section, we will discuss the intriguing properties and promising applications of 1,4‐azaborine‐based BN‐heteroarenes owing to the unique effect of BN‐incorporation.
3.1. Altering the Photophysical Properties
The different π‐electron distribution and unique dipole moment of 1,4‐azaborine as compared with benzene endow 1,4‐BN‐heteroarenes with appealing properties. To deeply understand this effect, Liu and co‐workers synthesized two kinds of donor–acceptor‐type 1,4‐azaborines 102 and 103, manifesting different photophysical properties compared with their isoelectronic counterparts 100 and 101 (Figure 3 ).[ 19 ] It was obvious that carbonaceous terphenyls 100 and 101 exhibited similar absorption (λ max: 272 and 270 nm) as well as emission (λ max: 365 and 370 nm) bands, which was attributed to the same conjugated backbone. In contrast, compounds 102 and 103 with polar 1,4‐azaborine moiety revealed significant differences in both the absorption and emission spectra. The absorption maximum of 102 was at 296 nm, slightly bathochromic‐shifted compared with 100, but hypochromic shifted than its isomer 103 (λ max: 313 nm). On the other hand, compound 102 revealed a broad emission band (from 350 to 600 nm) with the maximum at 482 nm, whereas compound 103 exhibited a relatively narrow emission band with the maximum at 359 nm. Meanwhile, due to the electronic effect of the substituents, 1,4‐azaborines 102 and 103 showed significantly different Stokes shifts: the former displayed a relatively large Stokes shift (13037 cm–1) compared with the carbon analogs (9367 cm–1 for 100 and 10010 cm–1 for 101), while the latter exhibited a dramatically decreased Stokes shift (4094 cm–1). Such results demonstrated that 103 has a relatively small structural reorganization between the ground state and the excited state. This example showcased how the incorporation of 1,4‐azaborine greatly affected the photophysical properties of conjugated materials.
Figure 3.
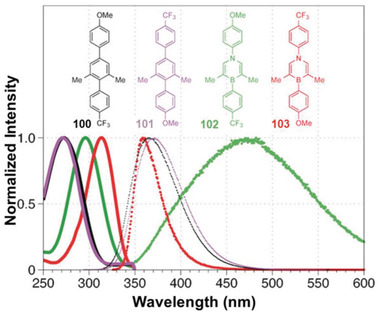
Normalized absorption (solid lines) and emission (dotted traces) spectra of 1,4‐azaborines 102 (green) and 103 (red) and their carbonaceous analogs 100 (black) and 101 (purple) in THF at 10 × 10‐6 m. Reproduced with permission.[ 19 ] Copyright 2016, Wiley‐VCH.
3.2. Bringing Excellent Chiroptical Properties
In recent years, helicene‐based materials have shown great potential in the field of chiral optoelectronic devices, for which significant chiroptical responses in the visible region with high dissymmetry factors are desirable.[ 43 ] Up to date, only very few helicenes exhibit dissymmetry factors up to 10−2 in the visible region, calling for new designs of helicene‐based chiroptical materials. In 2021, Wang and co‐workers reported the synthesis and chiroptical properties of N,B,N‐type 1,4‐azaborine‐embedded double [7]helicenes 69 (Figure 4a).[ 31 ] The synthetic route to compounds 69 was illustrated in Section 2.3. Compounds 69 exhibited excellent chiral stability with the isomerization barrier of 69a up to 67.52 kcal mol‐1. Each pair of enantiomers of 69a–c was successfully separated by chiral high‐performance liquid chromatography (HPLC) and their chiroptical properties were characterized. The circular dichroism (CD) spectra of compounds 69a–c covered the ultraviolet and the whole visible regions (from 300 to 700 nm) with the maximum absorption dissymmetry factors (|g abs|) reaching 0.033 at 502 nm, representing the highest value for helicene molecules in the visible range (Figure 4b–d).
Figure 4.
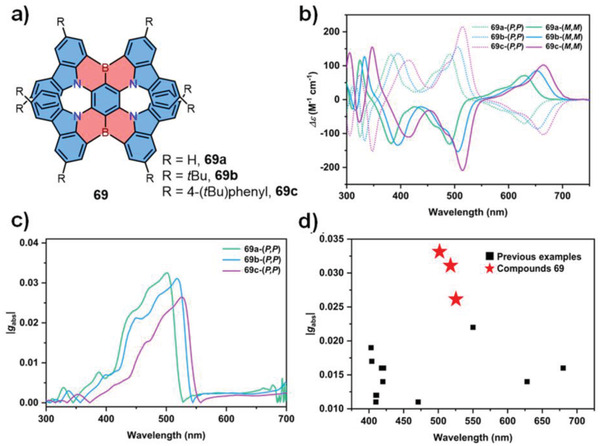
a) Chemical structures of compounds 69a–c. b) Circular dichroism spectra of 69a–c in dichloromethane solution (1 × 10–5 m). c) The corresponding |g abs| of (P,P)‐configuration calculated according to the equation g abs = Δε/ε. d) Summary of the |g abs| values of the reported helicenes and compounds 69a–c. Reproduced with permission.[ 31 ] Copyright 2021, American Chemical Society.
Theoretical calculations revealed that the transition with the largest |g abs| was assigned to the S0→S2 transition (HOMO→LUMO+1). For small organic molecules, the dissymmetry factors can be determined by the simplified equation g = 4cosθ| m |/| µ |. Therefore, larger | m | and cosθ as well as smaller | µ | can lead to higher g values. In compounds 69, the well‐separated molecular orbital distributions between HOMO and LUMO+1 were observed owing to the opposite resonance effect of boron and nitrogen in the 1,4‐azaborine cores, resulting in lower electric transition dipole moments | µ | for the S0→S2 transition. Meanwhile, the |cosθ| equaled 1 for all cases. As a result, the incorporation of 1,4‐azaborine core in helicenes 69 was helpful for their high |g abs|. In addition, strong intramolecular donor–acceptor interactions from the B and N atoms extended the absorption of light to the red region. Furthermore, compounds 69 displayed PLQYs up to 100%, and exhibited tunable circularly polarized luminescence (CPL) responses in the red/NIR region with the CPL brightness up to 40.0 M–1 cm–1, indicating the great potential of 69 as chiral optoelectronic materials. Given the excellent CPL performance of compounds 69 and their MR‐TADF characters, they might also function as promising candidates for CP‐OLED applications.
3.3. Applications As Novel Ligands for Metal Catalysis
In 2014, Liu and co‐workers reported the synthesis of 1,4‐azaborine‐embedded naphthalenes 17 (see Section 2.1).[ 14 ] Theoretical calculations revealed that the 1,4‐azaborine ring showed a substantial iminium resonance contribution, resulting in a significant donating feature of the C(3) atom (Scheme 17a). Furthermore, the electron‐rich C(3) atom together with the neighboring B atom promoted the formation of η2‐L‐type complex with Group 10 transition metals (e.g., Pt and Pd). The 1,4‐azaborine‐based complex stimulated the development of 1,4‐azaborine‐based phosphine ligand 17b for palladium‐catalyzed hydroboration of 1,3‐enyne, leading to preferable trans addition products. Given this preliminary result, Liu and co‐workers further investigated the selectivity of Pd‐catalyzed trans‐hydroboration reaction by using 1,4‐azaborines 27a and 27b as ligands (see Section 2.1 and Scheme 17b).[ 20a ] Under optimized conditions, 1,4‐azaborine 27a was found suitable for the hydroboration of terminal E‐1,3‐enyne with various electronically and sterically different substituents, affording trans‐products with high yields (70–93%) and selectivity (94:6 to 98:2 dr). On the other hand, hydroboration of internal 1,3‐enyne remained a great challenge for common metal catalysts because that they required the presence of the terminal alkyne proton to operate the catalytic cycle.[ 44 ] Excitingly, highly efficient internal 1,3‐enyne hydroboration was achieved by using 1,4‐azaborine 27b. Generally, higher trans‐hydroboration selectivity was observed when R1 were aryl groups (86:14 to 98:2 dr) compared with alkyl groups (81:19 to 97:3 dr). Furthermore, small steric hindrance of R 3 was favorable for high trans‐hydroboration selectivity. Note that this reaction could be scaled up to gram scale without erosion of stereoselectivity. In addition to hydroboration reaction, Liu and co‐workers also developed two 1,4‐azaborine‐based Senphos ligands 104 and 105, which displayed tremendous advantages in Pd‐catalyzed trans‐selective cyanoboration of 1,3‐enynes (Scheme 17c ).[ 20b ] In general, 1,4‐azaborines as ligands were favorable to form trans‐configuration in palladium‐catalyzed hydroboration and cyanoboration reactions, demonstrating the essence of developing 1,4‐azaborine‐based ligands for organic catalysis.
Scheme 17.
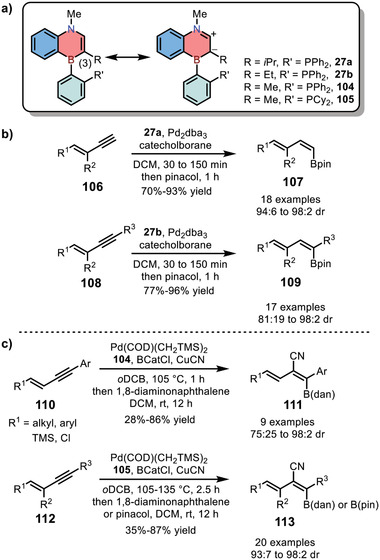
a) Chemical structures of 1,4‐azaborine‐based phosphine ligands. b) Pd‐catalyzed trans‐hydroboration and c) trans‐cyanoboration of 1,3‐enynes.
3.4. Precursors for Atomically Precise BN‐Doped Graphene Nanoribbons
Graphene nanoribbons (GNRs) are 1D graphene cutouts and have gained wide attention due to their potential applications as the next generation semiconductors.[ 45 ] Although BN‐doped graphene has been widely explored for optoelectronics and energy‐related applications, the synthesis of site‐specific BN‐doped GNRs with well‐defined structures have been a great challenge. In 2018, Kawai and co‐workers reported the bottom‐up synthesis of 1,4‐azaborine‐embedded armchair GNRs 116 and 119 (Scheme 18 ).[ 46 ] Precursor 114 was designed and synthesized from N‐(2‐bromophenyl)‐10‐chloro‐N‐phenylanthracen‐9‐amine. After deposition onto a Au(111) surface, the monomer underwent polymerization upon heating (200 °C) and then cyclodehydrogenation at a higher temperature (400 °C) to give GNRs. Because of the asymmetric character of the 1,4‐azaborine core, two different segments of GNRs were formed successfully via two different reaction paths. According to theoretical calculations, the GNRs lay on the gold surface with the relative adsorption height of −2 pm for boron and +4 pm for nitrogen. Therefore, the elemental character of 1,4‐azaborine cores was directly resolved by non‐contact atomic force microscopy (nc‐AFM). Such an elemental difference was correlated to the van der Waals radii as well as the local electron density. This elemental‐sensitive measurement marked an important step in the analysis of functionalized 2D carbon materials. This work indicated that sophisticated BN‐doped graphene nanostructures could be obtained through the bottom‐up precision synthesis by using rationally designed BN‐heteroarene precursors.
Scheme 18.
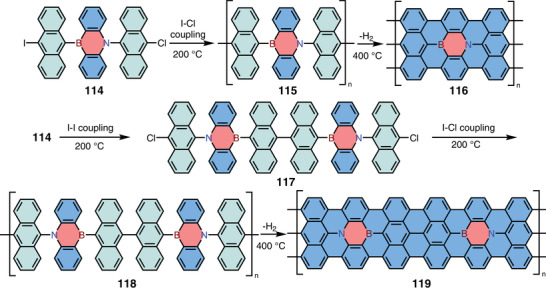
On‐surface synthesis of 1,4‐azaborine‐embedded GNRs.
3.5. Severing As Conducting Materials in Single‐Molecule Devices
Since the first single‐molecule conductance measurement by Reed and co‐workers in 1997,[ 47 ] a great number of π‐conjugated organic molecules have been employed as functional components for the development of single‐molecule devices.[ 48 ] Among them, heteroatom‐containing molecules have attracted wide attention due to their unique electronic structures as well as tunable electron transport properties.[ 49 ] However, there are rare examples that the electrons pass directly through the heterocycles. In 2021, González and co‐workers selected 1,4‐azaborines 121–122 as models, and studied their single‐molecule charge transport properties (Figure 5a).[ 50 ] The 1,4‐azaborine core was embedded in the center of the skeleton, and the linking groups were strategically located at the 1,4‐azaborine core, ensuring that the electron went through the fully conjugated 1,4‐azaborine ring.
Figure 5.
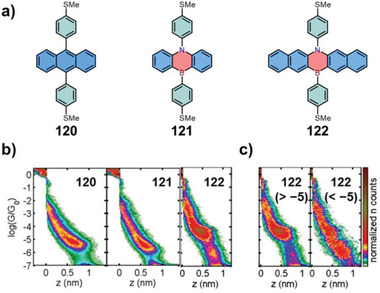
a) Chemical structures of compounds 120–122. b) 2D histograms for compounds 120–122. c) Independent 2D histograms of two separated groups for 122: traces without and with plateaus below log(G/G 0) = −5, respectively. Reproduced with permission.[ 50 ] Copyright 2021, Wiley‐VCH.
The synthesis of compounds 121 and 122 was achieved via the nucleophilic substitution method (Section 2.2). The single‐molecule conductance (G) measurements of 1,4‐azaborine 121 and its carbon analog 120 were performed firstly for understanding the effect of 1,4‐azaborine ring on the electron transport properties (Figure 5b). Both compounds exhibited a similar shape of conductance cloud with a broad and sloping conductance signal located between log(G/G 0) = −5 and −6. Meanwhile, a slight shift towards lower values was observed for 121. The Gaussian fitting of the conductance peaks revealed that the log(G/G 0) values were −5.3 ± 0.31 for 120 and −5.6 ± 0.33 for 121, respectively. The similar log(G/G 0) value of these two compounds demonstrated that the 1,4‐azaborine core not only permitted the electron transport through the skeleton, but also resulted in a minimum decrease of the conductance compared with that of the all‐carbon structure. Therefore, 1,4‐azaborines could be used as alternatives for investigating the single‐molecule conductance of their air‐sensitive carbon analogs. Then they further tested the single‐molecule conductance of the 1,4‐azaborine‐based pentacene 122. The 2D histogram of 122 displayed a well‐defined flat cloud of high conductance (80% of the total traces) at around log(G/G 0) = −4.5 as well as a less clear conductance cloud (20% of the total traces) below log(G/G 0) = −5. Nevertheless, a linker‐to‐linker configuration of the high conductance for 122 was excluded because of the short plateau length. Furthermore, the independent 2D histograms of two separated groups showed that the direct interaction of the electrodes with the central 1,4‐azaborine‐based pentacene unit was responsible for the observed high‐conductance plateaus for 122 (Figure 5c). Finally, the typical conductance of 122 was determined to be log(G/G 0) = −5.9 ± 0.40, comparable to compound 121, indicating that the lateral extension of the central 1,4‐azaborine had little effect on the conductance. This work provided a novel strategy for investigating the single‐molecule conductance of larger acenes by incorporating 1,4‐azaborine moieties.
3.6. Applications in MR‐TADF OLEDs
Recently, 1,4‐azaborine‐based PAHs have gained enormous attention owing to their intriguing advantages in MR‐TADF OLEDs. Due to the opposite resonance effect of boron and nitrogen atoms, 1,4‐azaborines can significantly separate HOMO and LUMO at different positions without introducing donor (D) or acceptor (A) groups. Compared with traditional DA‐type TADF systems, MR‐TADF materials usually reveal small reorganization energy to facilitate high radiative decay rates and low vibration frequency to suppress the nonradiative transitions, and thus exhibit stronger absorption oscillator strengths, smaller FWHMs, and higher PLQYs.[ 3e ] In this section, we do not aim at a comprehensive summary of the relevant advance, as there have been several reviews focusing on MR‐TADF emitters.[ 2 , 3 – e ] We hope to provide an overview of the reported structures as well as the device performance to shed light on the structure–property relationships of 1,4‐BN‐heteroarenes for high‐performance OLEDs. The structures that have been successfully employed for MR‐TADF OLED devices are summarized in Figure 6 . The performances of representative OLED devices employing MR‐TADF emitters are summarized in Figure 7 .
Figure 7.
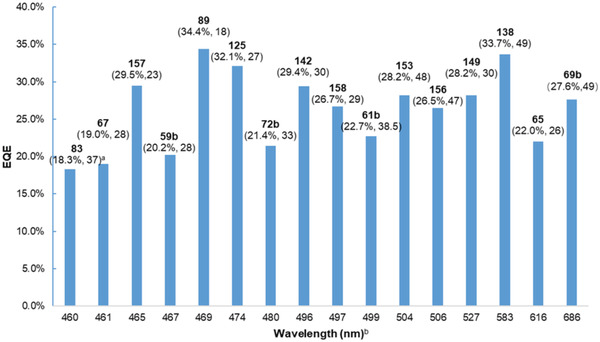
Representative device performances of previously reported MR‐TADF compounds. a) Maximum EQE and FWHM (nm) of the electroluminescence; b) Maximum electroluminescence wavelength.
In 2016, Hatakeyama and co‐workers reported the first N,B,N‐type 1,4‐azaborine‐based MR‐TADF materials 59a and 59b.[ 15 ] OLED devices based on these two compounds both exhibited ultrapure blue emission (λ max: 467 nm, FWHM: 28 nm for both two compounds) with high EQEs up to 20.2%. Although the device still suffered from serious efficiency roll‐off, this novel molecular design strategy offered an effective way to realize narrowband emission with promising device performance. To improve the EQE of N,B,N‐type 1,4‐azaborines as blue emitters, in 2018, Huang and co‐workers developed a carbazolyl‐substituted 1,4‐azaborine 125.[ 51 ] According to theoretical calculations, the introduction of peripheral carbazolyl group could effectively enhance MR‐effect and promote the radiative decay efficiency, and thus resulted in 125 with low efficiency roll‐off, an ultrahigh EQE (32.1%), and a small FWHM (27 nm). Furthermore, the potential of 125 as a host material for a yellow phosphorescent emitter was also demonstrated, and the device showed a significantly reduced turn‐on voltage of 3.2 V and a high EQE of 22.2%. Up to date, a variety of electron‐rich (or electron‐withdrawing) groups have been introduced onto this conjugated skeleton to optimize the device performance (Figure 6).[ 37 , 52 ]
Interestingly, in this V‐shaped skeleton, the number and positions of boron as well as nitrogen atoms showed limited effect on the emission wavelength. For example, in 2019, Hatakeyama and co‐workers reported B,N,B‐type 1,4‐azaborines 72 and investigated their potential as MR‐TADF materials.[ 32 ] Although compounds 72 were composed of two electron‐withdrawing boron atoms and only one electron‐rich nitrogen compared with the previously reported 1,4‐azaborines 59, only a slightly bathochromic shift in the emission spectra was observed with the maximum at 481 nm and 480 nm for 72a and 72b, respectively.
In order to achieve long‐wavelength emission of MR‐TADF materials, in 2019, Zhang and co‐workers developed a new carbazole‐fused backbone 61a and studied the influence of peripheral units, achieving narrowband green emission successfully.[ 27 ] Based on theoretical calculations, they found that a more delocalized HOMO/LUMO distribution and a slightly reduced energy gap (from 3.66 to 3.42 eV) were observed by replacing diphenylamine moieties in 59 with carbazole units. The bathochromic shift was further boosted by introducing electron‐withdrawing groups at the para position of boron, giving compounds 61a with an enhanced ICT character and reduced energy gaps (around 3.30 eV) for green emission without compromising the color fidelity, whereas the electron‐rich substituent displayed very limited modulating effect. 1,4‐BN‐heteroarenes 61a represented the first narrowband green MR‐TADF materials with the emission peaks of 493–501 nm, FWHMs of 32–40 nm and high EQEs up to 22.7%. In recent years, a series of carbazole‐fused 1,4‐azaborines were developed following the similar strategy, showing good color purity and device performance (Figure 6).[ 28 , 29 , 37 , 53 ] In 2020, Duan and co‐workers fused aza‐aromatics onto the 1,4‐azaborine framework and reported a pure‐green MR‐TADF material 149.[ 54 ] The target 1,4‐azaborine 149 showed nearly pure‐green emission with a relatively sharp peak at 522 nm, an FWHM of 28 nm, and an extremely high PLQY of 99.7%. The OLED devices employing 149 as the emitter exhibited a maximum EQE and power efficiency of 28.2% and 121.7 lm W−1, respectively, with an FWHM of merely 30 nm and Commission Internationale de l'Eclairage (CIE) coordinate y of 0.69.
In 2021, Li and co‐workers introduced chiral (R/S)‐octahydro‐binaphthol ((R/S)‐OBN) units into the carbazole‐fused 1,4‐BN‐heteroarene backbone and reported the first green MR‐TADF materials 142 and 143 with CPL signals.[ 53i ] CP‐OLEDs based on 142 and 143 exhibited green emissions with the maximum at 496 and 508 nm, narrow FWHMs of 30 nm and 33 nm, excellent EL performances with EQEs of 29.4% and 24.5%, and EL dissymmetry factors (g EL) of +1.43 × 10−3/−1.27 × 10−3 and +4.60 × 10−4/−4.76 × 10−4, respectively.
In addition to incorporating pendent groups onto the 1,4‐BN‐heteroarene core, expanding the conjugated plane of the rigid framework has also been demonstrated as a useful strategy to modulate their luminescent properties. In 2018, Hatakeyama and co‐workers synthesized three kinds of multiple 1,4‐azaborine‐embedded nanographenes 83–85 and demonstrated the potential of 83 as a blue MR‐TADF emitter (λ max: 460 nm, FWHM: 37 nm, EQE: 18.3%).[ 36 ] In 2021, they also reported a series of carbazole‐fused 1,4‐azaborines including 141 and 158 via the same strategy (Scheme 13b).[ 37 ] The OLED devices employing 141 and 158 as emitters exhibited narrowband sky‐blue (λ max: 477 nm, FWHM: 27 nm) and green (λ max: 497 nm, FWHM: 29 nm) emissions with high EQEs of 21.8% and 26.7%, respectively.
In 2019, Hatakeyama and co‐workers developed a π‐extended linear MR‐TADF material 89.[ 38 ] The vibronic coupling of 89 between the ground state (S0) and the singlet excited state (S1) as well as the energy gap between the S1 and the triplet excited state (T1) was minimized via the MR effect. OLED devices exhibited deep‐blue emission (λ max: 469 nm) with a FWHM of only 18 nm and an ultra‐high EQE up to 34.4%. In 2022, they further developed an expanded structure 91 based on the similar strategy.[ 39 ] The resonating π‐extension of 91 minimized the singlet–triplet energy gap and enabled rapid reverse intersystem crossing (RISC) with a rate constant (k RISC) of 4.4 × 105 s−1. The solution‐processed OLED devices employing 91 as an emitter exhibited intense sky‐blue emission peaking at 480 nm with an FWHM of 27 nm and a high EQE of 22.9%. In the same year, Kwon and co‐workers reported 1,4‐azaborines 159–161 by incorporating methyl groups or fluorine atoms on the framework of 89.[ 55 ] OLED devices employing 161 as the emitter achieved a high EQE up to 33.7%, CIE coordinates of (0.13, 0.06), and a narrow FWHM of 18 nm.
In 2021, Zhang and co‐workers reported 1,4‐BN‐heteroarenes 69a and 69b as the first deep‐red MR‐TADF emitters.[ 30 ] The 1,4‐azaborine cores were introduced to fundamentally overcome the luminescent boundary set by the “energy gap law”. The nonradiative transitions were greatly eliminated by the shallow potential energy surfaces due to the MR effect. The resulting heteroarenes 69a and 69b exhibited highly efficient deep‐red emission with PLQYs up to 100% and the maximum at 664 and 686 nm, respectively. OLED devices based on 69a and 69b showed small FWHMs of 48 nm and 49 nm and unprecedented high EQEs of 28.1% and 27.6%, respectively.
Recently, embedding 1,4‐azaborine cores in PAHs together with other heteroatoms (e.g., oxygen or sulfur) was found effective to modulate their emission properties.[ 56 ] In 2020, Hatakeyama and co‐workers designed a π‐extended pure‐green MR‐TADF material 150 by fusing a 1,4‐B,O six‐membered ring on the well‐studied 1,4‐azaborine core.[ 57 ] Furthermore, to realize solution‐processed OLED devices, two novel conjugated polymers with similar ionization potentials (5.61 and 5.77 eV) were also synthesized to facilitate charge recombination. The resulting devices fabricated with 150 showed green emission with the maximum at 506 nm, the CIE coordinate of (0.12, 0.63), an FWHM of 33 nm, and a high EQE up to 21.8%.
In 2021, Kido and co‐workers developed two green MR‐TADF materials 151 and 152 by inserting sp3‐carbon or oxygen into the skeleton.[ 58 ] The helical configuration of these two compounds could effectively suppress the aggregation‐induced fluorescence quenching, resulting in OLED devices with high EQEs of 20.3% and 23.3%, and FWHMs of 49 and 47 nm, respectively. Notably, MR‐TADF materials 151 and 153 with sp3‐carbon bridge were also reported by Yang and co‐workers independently in the same year.[ 59 ] The nonplanar framework in these two compounds significantly boosted the RISC process, resulting in superior device performances (EQEs up to 28.2%) without utilizing any sensitizer. Furthermore, the introduction of bulkier bis(acridan)phenylene groups in 153 were proved effective to boost the efficiency and alleviate roll‐off behavior (1.8% at 100 cd m−2).
In 2021, Yasuda and co‐workers developed MR‐TADF material 154 with 1,4‐B,S six‐membered rings showing sky‐blue emission (λ max: 478 nm, FWHM: 25 nm).[ 60 ] Through incorporation of two sulfur atoms, the spin–orbit coupling was effectively enhanced, which facilitated the spin‐flipping process between the excited triplet and singlet states, resulting in EQEs up to 21.0 %.
In 2021, Hatakeyama and co‐workers proposed an ultrapure deep‐blue MR‐TADF material 157 by replacing one nitrogen atom in 89 with an oxygen atom.[ 61 ] As compared with 89, OLED devices based on 157 exhibited a hypochromic‐shifted emission maximum at 465 nm, an increased FWHM of 23 nm, a decreased EQE of 29.5 %, a lower efficiency roll‐off (0.4%, 0.7%, and 2.6% at 10, 100, and 1000 cd m−2, respectively), and about 10 times longer device lifetime (LT50 = 314 and 31 h at 100 cd m−2,) because of the restricted π‐conjugation effect. In the same year, Yasuda and co‐workers introduced chalcogen (oxygen or sulfur) atoms into the same skeleton of 89 and developed three kinds 1,4‐azaborine‐based MR‐TADF emitters 167, 168, and 169.[ 62 ] Photophysical characterizations in conjunction with theoretical calculations demonstrated that introducing chalcogen (oxygen and sulfur) atoms can finely modulate the emission color while maintaining the narrow bandwidth (FWHM: 18–23 nm). The OLED devices based on these materials exhibited ultrapure blue EL emissions (λ max:445 to 463 nm) with CIE y coordinates in the range 0.04 to 0.08. Furthermore, the ultra‐fast RISC rate (k RISC ≈ 106 s−1) was observed in the sulfur‐doped compounds 168 and 169, which contributed to the high EQEs up to 26.9% and 26.8%, respectively, as well as the small efficiency roll‐offs.
In 2022, Lu and co‐workers incorporated one sulfur atom into the well‐studied backbone 136 and reported the asymmetric MR‐TADF material 174.[ 63 ] According to theoretical calculations, the sulfur atom in 174 can effectively enhance spin–orbit coupling (SOC) via the heavy atom effect, leading to fast k RISC over 1.0 × 105 s−1. The OLED devices employing 174 as emitters exhibited pure green emission (λ max: 516 nm) with high EQEs up to 32.8% without utilizing any sensitizer. Moreover, the devices sufficiently maintained high EQEs of over 23.5% under 1000 cd m−2, representing the highest value for all reported green MR‐TADF materials at the same luminescence.
In addition to the planar MR‐TADF materials, in 2021, Chou and co‐workers reported the helical MR‐TADF materials 155 and 156 for CP‐OLEDs. The asymmetric skeleton was composed of one sp3‐carbon bridge and a 1,4‐SN six‐membered ring.[ 64 ] Theoretical calculations revealed that the chiral moiety of two compounds directly participated in the frontier molecular orbitals (FMOs). The enantiomers of 155 and 156 were separated and employed in CP‐OLED devices, which displayed narrow FWHMs of 49/50 and 48/47 nm, high EQEs of 20.6%/19.0% and 22.0%/26.5%, and g EL of +3.7 × 10−3/−3.1 × 10−3 and +1.9 × 10−3/−1.6 × 10−3, respectively.
In recent years, modification of the 1,4‐azaborine core and the peripheries also provided opportunities to tune the emission color of MR‐TADF materials in a wide wavelength region. In 2020, Yasuda and co‐workers reported a series of 1,4‐azaborine‐based MR‐TADF materials 63, 65, 137, and 139.[ 28 ] These compounds allowed the systematic color‐tuning with narrowband emissions (FWHM: 26–54 nm) to cover the entire visible range, ranging from deep‐blue (63, λ max: 469 nm) to green (137, λ max: 515 nm) and to red (65, λ max: 616 nm). OLED devices employing these compounds as emitters exhibited high EQEs of 29.3% for 63, 31.8% for 137, 29.3% for 139, and 22.0% for 65, respectively. In 2021, the same group provided a facile strategy to modulate emission colors and synthesized 1,4‐azaborines 67, 134, and 135 with high PLQYs and color purity.[ 29 ] By introducing the electron‐withdrawing imine and electron‐donating amine moieties into the reported skeleton of 1,4‐azaborine 128, the resulting materials showed systematic hypochromic and bathochromic shifts in the emission spectra with the color ranging from deep‐blue (67, λ max: 461 nm) to green (134, λ max: 515 nm) and to yellow (135, λ max: 571 nm). OLED devices employing these compounds as emitters exhibited high EQEs of 19.0% for 67, 29.2% for 134, and 19.6% for 135, respectively.
4. Conclusions and Perspectives
In this review, we have discussed the synthetic strategies, the intriguing properties and promising applications of 1,4‐azaborine‐embedded conjugated materials. The synthetic strategies for air‐stable 1,4‐BN‐heteroarenes are generally divided into the following three types: 1) transition‐metal‐catalyzed reaction, 2) nucleophilic substitution, and 3) direct electrophilic borylation. The first two strategies are mainly used for constructing monocyclic and linear 1,4‐BN‐heteroarenes, whereas the third strategy has been widely utilized to build 1,4‐BN‐PAHs with various architectures. Meanwhile, the value of incorporating 1,4‐azaborine moieties in materials has been summarized, including the modulating effect on photophysical and chiroptical properties, the roles as novel ligands for metal catalysis and as precursors for atomically precise BN‐doped graphene nanoribbons, and the applications in MR‐TADF OLEDs. These advances demonstrate a bright future of 1,4‐azaborine‐based materials.
Despite the significant achievements, there are remaining challenges and opportunities that should draw attention in the future. Firstly, most of the 1,4‐BN‐heteroarenes are built up via the electrophilic borylation strategy, which still bears certain disadvantages, such as harsh reaction conditions (usually under high temperatures) and low regioselectivity. These drawbacks somewhat hamper the diversity of 1,4‐BN‐heteroarenes. Hence, it is still necessary to develop new facile strategies for constructing 1,4‐azaborine‐based PAHs with novel conjugated skeletons. Second, although 1,4‐BN‐heteroarenes have been widely explored as MR‐TADF materials, the emission wavelength only covers the region from 460 to 692 nm, and especially, NIR emission based on 1,4‐BN‐heteroarenes has remained elusive. Therefore, there is still a broad space to tune the molecular properties of MR‐TADF materials in the future. Finally, in recent five years, 1,4‐BN‐heteroarenes have mainly been used for MR‐TADF OLEDs, whereas only few other applications have been reported. The applications of 1,4‐BN‐heteroarenes beyond OLEDs should be further explored given their unique properties. We believe that this review would stimulate more interest in the synthetic chemistry of 1,4‐azaborines and promote the future development of 1,4‐azaborine‐based materials with deeper understanding of the structure‐property relationships.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to the financial support from the National Natural Science Foundation of China (Nos. 21901128 and 22071120), the National Key R&D Program of China (2020YFA0711500), and the Fundamental Research Funds for the Central Universities.
Biographies
Cheng Chen received his B.Sc. degree from Suzhou University of Science and Technology in 2017. In 2020, he completed his Master degree at East China University of Science and Technology. In the same year, he moved to Nankai University to pursue his Ph.D. degree under the supervision of Prof. Xiao‐Ye Wang. His research focuses on synthesis and properties of boron‐containing heteroarenes in the fields of optoelectronic and magnetic materials.

Cheng‐Zhuo Du received his B.Sc. degree from Hubei University in 2019. He is currently a Master student at Nankai University under the supervision of Prof. Xiao‐Ye Wang. His research interest mainly focuses on the design and synthesis of organic BN‐embedded conjugated materials and their optoelectronic applications.

Xiao‐Ye Wang received his B.Sc. degree from Nankai University in 2009, and obtained his Ph.D. degree from Peking University in 2014 under the supervision of Prof. Jian Pei. From 2014 to 2019, he was a postdoctoral researcher and a Humboldt Fellow in the group of Prof. Klaus Müllen at the Max Planck Institute for Polymer Research in Germany. In 2019, he moved to Nankai University to start his independent research as a professor of Chemistry. His research focuses on organic and nanocarbon materials for optoelectronic applications.

Chen C., Du C.‐Z., Wang X.‐Y., The Rise of 1,4‐BN‐Heteroarenes: Synthesis, Properties, and Applications. Adv. Sci. 2022, 9, 2200707. 10.1002/advs.202200707
Dedicated to Professor Klaus Müllen on the occasion of his 75th birthday
References
- 1.a) Wang X.‐Y., Yao X., Müllen K., Sci. China: Chem. 2019, 62, 1099; [Google Scholar]; b) Narita A., Wang X. Y., Feng X., Müllen K., Chem. Soc. Rev. 2015, 44, 6616; [DOI] [PubMed] [Google Scholar]; c) Wu J., Pisula W., Müllen K., Chem. Rev. 2007, 107, 718; [DOI] [PubMed] [Google Scholar]; d) Liu J., Feng X., Angew. Chem., Int. Ed. 2020, 59, 23386; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wang C., Dong H., Hu W., Liu Y., Zhu D., Chem. Rev. 2012, 112, 2208; [DOI] [PubMed] [Google Scholar]; f) Mei J., Diao Y., Appleton A. L., Fang L., Bao Z., J. Am. Chem. Soc. 2013, 135, 6724. [DOI] [PubMed] [Google Scholar]
- 2.a) Stępień M., Gońka E. b., Żyła M., Sprutta N., Chem. Rev. 2017, 117, 3479; [DOI] [PubMed] [Google Scholar]; b) Hirai M., Tanaka N., Sakai M., Yamaguchi S., Chem. Rev. 2019, 119, 8291; [DOI] [PubMed] [Google Scholar]; c) Wang X.‐Y., Yao X., Narita A., Müllen K., Acc. Chem. Res. 2019, 52, 2491; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Liu J., Feng X., Angew. Chem., Int. Ed. 2020, 59, 23386; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Borissov A., Maurya Y. K., Moshniaha L., Wong W. S., Żyła‐Karwowska M., Stępień M., Chem. Rev. 2022, 122, 565; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Campbell P. G., Marwitz A. J. V., Liu S.‐Y., Angew. Chem., Int. Ed. 2012, 51, 6074; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Teng J.‐M., Wang Y.‐F., Chen C.‐F., J. Mater. Chem. C 2020, 8, 11340; [Google Scholar]; h) Giustra Z. X., Liu S. Y., J. Am. Chem. Soc. 2018, 140, 1184; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Huang J., Li Y., Front. Chem. 2018, 6, 341; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Biagiotti G., Perini I., Richichi B., Cicchi S., Molecules 2021, 26, 6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Wang X.‐Y., Wang J.‐Y., Pei J., Chem. ‐ Eur. J. 2015, 21, 3528;25469827 [Google Scholar]; b) Wang J.‐Y., Pei J., Chin. Chem. Lett. 2016, 27, 1139; [Google Scholar]; c) Hall D., Suresh S. M., dos Santos P. L., Duda E., Bagnich S., Pershin A., Rajamalli P., Cordes D. B., Slawin A. M. Z., Beljonne D., Köhler A., Samuel I. D. W., Olivier Y., Zysman‐Colman E., Adv. Opt. Mater. 2019, 8, 1901627; [Google Scholar]; d) Kothavale S. S., Lee J. Y., Adv. Opt. Mater. 2020, 8, 2000922; [Google Scholar]; e) Madayanad Suresh S., Hall D., Beljonne D., Olivier Y., Zysman‐Colman E., Adv. Funct. Mater. 2020, 30, 1908677. [Google Scholar]
- 4. Stock A., Pohland E., Ber. Dtsch. Chem. Ges. 1926, 59, 2210. [Google Scholar]
- 5.a) Kranz M., Clark T., J. Org. Chem. 1992, 57, 5492; [Google Scholar]; b) Baranac‐Stojanović M., Chemistry 2014, 20, 16558. [DOI] [PubMed] [Google Scholar]
- 6.a) Dewar M. J. S., Kubba V. P., Pettit R., J. Chem. Soc. 1958, 3073; [Google Scholar]; b) Dewar M. J. S., Dietz R., J. Chem. Soc. 1959, 2728; [Google Scholar]; c) Dewar M. J. S., Kaneko C., Bhattacharjee M. K., J. Am. Chem. Soc. 1962, 84, 4884; [Google Scholar]; d) Dewar M. J. S., Marr P. A., J. Am. Chem. Soc. 1962, 84, 3782; [Google Scholar]; e) Dewar M. J. S., Grisdale P. J., J. Org. Chem. 1963, 28, 1759; [Google Scholar]; f) Dewar M. J. S., Poesche W. H., J. Am. Chem. Soc. 1963, 85, 2253; [Google Scholar]; g) Davies K. M., Dewar M. J. S., Rona P., J. Am. Chem. Soc. 1967, 89, 6294; [Google Scholar]; h) Dewar M. J. S., Jones R., J. Am. Chem. Soc. 1968, 90, 2137. [Google Scholar]
- 7. Marwitz A. J., Matus M. H., Zakharov L. N., Dixon D. A., Liu S. Y., Angew. Chem., Int. Ed. 2009, 48, 973. [DOI] [PubMed] [Google Scholar]
- 8.a) Helten H., Chem. ‐ Eur. J. 2016, 22, 12972; [DOI] [PubMed] [Google Scholar]; b) Bélanger‐Chabot G., Braunschweig H., Roy D. K., Eur. J. Inorg. Chem. 2017, 2017, 4353; [Google Scholar]; c) McConnell C. R., Liu S. Y., Chem. Soc. Rev. 2019, 48, 3436; [DOI] [PMC free article] [PubMed] [Google Scholar]; Abengózar d) A., García‐García P., Fernández‐Rodríguez M. A., Sucunza D., Vaquero J. J., in Advances in Heterocyclic Chemistry, Vol. 135 (Eds: Scriven E. F. V., Ramsden C. A.), Academic Press, San Diego, 2021, p. 197; [Google Scholar]; e) Bhattacharjee A., Davies G. H. M., Saeednia B., Wisniewski S. R., Molander G. A., Adv. Synth. Catal. 2021, 363, 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu S., Zakharov L. N., Liu S. Y., J. Am. Chem. Soc. 2011, 133, 20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maitlis P. M., J. Chem. Soc. 1961, 425. [Google Scholar]
- 11. Kranz M., Hampel F., Clark T., J. Chem. Soc., Chem. Commun. 1992, 1247. [Google Scholar]
- 12.a) Agou T., Kobayashi J., Kawashima T., Org. Lett. 2006, 8, 2241; [DOI] [PubMed] [Google Scholar]; b) Agou T., Kobayashi J., Kawashima T., Chem. Commun. 2007, 3204; [DOI] [PubMed] [Google Scholar]; c) Agou T., Kojima T., Kobayashi J., Kawashima T., Org. Lett. 2009, 11, 3534; [DOI] [PubMed] [Google Scholar]; d) Agou T., Arai H., Kawashima T., Chem. Lett. 2010, 39, 612; [Google Scholar]; e) Agou T., Sekine M., Kobayashi J., Kawashima T., Chem. ‐ Eur. J. 2009, 15, 5056. [DOI] [PubMed] [Google Scholar]
- 13. Braunschweig H., Damme A., Jimenez‐Halla J. O., Pfaffinger B., Radacki K., Wolf J., Angew. Chem., Int. Ed. 2012, 51, 10034. [DOI] [PubMed] [Google Scholar]
- 14. Xu S., Haeffner F., Li B., Zakharov L. N., Liu S. Y., Angew. Chem., Int. Ed. 2014, 53, 6795. [DOI] [PubMed] [Google Scholar]
- 15. Hatakeyama T., Shiren K., Nakajima K., Nomura S., Nakatsuka S., Kinoshita K., Ni J., Ono Y., Ikuta T., Adv. Mater. 2016, 28, 2777. [DOI] [PubMed] [Google Scholar]
- 16. Hong W. P., Lim H. N., Shin I., Synthesis 2021, 54, 570. [Google Scholar]
- 17.a) Saito S., Yamamoto Y., Chem. Rev. 2000, 100, 2901; [DOI] [PubMed] [Google Scholar]; b) Heller B., Hapke M., Chem. Soc. Rev. 2007, 36, 1085. [DOI] [PubMed] [Google Scholar]
- 18. Schäfer M., Beattie N. A., Geetharani K., Schäfer J., Ewing W. C., Krahfuß M., Hörl C., Dewhurst R. D., Macgregor S. A., Lambert C., Braunschweig H., J. Am. Chem. Soc. 2016, 138, 8212. [DOI] [PubMed] [Google Scholar]
- 19. Liu X., Zhang Y., Li B., Zakharov L. N., Vasiliu M., Dixon D. A., Liu S. Y., Angew. Chem., Int. Ed. 2016, 55, 8333. [DOI] [PubMed] [Google Scholar]
- 20.a) Xu S., Zhang Y., Li B., Liu S. Y., J. Am. Chem. Soc. 2016, 138, 14566; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Y., Li B., Liu S. Y., Angew. Chem., Int. Ed. 2020, 59, 15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Igarashi T., Tobisu M., Chatani N., Angew. Chem., Int. Ed. 2017, 56, 2069. [DOI] [PubMed] [Google Scholar]
- 22. Wu T. L., Lo S. H., Chang Y. C., Huang M. J., Cheng C. H., ACS. Appl. Mater. Interfaces 2019, 11, 10768. [DOI] [PubMed] [Google Scholar]
- 23. Nagata M., Oshiro T., Mizuhata Y., Tokitoh N., Hosoya T., Yamada S., Konno T., Fukumoto H., Kubota T., Agou T., Bull. Chem. Soc. J. 2021, 94, 21. [Google Scholar]
- 24. Ishikawa Y., Suzuki K., Hayashi K., Nema S. Y., Yamashita M., Org. Lett. 2019, 21, 1722. [DOI] [PubMed] [Google Scholar]
- 25. Chinnapattu M., Sathiyanarayanan K. I., Iyer P. S., RSC Adv. 2015, 5, 37716. [Google Scholar]
- 26. Iqbal S. A., Pahl J., Yuan K., Ingleson M. J., Chem. Soc. Rev. 2020, 49, 4564. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., Zhang D., Wei J., Liu Z., Lu Y., Duan L., Angew. Chem., Int. Ed. 2019, 58, 16912. [DOI] [PubMed] [Google Scholar]
- 28. Yang M., Park I. S., Yasuda T., J. Am. Chem. Soc. 2020, 142, 19468. [DOI] [PubMed] [Google Scholar]
- 29. Yang M., Shikita S., Min H., Park I. S., Shibata H., Amanokura N., Yasuda T., Angew. Chem., Int. Ed. 2021, 60, 23142. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y., Zhang D., Huang T., Gillett A. J., Liu Y., Hu D., Cui L., Bin Z., Li G., Wei J., Duan L., Angew. Chem., Int. Ed. 2021, 60, 20498. [DOI] [PubMed] [Google Scholar]
- 31. Li J. K., Chen X. Y., Guo Y. L., Wang X. C., Sue A. C., Cao X. Y., Wang X. Y., J. Am. Chem. Soc. 2021, 143, 17958. [DOI] [PubMed] [Google Scholar]
- 32. Oda S., Kawakami B., Kawasumi R., Okita R., Hatakeyama T., Org. Lett. 2019, 21, 9311. [DOI] [PubMed] [Google Scholar]
- 33. Knöller J. A., Meng G., Wang X., Hall D., Pershin A., Beljonne D., Olivier Y., Laschat S., Zysman‐Colman E., Wang S., Angew. Chem., Int. Ed. 2020, 59, 3156. [DOI] [PubMed] [Google Scholar]
- 34. Nakatsuka S., Gotoh H., Kinoshita K., Yasuda N., Hatakeyama T., Angew. Chem., Int. Ed. 2017, 56, 5087. [DOI] [PubMed] [Google Scholar]
- 35. Gotoh H., Nakatsuka S., Tanaka H., Yasuda N., Haketa Y., Maeda H., Hatakeyama T., Angew. Chem., Int. Ed. 2021, 60, 12835. [DOI] [PubMed] [Google Scholar]
- 36. Matsui K., Oda S., Yoshiura K., Nakajima K., Yasuda N., Hatakeyama T., J. Am. Chem. Soc. 2018, 140, 1195. [DOI] [PubMed] [Google Scholar]
- 37. Oda S., Kumano W., Hama T., Kawasumi R., Yoshiura K., Hatakeyama T., Angew. Chem., Int. Ed. 2021, 60, 2882. [DOI] [PubMed] [Google Scholar]
- 38. Kondo Y., Yoshiura K., Kitera S., Nishi H., Oda S., Gotoh H., Sasada Y., Yanai M., Hatakeyama T., Nat. Photonics 2019, 13, 678. [Google Scholar]
- 39. Oda S., Kawakami B., Yamasaki Y., Matsumoto R., Yoshioka M., Fukushima D., Nakatsuka S., Hatakeyama T., J. Am. Chem. Soc. 2022, 144, 106. [DOI] [PubMed] [Google Scholar]
- 40. Suresh S. M., Duda E., Hall D., Yao Z., Bagnich S., Slawin A. M. Z., Bässler H., Beljonne D., Buck M., Olivier Y., Köhler A., Zysman‐Colman E., J. Am. Chem. Soc. 2020, 142, 6588. [DOI] [PubMed] [Google Scholar]
- 41. Mitsudo K., Shigemori K., Mandai H., Wakamiya A., Suga S., Org. Lett. 2018, 20, 7336. [DOI] [PubMed] [Google Scholar]
- 42. Zhang J. J., Yang L., Liu F., Fu Y., Liu J., Popov A. A., Ma J., Feng X., Angew. Chem., Int. Ed. 2021, 60, 25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.a) Gingras M., Felix G., Peresutti R., Chem. Soc. Rev. 2013, 42, 1007; [DOI] [PubMed] [Google Scholar]; b) Dhbaibi K., Favereau L., Crassous J., Chem. Rev. 2019, 119, 8846; [DOI] [PubMed] [Google Scholar]; c) Hasegawa M., Nojima Y., Mazaki Y., ChemPhotoChem 2021, 5, 1042. [Google Scholar]
- 44. Sasaki Y., Horita Y., Zhong C., Sawamura M., Ito H., Angew. Chem., Int. Ed. 2011, 50, 2778. [DOI] [PubMed] [Google Scholar]
- 45.a) Wang X., Ouyang Y., Li X., Wang H., Guo J., Dai H., Phys. Rev. Lett. 2008, 100, 206803; [DOI] [PubMed] [Google Scholar]; b) Li X., Wang X., Zhang L., Lee S., Dai H., Science 2008, 319, 1229; [DOI] [PubMed] [Google Scholar]; c) Jiao L., Wang X., Diankov G., Wang H., Dai H., Nat. Nanotechnol. 2010, 5, 321; [DOI] [PubMed] [Google Scholar]; d) Wang X. Y., Narita A., Müllen K., Nat. Rev. Chem. 2018, 2, 0100; [Google Scholar]; e) Ruffieux P., Wang S., Yang B., Sánchez‐Sánchez C., Liu J., Dienel T., Talirz L., Shinde P., Pignedoli C. A., Passerone D., Dumslaff T., Feng X., Müllen K., Fasel R., Nature 2016, 531, 489. [DOI] [PubMed] [Google Scholar]
- 46. Kawai S., Nakatsuka S., Hatakeyama T., Pawlak R., Meier T., Tracey J., Meyer E., Foster A. S., Sci. Adv. 2018, 4, eaar7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reed M. A., Zhou C., Muller C. J., Burgin T. P., Tour J. M., Science 1997, 278, 252. [Google Scholar]
- 48.a) Xiang D., Wang X., Jia C., Lee T., Guo X., Chem. Rev. 2016, 116, 4318; [DOI] [PubMed] [Google Scholar]; b) Ke G., Duan C., Huang F., Guo X., InfoMat 2019, 2, 92; [Google Scholar]; c) Xin N., Guan J., Zhou C., Chen X., Gu C., Li Y., Ratner M. A., Nitzan A., Stoddart J. F., Guo X., Nat. Rev. Phys. 2019, 1, 211; [Google Scholar]; d) Bai J., Li X., Zhu Z., Zheng Y., Hong W., Adv. Mater. 2021, 33, 2005883; [DOI] [PubMed] [Google Scholar]; e) Chen Y., Huang L., Chen H., Chen Z., Zhang H., Xiao Z., Hong W., Chin. J. Chem. 2021, 39, 421. [Google Scholar]
- 49.a) Miguel D., Álvarez de Cienfuegos L., Martín‐Lasanta A., Morcillo S. P., Zotti L. A., Leary E., Bürkle M., Asai Y., Jurado R., Cárdenas D. J., Rubio‐Bollinger G., Agraït N., Cuerva J. M., González M. T., J. Am. Chem. Soc. 2015, 137, 13818; [DOI] [PubMed] [Google Scholar]; b) Dell E. J., Capozzi B., Xia J., Venkataraman L., Campos L. M., Nat. Chem. 2015, 7, 209; [DOI] [PubMed] [Google Scholar]; c) Cai Z., Lo W.‐Y., Zheng T., Li L., Zhang N., Hu Y., Yu L., J. Am. Chem. Soc. 2016, 138, 10630; [DOI] [PubMed] [Google Scholar]; d) Ismael A. K., Wang K., Vezzoli A., Al‐Khaykanee M. K., Gallagher H. E., Grace I. M., Lambert C. J., Xu B., Nichols R. J., Higgins S. J., Angew. Chem., Int. Ed. 2017, 56, 15378; [DOI] [PubMed] [Google Scholar]; e) O'Driscoll L. J., Hamill J. M., Grace I., Nielsen B. W., Almutib E., Fu W. H. Y., Lambert C. J., Jeppesen J. O., Chem. Sci. 2017, 8, 6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palomino‐Ruiz L., Rodríguez‐González S., Fallaque J. G., Márquez I. R., Agraït N., Díaz C., Leary E., Cuerva J. M., Campaña A. G., Martín F., Millán A., González M. T., Angew. Chem., Int. Ed. 2021, 60, 6609. [DOI] [PubMed] [Google Scholar]
- 51. Liang X., Yan Z. P., Han H. B., Wu Z. G., Zheng Y. X., Meng H., Zuo J. L., Huang W., Angew. Chem., Int. Ed. 2018, 57, 11316. [DOI] [PubMed] [Google Scholar]
- 52.a) Kim J. H., Chung W. J., Kim J., Lee J. Y., Mater. Today Energy 2021, 21, 100792; [Google Scholar]; b) Wang Y., Di K., Duan Y., Guo R., Lian L., Zhang W., Wang L., Chem. Eng. J. 2022, 431, 133221; [Google Scholar]; c) Han S. H., Jeong J. H., Yoo J. W., Lee J. Y., J. Mater. Chem. C 2019, 7, 3082; [Google Scholar]; d) Wang Y., Duan Y., Guo R., Ye S., Di K., Zhang W., Zhuang S., Wang L., Org. Electron. 2021, 97, 106275. [Google Scholar]
- 53.a) Xu Y., Cheng Z., Li Z., Liang B., Wang J., Wei J., Zhang Z., Wang Y., Adv. Opt. Mater. 2020, 8, 1902142; [Google Scholar]; b) Xu Y., Li C., Li Z., Wang Q., Cai X., Wei J., Wang Y., Angew. Chem., Int. Ed. 2020, 59, 17442; [DOI] [PubMed] [Google Scholar]; c) Lee Y. T., Chan C. Y., Tanaka M., Mamada M., Balijapalli U., Tsuchiya Y., Nakanotani H., Hatakeyama T., Adachi C., Adv. Electron. Mater. 2021, 7, 2001090; [Google Scholar]; d) Liu Y., Xiao X., Ran Y., Bin Z., You J., Chem. Sci. 2021, 12, 9408; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Qi Y., Ning W., Zou Y., Cao X., Gong S., Yang C., Adv. Funct. Mater. 2021, 31, 2102017; [Google Scholar]; f) Xu S., Yang Q., Zhang Y., Li H., Xue Q., Xie G., Gu M., Jin J., Huang L., Chen R., Chin. Chem. Lett. 2021, 32, 1372; [Google Scholar]; g) Xu T., Liang X., Xie G., Front. Chem. 2021, 9, 691172; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Xu Y., Li C., Li Z., Wang J., Xue J., Wang Q., Cai X., Wang Y., CCS Chem. 2021, 3, 2077; [Google Scholar]; i) Xu Y., Wang Q., Cai X., Li C., Wang Y., Adv. Mater. 2021, 33, 2100652; [DOI] [PubMed] [Google Scholar]; j) Zhang Y., Wei J., Zhang D., Yin C., Li G., Liu Z., Jia X., Qiao J., Duan L., Angew. Chem., Int. Ed. 2022, 61, 202113206; [DOI] [PubMed] [Google Scholar]; k) Qiu Y., Xia H., Miao J., Huang Z., Li N., Cao X., Han J., Zhou C., Zhong C., Yang C., ACS Appl. Mater. Interfaces 2021, 13, 59035. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y., Zhang D., Wei J., Hong X., Lu Y., Hu D., Li G., Liu Z., Chen Y., Duan L., Angew. Chem., Int. Ed. 2020, 59, 17499. [DOI] [PubMed] [Google Scholar]
- 55. Rayappa Naveen K., Lee H., Braveenth R., Joon Yang K., Jae Hwang S., Hyuk Kwon J., Chem. Eng. J. 2022, 432, 134381. [Google Scholar]
- 56.a) Park J., Lim J., Lee J. H., Jang B., Han J. H., Yoon S. S., Lee J. Y., ACS Appl. Mater. Interfaces 2021, 13, 45798; [DOI] [PubMed] [Google Scholar]; b) Hua T., Zhan L., Li N., Huang Z., Cao X., Xiao Z., Gong S., Zhou C., Zhong C., Yang C., Chem. Eng. J. 2021, 426, 131169. [Google Scholar]
- 57. Ikeda N., Oda S., Matsumoto R., Yoshioka M., Fukushima D., Yoshiura K., Yasuda N., Hatakeyama T., Adv. Mater. 2020, 32, 2004072. [DOI] [PubMed] [Google Scholar]
- 58. Liu G., Sasabe H., Kumada K., Matsunaga A., Katagiri H., Kido J., J. Mater. Chem. C 2021, 9, 8308. [Google Scholar]
- 59. Jiang P., Zhan L., Cao X., Lv X., Gong S., Chen Z., Zhou C., Huang Z., Ni F., Zou Y., Yang C., Adv. Opt. Mater. 2021, 9, 2100825. [Google Scholar]
- 60. Nagata M., Min H., Watanabe E., Fukumoto H., Mizuhata Y., Tokitoh N., Agou T., Yasuda T., Angew. Chem., Int. Ed. 2021, 60, 20280. [DOI] [PubMed] [Google Scholar]
- 61. Tanaka H., Oda S., Ricci G., Gotoh H., Tabata K., Kawasumi R., Beljonne D., Olivier Y., Hatakeyama T., Angew. Chem., Int. Ed. 2021, 60, 17910. [DOI] [PubMed] [Google Scholar]
- 62. Park I. S., Yang M., Shibata H., Amanokura N., Yasuda T., Adv. Mater. 2022, 34, 2107951. [DOI] [PubMed] [Google Scholar]
- 63. Liu F., Cheng Z., Jiang Y., Gao L., Liu H., Liu H., Feng Z., Lu P., Yang W., Angew. Chem., Int. Ed. 2022, 10.1002/anie.202116927. [DOI] [PubMed] [Google Scholar]
- 64. Wu X., Huang J. W., Su B. K., Wang S., Yuan L., Zheng W. Q., Zhang H., Zheng Y. X., Zhu W., Chou P. T., Adv. Mater. 2022, 34, 2105080. [DOI] [PubMed] [Google Scholar]


