Abstract
The existing knowledge about morbidity in adults with Rubinstein-Taybi syndrome (RTS) is limited and detailed data on their natural history and response to management are needed for optimal care in later life. We formed an international, multidisciplinary working group that developed an accessible questionnaire including key issues about adults with RTS and disseminated this to all known RTS support groups via social media. We report the observations from a cohort of 87 adult individuals of whom 43 had a molecularly confirmed diagnosis. The adult natural history of RTS is defined by prevalent behavioural/psychiatric problems (83%), gastrointestinal problems (73%) that are represented mainly by constipation; and sleep problems (62%) that manifest in a consistent pattern of sleep apnoea, difficulty staying asleep and an increased need for sleep. Furthermore, over than half of the RTS individuals (65%) had skin and adnexa-related problems. Half of the individuals receive multidisciplinary follow-up and required surgery at least once, and most frequently more than once, during adulthood. Our data confirm that adults with RTS enjoy both social and occupational possibilities, show a variegated experience of everyday life but experience a significant morbidity and ongoing medical issues which do not appear to be as coordinated and multidisciplinary managed as in paediatric patients. We highlight the need for optimal care in a multidisciplinary setting including the pivotal role of specialists for adult care.
Subject terms: Genetics research, Prognosis
Introduction
In the present times progress in diagnostics, knowledge and management abilities allows us with increasing frequency to care for older individuals with genomic developmental disorders. One of the archetypical developmental disorders is Rubinstein-Taybi syndrome (RTS) [1], which is most characterised by intellectual disability, growth disturbances, abnormalities of the distal limbs and a typical face [2].
The literature on morbidity in adults with RTS is limited which highlights the need for more data on their natural history and management in later life. Hennekam et al. [3] report on the limited knowledge about the adult clinical presentation based on the lack of ascertainment of older individuals, and mention, for the first time, obstipation, upper and lower respiratory infections, mild allergic disorders, recurrent cystitis and sleep problems as specific problems of the RTS adulthood.
Stevens et al. [4] report that 17% of families experience difficulties in transitioning from paediatric to adult medical care. The changes over time in various organ systems during and after transition into adulthood that has been suggested in several publications [5–9] stress the importance of this information for optimal care.
Typically, gathering information on adults with developmental disorders is difficult as care is more fragmented and less coordinated among medical specialties, and controls are more irregular compared to care of children with these disorders by paediatricians. There may also be the perception by adult specialists that management of medical problems has already been offered in paediatric age and if not solved by adolescence, then there is little that can be done in adulthood. In addition, adult specialists are not as familiar with syndromic developmental disorders as paediatricians are. We reasoned that there are national support groups for families with RTS on social media and these represent a virtual meeting point to share experiences and information, also on adults. Therefore, we developed an accessible questionnaire about adults with RTS which was disseminated on relevant social media. We present here the analysis of the information collected this way and compare this to the pertinent literature.
Methods
We formed an international, multidisciplinary working group constituting eight participants in seven countries from Europe and North America. The group consisted of five clinicians, one scientist and two patient-group representatives. Discussions occurred via video conference calls, e-mail communications and file exchanges. We performed a systematic literature review using as MeSH term “Rubinstein-Taybi syndrome”, selected all publications with molecularly confirmed individuals, and evaluated publications for data on adult individuals with RTS. To identify key issues that should be included in the questionnaire we contacted all known support groups on RTS via e-mail and social media. In this way consensus was obtained about the content of the questionnaire.
Questionnaire
The questionnaire consisted of 61 questions structured across nine thematic areas: general information, gastrointestinal, skin, sleep, behavioural or psychiatric, other medical problems, surgery, everyday life, and medical follow-up. The type of question was tailored to the type of information requested and included demographic, multiple choice (with single or multiple options according to the context), ranking and open-ended questions (see Supplementary Information). The English questionnaire was translated by machine-translation (Deep-L©) and proofed by native language speakers in Dutch, French, German, Italian and Spanish. The questionnaire was disseminated in social media platforms (Facebook© and Twitter©) and as part of the UK RTS group regular newsletter with the specification that it was meant for families or individuals with RTS older than 16 years of age. Reminders for contribution were sent 1 month after dissemination. Answers were analysed using Excel© and simple frequency or percentage analysis. Free text comments which contributed additional information are presented in Supplementary Table 1.
Results
Demographic data
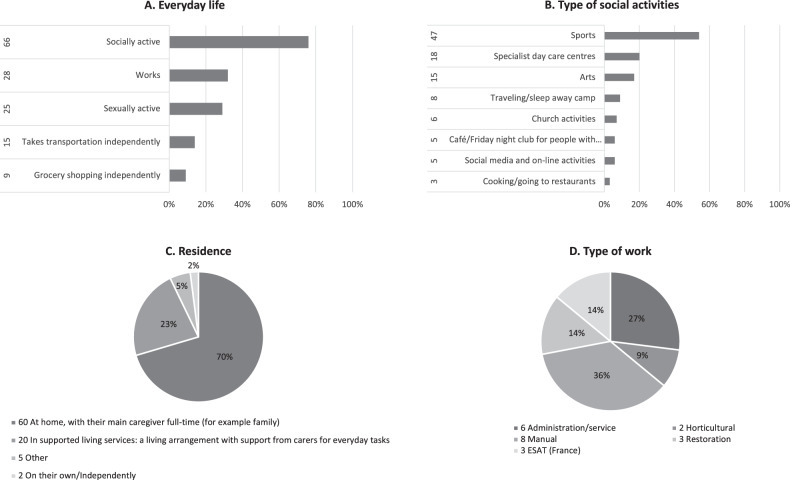
Completed questionnaires were returned from 87 responders living in 12 countries: the Netherlands, Belgium, USA, England, Canada, Northern Ireland, Spain, Puerto Rico, Venezuela, France, Italy, and Germany. All responders were first-degree family members of a reportedly affected individual. The gender distribution of the affected individuals was 40 women/47 men. The age distribution is shown in Supplementary Fig. S1A (median 26, average 37; the oldest, living, RTS individual was 54 years old whilst the oldest, molecularly confirmed one, was 50 years old). Further demographic data about diagnosis achieved by age and by genetic confirmation is illustrated in Supplementary Fig. S1A,B, respectively. The level of independence achieved in adulthood is explored in Fig. 1A while a description of their social activities and occupations are shown in Fig. 1B, D, respectively. Residential arrangements are illustrated in Fig. 1C.
Fig. 1. Adult RTS reported everyday life.
Adult RTS reported A Everyday life, B Type of social activities, C Residence and D Type of work.
Clinical presentation and medical management
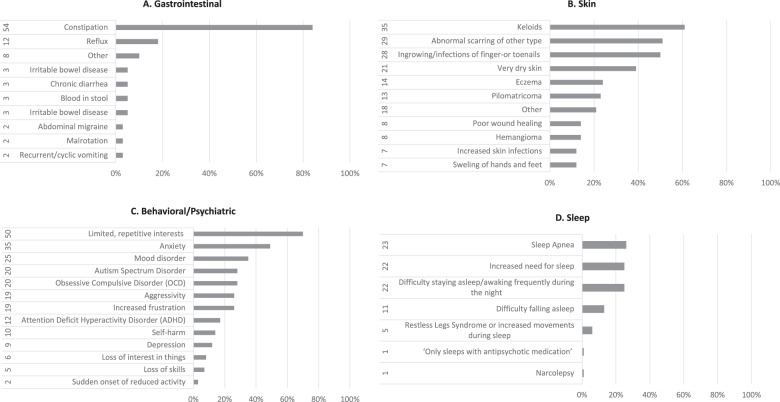
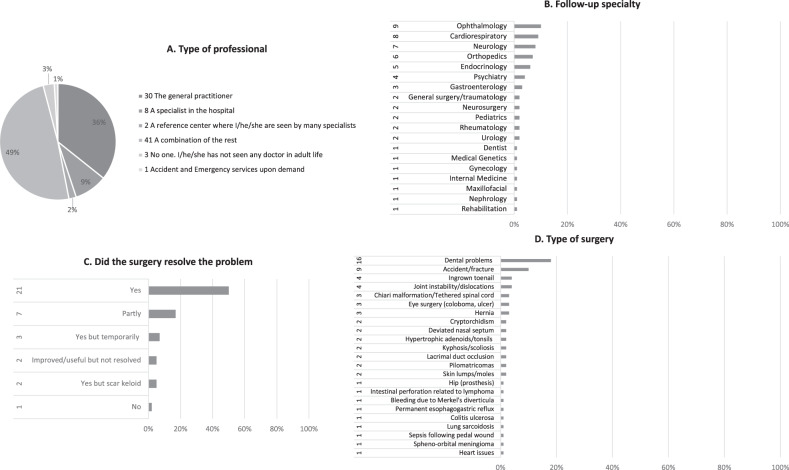
The responders contributed those medical problems which impacted the quality of life in adulthood for 74 individuals (85%): on thirteen occasions these affected one body system (17%) while for the rest (61/74, 82%) this involved multiple body systems. These were distributed in: 83% (72/87) behavioural/psychiatric problems, 73% (64/87) gastrointestinal, 65% (57/87) skin and 62% (54/87) sleep. The reported distribution of the major signs and symptoms for each of these systems is shown in Fig. 2. The patterns of the most frequent symptoms by body system (constipation, keloids and increased need for sleeping) are delineated in Table 1. In Fig. 3 responders classify the onset of the behavioural/psychiatric symptoms (Fig. 3A) but also the most helpful management (Fig. 3B) and those problems that are most important in influencing everyday life (Fig. 3C). Seventy-four (85%) responders contributed a pattern of further problems which are included in the comparison with previous attempts to delineate an adult RTS natural history (Table 2). Rare other problems reported once, are listed in Supplementary Table 2. Details about the medical follow-up are illustrated in Fig. 4 regarding types of professional (Fig. 4A) and of specialties involved (Fig. 4B). The frequency of health checks ranged from once a year (35/87, 40%) to once every 2 or 3 years (3/87, 3%); one-third of individuals (27/87, 31%) are followed-up only if there are specific concerns, 17% (15/87) twice a year and on four occasions (4%) the individuals required follow-up multiple times a year. Almost half (42/87, 48%) of RTS individuals had surgery at least once in adulthood and more than half of those (24) had surgery more than once (11 twice, 6 thrice and 7 multiple times). For those individuals who had surgery, the responders classify the outcome in Fig. 4C. The type of problems for which surgery was required are detailed in Fig. 4D.
Fig. 2. Summary of adult RTS morbidity by body system.
A Gastrointestinal, B skin, C behavioural/psychiatric and D sleep.
Table 1.
Patterns of major medical problems.
| CONSTIPATION (n = 54) | |
|---|---|
| A. Frequency of stools | Number (percentage of total replies) |
| Every other day/3–4 times per week | 19 (35%) |
| Almost everyday | 10 (19%) |
| Twice/day | 9 (14%) |
| Once/day | 4 (7%) |
| Variable | 3 (5%) |
| B. Management (multiple options possible) | |
| Medications | 29 (43%) |
| Dulcolax, Forlax, Exlax, Miralax, Sodium picosulphate | 13 (24%) |
| Movicol, Macrogol, Metamucil | 4 (7%) |
| A specific diet | 19 (35%) |
| 17 Diet based on foods with lots of fibres | 17 (31%) |
| 2 fibres in supplements | 2 (4%) |
| Other | 13 (24%) |
| Regular exercise | 2 (4%) |
| Regular meals | 1 (2%) |
| Colonic irrigation | 1 (2%) |
| A combination of 2 options | 13 (24%) |
| C. Most helpful management/treatment | |
| Exlax®, Dulcolax®, Forlax®, Miralax®, Sodium picosulphate | 10 (19%) |
| Diet based on foods with lots of fibres or fibre supplements | 7 (13%) |
| Movicol®, Macrogol® | 6 (11%) |
| None | 5 (9%) |
| Colonic irrigation | 1 (2%) |
| Meal/toilet routine | 1 (2%) |
| KELOIDS (n = 35) | |
|---|---|
| Itching | 20 (57%) |
| Which influence daily life | 11 (30%) |
| Management/treatment | |
| Steroids | 9 (23%) (in 3 most helpful) |
| Non-steroid creams | 4 (11%) |
| Surgery | 1 (3%) |
| Menthol powder | 1 (3%) |
| INCREASED NEED FOR SLEEPING (n = 22) | |
|---|---|
| Mood change if not allowed to sleep | 3 (14%) |
| Daytime | 1 (4%) |
| Need to sleep immediately after lunch | 1 (4%) |
| “Often gets up at night and sleeps sitting up on sofa” | 1 (4%) |
| Daily requirement | 10–14 h |
Fig. 3. Pattern of RTS behavioural or psychiatric problems.
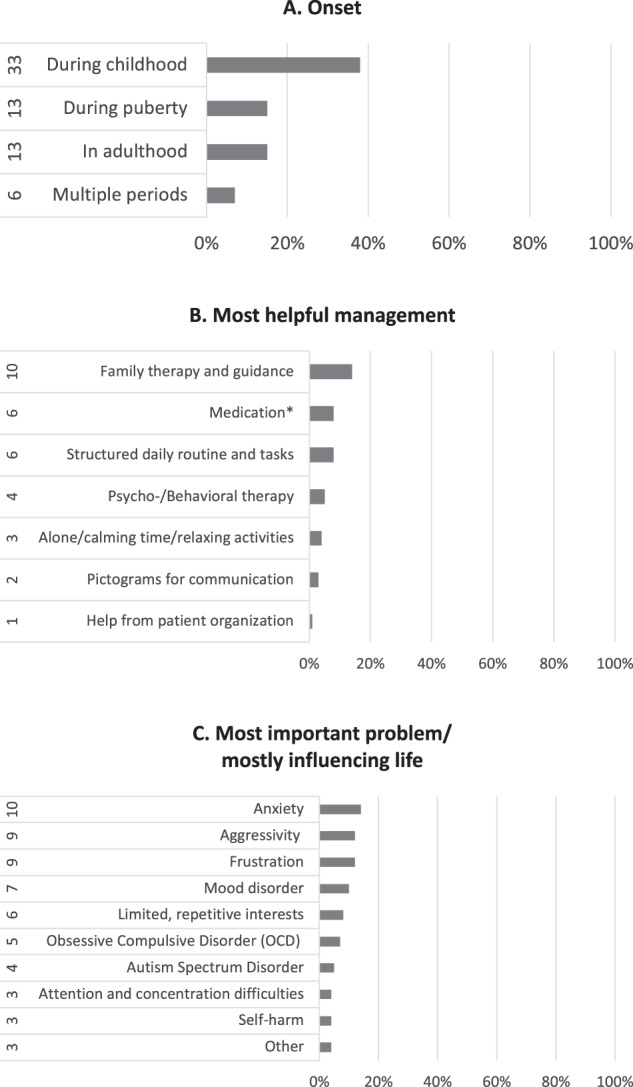
A Onset, B most helpful management and C most important problem/mostly influencing life.
Table 2.
Comparison with literature data.
| Stevens et al. [4] | This cohort | |
|---|---|---|
| Gastrointestinal problems | ||
| Feeding difficulties | 53% | 14% |
| Eosinophilic esophagitis | 2/61, 3% | 5% |
| Binge eating | 52% | 36% |
| Behavioural or psychiatric problems (also [13–15]) | ||
| Aggression towards others | 22% | 26% |
| Anxiety | 33% | 49% |
| Autism Spectrum Disorder | 60% | 28% |
| Attention Deficit Hyperactivity Disorder (ADHD) | ~70% | 17% |
| Self-harm/Aggression towards themselves | 32% | 14% |
| Intolerance of noise and crowds | 62% | 44% |
| Worsening or progression of behaviour | 37% | The majority |
| Medication because of behavioural problem | 15/61, 24.5% | 26% |
| Musculoskeletal (also [22]) | ||
| Spinal curvature | 30/62, 48% | 3% |
| Joint problems including dislocated patellae and hypermobility | Up to 46% | 5% |
| Sensory organs | ||
| Visual difficulties | 79% | 44% |
| Hearing loss | 18/61, 30% | 15% |
| Skin and adnexa | ||
| Keloids (also [23]) | 57% | 61% |
| Infections/ingrowing nails that require treatment (also [24]) | 19/62, 30% | 50% |
| Hypohidrosis | 38% | – |
| Dental problems | Up to 80% | 18% |
| Sleep difficulties | 28% | 42% |
| Sleep apnoea | 15/61, 25% | 26% |
| Cardiac problems (also [25]) | 11/62, 18% | 12% |
| Hypertension | 10% | 9% |
| Genitourinary problems (also [12]) | 35% | 4% |
| Endocrine problems | ||
| Hypothyroidism | 11% | – |
| Diabetes mellitus | 5% | 4% |
| Hypoglycaemia | 5% | – |
| Menopause | 2 individuals | 10%a |
| Osteoporosis | – | 8% |
| Other | ||
| Frequent infections | 17% | – |
| Seizures | 5% | 1 individual |
| Anaesthesia complications | 14% | – |
| High pain threshold | na | 57% |
| Decreased mobility/any difficulties with moving around (also ref [8]) | 32% | 50% |
| Issues related to learning disability incl. the requirement of constant supervision or very low level of independence for daily activities | na | 11% |
| Immunology-related problems (refs. [26–28]) | 3 individuals | – |
| Overweight | 25% | 1 individual |
| Tumour/cancer (refs. [9, 29–31]) | 6/61,10% | 1 individual |
| Pilomatrixomas | 17% | 23% |
| Multidisciplinary medical follow-up | The majority | 50% |
| Surgery (all types) | na | 50% |
| Spinal surgery | 10/61, 16% | 2% |
| Surgery for dislocated patellae/other joint instability | 10/61, 16% | 4/85% |
| Adenotonsillectomy | 11/61, 18% | 1 individual |
| Surgery for congenital heart defects | 9/61, 15% | – |
na not available.
aOf women.
Fig. 4. Medical follow-up and surgery details.
A Type of professional, B follow-up specialty, C did the surgery resolve the problem and D type of surgery.
Discussion
This is the first international attempt at collecting and analysing relevant information about the adult RTS morbidity and its experience. We report the observations from the largest, molecularly (n = 43) or clinically (n = 87) confirmed cohort of adult individuals with RTS to date. The adult natural history of RTS is defined by prevalent (83%) behavioural/psychiatric problems that are variable but include frequently anxiety, aggressivity, frustration and/or a mood disorder; gastrointestinal problems (73%) that are represented mainly by constipation and several other problems in much lower frequency; many, variable problems that affect skin and adnexa in over than half (65%) of the RTS individuals and sleep problems (62%) that manifest in a consistent pattern of sleep apnoea, difficulty staying asleep and an increased need for sleep. This rich morbidity is further complicated by further concerns identified in approximately half of the replies as a pattern of high pain threshold, decreased mobility, hypersensitivity to noise and crowded places, and vision difficulties (Table 2). Half of the individuals require multidisciplinary follow-up and required surgery at least once, and most frequently more than once, during adulthood (Fig. 4).
Ascertainment and assessment approaches and methods are different when compared to previous systematic attempts and, thus, a formal statistical analysis could not be performed [4, 5, 8, 9]. Still, a number of conclusions can be drawn (Table 2). A few observations show considerable consistency, arguing for a true correlation with the adult natural history of RTS: eosinophilic esophagitis, aggression towards others, requirement of medication for the behavioural problems, keloids, sleep apnoea, hypertension, and diabetes mellitus. The observations in our cohort evidence a decreased incidence in adulthood regarding spinal curvature, joint problems, visual and hearing difficulties, dental problems, genitourinary problems, and overweight. This could represent a positive effect of improving general healthcare management of these issues in the wider population, independently from an RTS diagnosis. The decrease of the reported incidence of tumours could be secondary to an ascertainment bias of the previous publications that reported a high incidence. A few previous consistent observations were not confirmed in our cohort: hypohidrosis, hypothyroidism, hypoglycaemia, and anaesthesia complications [4]. Also, previous individual reports of cervical spine anomalies [10], myositis ossificans traumatica [11], and chronic renal failure of unknown cause [12] were not confirmed in our cohort.
Hennekam et al. [13] first document, in 1992, variable behavioural and psychiatric problems in RTS adults. Levitas and Reid [14] report, in 1998, 13 adult RTS individuals with psychiatric diagnoses (Tourette; mood disorder, OCD, chronic tic, trichotillomania, bipolar, major depressive disorder, psychotic features, hallucinosis, delusions). They also document side effects of anti-DOPA drugs whilst Hellings et al. [15] report that patients responded well to the GABAergic drug DVP, showing a lasting response of a year. What Stevens et al. [4] describe as a consistent report of worsening in behaviours (37%), is described by the present families as an evolution of the behavioural phenotype over time. Anxiety begins in childhood and persists into adulthood. Around puberty and/or transition to adulthood there is more aggressivity, frustration and sudden mood changes. In later adulthood this pattern changes again to a loss in interests and sometimes depression (Fig. 3 and Supplementary Table 1). Our observations agree with previous ones [7, 16, 17] and highlight the need to follow up patients with RTS and, if necessary, refer to psychiatry. Moreover, our observations are similar to behavioural findings in other developmental disorders caused by disruptions of epigenetic regulator genes both regarding the high incidence of a specific pattern of rigid, repetitive, and inflexible behaviours and emotional dysregulation [18] but also age-dependent progression [19]. The use of medication (Diazepam©, Risperdal©, Methylphenidate©, sleep tablets) is the second most frequent management amongst our responders following a combination with psychotherapy and/or a change of environment. It is also classified as the second most helpful management whilst the first is family therapy and home guidance (Fig. 3B). This may represent a difference in the approach regarding treatment and management but also residential choices (inclusion in the original household) over the years towards individuals with developmental disorders that predispose to psychiatric disturbances.
Ours is the first systematic report of skin and adnexa-related problems in adult RTS individuals (Fig. 2B). These are mainly progressive manifestations: keloids, abnormal scarring of other type, ingrowing/infections of finger-or toenails and poor wound healing.
Boot et al. [9], based on both a nationwide, Dutch population-based study and a literature review, conclude that RTS individuals are at increased risk to develop meningiomas and pilomatrixomas, but not for other types of tumours, and do not find a genotype-phenotype correlation. They conclude that tumour surveillance below 40 years old is not indicated but miss sufficient data to draw conclusions for older patients. Our questionnaire offered a clear distinction between pilomatrixomas and other tumours. Amongst our responders there were 13 individuals with pilomatrixomas (15%) but only on one occasion a (non-further-detailed) “cancer” was mentioned as a health concern, whilst one further patient had surgery for a sphenoid-orbital meningioma (Table 2). Our data confirms that pilomatrixomas and meningiomas are the most frequent tumours in RTS individuals throughout their lives. However, the number of older patients (above 40 years of age) is small (n = 19, Supplementary Fig. S1) in our study as well, so cannot contribute further data about tumour surveillance.
There has been significant progress both in the clinical management of adult individuals with syndromic developmental disorders and in the general acceptance of their social roles since Stevens et al. [20] first discussed fertility and occupation in RTS adult individuals. Wiley et al. [6] brought up developmentally appropriate sexual education strategies which should be encouraged throughout the lifespan and as part of a universal programme of transition to adulthood. Our data confirm that RTS adults enjoy both social and occupational possibilities and show a variegated experience of everyday life (Fig. 1). Nevertheless, the results of the survey show significant morbidity and ongoing medical issues in adult RTS patients that do not appear to be as coordinated and multidisciplinary managed as in paediatric patients (Fig. 4).
When compared with global health data estimates from the World Health Organization [21], the global top 10 causes of mortality in high-income countries (ischaemic heart disease, dementias, stroke, trachea, bronchus and lung cancers, chronic obstructive pulmonary disease, colon and rectum cancers, kidney diseases, hypertensive heart disease and diabetes mellitus) are underrepresented in the reported observations from this cohort. Despite the relatively small number of our cohort, our data do not evidence an increased risk for the general major diseases of adulthood in someone with RTS and we believe that this is useful information for counselling families.
An important limitation of our approach is that, in a significant proportion of cases, our responders do not declare a molecularly confirmed RTS diagnosis. The likely explanation is that these now adult patients may have missed the opportunity for genetic testing during childhood and the diagnosis was made on clinical grounds. Still, this is most probably the correct diagnosis, taking into consideration that RTS has a well recognisable phenotype and that the reported medical history fits with our knowledge about the condition. Moreover, this survey also offered the opportunity to address this issue and offer genetic testing to interested families within the framework of ERN-ITHACA. Other limitations are selection or ascertainment bias (data were reported mostly by a first-degree family member). Our cohort was not large and data were aggregated and not stratified by country, and was organisational regarding type of work and activities and medical care. The genotype-related distribution of individuals in this study (7 EP300 individuals and 36 CREBBP ones) does not allow for statistical comparison that would hint towards a genotype-natural history correlation. Yet this is an important issue to address in future studies of larger cohorts of individuals with molecularly confirmed RTS. We believe that our survey generated a wealth of useful messages for families with RTS individuals. We highlight the need for optimal care in a multidisciplinary setting including the pivotal role of specialists for adult care. Finally, we create a standard for future data collections with the hope to increase the number of adult observations within the RTS community.
Supplementary information
Acknowledgements
We thank all the RTS individuals and their families for their time and dedication in providing us with this invaluable information. This work has been generated within the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (ERN-ITHACA) [EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/79516].
Author contributions
All authors acquired data and approved the final version of the manuscript. SH and SGM played an important role in interpreting the results. SD and RCMH conceived and designed the work that led to the submission, drafted and revised the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Ethical approval
This investigation was performed in accordance with the Declaration of Helsinki. Reported information was provided voluntarily by all participants filing in the questionnaire.
Footnotes
SD, SH, KV, DL, and RCMH are members of ERN-ITHACA.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01097-8.
References
- 1.Rubinstein JH, Taybi H. Broad thumbs and toes and facial abnormalities. Am J Dis Child. 1963;105:588–608. doi: 10.1001/archpedi.1963.02080040590010. [DOI] [PubMed] [Google Scholar]
- 2.Hennekam RC. Rubinstein-Taybi syndrome. Eur J Hum Genet. 2006;14:981–5. doi: 10.1038/sj.ejhg.5201594. [DOI] [PubMed] [Google Scholar]
- 3.Hennekam RC, Van Den Boogaard MJ, Sibbles BJ, Van Spijker HG. Rubinstein-Taybi syndrome in the Netherlands. Am J Med Genet. 1990;6:17–29. doi: 10.1002/ajmg.1320370604. [DOI] [PubMed] [Google Scholar]
- 4.Stevens CA, Pouncey J, Knowles D. Adults with Rubinstein-Taybi syndrome. Am J Med Genet A. 2011;155A:1680–4. doi: 10.1002/ajmg.a.34058. [DOI] [PubMed] [Google Scholar]
- 5.van Genderen MM, Kinds GF, Riemslag FC, Hennekam RC. Ocular features in Rubinstein-Taybi syndrome: investigation of 24 patients and review of the literature. Br J Ophthalmol. 2000;84:1177–84. doi: 10.1136/bjo.84.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiley S, Swayne S, Rubinstein JH, Lanphear NE, Stevens CA. Rubinstein-Taybi syndrome medical guidelines. Am J Med Genet A. 2003;119A:101–10. doi: 10.1002/ajmg.a.10009. [DOI] [PubMed] [Google Scholar]
- 7.Yagihashi T, Kosaki K, Okamoto N, Mizuno S, Kurosawa K, Takahashi T, et al. Age-dependent change in behavioral feature in Rubinstein-Taybi syndrome. Congenit Anom. 2012;52:82–6. doi: 10.1111/j.1741-4520.2012.00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Milani D, Manzoni FM, Pezzani L, Ajmone P, Gervasini C, Menni F, et al. Rubinstein-Taybi syndrome: clinical features, genetic basis, diagnosis, and management. Ital J Pediatr. 2015;41:4–12. doi: 10.1186/s13052-015-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boot MV, van Belzen MJ, Overbeek LI, Hijmering N, Mendeville M, Waisfisz Q, et al. Benign and malignant tumors in Rubinstein-Taybi syndrome. Am J Med Genet A. 2018;176:597–608. doi: 10.1002/ajmg.a.38603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Kurosawa K, Masuno M, Okuzumi S, Kondo S, Miyama S, et al. Congenital anomaly of cervical vertebrae is a major complication of Rubinstein-Taybi syndrome. Am J Med Genet A. 2005;135:130–3. doi: 10.1002/ajmg.a.30708. [DOI] [PubMed] [Google Scholar]
- 11.Utumi ER, Pedron IG, Zambon CE, Neto NP, Rocha AC. Rare occurrence of myositis ossificans traumatica in a patient with Rubinstein-Taybi syndrome. J Oral Maxillofac Surg. 2010;68:2616–22. doi: 10.1016/j.joms.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Cotsirilos P, Taylor JC, Matalon R. Dominant inheritance of a syndrome similar to Rubinstein-Taybi. Am J Med Genet. 1987;26:85–93. doi: 10.1002/ajmg.1320260115. [DOI] [PubMed] [Google Scholar]
- 13.Hennekam RC, Baselier AC, Beyaert E, Bos A, Blok JB, Jansma HB, et al. Psychological and speech studies in Rubinstein-Taybi syndrome. Am J Ment Retard. 1992;96:645–60. [PubMed] [Google Scholar]
- 14.Levitas AS, Reid CS. Rubinstein-Taybi syndrome and psychiatric disorders. J Intellect Disabil Res. 1998;42:284–92. doi: 10.1046/j.1365-2788.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 15.Hellings JA, Hossain S, Martin JK, Baratang RR. Psychopathology, GABA, and the Rubinstein-Taybi syndrome: a review and case study. Am J Med Genet. 2002;114:190–5. doi: 10.1002/ajmg.10156. [DOI] [PubMed] [Google Scholar]
- 16.Nayak RB, Lakshmappa A, Patil NM, Chate SS, Somashekar L. Rubinstein-taybi syndrome with psychosis. Indian J Psychol Med. 2012;34:184–6. doi: 10.4103/0253-7176.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite J, Moss J, Beck SR, Richards C, Nelson L, Arron K, et al. Repetitive behavior in Rubinstein-Taybi syndrome: parallels with autism spectrum phenomenology. J Autism Dev Disord. 2015;45:1238–53. doi: 10.1007/s10803-014-2283-7. [DOI] [PubMed] [Google Scholar]
- 18.Chan AJS, Cytrynbaum C, Hoang N, Ambrozewicz PM, Weksberg R, Drmic I, et al. Expanding the neurodevelopmental phenotypes of individuals with de novo KMT2A variants. NPJ Genom Med. 2019;4:9–18. doi: 10.1038/s41525-019-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groves L, Oliver C, Moss J. Behaviour across the lifespan in Cornelia de Lange syndrome. Curr Opin Psychiatry. 2021;34:112–7. doi: 10.1097/YCO.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 20.Stevens CA, Carey JC, Blackburn BL. Rubinstein-Taybi syndrome: a natural history study. Am J Med Genet. 1990;6:30–37. doi: 10.1002/ajmg.1320370605. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. The top 10 causes of death. 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 22.Marion RW, Garcia DM, Karasik JB. Apparent dominant transmission of the Rubinstein-Taybi syndrome. Am J Med Genet. 1993;46:284–7. doi: 10.1002/ajmg.1320460309. [DOI] [PubMed] [Google Scholar]
- 23.Siraganian PA, Rubinstein JH, Miller RW. Keloids and neoplasms in the Rubinstein-Taybi syndrome. Med Pediatr Oncol. 1989;17:485–91. doi: 10.1002/mpo.2950170526. [DOI] [PubMed] [Google Scholar]
- 24.Balci S, Ali Ergün M, Lechno S, Bartsch O. Rubinstein-Taybi syndrome in first cousins with different de novo mutations. Am J Med Genet A. 2010;152A:1036–8. doi: 10.1002/ajmg.a.33259. [DOI] [PubMed] [Google Scholar]
- 25.Fischer S, Bäzner H, Henkes H. Cervical artery dissection in a young patient with Rubinstein-Taybi syndrome. Clin Neuroradiol. 2013;23:41–4. doi: 10.1007/s00062-011-0100-7. [DOI] [PubMed] [Google Scholar]
- 26.Kim CJ, Nam JH, Chung HY, Kook JH, Kim SY, Woo YJ. Kimura disease in a patient with Rubinstein-Taybi syndrome. Pediatr Int. 2004;46:609–11. doi: 10.1111/j.1442-200x.2004.01941.x. [DOI] [PubMed] [Google Scholar]
- 27.Naimi DR, Munoz J, Rubinstein J, Hostoffer RW., Jr Rubinstein-Taybi syndrome: an immune deficiency as a cause for recurrent infections. Allergy Asthma Proc. 2006;27:281–4. doi: 10.2500/aap.2006.27.2864. [DOI] [PubMed] [Google Scholar]
- 28.Herriot R, Miedzybrodzka Z. Antibody deficiency in Rubinstein-Taybi syndrome. Clin Genet. 2016;89:355–8. doi: 10.1111/cge.12671. [DOI] [PubMed] [Google Scholar]
- 29.Miller RW, Rubinstein JH. Tumors in Rubinstein-Taybi syndrome. Am J Med Genet. 1995;56:112–5. doi: 10.1002/ajmg.1320560125. [DOI] [PubMed] [Google Scholar]
- 30.Bayle P, Bazex J, Lamant L, Lauque D, Durieu C, Albes B. Multiple perforating and non perforating pilomatricomas in a patient with Churg-Strauss syndrome and Rubinstein-Taybi syndrome. J Eur Acad Dermatol Venereol. 2004;18:607–10. doi: 10.1111/j.1468-3083.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 31.Papathemeli D, Schulzendorff N, Kohlhase J, Göppner D, Franke I, Gollnick H. Pilomatricomas in Rubinstein-Taybi syndrome. J Dtsch Dermatol Ges. 2015;13:240–2. doi: 10.1111/ddg.12504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.