Abstract
Purpose
Gouty arthritis could be triggered by the deposition of monosodium uric acid (MSU) crystals. Palmatine (PAL), a protoberberine alkaloid, has been proven to possess compelling health-beneficial activities. In this study, we aimed to explore the effect of PAL on LPS plus MSU crystal-stimulated gouty arthritis in vitro and in vivo.
Methods
PMA-differentiated THP-1 macrophages were primed with LPS and then stimulated with MSU crystal in the presence or absence of PAL. The expression of pro-inflammatory cytokines and oxidative stress-related biomarkers and signal pathway key targets were determined by ELISA kit, Western blot, immunohistochemistry and qRT-PCR, respectively. In addition, the anti-inflammatory and antioxidant activities of PAL on MSU-induced arthritis mice were also evaluated.
Results
The results indicated that PAL (20, 40 and 80 μM) dose-dependently decreased the mRNA expression and levels of pro-inflammatory cytokines (interleukin-1beta (IL-1β), IL-6, IL-18 and tumor necrosis factor alpha (TNF-α)). The levels of superoxide dismutase (SOD) and glutathione (GSH) were remarkably enhanced, while the level of malondialdehyde (MDA) was reduced. Western blot analysis revealed that PAL appreciably inhibited NF-κB/NLRP3 signaling pathways through inhibiting the phosphorylation of p-65 and IκBα, blocking the expression of NLRP3, ASC, IL-1β and Caspase-1, as well as enhancing the antioxidant protein expression of Nrf2 and HO-1. In vivo, PAL attenuated MSU-induced inflammation in gouty arthritis, as evidenced by mitigating the joint swelling, and decreasing the productions of IL-1β, IL-6, IL-18, TNF-α and MDA, while enhancing the levels of SOD and GSH. Moreover, PAL further attenuated the infiltration of neutrophils into joint synovitis.
Conclusion
PAL protected against MSU-induced inflammation and oxidative stress via regulating the NF-κB/NLRP3 and Nrf2 pathways. PAL may represent a potential candidate for the treatment of gouty arthritis.
Keywords: palmatine, NF-κB/Nrf2 signal pathways, NLRP3 inflammasome, gouty arthritis
Introduction
In recent years, gouty arthritis or gout is one of the most common metabolic diseases. The number of gouty patients is gradually increasing with the aging of the population and the change in lifestyle, especially men and postmenopausal women.1 They usually exhibit extreme pain, erythema, redness and joint swelling.2 If the joint is not treated in time, it will become stiff and deformed, which could seriously increase their financial burden, affect their quality of life and may even lead to incapacity of action.3
The hallmark for the diagnosis of this disorder is the precipitation of monosodium urate (MSU) crystals in the joint or periarticular tissues.4 NLRP3 inflammasome has received particular attention in the development of MSU-induced gout arthritis.4 It is composed of NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC), and pro-caspase-1.5 The activation of NLRP3 inflammasome requires a two-step process.6 The first priming signal is that lipopolysaccharide (LPS) activates nuclear factor-κB (NF-κB) signal pathway, which promotes the expression of inflammasome components, such as NLRP3 and pro‐IL‐1β.7 The second activation signal is that monosodium urate (MSU) crystals are taken up by macrophages, which promotes the assembly and activation of the NLRP3 inflammasome.5
Furthermore, MSU-induced gouty inflammation was mediated by specific mechanisms that were involved in nuclear factor-erythroid 2-related factor 2 (Nrf2) signal pathway, which has a negative regulatory effect on the activation of NLRP3 inflammasome.8–10 Collectively, this provides a direction for understanding the pathogenesis of gouty arthritis and looking for drugs with anti-inflammatory and antioxidant properties for targeted therapy.
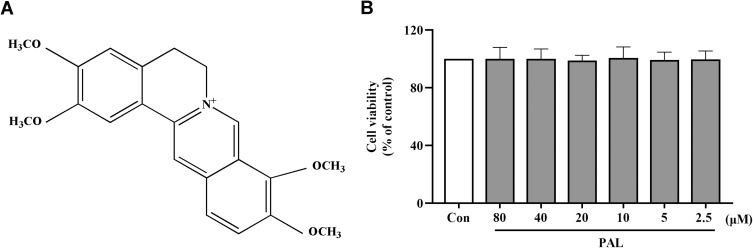
Palmatine (C21H25NO4, PAL, Figure 1A), one of the major active protoberberine alkaloids in Cortex Phellodendri,11 exists in medicinal plants like Corydalis yanhusuo and Aristolochiae Herba, etc.12 PAL has been used as an anti-inflammatory agent to cure gynecological inflammation, enteritis, and conjunctivitis in clinical practice.13,14 Furthermore, it also has a wide range of health-beneficial effects in the treatment of gastric ulcer, skin cancer and ulcerative colitis due to its anti-inflammatory and antioxidant properties mediated by NF-κB/Nrf2 signal pathways and NLRP3 inflammasome.15–17
Figure 1.
(A) Chemical structure of PAL. (B) Effect of PAL on cell viability of THP-1 cells. Data are shown as mean ± SD (n = 3).
Furthermore, PAL has been found to exert protective effect on the experimental osteoarthritis rabbit model and possess potential antalgic effect.18,19 It has been also found to be a xanthine oxidase inhibitor with potential application in the treatment of hyperuricemia.20 However, whether PAL could alleviate inflammatory response on MSU-induced gouty arthritis was unclear. Therefore, the present study made a pioneering endeavor to explore the potential effect and underlying mechanism of PAL in LPS plus MSU crystal-induced inflammation in THP-1 cells, and MSU crystal-induced gouty arthritis in mice.
Materials and Methods
Materials and Reagents
Palmatine (PAL, purity >98%, the chemical structure is shown in Figure 1A) was provided by Xi’an Ruilin Pharmaceutical (Xi’an, China). Colchicine (Col, purity >98%, MB1063) was purchased from Dalian Meilun Biotechnology Co., LTD (Dalian China). Lipopolysaccharide (LPS, L4391), phorbol myristate acetate (PMA, P1585) and monosodium urate (MSU, U2875) were obtained from Sigma-Aldrich (St. Louis, MO, USA). RPMI1640 media, fetal bovine serum (FBS) and phosphate‐buffered saline (PBS) were purchased from Gibco (Grand Island, NY, USA).
Cell Culture and Viability Assay
THP-1 cells were obtained from the Central South University (Hunan, China), which has obtained the qualification of national laboratory qualification (measurement certification, certificate number: 170021002479) and the laboratory accreditation certificate of China National Accreditation Service for Conformity Assessment (certificate number: CNAS L10220). Cells were cultured in RPMI-1640 medium supplemented with 10% FBS, and 1% penicillin streptomycin at 37°C and 5% (v/v) CO2. THP-1 cells were differentiated into macrophages by incubation with 50 nM of PMA for 24 h. In order to assess the effect of PAL on THP-1 cells, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assay was performed as previously described.21
Briefly, cells were seeded in a 96-well plate at a density of 2×105 cells/well until they reached a density of 90%. Various concentrations of PAL (2.5–80 μM) were added to each well for 24 h. PAL was dissolved in DMSO and the final concentration of DMSO was 0.1%. Thereafter, the medium was decanted, and 20 μL solution (5 mg/mL in PBS buffer) was replaced. After incubation at 37°C for 4 h, the supernatant was removed and 150 μL DMSO was added to each well for 15 min at 37°C. To calculate the relative cell viability, the plate was determined by spectrometry at 570 nm under gentle shaking and the relative viability in untreated control was designed to be 100%.
Cell Stimulation
THP-1 cells were first primed with LPS stimulation (500 ng/mL) for 4 h. And then PAL (20, 40, 80 μM) was added to the cell culture medium for 1 h, followed by stimulation with MSU (200 μg/mL) for 5 h. Col (1 μM) served as the positive control according to the previous report.21
Animals
KM male mice (6–8 weeks old), weighing 20 ± 2 g, were bought from Laboratory Animal Center of Guangzhou University of Chinese Medicine (GZUCM, Guangzhou, China). In order to acclimate to the new environment, all animals were housed under a light/dark cycle for 12 h at 22–24°C in the specific pathogen-free (SPF) environment, and given free access to food and water for the duration of the experiment. The experimental procedures were performed according to the guidelines approved by the Institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine (NO. 20210113001).
Measurement of Inflammatory Cytokines and Oxidative Stress
The supernatants of the cells and serum were collected to determine the levels of TNF-α (#ml002095), IL-1β (#ml063132), IL-18 (#ml002294) and IL-6 (#ml063159) by mouse enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). In addition, the cell lysate was used for the assessment of the enzymatic antioxidants of superoxide dismutase (SOD, #A001-3), glutathione (GSH, #A006-2) and the levels of malondialdehyde (MDA, #A003-1), by using corresponding commercially available assay kits (Jiancheng Company, Nanjing, China).
Quantitative Real-Time PCR
Total RNA was extracted from THP-1 cells with Trizol reagent according to the manufacturer’s instructions. Total RNA was reversely transcribed into cDNA with the PrimerScript RT reagent kit (#R222-01, Vazyme Biotech Co. Ltd., Nanjing, China). Gene primers were designed by using NCBI/primer-BLAST tool software and synthesized by Shanghai Bioengineering (Shanghai, China) (Table 1). Afterwards, cDNA was used for real-time PCR amplification as follows: 95°C for 3 min, followed by 39 cycles at 95°C for 10 sec, 60°C for 10 sec and 72°C for 20 sec, and a final single cycle at 95°C for 10 sec. The target gene expression levels were calculated using the 2−ΔΔCt method and GAPDH was used as a reference for normalization.
Table 1.
Primer Sequences
| Gene | Sequence (5’ to 3’) |
|---|---|
| IL-1β | Forward GGACAGGATATGGAGCAACAAGTGG Reverse TCATCTTTCAACACGCAGGACAGG |
| TNF-α | Forward AGCCCTGGTATGAGCCCATCTATC Reverse TCCCAAAGTAGACCTGCCCAGAC |
| IL-6 | Forward GACAGCCACTCACCTCTTCAGAAC Reverse GCCTCTTTGCTGCTTTCACACATG |
| IL-18 | Forward TTGACCAAGGAAATCGGCCTC Reverse GCCATACCTCTAGGCTGGCT |
| GAPDH | Forward GGTCGGAGTCAACGGATTTG Reverse GGAAGATGGTGATGGGATTTC |
Western Blot Analysis
Cells were harvested and lysed in ice-cold RIPA (Radio-Immunoprecipitation Assay) buffer mixed with cocktail and PMSF for 30 min. The BCA protein assay kit (BB-3401, Shanghai Beibo Biotechnology, Shanghai, China) was used to measure the protein concentration. Then, equal amounts of protein were resolved by 7.5% to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes.
After blocked with 5% skim milk for 2 h at room temperature, the membranes were incubated with appropriate primary antibodies against p-NF-κB p65 (CST, #3033S, 1:1000), NF-κB p65 (CST, #8242S, 1:1000), p-IKB-α (GeneTex, #32224, 1:1000), IKB-α (GeneTex, #110521, 1:1000), NLRP3 (Abcam, #ab263889, 1:1000), ASC (Affinity, #DF6304, 1:500), IL-1β (Affinity, #AF5103, 1:1000), caspase-1 (Affinity, #AF5418, 1:1000), Nrf2 (Affinity, #AF0639, 1:1000), HO-1 (Affinity, #AF5393, 1:1000) and β-actin (Affinity, #AF7018, 1:1000) overnight at 4°C. Subsequently, membranes were incubated with secondary HRP-conjugated goat anti-rabbit IgG (Immunoglobulin G, Affinity, #072102, 1:5000) for 1 h. Protein bands were visualized using the enhanced chemiluminescence (ECL) (Tanon 4200SF, China) detection system. The intensity of the bands was assessed using the ImageJ software (National Institutes of Health, Bethesda, MA, USA) tool.
MSU-Induced Gouty Arthritis Model
Gouty arthritis model in mice was established as previously described.22,23 Sixty mice were randomly divided into the following six groups: Control group, Model group, Col (1 mg/kg) group, PAL-L (25 mg/kg) group, PAL-M (50 mg/kg) group and PAL-H (100 mg/kg) group. Before injecting MSU, PAL (dissolved in 0.9% NaCl) or Col (dissolved in PBS) was orally administered to the treatment group, respectively.
The dosages of PAL were selected according to our preceding report and our pilot trial.24 Col served as a positive control.25 One hour later, the MSU crystal suspension (0.1 mg/20 μL of PBS per mouse) or PBS alone was injected in the left knee joint of each mouse. The joint swelling of each mouse at different time points was measured with an electronic caliper. After 24 h, the joint tissues were homogenized in RIPA buffer, and then centrifuged at 12,000 rpm for 15 min. The supernatants were used for TNF-α, IL-1β, IL-18 and IL-6 assays.
H&E Staining
At the end of the experiment, the joint tissues were fixed in 4% paraformaldehyde for more than 24 h. Then the samples were embedded in paraffin, which were sectioned into 5 µm for hematoxylin-eosin (HE) staining. The histologic score was performed according to the previous report.26
Immunohistochemistry
The sample sections were deparaffinized, rehydrated with different concentrations of ethanol, and washed with PBS. Then the slides were incubated with primary antibodies at 4°C overnight. After that, tissue slices were incubated with HRP-conjugated secondary antibodies and stained with 3,3′-diaminobenzidine (DAB), followed by hematoxylin counterstaining.
Statistical Analysis
Data were presented as mean ± standard deviation (SD). The differences between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by LSD or Dunnett’s test using SPSS software (version 20.0, SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Assessment of Cell Toxicity of PAL on THP-1 Cells
As shown in Figure 1B, PAL at a concentration up to 80 µM did not show significant cytotoxic effect on the viability of THP-1 cells after incubation for 24 h (P > 0.05). Therefore, 20, 40 and 80 μM of PAL were used in the subsequent experiments according to our pre-experiment results. 1 μM of Col was used as the positive control according to previous reports.21,27
Effect of PAL on the Levels of IL-1β, TNF-α, IL-6 and IL-18 in THP-1 Cells
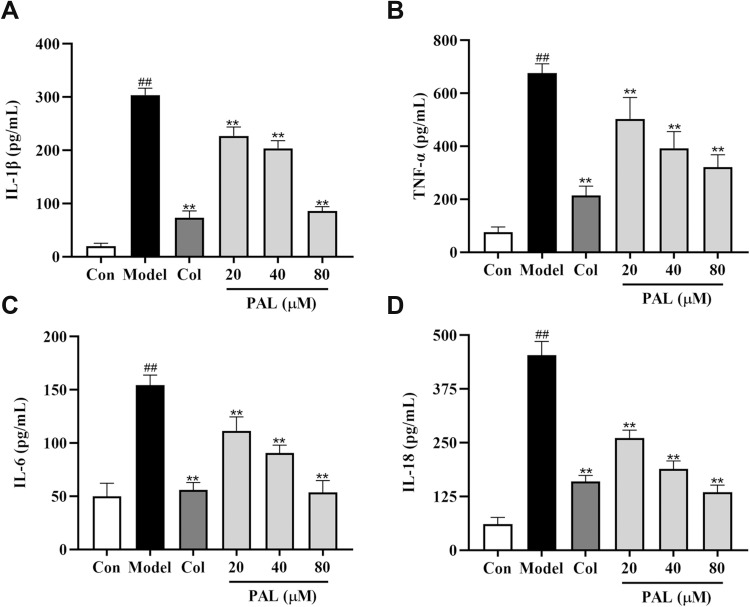
As shown in Figure 2, treatment with LPS (500 ng/mL) and MSU (200 μg/mL) significantly increased IL-1β, TNF-α, IL-18 and IL-6 productions in the supernatant (all P < 0.01), as compared to the control group. Nevertheless, these changes were dose-dependently reversed by PAL and Col (all P < 0.01). The above results indicated that the anti-inflammatory effect of PAL might be associated with its regulation of the productions of pro-inflammatory mediators.
Figure 2.
Effects of PAL and Col (1 µM) on the levels of IL-1β (A), TNF-α (B), IL- 6 (C) and IL-18 (D) in LPS plus MSU-activated THP-1 cells. Data are shown as mean ± SD (n = 3); ##P < 0.01 vs Control group; **P < 0.01 vs Model group.
Effect of PAL on IL-1β, TNF-α, IL-6 and IL-18 mRNA Expression in THP-1 Cells
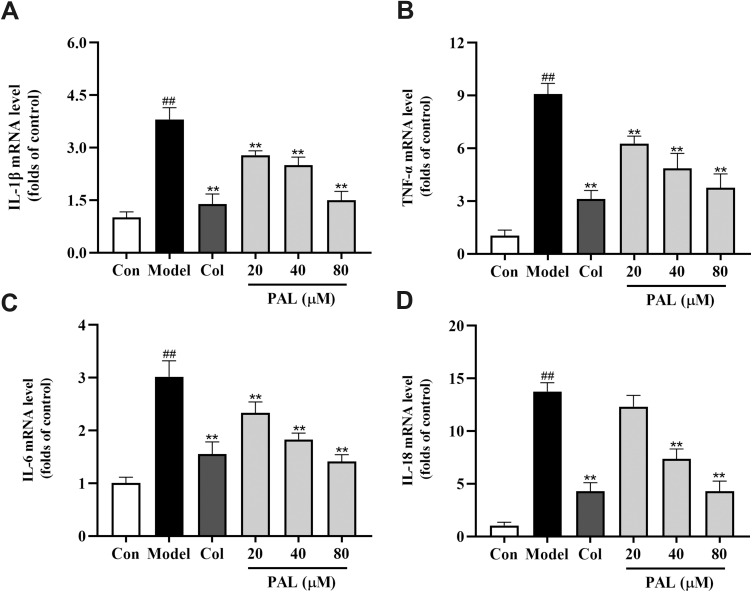
To further investigate the inhibitory effect of PAL on the mRNA expression of IL-1β, TNF-α, IL-6 and IL-18, RT-PCR analysis was performed. As illustrated in Figure 3, in contrast to the control group, the expression of IL-1β, TNF-α, IL-6 and IL-18 was significantly upregulated (all P < 0.01). Meanwhile, these changes were strikingly reversed by PAL and Col (P < 0.01) in a dose-dependent manner.
Figure 3.
Effects of PAL and Col (1 µM) on the mRNA expression of IL-1β (A), TNF-α (B) IL-6 (C) and IL-18 (D) in LPS plus MSU-activated THP-1 cells. Data are shown as mean ± SD (n = 3); ##P < 0.01 vs Control group, **P < 0.01 vs Model group.
Effect of PAL on the Levels of SOD, MDA and GSH in THP-1 Cells
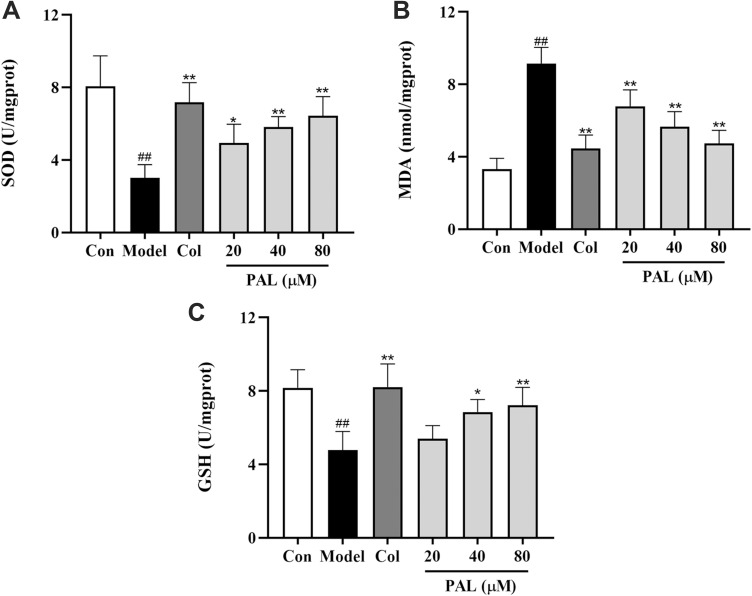
As shown in Figure 4, the production of MDA was also dramatically increased in the model group in parallel to that of the control group (P < 0.01). On the contrary, the levels of antioxidant enzymes (SOD and GSH) were significantly suppressed relative to those of the control group (all P < 0.01). However, treatment with PAL significantly elevated the levels of SOD and GSH in a dose-dependent manner. Notably, there was no significant difference between the effects of PAL (80 µM) and Col in LPS plus MSU-induced THP-1 cells.
Figure 4.
Effects of PAL and Col (1 µM) on the levels of SOD (A), MDA (B) and GSH (C) in LPS plus MSU-activated THP-1 cells. Data are shown as mean ± SD (n = 3); ##P < 0.01 vs Control group; *P < 0.05, **P < 0.01 vs Model group.
Effect of PAL on NF-κB/NLRP3 and Nrf2 Signaling Pathways in THP-1 Cells
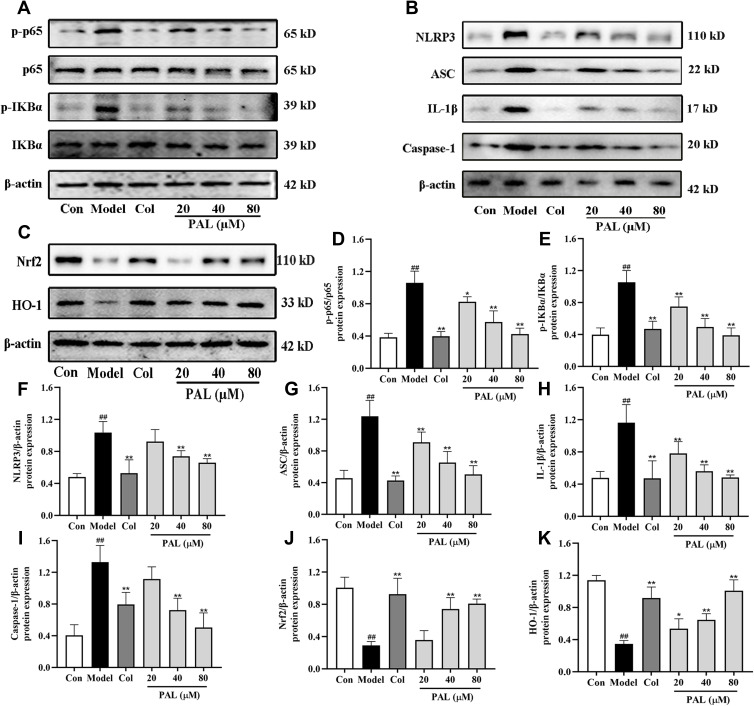
When macrophages are exposed to LPS, it can activate NF-κB signaling pathway and release multiple pro-inflammatory factors.28 To further explore whether the anti-inflammatory effect of PAL was associated with the two pathways, the expression of several key proteins was examined. As shown in Figure 5A, B and D-I, the expression of p-p65, p-IKBα, NLRP3, ASC, IL-1β and Caspase-1 (all P < 0.01) was dramatically increased in the model group. Nevertheless, treatment with PAL at various concentrations blocked their expression to different extents, and dose-dependently arrested the activation of NF-κB and NLRP3.
Figure 5.
Effects of PAL and Col (1 µM) on NF-κB/NLRP3 and Nrf2 signaling pathways in LPS plus MSU-activated THP-1 cells. (A) Representative Western blotting images of p-p65, p65, p-IKBα, IKBα and β-actin. (B) Representative Western blotting images of NLRP3, ASC, IL-1β, Caspase-1 and β-actin. (C) Representative Western blotting images of Nrf2, HO-1 and β-actin. Changes in the relative protein expression levels of p-p65/p65 (D), p-IKBα/IKBα (E), NLRP3 (F), ASC (G), IL-1β (H), Caspase-1 (I), Nrf2 (J) and HO-1 (K). Data are shown as mean ± SD (n = 3); ##P < 0.01 vs Control group; *P < 0.05, **P < 0.01 vs Model group.
To further explore the mechanism underlying the protective effect of PAL on LPS plus MSU-induced oxidative stress, the protein expression of Nrf2 and HO-1 was measured. As shown in Figure 5C, J and K, the protein expression of Nrf2 and HO-1 in the model group was significantly down-regulated when compared with that of the normal control group. However, PAL significantly restored the protein expression of Nrf2 and HO-1 (P < 0.05). In general, these results suggest that the protective effect of PAL against oxidative damage was associated with the activation of Nrf2/HO-1 antioxidant response. Collectively, the therapeutic effect of PAL might be related to suppression of NF-κB/NLRP3 activation and Nrf2 pathway.
Effect of PAL on Knee Joint Inflammation in Mice
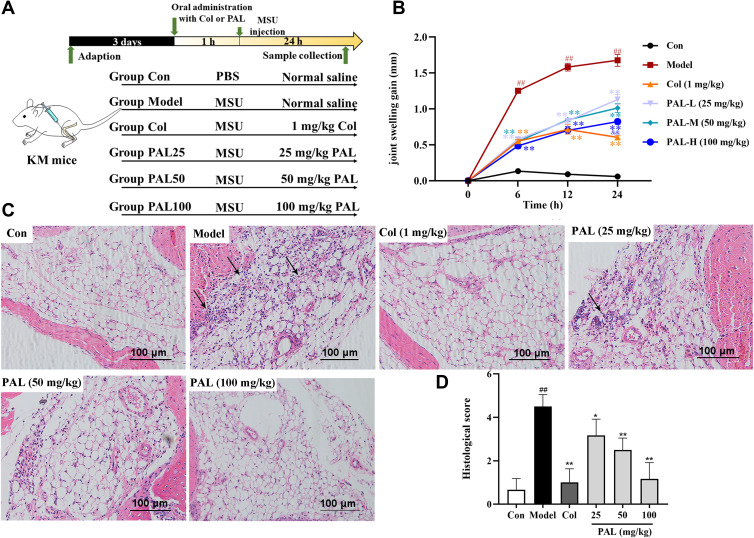
We then investigated the role of PAL and Col in MSU-induced gouty arthritis. After mice were orally administrated with different concentrations of test articles, MSU crystals (0.1 mg) were injected into the knee joints of mice (Figure 6A). As shown in Figure 6B, there was an obvious swelling in the model group after injection of MSU. However, in the PAL and Col treatment groups, the swelling was significantly decreased.
Figure 6.
PAL suppressed the MSU-induced gouty arthritis. Either 25 mg/kg (L), 50 mg/kg (M) and 100 mg/kg (H) of PAL, PBS (Con) or 1 mg/kg of Col (positive control group) was orally administered to mice. After 1 h, either monosodium urate (MSU) crystal in 0.1 mg/10 µL of PBS or PBS alone was injected into the left knee joint of each mouse. PAL decreased the MSU crystal-induced acute gout inflammation in mice: (A) Schematic diagram of in vivo experimental protocol. (B) Joint swelling gain at different time points. (C) Hematoxylin and eosin staining of leukocytes in joint tissues (black arrow). (D) Histologic score. Data are expressed as mean ± SD (n = 6), ##P < 0.01 vs Control group; *P < 0.05, **P < 0.01 vs Model group.
The infiltration of a large number of neutrophils into the joint space has been deemed as an obvious feature of gouty arthritis.29 As shown in the histological examination (Figure 6C), in the model group, there were obvious neutrophil cells recruited to synovium and joint cavity caused by MSU crystals. However, oral gavage of 25, 50 and 100 mg/kg PAL significantly attenuated neutrophil infiltration in the synovial cavity. The microscopic score (Figure 6D) of PAL groups (25, 50 and 100 mg/kg) was also observably lowered in a dose-dependent manner (all P < 0.01). Col was also observed to significantly reverse the MSU-induced neutrophil infiltration.
Evaluation of MSU-Induced Inflammation in Knee Gouty Arthritis
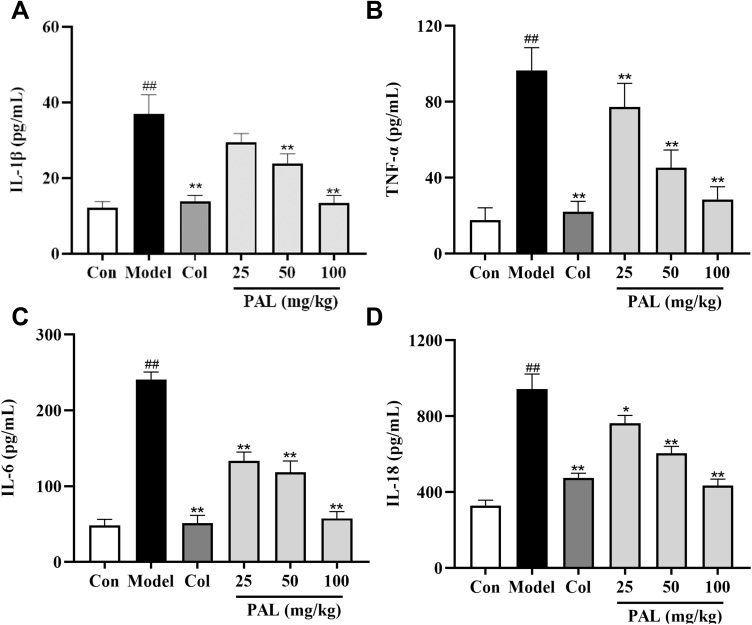
As depicted in Figure 7, PAL substantially suppressed the increase of IL-1β, TNF-α, IL-6 and IL-18 levels (all P < 0.01) in the joint tissues injected with MSU crystals as compared to the model group. Notably, there was no significant difference between the anti-inflammatory effects of PAL (100 mg/kg) and Col in joint tissues.
Figure 7.
Levels of IL-1β (A), TNF-α (B), IL-6 (C) and IL-18 (D) in joint tissues, as measured by ELISA. Data are expressed as mean ± SD (n = 6), ##P < 0.01 vs Control group; *P < 0.05, **P < 0.01 vs Model group.
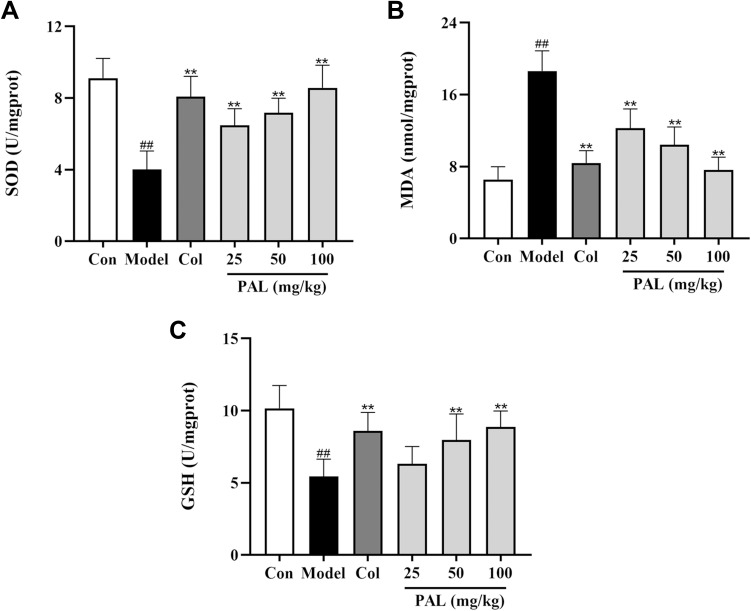
Effect of PAL on the Levels of SOD, MDA and GSH in Mice
As shown in Figure 8, compared with the control group, the production of MDA was dramatically increased in the model group (P < 0.01). On the contrary, the levels of antioxidant enzymes (SOD and GSH) were significantly suppressed relative to those of the control group. However, treatment with PAL significantly reduced the level of MDA (all P < 0.01), elevated the activities of SOD and GSH in a dose-dependent manner. It was noteworthy that the effect of PAL (100 mg/kg) was comparable to that of Col in joint tissues.
Figure 8.
Effects of PAL and Col (1 mg/kg) on the levels of SOD (A), MDA (B) and GSH (C) in joint tissue. Data are shown as mean ± SD (n = 6); ##P < 0.01 vs Control group; **P < 0.01 vs Model group.
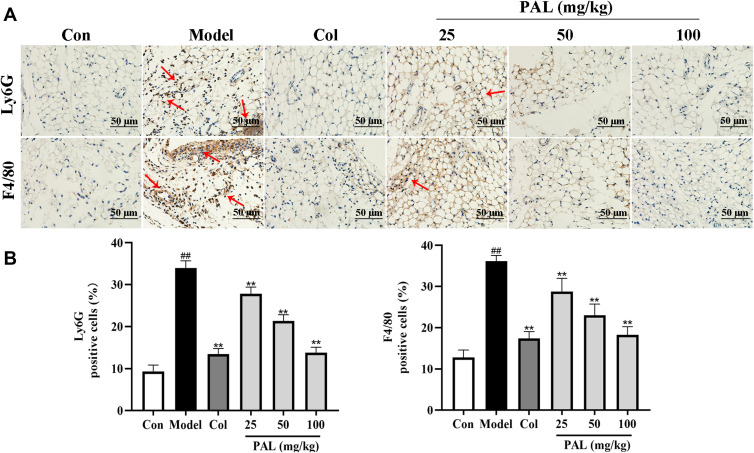
Effect of PAL on the Expression of Ly6G and F4/80 in the Knee Synovium
Knee joint sections were stained with Ly6G and/or F4/80 antibody to analyze neutrophil and macrophage. The immunohistochemical results showed that MSU recruited more neutrophil and macrophage to knee synovium, whereas PAL and Col effectively reduced the infiltration of neutrophil and macrophage (Figure 9). The above results showed that PAL possessed appreciable inhibitory effect on MSU-induced arthritis, and the effect of PAL (100 mg/kg) appeared close to that of Col. Our results indicated that PAL could alleviate the symptoms of gouty arthritis by reducing the infiltration of immune cell.
Figure 9.
Effects of PAL and Col (1 mg/kg) on the inflammatory cell infiltration in vivo. (A) Representative immunohistochemistry images of knee joint sections stained with Ly6G or F4/80 (red arrow). (B) The percentage of Ly6G or F4/80 positive cells relative to total cells was calculated. Data are shown as mean ± SD (n = 3). ##P < 0.01 vs Control group; **P < 0.01 vs Model group.
Discussion
In the past few decades, the incidence of gouty arthritis has increased all over the word.30 Currently, colchicine and corticosteroids are the most frequently used drugs for the prevention and treatment of gouty arthritis.31 However, clinical studies have found that these agents usually cause many undesirable side effects in patients, including gastrointestinal bleeding, gastrointestinal toxicity, and nephrotoxicity.32
As an acute inflammatory disease, gout has seriously affected the quality of life of patients. Some inflammatory factors, such as IL-1β and IL-18, have been found to play essential roles in the occurrence and development of acute gouty arthritis.33 In macrophages, the inactive precursors IL-1β and IL-18 need to be cleaved by caspase-1 to become active form.4 Both TNF-α and IL-6 are significantly related to the pathogenesis of MSU-induced inflammatory response, whose productions can be enhanced by IL-1β.33 Compared with healthy controls, patients with gout have higher levels of inflammatory factors in serum and other tissues,34,35 which could further accelerate inflammatory reaction. Previous studies have suggested that PAL has anti-inflammatory effect in multiple diseases.24,36 Therefore, we evaluated the potential role of PAL in LPS plus MSU-stimulated THP-1 cells. The ELISA and qRT-PCR assays showed that PAL dose-dependently reduced the productions and mRNA expression of IL-1β, IL-18, TNF-α and IL-6 as compared with the model group. Interestingly, the effect of PAL (80 μM) was similar to that of Col, suggesting that PAL also had appreciable anti-inflammatory effect.
It has been reported that gout is accompanied by the activation of NF-κB signaling pathway,37 which can regulate the expression of NLRP3 inflammasome.38,39 Therefore, to further explore the anti-inflammatory mechanism of PAL, we investigated whether PAL would affect NLRP3 inflammasome activation or NF-κB pathway.
NF-κB serves as a crucial initial step that triggers expression of NLRP3 inflammasome components by upregulating the expression of NLRP3, pro‐IL‐1β and pro-IL-18.4 Yan et al have demonstrated that PAL exerted anti-inflammatory effect in goat endometrial epithelial cells, at least in part, via regulation of the NF-κB pathway.16 In the present study, our results indicated that PAL dose-dependently inhibited the activation of NF-κB pathway by blocking the phosphorylation of p-65 and IKB-α.
Zhong et al have reported that baicalin, an inhibitor of NLRP3, could effectively attenuate MSU crystal-induced inflammatory response in vivo.22 Our previous study has indicated that PAL exhibited anti-inflammatory activity by downregulating the expression of NLRP3-related proteins in ulcerative colitis and THP-1 cells.24 As a follow-up study, we further investigated the effect of PAL on NLRP3 inflammasome. Consistent with previous study, treatment with PAL dramatically suppressed the activation of NLRP3 inflammasome in LPS plus MSU-primed macrophages, as evidenced by the decreased protein expression of NLRP3, ASC, IL-1β and Caspase-1. Taken together, the therapeutic effect of PAL might be related to the inhibition of NLRP3 inflammasome assembly and the activation of NF-κB pathway. Importantly, it was noteworthy that PAL (80 μM) exhibited similar anti-inflammatory effect to Col.
Multiple studies have shown that the inflammatory response is closely related to the oxidative stress.40 Nrf2 signal pathway could alleviate oxidative stress by promoting its downstream antioxidant enzymes, such as HO-1, SOD and GSH.41,42 Previous report has suggested that transfection with the Nrf2 plasmid greatly inhibited the NLRP3 expression at both the mRNA and protein levels, thereby inhibiting the priming step of NLRP3 inflammasome activation.10 In this study, PAL treatment was found to significantly increase SOD and GSH levels, reduce MDA content and restore the protein expression of Nrf2 and HO-1. Interestingly, the effect of PAL was comparable to that of Col.
We then investigated whether PAL could exert beneficial effect in gout mouse model. Results indicated that MSU crystal injection led to the joint swelling, increased the productions of IL-1β, IL-18, TNF-α and IL-6, elevated the levels of SOD and GSH, reduced MDA content and accented neutrophil infiltration in joint tissues as shown by histologic examination. While PAL effectively decreased the joint swelling to almost normal levels and significantly suppressed the productions of IL-1β, IL-18, TNF-α and IL-6 in joint tissues. There was no significant difference between the effect of PAL (100 mg/kg) and Col. Excessive neutrophil infiltration in the synovial space can cause severe pain and swelling.43 The results of histological analysis showed that PAL blocked the infiltration of inflammatory cell into synovium and joint cavity. Immunohistochemistry was used to detect the cell subsets of macrophage and neutrophil in joint synovium of arthritic mice. The results also indicated that PAL could reduce proinflammatory cell infiltration. This result was consistent with the in vitro data, indicating that PAL had therapeutic effect in the mouse model of gouty arthritis.
Together, the results obtained clearly indicated that PAL could effectively alleviate the inflammatory response and oxidative stress in THP-1 cells and gouty arthritis mouse model, which provided experimental evidence for the traditional application of Cortex Phellodendri in the treatment of gout. In the future, more investigations are needed to improve our understanding of PAL. The present study solely focused on acute gouty arthritis of the knee, further researches on the mechanisms underlying chronic gouty arthritis were merited.
Conclusion
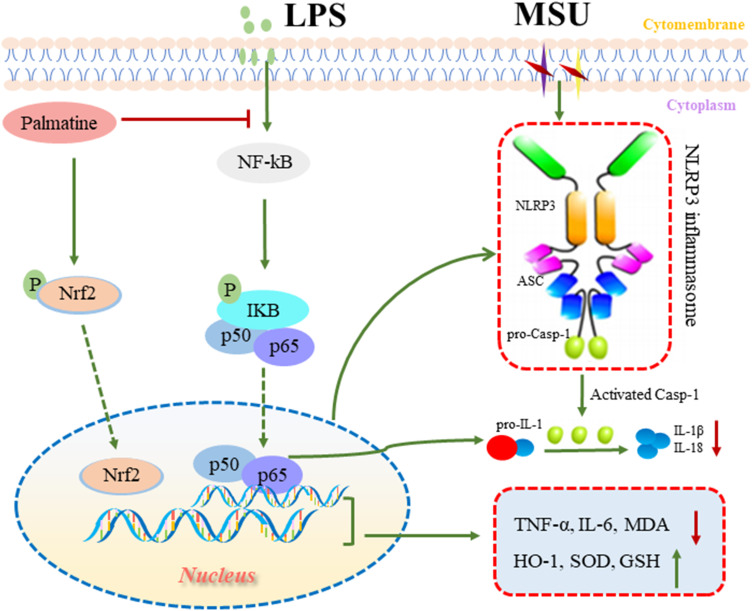
In summary, PAL exerted anti-inflammatory and antioxidant effects, at least in part, by inhibiting NF-κB/NLRP3 activation and Nrf2 pathway in LPS plus MSU-stimulated THP-1 cells. Furthermore, PAL effectively prevented acute gouty arthritis induced by MSU in KM mice (Figure 10). Collectively, our results revealed that PAL had potential to be further developed into a promising therapeutic agent against gouty arthritis. Further investigations on the in-depth studies were warranted to provide more detailed mechanism.
Figure 10.
Summary scheme of the mechanisms underlying the beneficial effect of PAL in ameliorating MSU-induced inflammation and oxidative stress. On the one hand, PAL inhibited the activation of NF-κB/NLRP3 signaling to ameliorate inflammation response. On the other hand, PAL activated Nrf2 and promoted its nuclear translocation, thereby alleviating oxidative stress.
Funding Statement
The work was supported by Grants from National Natural Science Foundation of China (Nos. 82074082 & 82104472), Guangdong Natural Science Foundation (Nos. 2021A1515011490 & 2019A1515010819 & 2022A1515011706), Characteristic Cultivation Program for Subject Research of Guangzhou University of Chinese Medicine (No. XKP2019007), Guangzhou Liwan Disrtrict Science and Technology Project (No. 202201005), Key Program for Subject Research of Guangzhou University of Chinese Medicine (No. XK2019002) and Chinese Medicine Bureau of Guangdong Province (No. 20201134).
Disclosure
The authors declare no conflict of interest.
References
- 1.Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 Suppl 5:S9-S12. doi: 10.3949/ccjm.75.suppl_5.s9 [DOI] [PubMed] [Google Scholar]
- 2.Liu HJ, Pan XX, Liu BQ, et al. Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J Neuroinflammation. 2017;14(1):74. doi: 10.1186/s12974-017-0849-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10(5):271–283. doi: 10.1038/nrrheum.2014.32 [DOI] [PubMed] [Google Scholar]
- 4.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol. 2017;13(11):639–647. doi: 10.1038/nrrheum.2017.155 [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 6.Major TJ, Dalbeth N, Stahl EA, et al. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2018;14(6):341–353. doi: 10.1038/s41584-018-0004-x [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Zhang D, Hu D, et al. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;103:115–124. doi: 10.1016/j.molimm.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Tsai PY, Ka SM, Chang JM, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51(3):744–754. doi: 10.1016/j.freeradbiomed [DOI] [PubMed] [Google Scholar]
- 9.Ka SM, Lin JC, Lin TJ, et al. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res Ther. 2015;17(1):331. doi: 10.1186/s13075-015-0844-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu XT, Zhang X, Ding Y, et al. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid Redox Signal. 2017;26(1):28–43. doi: 10.1089/ars.2015.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H, Wang HY, Zhang A, et al. Chemical Discrimination of Cortex Phellodendri amurensis and Cortex Phellodendri chinensis by Multivariate Analysis Approach. Pharmacogn Mag. 2016;12(45):41–9. doi: 10.4103/0973-1296.176023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commission, C. P. J. P. s. M. P. H. Pharmacopoeia of the People’s Republic of China.
- 13.Zhou JT, Li CL, Tan LH, et al. Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: investigation on the Potential Mechanism. PLoS One. 2017;12(1):e0168944. doi: 10.1371/journal.pone.0168944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathan NB, Parvez A, Bader A, et al. Synthesis, characterization, crystal structure determination and biological screening of novel N-1 and C5 alkyl substituted scaffolds of pyrimidine. Eur J Med Chem. 2015;103:594–599. doi: 10.1016/j.ejmech.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 15.Chen GR, Xu YB, Jing J, et al. The anti-sepsis activity of the components of Huanglian Jiedu Decoction with high lipid A-binding affinity. Int Immunopharmacol. 2017;46:87–96. doi: 10.1016/j.intimp.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 16.Yan BQ, Wang DS, Dong SW, et al. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol. 2017;45:194–200. doi: 10.1016/j.intimp.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Tang CL, Hong JM, Hu CY, et al. Palmatine Protects against Cerebral Ischemia/Reperfusion Injury by Activation of the AMPK/Nrf2 Pathway. Oxid Med Cell Longev. 2021:6660193. doi: 10.1155/2021/6660193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, Liu L, Guan S, et al. Palmatine alleviates hyperalgesia by inhibiting the expression of calcitonin gene-related peptide in the trigeminal ganglion of rats with chronic constriction injury of the infraorbital nerve. Br J Oral Maxillofac Surg. 2020;58(4):443–450. doi: 10.1016/j.bjoms.2020.01.031 [DOI] [PubMed] [Google Scholar]
- 19.Zhou XD, Lin XL, Xiong Y, et al. Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: a possible mechanism of inhibiting the Wnt/β-catenin and Hedgehog signaling pathways. Int Immunopharmacol. 2016;34:129–138. doi: 10.1016/j.intimp.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 20.Orhan IE, Deniz FSS. Natural Products and Extracts as Xantine Oxidase Inhibitors – a Hope for Gout Disease? Curr Pharm Des. 2021;27(2):143–158. doi: 10.2174/1381612826666200728144605 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Xu DQ, Sun DY, et al. Curcumin ameliorates monosodium urate‐induced gouty arthritis through Nod‐like receptor 3 inflammasome mediation via inhibiting nuclear factor‐kappa B signaling. J Cell Biochem. 2019;120(4):6718–6728. doi: 10.1002/jcb.27969 [DOI] [PubMed] [Google Scholar]
- 22.Amaral FA, Costa VV, Tavares LD, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64(2):474–484. doi: 10.1002/art.33355 [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Lee HE, Moon SJ, et al. Direct binding to NLRP3 pyrin domain is a novel strategy to prevent NLRP3-driven inflammation and gouty arthritis. Arthritis Rheumatol. 2020;72(7):1192–1202. doi: 10.1002/art.41245 [DOI] [PubMed] [Google Scholar]
- 24.Mai CT, Wu MM, Wang CL, et al. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol Immunol. 2019;105:76–85. doi: 10.1016/j.molimm.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Lin YQ, Dou JP, et al. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Prolif. 2020;53(9):e12868. doi: 10.1111/cpr.12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AS, Richards PJ, Thomas E, et al. Interferon- protects against the development of structural damage in experimental arthritis by regulating polymorphonuclear neutrophil influx into diseased joints. Arthritis Rheum. 2007;56(7):2244–2254. doi: 10.1002/art.22732 [DOI] [PubMed] [Google Scholar]
- 27.Dinesh P, Rasool MK. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int Immunopharmacol. 2017;44:26–37. doi: 10.1016/j.intimp.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Zhang LY, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cumpelik A, Ankli B, Zecher D, et al. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann Rheum Dis. 2016;75(6):1236–1245. doi: 10.1136/annrheumdis-2015-207338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–2052. doi: 10.1016/S0140-6736(16)00346-9 [DOI] [PubMed] [Google Scholar]
- 31.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707 [DOI] [PubMed] [Google Scholar]
- 32.Keenan RT, O’Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124(2):155–163. doi: 10.1016/j.amjmed.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 33.Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1β, and gout: is there a link? Semin Immunopathol. 2013;35(4):501–512. doi: 10.1007/s00281-013-0361-0 [DOI] [PubMed] [Google Scholar]
- 34.Cavalcanti NG, Marques CD, Lins T Lins TUUbiratan L, et al. Cytokine Profile in Gout: inflammation Driven by IL-6 and IL-18? Immunol Invest. 2016;45(5):383–395. doi: 10.3109/08820139.2016.1153651 [DOI] [PubMed] [Google Scholar]
- 35.Choe JY, Choi CH, Park KY, et al. High-mobility group box 1 is responsible for monosodium urate crystal-induced inflammation in human U937 macrophages. Biochem Biophys Res Commun. 2018;503(4):3248–3255. doi: 10.1016/j.bbrc.2018.08.139 [DOI] [PubMed] [Google Scholar]
- 36.Ma H, Zhang YF, Wang JX, et al. Palmatine attenuates LPS-induced inflammatory response in mouse mammary epithelial cells through inhibiting ERK1/2, P38 and Akt/NF-кB signalling pathways. J Anim Physiol Anim Nutr (Berl). 2021;105(1):183–190. doi: 10.1111/jpn.13440 [DOI] [PubMed] [Google Scholar]
- 37.Cao Y. Icariin alleviates MSU-induced rat GA models through NF-κB/NALP3 pathway. Cell Biochem Funct. 2021;39(3):357–366. doi: 10.1002/cbf.3598 [DOI] [PubMed] [Google Scholar]
- 38.Fann YW, Lim YA, Cheng YL, et al. Evidence that NF-κB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol Neurobiol. 2018;55(2):1082–1096. doi: 10.1007/s12035-017-0394-9 [DOI] [PubMed] [Google Scholar]
- 39.Ghonime MG, Shamaa OR, Das S, et al. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol. 2014;192(8):3881–3888. doi: 10.4049/jimmunol.1301974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee TS, Tsai HL, Chau LY. Induction of Heme Oxygenase-1 Expression in Murine Macrophages Is Essential for the Anti-inflammatory Effect of Low Dose 15-Deoxy-Δ12,14-prostaglandin J2*. J Biol Chem. 2003;278(21):19325-19330. doi: 10.1074/jbc.M300498200 [DOI] [PubMed] [Google Scholar]
- 42.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247.doi: 10.1007/s00018-016-2223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yahia SA, Zeller V, Desplaces N, et al.Crystal-induced arthritis after arthroplasty: 7 cases. Joint Bone Spine. 2016;83(5):559–562. doi: 10.1016/j.jbspin.2016.01.006 [DOI] [PubMed] [Google Scholar]