Abstract
Introduction:
Cardiorespiratory fitness is not limited by pulmonary mechanical reasons in the majority of adults. However, the degree to which lung function contributes to exercise response patterns among ostensibly healthy individuals remains unclear.
Methods:
We examined 2314 Framingham Heart Study participants who underwent cardiopulmonary exercise testing (CPET) and pulmonary function testing. We investigated the association of FEV1, FVC, FEV1/FVC and DLCO with the primary outcome of peak VO2, along with other CPET parameters using multivariable linear regression. Finally, we investigated the association of total and peripheral pulmonary blood vessel volume with peak VO2.
Results:
We found lower FEV1, FVC and DLCO were associated with lower peak VO2. For example, a one-liter lower FEV1 and FVC were associated with 7.1% (95% CI: 5.1%, 9.1%) and 6.0% (95% CI: 4.3%, 7.7%) lower peak VO2, respectively. By contrast, FEV1/FVC ratio was not associated with peak VO2. Lower lung function was associated with lower oxygen uptake efficiency slope oxygen pulse slope, VO2 at AT, VE at AT and breathing reserve. In addition, lower total and peripheral pulmonary blood vessel volume were associated with a lower peak VO2.
Conclusion:
In a large, community-based cohort of adults, we found lower FEV1, FVC and DLCO were associated with lower exercise capacity, as well as oxygen uptake efficiency slope and ventilatory efficiency. In addition, lower total and peripheral pulmonary blood vessel volume were associated with lower peak VO2. These findings underscore the importance of lung function and blood vessel volume as contributors to overall exercise capacity.
Keywords: Pulmonary Function Testing, Cardiopulmonary Exercise Testing, Pulmonary Blood Vessel Volume, Peak oxygen consumption
Introduction:
Exercise capacity as measured by cardiopulmonary exercise testing is a powerful predictor of clinical outcomes across both health and disease.1-4 Global exercise capacity integrates the entire O2 cascade through which oxygen transits from mouth to mitochondria in order to support performance of physical activity. While studies primarily in referral populations have often related resting pulmonary function tests to impairment in exercise capacity among individuals with established lung disease, less is known about the impact of lung structure and function on overall fitness in relatively healthy community cohort without overt lung disease. Whether the association of resting lung function with exercise capacity is driven by limitations in pulmonary performance including gas exchange (ventilatory efficiency), or may also be associated with limitations in cardiac performance including heart rate response or changes in pulmonary vasculature (as measured by pulmonary blood vessel volume) has not been fully explored.
While prior studies have demonstrated that lung function as measured by FEV1 is associated with peak VO2 in healthy and elderly individuals, the exact contributions of resting pulmonary function to exercise physiology including cardiac and pulmonary performance remain incompletely understood.5,6 Determining if resting lung function may be associated with specific physiological measures of exercise response as well as lung structure may be of direct clinical relevance. For example, oxygen uptake efficiency slope (OUES), an effort-independent measure that integrates O2 uptake augmentation and ventilatory response and predicts outcomes in patients with conditions such as heart failure. However, OUES has not been investigated in relation to lung structure and function in a community cohort.7 Furthermore, ventilatory anaerobic threshold has been ascribed to cardiovascular limitations in sustaining aerobic metabolism, though the proportion of breathing reserve utilized across pulmonary function test distributions in the community remains largely unexplored. In addition, prior studies within the FHS cohort have demonstrated that lower FEV1, FVC and DLCO are associated with a lower total and peripheral pulmonary vasculature, yet the relationship of pulmonary vasculature to peak VO2 has not yet been analyzed.8
Thus, we sought to further explore the association of lung function with multi-dimensional cardiopulmonary exercise response and the relationship of static computed tomography (CT) imaging of total and peripheral pulmonary vasculature volume with peak VO2. We hypothesized that lower lung function would be associated with lower cardiorespiratory fitness and that lower total and/or peripheral pulmonary blood vessel volume (which may indicate vascular pruning) would be associated with lower peak VO2.
Methods:
Study sample:
Participants from the Framingham Heart study who were a part of the Generation Three, Omni Generation Two and New Offspring cohorts were included in this study.9,10 Participants without a medical contraindication to exercise underwent cardiopulmonary exercise testing during their third examination (2016-2019; N = 3117). Among these, 2800 had available pulmonary function testing performed at the second examination (2008-2011). We excluded participants with sub-maximal exercise defined as peak respiratory exchange ratio (RER) <1.0 (N = 43), history of heart failure (N = 1), history of pulmonary embolism (N = 6), history of lung cancer (N = 7) or missing at least one PFT measurement (N=396) or key outcome values (N = 24) or covariates (N = 9), resulting in a final sample size of N=2314. In addition, Framingham physical activity index (PAI) was collected on all participants. The PAI is a composite score based upon hours spent performing each activity and the weight factor derived from the estimated oxygen consumption for each activity.11 PAI has been shown to predict incident cardiovascular disease within the FHS.11 All participants provided informed consent and the study was approved by the Massachusetts General Hospital and Boston Medical Center Institution Review Boards.
Cardiopulmonary Exercise Testing
Participants underwent upright cycle ergometer exercise testing (Lode, the Netherlands) and breath-by-breath gas exchange measurement (MedGraphics, St. Paul, MN).12 After completion of at least two minutes of resting gas exchange measurements, participants performed three minutes of unloaded exercise followed by maximal effort-limited incremental ramp exercise using 15 or 25 Watt/min ramps. 12 Peak VO2 values were determined by the highest 30-second median during the final 90 seconds of exercise. Additional CPET measures are further described in the supplement.
Pulmonary Function Testing and Pulmonary Blood Vessel Volume
Pulmonary function tests were conducted during exam cycle two.13 Spirometry and diffusion capacity were performed using the Collins Classic Pulmonary Function Laboratory system (Ferraris, Respiratory, Ayer, MA).12 Spirometric measurements were performed according to the American Thoracic Society standards.14
In addition to lung function measurements, a subset of the cohort (N=1389) underwent computed tomography (CT) scans of the thorax between 2008-2011, with further details included in the supplement.
Pulmonary blood vessel volumes measured from the same CT scans were available for 867 participants. We measured total blood vessel volume (TBV) within the pulmonary vasculature. The volume of the entire vessel includes the vascular wall and lumen and includes both arterial and venous vessels. Small intraparenchymal vessels were defined as less than 5mm2 in cross section, BV5. The ratio of BV5/TBV was calculated as an indicator of vascular pruning.13 These measures previously were shown to correspond with histological pulmonary vascular volumes.15 Additional details are included in the supplement.13
Statistical Analysis
Baseline clinical characteristics, lung function measures, and CPET measures were summarized using frequencies, or means and standard deviations as appropriate. Cross sectional associations of lung function (FEV1, FVC, FEV1/FVC and DLCO) with CPET measurements were evaluated using multivariable linear regression with peak VO2 expressed in mL/min/kg as the primary outcome. Peak VO2, VO2 at AT and oxygen pulse were log transformed to accommodate their skewed distributions and heteroscedasticity. In addition, least squares means (LSMEANs) of peak VO2 adjusted for age, sex, height and weight across quartiles of lung function were calculated.
All lung function measures and CPET outcomes were standardized (to mean 0, variance 1) to facilitate effect size comparison across variables. The analyses were adjusted for age, sex, smoking status (never, former, current), hypertension (defined as use of hypertension medications or sbp≥130mmHg pr dbp≥80mmHg), height (inches), weight (kg),diabetes mellitus (defined as fasting glucose ≥126 mg/dl, non-fasting glucose ≥ 200mg/dl, or the use of antidiabetic medications) and Framingham cohort (Generation Three, Omni Generation Two and New Offspring).
In exploratory analysis, cross sectional associations of CT-based measures of lung blood vessel volumes with peak VO2 was performed using multivariable linear regression (adjusted for same covariates as primary analysis). P-values were adjusted using a Bonferroni correction to address multiple testing and were evaluated at a 5% level of significance. In addition, least squares means of peak VO2 adjusted for age, sex, height and weight across quartiles lung blood vessel volumes were calculated. Analyses were conducted using R (The R Foundation for Statistical Computing, version 4.0.3; http://www.rproject.org). and SAS version 9.4 (Cary, NC).
Results:
We studied a total of 2314 participants with mean age of 54±9 years and 54% women. Clinical characteristics are described in Table 1. In brief, the average BMI was 28±5 kg/m2 and 48% had hypertension and 8% diabetes mellitus. In regard to smoking status, 33% were former smokers and 6% were current smokers. The majority of the participants had normal lung function with mean % predicted FEV1 102 ± 14, % predicted FVC 105 ± 13, FEV1/FVC 0.77 ± 0.06 and % predicted DLCO 97 ± 14.
Table 1:
Baseline Participant Characteristics
| Baseline Demographics | N= 2314 |
|---|---|
| Clinical Characteristics | |
| Age, years | 54 (9) |
| Caucasian, N (%) | 2110 (91) |
| BMI, kg/m2 | 28.2 (5.4) |
| Smoking status | |
| Never, N(%) | 1420 (61) |
| Former, N(%) | 758 (33) |
| Current, N(%) | 136 ( 6) |
| Hypertension, N(%) | 1104 (48) |
| Diabetes, N(%) | 177 ( 8) |
| Emphysema on imaging, N (%) | 65 (5) |
| ILA on imaging, N (%) | 20 (1) |
| Physical Activity Score (PAI), mean (SD) | 34 (6) |
| Pulmonary Function Measures | |
| FEV1 (L) | 3.40 (0.76) |
| % predicted FEV1 | 102 (14) |
| FVC (L) | 4.43 (0.99) |
| % Predicted FVC | 105 (13) |
| FEV1/FVC | 0.77 (0.06) |
| % Predicted FEV1/FVC | 95 (7) |
| DLCO, mL/min/mm Hg | 26.29 (6.06) |
| % Predicted DLCO | 97 (14) |
| CPET Measures | |
| Peak VO2 (mL/min/kg) | 22.9 (6.80) |
| OUES (mL/min/log(L/min)) | 1969 (582) |
| VO2 at AT (mL/min/kg) | 12.6 (3.6) |
| % predicted peak VO2 | 95.5 (19.9) |
| Aerobic Efficiency (mL/W/min) | 8.95 (0.95) |
| VO2/work (mL.min−1.W−1) | 10.96 (1.0) |
| Peak Borg Score | 6.81 (1.84) |
| % predicted maximum heart rate | 91 (10) |
| VE at AT ((L/min) | 24.7 (7.50) |
| Peak RER | 1.22 (0.10) |
| Oxygen pulse slope (VO2/HR) | 19.3 (7.40) |
| Breathing reserve at AT | 79.0 (5.2) |
| Breathing reserve at peak exercise (%) | 43.3(15.24) |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusion capacity of the lungs for carbon monoxide; VO2: maximum oxygen consumption; OUES, oxygen uptake efficiency slope; VE at AT, minute ventilation at anaerobic threshold; RER, respiratory exchange ratio
PAI is a composite score of hours spent performing an activity and weighted oxygen consumption of the activity as previous described by Kannel et al. 11
Lower lung function is associated with lower exercise capacity
During CPET, participants on average achieved 91% of predicted maximum heart rate with an average RER of 1.22 indicating maximal effort exercise. Across all participants, the mean peak VO2 was 22.9±6.8 ml/kg/min, and the majority (98%) exercised to a normal breathing reserve (>20%). In this setting we found that lower FEV1 was associated with lower exercise capacity. For example, the least squares mean of peak VO2, adjusted for age, sex, height and weight, was 20.3 ml/kg/min (95% CI: 19.9, 20.7) in the lowest quartile and 23.7 ml/kg/min (95% CI: 23.1, 24.2) among participants in the highest quartile of FEV1 (Figure 1, Ptrend = <0.001). Similarly, lower FVC and DLCO were associated with worse peak VO2 (Ptrend = <0.001). By contrast, peak VO2 appeared similar across FEV1/FVC ratio quartiles.
Figure 1. Peak VO2 across quartiles of lung function measurements.
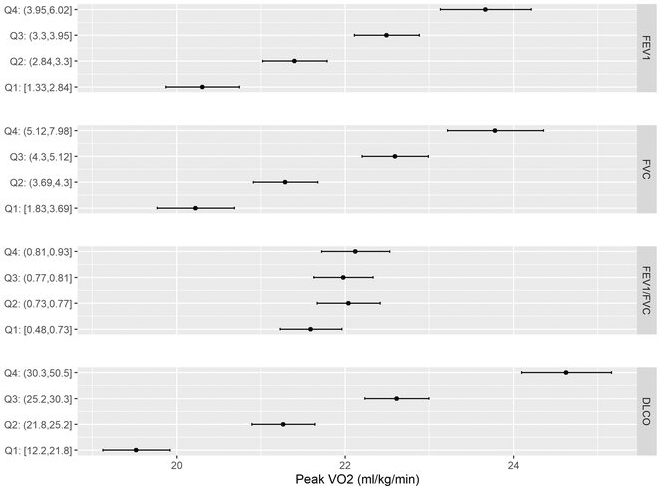
Forest plots displaying the peak VO2least squares means adjusted for age, sex, height and weight across quartiles of FEV1, FVC, FEV1/FVC, and DLCO. The black points represent the quartile mean peak VO2.
We next examined the association of continuous lung function measures and peak VO2 using multivariable regression models. We found that lower FEV1, FVC and DLCO were associated with lower peak VO2 and % predicted peak VO2 with multivariable adjustment (Table 2). For example, a one liter lower FEV1 and FVC were associated with a 7.1% (95% CI: 5.1%, 9.1%) and 6.0% (95% CI: 4.3%, 7.7%) lower peak VO2, respectively. Similarly, a 5 mL/min/mmHg lower DLCO was associated with a 7.1% (95% CI: 15.9%, 8.2%) lower peak VO2. FEV1/FVC ratio was not statistically significantly associated with peak VO2 (Figure 2). In a sensitivity analysis including only participants with % predicted peak VO2 > 85% (N=1560), similar associations were found that lower FEV1, FVC and DLCO were associated with lower peak VO2 after adjustment for confounders. In addition, after excluding individuals with restrictive lung disease, the association of FEV1, FVC and DLCO with peak VO2 was similar. To assess the sensitivity of our estimates to smoking status, we removed current and former smokers and the results were similar with lower FEV1, FVC and DLCO associated with lower peak VO2.
Table 2:
Association of lung function with CPET variables
| FEV1 | FVC | FEV1/FVC | DLCO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta est. | Se | p-value | Beta est. | se | p-value | Beta est. | se | p-value | Beta | se | p-value | |
| Peak VO2 | 0.18 | 0.03 | <0.001 | 0.20 | 0.03 | <0.001 | 0.02 | 0.02 | >0.99 | 0.28 | 0.02 | <0.001 |
| OUES | 0.12 | 0.02 | <0.001 | 0.14 | 0.03 | <0.001 | −0.001 | 0.01 | >0.99 | 0.28 | 0.02 | <0.001 |
| % predicted peak VO2 | 0.25 | 0.04 | <0.001 | 0.27 | 0.04 | <0.001 | 0.02 | 0.02 | >0.99 | 0.40 | 0.03 | <0.001 |
| VO2 at AT | 0.13 | 0.03 | <0.001 | 0.16 | 0.03 | <0.001 | −0.004 | 0.02 | >0.99 | 0.30 | 0.03 | <0.001 |
| O2 pulse slope | 0.09 | 0.03 | 0.008 | 0.10 | 0.03 | 0.007 | 0.003 | 0.01 | >0.99 | 0.16 | 0.02 | <0.001 |
| VO2/work | −0.07 | 0.03 | 0.99 | −0.06 | 0.03 | >0.99 | −0.02 | 0.02 | >0.99 | 0.05 | 0.03 | >0.99 |
| %predicted HR | 0.09 | 0.04 | 0.53 | 0.11 | 0.04 | 0.14 | −0.01 | 0.02 | >0.99 | 0.19 | 0.03 | <0.001 |
| VE AT | 0.16 | 0.03 | <0.001 | 0.20 | 0.03 | <0.001 | −0.01 | 0.02 | >0.99 | 0.22 | 0.03 | <0.001 |
| Peak RER | 0.08 | 0.04 | >0.99 | 0.02 | 0.04 | >0.99 | 0.06 | 0.02 | 0.11 | −0.09 | 0.03 | 0.41 |
| BR AT | 0.75 | 0.03 | <0.001 | 0.59 | 0.04 | <0.001 | 0.25 | 0.02 | <0.001 | −0.03 | 0.03 | >0.99 |
| BR peak | 0.51 | 0.03 | <0.001 | 0.40 | 0.04 | <0.001 | 0.17 | 0.02 | <0.001 | −0.04 | 0.03 | >0.99 |
Analyses were adjusted for age, sex, smoking status (never, current former), hypertension, height (inches), weight (kg), diabetes mellitus, and Framingham cohort. P-values include Bonferroni correction to account for multiple testing and were evaluated at a 5% level of significance. Beta represents the standard-deviation difference in response variable (CPET measures) per 1-standard deviation change in predictor variable (raw lung function measure).
Abbreviations: VO2: maximum oxygen consumption; OUES, oxygen uptake efficiency slope; VE at AT, minute ventilation at anaerobic threshold; RER, respiratory exchange ratio
Figure 2: Unadjusted associations of lung function measurements with peak oxygen consumption.
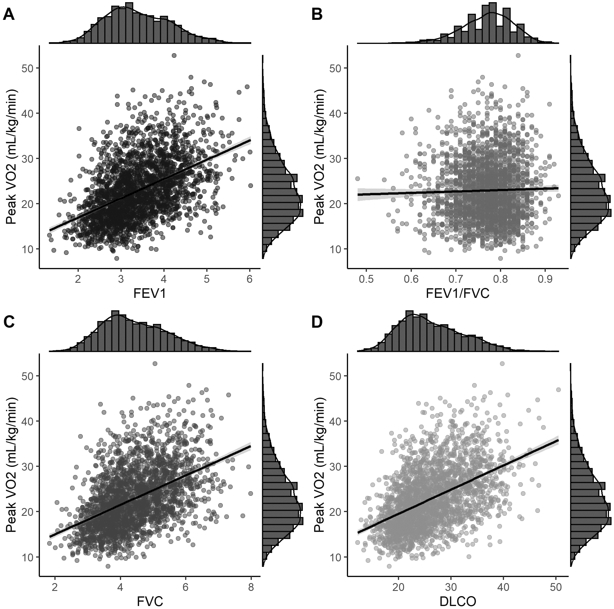
Scatter plots displaying the relationship of peak VO2 within FEV1 (Panel A), FVC (Panel B), FEV1/FVC (Panel C), and DLCO (Panel D). An unadjusted linear fit to the datapoints is included with a 95% confidence bands. Histograms of each variable are included on the margins.
Raw data display of A. FEV1, B. FVC, C. FEV1/FVC D. DLCO with relative peak VO2 with the dots representing individual participants
In order to investigate physical activity as a potential confounder, we performed additional analyses accounting for physical activity index (PAI) in multivariable models. We found similar findings as the main analysis with lower FEV1, FVC and DLCO associated with lower peak VO2. In addition, to further address the effect of age, we assessed age by quartile within the cohort and fit a model with an interaction between the age quartiles and pulmonary function (FEV1, FVC, and DLCO). For the pulmonary function variables, the interaction with age was not significant (See Supplemental Table 1).
Reduced breathing reserve is associated with higher peak VO2
Interestingly, participants with a reduced breathing reserve (≤20%; n=180) had a higher peak VO2 compared with those with a preserved breathing reserve (>20%; n= 2134), with mean peak VO2 31.2±6.8 and 22.2±6.3 ml/kg/min, respectively. The reduced breathing reserve group had a slightly higher FEV1 at 3.54 L (versus 3.39 L), demonstrating the reduced breathing reserve is being driven more by a higher VE rather than a lower maximum voluntary ventilation (MVV). Among the sample studied, 187 (8%) met criteria for obstructive lung disease and 53 (2%) for restrictive lung disease. For participants who met criteria for GOLD Stage 1 (N=140) and 2 (N=47) the mean peak VO2 was 22.9± 7.02 ml/kg/min and 19.3± 5.9 ml/kg/min, respectively. By contrast, those with restrictive lung disease (N=53) had a mean peak VO2 of 17.4 ± 3.8 ml/kg/min.
As a sensitivity analysis, the peak breathing reserve was compared between individuals with a RER<1.0 (N=43) and RER >1.0 (N=2314) with the mean as 65.8± 15.2% and 43.3±10.1%, respectively.
Lower lung function is associated with lower cardiopulmonary performance during exercise
In secondary analyses, using multivariable-adjusted linear regression analyses, we examined the relationship of lung function with other metrics of cardiopulmonary function with exercise. We found that lower FEV1, FVC and DLCO were associated with lower OUES and oxygen pulse slope (Table 2). With respect to chronotropic response, we found that lower DLCO was associated with lower % predicted HR achieved, whereas none of the spirometry-based measures were associated with HR response.
When examining respiratory performance, a lower FEV1, FVC and DLCO were associated with a lower VO2 at AT and VE at AT (p<0.001 for all). In addition, a lower FEV1, FVC and FEV1/FVC were associated with a lower breathing reserve at AT and peak (p<0.001 for all). These results are not unexpected, as FEV1 is utilized for MVV calculation and VE is a parameter in the breathing reserve equation.
Lower pulmonary blood vessel volume and emphysema on CT imaging is associated with lower peak VO2
Among the subset of N=1389 with CT measures, we found very few with evidence of radiographic abnormalities in lung parenchyma. This included 65 participants with evidence of emphysema on CT imaging with mean peak VO2 was 20.8 ± 5.3 ml/kg/min, compared with peak VO2 of 22.5± 6.7 ml/kg/min for those without disease (N=860). There were 20 participants with interstitial lung abnormalities (ILA) on CT imaging, with a mean peak VO2 of 23.1 ± 7.9 ml/kg/min, similar to those without disease (N=905) (22.4± 6.6 ml/kg/min).
When pulmonary blood vessel volume was examined, those in the lower quartile of TBV and peripheral blood vessel volume (BV5) had lower peak VO2 least squares means TBV lowest quartile LSMEAN: 20 ml/kg/min (95% CI: 19.4, 20.7); BV5 lowest quartile LSMEAN:20.9 ml/kg/min (95% CI: 20.3. 21.5)) in comparison to the highest quartile (TBV highest quartile LSMEAN: 22.9 ml/kg/min (95% CI: 22.2, 23.6); BV5 highest quartile LSMEAN: 22.1 ml/kg/min (95% CI: 21.5, 22.8) (p<0.001 for both)) (Figure 3). In regression analysis, a one mL lower TBV, was associated with a 0.18% (95% CI: 0.12%,0.24%) lower peak VO2 (mL/kg/min) and a one mL lower BV5 was associated with a 0.29% (95% CI: 0.15%, 0.43%) lower peak VO2 (Table 3). In addition, each SD lower BV5/TBV was associated with a higher peak VO2, although no relationship was seen with BV5/lung volume and peak VO2.
Figure 3. Peak VO2 across quartiles of CT pulmonary vasculature volumes.
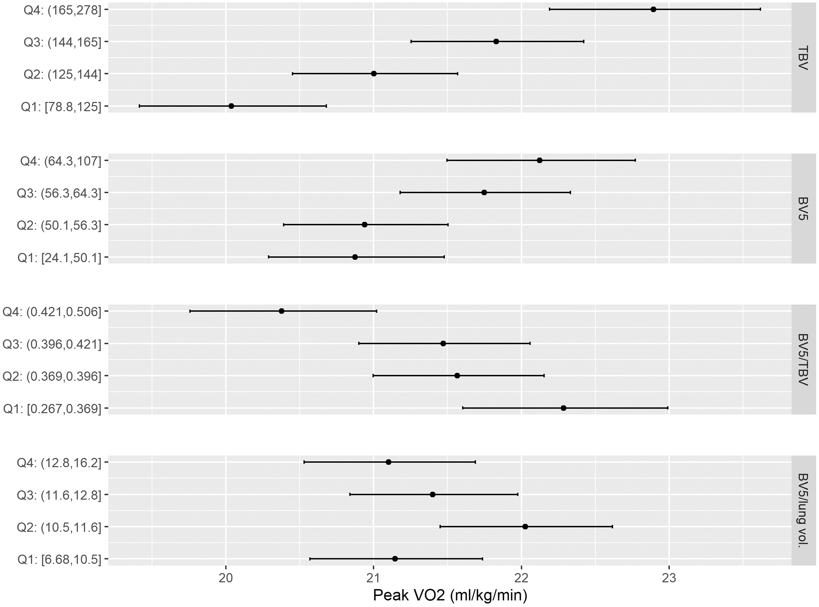
Forest plots displaying the peak VO2 least squares means adjusted for age, sex, height and weight across quartiles of BV5, TBV, BV5/TBV, and BV5/total lung volume. The black points represent the quartile mean peak VO2.
Table 3:
Association of pulmonary vasculature volumes with peak oxygen consumption and OUES
| Variable | TBV | BV5 | BV5/TBV | BV5/Total lung volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta est. | se | p-value | beta est. | se | p-value | beta est. | se | p-value | beta est. | se | p-value | |
| Peak VO2 | 0.18 | 0.03 | <0.001 | 0.11 | 0.027 | <0.001 | −0.09 | 0.03 | 0.007 | −0.02 | 0.02 | 0.54 |
| OUES | 0.2 | 0.03 | <0.001 | 0.13 | 0.024 | <0.001 | −0.08 | 0.03 | 0.005 | −0.01 | 0.02 | 0.70 |
Analyses were adjusted for age, sex, smoking status (never, current former), hypertension, height (inches), weight (kg), diabetes mellitus, and Framingham cohort. Effect sizes were expressed as the standard-deviation difference in dependent variable (peak VO2, OUES) per 1-standard deviation change in independent variable (blood vessel volume).
Abbreviations: TBV: total pulmonary blood vessel volume; BV5: peripheral pulmonary blood vessel volume; VO2: maximum oxygen consumption; OUES: oxygen uptake efficiency slope
In order to investigate physical activity as a potential confounder, we performed additional analyses accounting for physical activity index (PAI) in multivariable models. We found similar findings as the main analysis with lower TBV and BV5 associated with lower peak VO2.
In addition, to further address the effect of age, we assessed age by quartile within the cohort and fit a model with an interaction between the age quartiles and pulmonary blood vessel measurements (BV5 and TBV). For the pulmonary blood vessel measurements variables, the interaction with age was not significant (Supplemental Table 1).
Discussion
The primary findings in this study are threefold: first, among a large sample of community-dwelling adults, we found that lower lung function as ascertained by FEV1, FVC and DLCO were associated with lower exercise capacity. By contrast, there was no association of FEV1/FVC ratio with exercise capacity, suggesting that restrictive but not obstructive physiology may be an important determinant of overall fitness among ostensibly healthy individuals. Second, we found that beyond peak VO2 as an integrated measure of fitness, lung function measures were also associated with specific aspects of cardiovascular and respiratory performance, including OUES, oxygen pulse slope, VO2 at AT, VE at AT and breathing reserve. These findings indicate that the association of lung function and exercise capacity may be related to multiple specific exercise abnormalities in cardiopulmonary performance, including effort-independent measures and measurements taken prior to peak exercise capacity. Lastly, we found that lower radiographic pulmonary vasculature volume as assessed by TBV and BV5 was also associated with lower exercise capacity. Taken together, these findings suggest that lung function, even within the normal range, and with preserved breathing reserve has an effect on exercise capacity.
These results expand upon prior studies that have demonstrated a positive association between peak VO2 and FEV1 in healthy adults and the elderly by demonstrating the association extends to FVC and DLCO and does not include FEV1/FVC. 5,6,16 Our findings that lower FEV1 and FVC, demonstrating a restrictive like physiology, is associated with lower exercise capacity is in agreement with prior studies demonstrating that this pattern of lung function decline in a healthy cohort is associated with higher rates of cardiovascular disease development.17 For example, healthy participants defined as rapid decliners in FEV1 and FVC over a one-year period (average 3% change in spirometry) had a 4-fold increased risk in incident heart failure over the same time period.17 Our results demonstrating an association of lung function with the gold standard measurement for functional capacity, peak VO2, further highlights the interplay of cardiac and pulmonary systems as lower cardiorespiratory fitness is associated with higher rates of cardiovascular disease and all-cause mortality and draws attention to the use of pulmonary function testing as a key additional element of not only lung disease but early detection of cardiac disease.18
Given VO2 can be limited by pulmonary diffusing capacity, maximal cardiac output, oxygen carrying capacity of blood or peripheral extraction, we examined additional cardiopulmonary exercise variables to help further characterize the interplay of exercise capacity with pulmonary function. We found that lower OUES was associated with lower pulmonary function. OUES has advantages in comparison to peak VO2, in that it is an accurate, reproducible, and objective measure of functional capacity at submaximal exercise levels.7 Given OUES and peak VO2 have previously been shown to be correlated, it is not surprising the same lung function relationships were seen with OUES and peak VO2, with the results suggesting that in those who fail to achieve peak performance the lung function measurements are still related to cardiopulmonary performance.7 We also found that lower FEV1, FVC and DLCO were associated with a lower oxygen pulse slope. This highlights that the relationship between lung function and exercise capacity persists even after adjusting for heart rate.
The relationship of pulmonary diffusion capacity with peak VO2 may reflect decreased need for ventilation for CO2 and O2 transfer and/or increased pulmonary capillary blood volume. Indeed, we found that DLCO was related to OUES and that CT scan-based measures of pulmonary blood volume were related to peak VO2. To better understand this relationship, we examined the association of pulmonary blood vessel volume with functional capacity. Similar to DLCO, we observed that lower TBV and BV5 were associated with a lower peak VO2. However, the magnitude of the association of TBV to peak VO2 is substantially larger than the association for BV5, which results in the observed inverse association of lower BV5/TBV with higher peak VO2. These results indicate that the total detectable blood vessel volume on CT imaging at rest is most strongly linked to higher exercise tolerance, which, given that the pulmonary circulation must accommodate a five-fold increase in blood flow from rest to peak exercise, that TBV may be an indicator of greater vascular capacitance/potential for distensibility.19 Therefore, a higher TBV at rest may identify individuals with a greater potential for additional vessel recruitment and therefore oxygen extraction with exercise. In addition, the caliber of pulmonary vasculature has been graded by the pulmonary transit of agitated contrast (PTAC) during exercise, with larger vessels having more PTAC. 20 Individuals with greater PTAC at peak exercise have greater peak VO2, higher cardiac output and lower pulmonary vasculature resistance (PVR).20 This suggests when transitioning from resting to exercise, a larger appearance of the total pulmonary vasculature may indicate an increased distensibility during exercise, allowing greater blood flow and reduction in right ventricular afterload. We acknowledge that the current study is not able to ascertain these important physiological aspects of pulmonary vascular function, but rather assessed pulmonary vascular anatomy and volumes as a non-invasive imaging measure. Our findings suggest that a possible explanation for the relationship of lung function with peak VO2 extends beyond involvement in cardiac output and is related to pulmonary vascular volume at baseline.
To address potential confounding from baseline physical activity, additional analyses including PAI were included in the model and similar results that lower lung function and lower pulmonary blood vessel volume were associated with lower peak VO2 remained. For example, we found that DLCO was related to exercise capacity. As DLCO increases, less minute ventilation is required to transfer oxygen (OUES) and CO2 (VE/VCO2 slope) indicating higher efficiency of gas exchange during exercise.12 DLCO and TBV at rest are indicators of lung’s ability to accommodate blood volume, a property that must acutely adapt during incremental exercise with requisite augmentation of thoracic blood volume to support cardiac output augmentation.
There are some limitations of the study. The FHS allowed us to study the gold standard measurement of cardiorespiratory fitness through collection of peak VO2, however invasive hemodynamic measurements and arterial blood gases were not available. The collection of lung function and structure measurements (years 2008-2011) occurred prior to CPET measurements (years 2016-2019), which would have been expected to bias our results toward the null. Participants were from a predominantly Caucasian background, limiting potential generalizability of the results. Finally, future studies accounting for baseline physical activity using objective measures will be important to better understand the association of lung function and pulmonary blood vessel volume with functional capacity. Strengths of our study include a large cohort of community-dwelling adults with rigorous cardiopulmonary phenotyping including careful measurement of pulmonary function, CPET, and CT imaging of lung parenchyma and pulmonary vascular volumes.
Our findings indicate that subclinical and subtle decline in lung function can adversely impact exercise capacity as measured by peak VO2. While differences in exercise capacity between individuals is often ascribed to cardiac performance alone or degree of exposure to physical activity, our findings frame the importance of investigating the pathobiologic underpinnings of how the entire spectrum of lung structure and function impacts functional capacity. Our findings also highlight the importance of promoting lung health, including potential screening and identification of high-risk individuals for functional decline, as a way of optimizing peak VO2, which is known to be a potent prognostic predictor in referral populations and in the general population. These preventative strategies would include removal of pulmonary toxic behaviors such as smoking as well as exercise programs focused on respiratory muscle strength.
Conclusion
In a large community-based sample of whom the majority had preserved breathing reserve, we found a lower FEV1, FVC and DLCO were associated with a lower peak VO2. By contrast, FEV1/FVC ratio was not associated with peak VO2, suggesting a restrictive physiology pattern was more closely tied to functional capacity. Further, we observed that lower FEV1, FVC, and DLCO were associated with integrated measures of both cardiac performances including OUES and oxygen pulse slope, as well as pulmonary performances including VO2 at AT, VE at AT and breathing reserve at AT and peak. Lastly lower total and peripheral pulmonary vasculature volume as measured by CT imaging were associated with lower peak VO2. These findings underscore the importance of lung structure and function as contributors to overall functional capacity, even in the absence of abnormal breathing reserve.
Supplementary Material
Funding:
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contracts N01-HC-25195, HHSN268201500001I, and 75N92019D00031) and by NIH grants F32 HL143819 (A.J.S.); K23-HL138260 (MN); K23-HL136905(F.N.R); R01 HL134893 (J.E.H.); R01 HL140224 (J.E.H.); K24 HL153669 (J.E.H.) 1R01HL131029 (RSV and GDL), R01HL 151841 (GDL), and AHA grant 15GPSGC24800006 (GDL). RSV is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. AJS is supported by the American Lung Association.
Footnotes
Publisher's Disclaimer: “This is an author-submitted, peer-reviewed version of a manuscript that has been accepted for publication in the European Respiratory Journal, prior to copy-editing, formatting and typesetting. This version of the manuscript may not be duplicated or reproduced without prior permission from the European Respiratory Society. The publisher is not responsible or liable for any errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final, copy-edited, published article, which is the version of record, is available without a subscription 18 months after the date of issue publication.”
Declarations of Interest: Dr. Ho has received research support from Gilead Sciences and Bayer AG, and research supplies from EcoNugenics. Dr. San Jose Estepar has contracts with Lung Biotechnology, Insmed and Boeringer Ingelheim, receives consulting fees from Leuko Labs and has stock options in Qunatitative Imaging Solutions.
Take home message: Lower FEV1, FVC and DLCO were associated with lower exercise capacity, as well as oxygen uptake efficiency slope and ventilatory efficiency. In addition, lower total and peripheral pulmonary blood vessel volume were associated with lower peak VO2.
References
- 1.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290(12):1600–1607. [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. [DOI] [PubMed] [Google Scholar]
- 3.Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife Cardiorespiratory Fitness and the Long-Term Risk of Mortality: 46 Years of Follow-Up. J Am Coll Cardiol. 2018;72(9):987–995. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr., Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. [DOI] [PubMed] [Google Scholar]
- 5.Rasch-Halvorsen O, Hassel E, Langhammer A, Brumpton BM, Steinshamn S. The association between dynamic lung volume and peak oxygen uptake in a healthy general population: the HUNT study. BMC Pulm Med. 2019;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassel E, Stensvold D, Halvorsen T, Wisloff U, Langhammer A, Steinshamn S. Association between pulmonary function and peak oxygen uptake in elderly: the Generation 100 study. Respir Res. 2015;16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba R The oxygen uptake efficiency slope and its value in the assessment of cardiorespiratory functional reserve. Congest Heart Fail. 2000;6(5):256–258. [DOI] [PubMed] [Google Scholar]
- 8.Synn AJ, Li W, San Jose Estepar R, et al. Radiographic pulmonary vessel volume, lung function and airways disease in the Framingham Heart Study. Eur Respir J. 2019;54(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–1335. [DOI] [PubMed] [Google Scholar]
- 10.Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring). 2014;22(11):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139(8):857–861. [PubMed] [Google Scholar]
- 12.Nayor M, Xanthakis V, Tanguay M, et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in Patients With Preserved Left Ventricular Systolic Function. Circ Heart Fail. 2020;13(5):e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estepar RS, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. [DOI] [PubMed] [Google Scholar]
- 15.Rahaghi FN, Argemi G, Nardelli P, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019;54(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancox RJ, Rasmussen F. Does physical fitness enhance lung function in children and young adults? Eur Respir J. 2018;51(2). [DOI] [PubMed] [Google Scholar]
- 17.Silvestre OM, Nadruz W Jr., Querejeta Roca G, et al. Declining Lung Function and Cardiovascular Risk: The ARIC Study. J Am Coll Cardiol. 2018;72(10):1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134(24):e653–e699. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey JA. J.B. Wolffe memorial lecture. Is the lung built for exercise? Med Sci Sports Exerc. 1986;18(2):143–155. [PubMed] [Google Scholar]
- 20.La Gerche A, MacIsaac AI, Burns AT, et al. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol (1985). 2010;109(5):1307–1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


