Change history
2/1/2023
An Editorial Expression of Concern to this paper has been published: 10.1007/s13659-023-00370-7
Abstract
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is an orange-red, lipophilic keto-carotenoid pigment. It is majorly found in marine ecosystems particularly in aquatic animals such as salmon, shrimp, trout, krill, crayfish, and so on. It is also synthesized in microalgae Heamatococcus pluvialis, Chlorococcum, Chlorella zofingiensis, red yeast Phaffia rhodozyma and bacterium Paracoccus carotinifaciens. Some aquatic and terrestrial creatures regarded as a primary and secondary sources of the astaxanthin producing and accumulating it through their metabolic pathways. Astaxanthin is the powerful antioxidant, nutritional supplement as well as promising therapeutic compound, observed to have activities against different ravaging diseases and disorders. Researchers have reported remarkable bioactivities of astaxanthin against major non-communicable chronic diseases such as cardiovascular diseases, cancer, diabetes, neurodegenerative, and immune disorders. The current review discusses some structural aspects of astaxanthin. It further elaborates its multiple potencies such as antioxidant, anti-inflammatory, anti-proliferative, anti-cancer, anti-obese, anti-diabetic, anti-ageing, anti-TB, anti-viral, anti-COVID 19, neuro-protective, nephro-protective, and fertility-enhancing properties. These potencies make it a more precious entity in the preventions as well as treatments of prevalent systematic diseases and/or disorders. Also, the review is acknowledging and documenting its powerful bioactivities in relation with the pharmaceutical as well as nutraceutical applicability.
Graphical Abstract
Keywords: Astaxanthin, Nutritional supplement, Therapeutic compound, Chronic diseases, Fertility enhancer
Introduction
Carotenoids are a large class of bio-pigments found universally in plants, algae, fungi, and bacteria in yellow, orange and red shades. Terpenoids is one of the subfamily of carotenoids containing more than 55,000 varied structures. It is a well-known group of natural secondary metabolites having pharmaceutical, nutraceutical, and seasoning properties [1–3]. Prior studies have shown significant role of carotenoids on human and animal health [4]. Some well-known bio-functions of carotenoids are vitamin A conversion and immunity boost up. It is also useful in the protections by scavenging of free radicals in some lethal non-communicable diseases viz, Cardiovascular diseases, Cancer, Respiratory diseases, Diabetes [5, 6]. Since carotenoids cannot be synthesized by animals and is need to be provided as the dietary supplements for the wellness purposes [7].
In recent times, hectic lifestyle has increased the prevalence of many chronic diseases. Consequently, people are trying to manage their lifestyle habits with the help of some preventive healthcare supplements and that stands a driving force of market growth in pharmaceutical and nutraceutical industries. Emerging economic countries such as China, India and South America have been anticipated for offering growth opportunities, predominantly in the nutraceutical and cosmetic industries for the next six years in the global market [8].
Earlier, people used to have medicines and pharmaceutical products whenever they had some illnesses. However, during the last few years, people throughout the world are becoming more self-aware and health conscious. Such habits of the people have been enhanced more after COVID-19 pandemic. They are now very much curious and are giving more importance to maintaining their good health and wellnesses. With this perspective, the health and wellness industries are going to get uplifted in coming years. The wellness is described as a process that an individual follows to achieve top mental health and physical wellbeing. Which can be attained by developing a good eating habit, along with an active lifestyle. Exactly here the role of certain dietary supplementation get involved. The researchers are actively exploring more and more superfoods in this context [8].
It is being brainstormed, that certain natural bioactive compounds like carotenoids may possesses the potency of fulfilling such nutritional gaps and hence, are getting explored curiously for it. The carotenoid named, astaxanthin (AST) is top listed in those bioactive compounds. Which is a tetra-terpenes with cyclic C40 scaffold with eight isoprene units. In global market, AST acquires third position as the most important carotenoid after β-carotene and lutein. Perhaps the trade market of AST has been expected to be valued at USD 647 million in 2021 and estimated to the peak up to USD 965 million by the year 2026 [4].
Chemistry of astaxanthin
Astaxanthin is a strong lipophilic keto-carotenoid [3,3′-dihydroxy-β,β′-carotene-4,4′-dione] insoluble in aqueous solutions while soluble in nonpolar solvents such as dichloromethane (~ 30 g/L), chloroform (~ 10 g/L), dimethylsulfoxide (~ 0.5 g/L) and acetone (~ 0.2 g/L) (Table 1). The AST shows close structural resemblance to other carotenoids such as β-carotene, zeaxanthin and lutein. Its absorption spectrum reveals a conjugated polyene structure with λmax of 489, 478, and 480 nm in chloroform, ethanol, and acetone respectively [7]. It is an optically active compound; as its structure is made up of long nonpolar conjugated polyene backbone and two polar β-ionone rings at each side [9]. Each ring holds one hydroxyl (–OH) and one keto (C=O) moiety actually imparts a good antioxidant property to it. The polar-nonpolar-polar linear structural array of the astaxanthin enable it to bind and span the cell membrane. Thus, AST’s chemical structure and its orientation in plasma membrane (Fig. 1) favor counterbalancing of reactive oxygen (ROS) as well as reactive nitrogen species (RONS) [10]. AST is well recognized for its antioxidant potencies. It is 110 times potent antioxidant than vitamin E, 560 times than green tea catechins, 800 times than Co-Q 10, 3000 times than resveratrol and 6000 times than vitamin C [11–13]. It is strongly supposed as a completely unique antioxidant and a potent bioactive compound as it shows three concurrent novel peculiarities i.e., powerful anti-oxidation property, safe for human use and acquires best position within the cell membrane [13].
Table 1.
Chemical properties of astaxanthin
| CAS-No | 472-61-7 |
| E-No | E161j |
| Doc. No. (21 CFR) | 73.35 |
| MF | C40H52O4 |
| MW | 596.8 g mol.−1 |
| Density | 1.07 g/cm3 |
| MP | 216 °C |
| BP | 774 °C |
Fig. 1.
Orientation of astaxanthin, β-carotene, vitamin C and vitamin E in plasma membrane
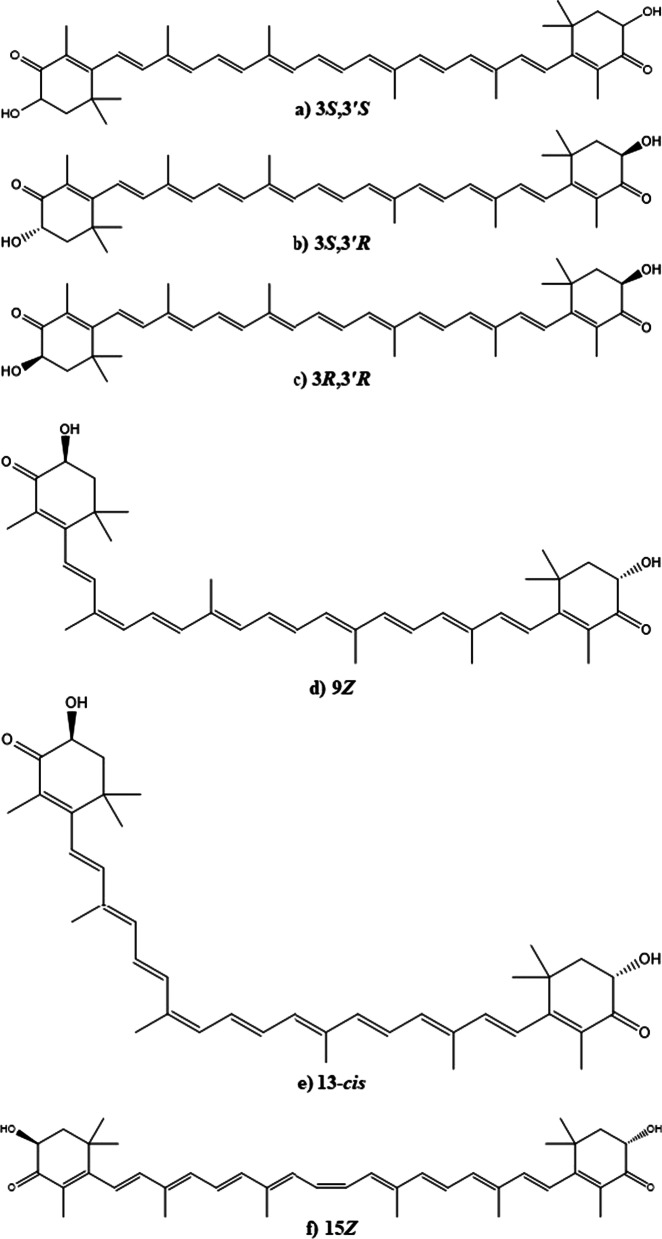
Structurally, AST is present in trans and cis (E and Z) isomeric forms (Fig. 2). Whereas, all trans (all E-AST) isomers are more stable and therefore those are abundantly found in the nature. Three different stereoisomers namely 3S,3′S; 3S,3′R (meso); 3R,3′R; results due to presence of chiral centers at C-3 and C-3′ on β-ionone rings. Amongst them, the all trans 3S,3′S isomer is commonly seen in nature. Its esterification can be possible because of hydroxyl (-OH) groups on C-3 and C-3′ positions, which enables its increased solubility, absorbability and transport within the cells. Either one or both hydroxyl (-OH) groups can bind to various fatty acids and produce mono or di-esters. AST can also be present in a free form where hydroxyl (-OH) groups remain to be esterified and even it forms a bonding with proteins or lipoproteins in the cell [9, 14]. The change in molecular environment leads to change in an absorbance of AST. For example, in the case of lobsters carotenoprotein complex is observed to be greenish blue rather than reddish orange [9].
Fig. 2.
Structures of optical isomers (a–c) and geometric isomers (d–f) of the astaxanthin
Synthetic astaxanthin
Chemically derived AST is heading the market over natural AST [15]. Basically, the manufacturing of synthetic AST is more complex when it comes to pharmaceutical standards. However, researchers have succeeded in the artificial synthesis of 3S,3′S enantiomer; mimicking natural AST [16]. Further, derivatives are being developed for clinical trials studies. Disodium disuccinate astaxanthin (DDA), a synthetic form of AST manufactured by Cardax Pharmaceuticals and reported to be used in experimental models of myocardial ischemia and reperfusion [17]. It’s easy to use by intravenous or oral way, because of good aqueous solubility [18]. Since DDA is not available from the manufacturers, another derivative of AST has been launched by Cardax Pharmaceuticals i.e. pro-drug Heptax/XanCor, CDX-085 [19]. The pro-drug was claimed for the treatment of liver inflammatory diseases, reduction of triglycerides, prevention of re-thrombosis and amelioration of the metabolic syndrome [20]. The CDX-085 compound shows greater water solubility as well as bio-availability than DDA and the natural AST derived from H. pluvialis [18, 21].
Although the synthetic AST has better applicability, people prefer to use natural AST produced by microorganisms using biotechnological processes [22]. Due to the more demand of natural AST by the health conscious people, the industries like pharmaceutical, nutraceutical, feed, and cosmetics use only natural AST because of its biosafety [21] (Fig. 3).
Fig. 3.
Astaxanthin applications in various industries
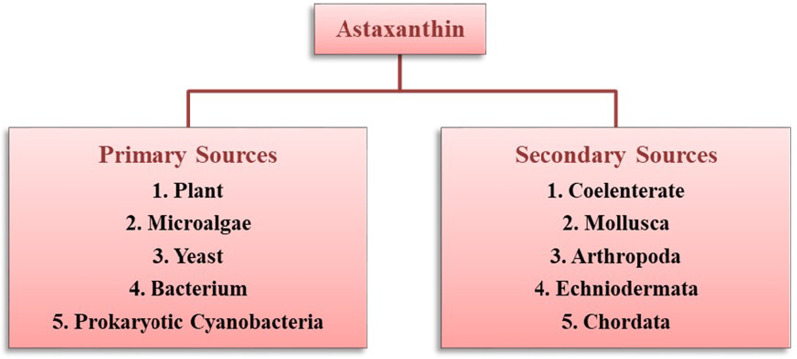
Natural astaxanthin
The known primary sources of natural AST includes certain, plants, algae, fungi, yeast and bacteria. More precisely, the sources may include flowering plant (Adonis), microalgae (Haematococcus pluvialis, Chlorella zofingiensis, Chlorococcum sp.), yeast (Phaffia rhodozyma) and bacteria (Agrobacterium aurantiacum, Paracoccus carotinifaciens) [14, 23] (Fig. 4). The AST biosynthesis occurs by de nono pathway in their natural systems including. AST is ubiquitously found in marine ecosystems like salmon, shrimp, crayfish, lobster, krill, Arctic char, brook trout (red trout), Pagrus major (red bream), red snapper (Lutjanus campechanus), copepod, and asteroidean. The feathers of birds like quail, flamingo and scarlet ibis could also own AST [21, 24]. These marine animals are unable to synthesize AST by biochemical ways, and therefore they do take and accrue AST from zooplanktons including algae and other microbes [9, 14, 25].
Fig. 4.
Natural sources of astaxanthin production
Primary sources
The genes required for astaxanthin biosynthesis can be observed in some natural producers such as plant, microalgae, yeast, bacteria, etc. Therefore, these are considered as the primary sources and have been elaborated in the following section.
Plant derived astaxanthin
As per earlier reports, AST accumulation was observed in plants of Adonis species with an AST biosynthesis pathway wholly distinct from that of bacteria and green algae [26]. The red–orange flowers of the genus Adonis from Ranunculaceae family are well-known for AST production. The species like A. aertivalis, A. amurensis, A. annua have been studied so far. According to Mawson R., a strain A. aertivalis is an abundant source of valuable AST. It can give 200–350 μg of AST from an average of 18–22 petals of a single flower [27]. In A. annua flower petals, the amount of AST reported is about 1% of its dry weight [28, 29]. As per the recent studies of Li Y., AST extracted from the A. amurensis flower petals was accounted for 3.31% of the dry weight [30].
In addition, one of the classes of Bryophyta known as liverworts (small green terrestrial plants) has been recently reported to produce AST. The AST has been detected in the liverwort gametophyte by a Thin Layer Chromatography (TLC) of the same [31–33]. This may suggest that there might be many more plant sources yet to be explored for AST productions.
Microbial derived astaxanthin
Microalgae, Haematococcus Among different microbial sources, Haematococcus pluvialis is considered as the most promising one; therefore, it is widely exploited for the natural AST production. H. pluvialis (class -Chlorophyceae; order- Volvocales; family- Haematococcaceae) is a unicellular eukaryotic photosynthetic green microalgae generally found in freshwater [34]. Currently, the highest commercial AST production takes place from the H. pluvialis with 3.8%–5% of its dry weight [35–38]. The produced AST is present with 73% trans-AST and 27% as cis-AST and predominantly in 3S,3′S form [39–42]. The United States Food and Drug Administration (USFDA) have recommended H. pluvialis derived AST as a “GRAS” (Generally recognized as safe) in 1999 and can be considered as a nutraceutical compound for consumptions [43]. However, the European Food Safety Authority (EFSA) has approved daily intake AST for humans is 0.034 mg/kg and can be used in dietary supplements in the form of nutraceuticals [44–46].
Red yeast Phaffia (Xanthophyllomyces dendrorhous) Phaffia rhodozyma (class- Tremellomycetes; order- Cystofilobasidiales; family- Cystofilobasidiaceae) has also been recognized as another good source of natural AST producers [47]. P. rhodozyma is reported to produce 0.5% AST of its dry weight [48, 49]. Generally, free and non-esterified 3R,3′R AST is synthesized. The red yeast’s AST is considered as GRAS by USFDA and has been certified as a colour additive in fish feed supplements by FDA [38, 50, 51]. Recently, another yeast (Rhodosporidium toruloides) was reported as a new source of AST and in coming years, the source will be more explored for its productions and other properties [46, 52].
Bacteria:
Paracoccus: Paracoccus carotinifaciens (class- Alphaproteobacteria; order- Rhodobacterales; family- Rhodobacteraceae) is an anaerobic Gram-negative bacterium that shows a wide range of carotenoid synthesis abilities [53]. P. carotinifaciens is reported to produce 2.2% AST of its dry weight in non-esterified form [54, 55]. The stereo-isometric form i.e., 3S,3′S produced by this bacterium is similar to that of H. pluvialis form. And many human trials revealed that bacterium derived AST is considered as safe for human consumption [45, 46, 56]. So the source is being more explored by multiple research groups for enhanced productions and more applicability.
Prokaryotic cyanobacteria: AST was also found in a limited number of cyanobacteria. It is synthesized from β-carotene via zeaxanthin and canthaxanthin by carotene β-ketolase (BKT/CrtW/CrtO) and CHYB/CrtZ/CrtR [57].
Secondary sources
Secondary sources of AST includes some aquatic and terrestrial animals, which cannot synthesize it through their metabolism. However, they can acquire it from their food chain. Some of the secondary sources of AST have been highlighted in the following section.
Coelenterate
AST is observed in tissues of certain marine animal, which actually originates from their dietary zooplankton [58, 59]. Two unique metabolites of AST i.e., 2-nor-astaxanthin and actinioerythrin were reported in sea anemones, Actinia equina and Tealia feline [60].
Mollusca
In a freshwater snail Pomacea canaliculata, an experiment with feeding of carotenoids have showed that the β-carotene was oxidatively metabolized to AST (3S,3ʹS) [60, 61]. In the food chain of algae to sea angel, Clione limacina, AST is found to be produced from the oxidative metabolism of β-carotene, β-cryptoxanthin and zeaxanthin. However, 7,8-didehydroastaxanthin and 7,8,7ʹ,8ʹ-didehydroastaxanthin were seem to be synthesized from diatoxanthin and alloxanthin respectively [60].
Arthropoda
Many crustaceans can also synthesize AST from β-carotene, ingested in dietary algae, via different metabolic intermediates such as echinenone, 3-hydroxyechinenone, canthaxanthin, and adonirubin. In Penaeus japonicas, racemization of AST was observed when these prawns were fed with [3H]-labeled AST (3S,3ʹS) [62]. As a result, the isotopic AST converted to (3R,3ʹR)-meso, and (3S,3ʹS)-isomers at an approximate ratio of 1:2:1. Among this metabolic conversion, iso-astaxanthin (4,4ʹ-dihydroxy-ε,ε-carotene-3,3ʹ-dione) was the key intermediate [60].
In Orthoptera, particularly grasshoppers do possess AST and adonirubin as mixtures of optical isomers [63]. However, in locusts, it was synthesized from β-carotene.
In Lepidoptera, in the pupae of swallowtail Papilio xuthus, zeaxanthin metabolism results in AST (3S,3ʹS) via adonixanthin. Whereas orange green shade of pupae is possibly due to AST, fritschiellaxanthin, and papirioerythrinon metabolites [64].
In spider Nephila clavata (Arachnida), AST is present as one of the major keto-carotenoid [65]. As per the report of Veeman, two-spotted spider mite i.e. Tetranychus urticae contains a series of keto-carotenoids such as adonirubin, astaxanthin and 3-hydroxyechinenone [66]. During long nights of lower temperatures, when female spider mites enter into a facultative diapause condition characterized by the termination of reproduction, visible changes in the body colour from faint yellow to red–orange shade occurs. Such transition results due to the accumulation of keto-carotenoids, such as AST. Hence, AST was proposed to protect from physical stress caused by overwintering [60].
As per the recent reports of Wybouw and co-workers, the knockout technique revealed that the endogenous CYP384A1 gene of Tetranychus urticae (plant feeding mite) is positively code for carotenoid ketolase, which synthesizes AST [33, 67].
Echinodermata
Starfishes are carnivorous in nature and they feed on small crustaceans and bivalves. In the marine system, carotenoids mainly transfer from zooxanthellae (dinoflagellate algae) to starfish via corals. The starfish Acanthaster planci eats corals. Whereas, coral Acropora japonica shows symbiotic association with zooxanthellae and so carotenoids easily get absorbed from them and accumulate in corals without any changes. By the end of these oxidative metabolic transformations, 7,8-didehydroastaxanthin and AST were found as major keto-carotenoids in starfish [59, 68, 69].
AST was also observed in the gonads of sea cucumbers. One of the known sea cucumber Plesiocolochirus minutus was found to own 9Z,9ʹZ configured tetrahydroastaxanthin and which has been considered to be a metabolite of AST [70].
Chordata
Oxidative metabolism of zeaxanthin in Cyprinidae fish leads to the production of AST (3S,3ʹS) via adonixanthin and idoxanthin [60]. Whereas in Reptilia, it was found in various species of lizards. While in the zebra finch, it was a major carotenoid in feathers and beak along with other keto-carotenoids, such as 4-ketolutein, adonirubin, and canthaxanthin [71]. The red colour shade in the feathers of American flamingos is due to AST, derived from dietary micro-crustaceans [72]. Among different carotenoids, it was also observed in the red plumage of caciques and meadowlarks (Icteridae) [71].
The pompadour cotinga (Xipholena punicea), a species from the Amazonian rainforest shows interesting structural carotenoids such as 3-methoxy-4-keto-β-ring and 2,3-didehydro-3-methoxy-4-keto-β-ring moieties along with canthaxanthin and AST [73, 74].
Astaxanthin biosynthesis factors
Primarily β-carotene forms a precursor for AST synthesis and is catalyzed by β-carotene ketolase and hydroxylase to canthaxanthin and zeaxanthin metabolites respectively. Previous investigations say that, declined photosynthetic activity or a limited oxygen evolution triggers accumulation of AST. The damaged PS-II complex generates limited O2 which acts as the principal factor for AST pigment synthesis [75–79]. Therefore, the amount of AST production is inversely proportional to photosynthetic events, despite the amount of chlorophyll and PS-II reaction center remains constant [78, 80].
The photosynthetic inequity between input energy from the light adsorption by an antennae and output energy in the form of CO2 fixation gets quenched and it produces reactive oxygen species (ROS). So, earlier studies reveals that the carotenoids certainly will prevent excessive injuries caused by ROS; directly quenching triplet chlorophyll (3-Chl) or singlet oxygen (1O2) produced from photodynamic reactions [81–83]. In contrast, when CO2 fixation gets restricted by stress, environmental surroundings such as nutrient famine, cold/high temperatures, high salinity or low CO2 accessibility, the production of these ROS could occur even at moderate light intensity because of an energy spare [84, 85]. While under nutrient starvation, O2 is possibly the most effective ROS species that might take part for AST accumulation [86, 87]. ROS could also stimulate the expression of genes coding for carotenogenesis enzymes [88, 89].
The higher light intensity and nutrient stress; permutated with NaCl or sodium acetate had shown boosted production of total carotenoids and total AST content to 32 mg/g and 24.5 mg/g of dry biomass, respectively [90, 91].
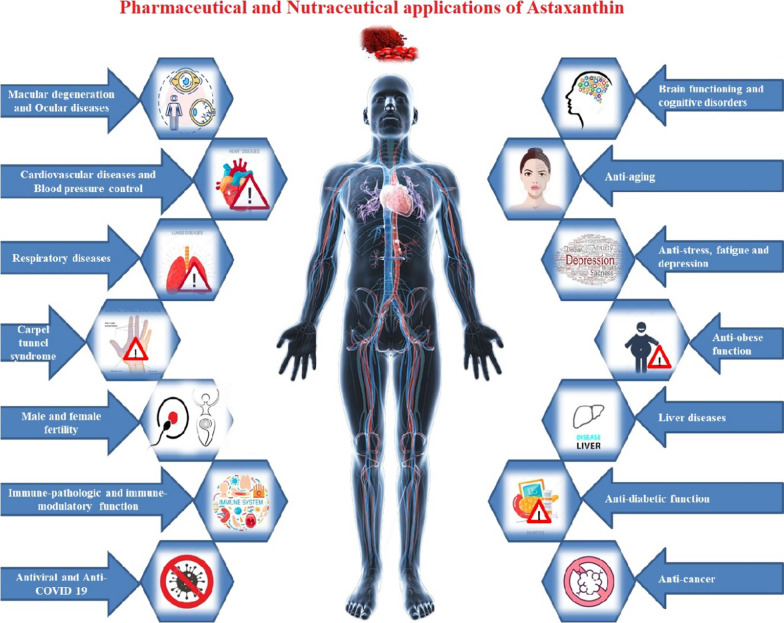
Pharmaceutical potential of astaxanthin in chronic diseases
The strong antioxidant property of AST has been allied to various biological functions was shown in animal and human clinical trials. It has promising applications in human health and nutritions [92–96]. Some of the remarkable applications of it have been discussed below.
Cardiovascular diseases
Cardiovascular diseases (CVDs) are a prominent cause of deaths worldwide. According to WHO, about 17.9 million people died from CVDs, signifying 32% of all global deaths, in 2019. Among those, 85% of deaths were simply due to heart attack and stroke [97].
Many risk factors eventually contribute to CVDs such as obesity, hypertension, dyslipidemia, glucose metabolism dysfunctions, oxidative stress, elevated levels of reactive oxygen species (ROS) and reactive nitrogen species (RONS) and inflammation [98–100]. But these risk factors can be controlled by managing and adapting healthy lifestyles, correcting biological clocks and having supplements enriched nutritious diets. As per earlier reports, the oxidative stress and inflammations are correlated and mutually contributed in the initial dealings of the CVDs. Those can be restricted by the influence of antioxidants like AST, which can modulate redox balance, regulate inflammatory responses and control lipid as well as glucose metabolism [101–105]. Such significant abilities of AST could help in preventing disorders like dyslipidemia, arterial hypertension and atherosclerosis as well [106].
Molecular and cellular mechanism of actions of AST has revealed its potential in preclinical and clinical studies in prevention and treatment of CVDs. By reducing mitochondrial and cellular oxidative stress, elevating antioxidant cascades and related enzymes, protecting blood rheological parameters, equilibrating mitochondrial dynamics, biogenesis and mitophagy, it can manage homeostasis too [10, 107, 108]. It also possesses the potential worth in setting of ischemia–reperfusion and controls glucose as well as lipid metabolism by reducing lipid peroxidation and re-thrombosis after thrombolysis [105, 109, 110].
Blood pressure control
Administration of AST has lowered BP in the rats, which was elevated there due to sucrose intake and insulin resistance [111]. Consequently, in hypertensive rats (SHRs) observed for lowering of blood pressure and hence delay in the frequencies of strokes [112, 113]. However, this antihypertensive effect is still not fully known and it needs to be studied comprehensively in future [105].
According to recent report of Mashhadi N.S., AST supplemented in type 2 diabetic patients were recorded for increased glucose metabolism, lowered visceral fat deposition (11.2 ± 3.4 vs. 11.85 ± 3.8% compared with placebo; P < 0.05) and decreased systolic blood pressure (132 ± 18 vs. 133 ± 19 and 143 ± 27 mmHg compared with placebo and baseline, respectively; P < 0.05) [114]. The factors such as blood plasma velocity and vasodilation can directly affect the vascular resistance, possibly contributing to hypertension and producing cardiac complications such as myocardial hypertrophy [115].
In experimental studies on SHR rats shown reduced systolic blood pressure and persuaded significant histological alterations in the aorta along with decreased vascular stiffness when supplemented with AST [102, 108, 116]. However, in stroke-prone SHR rats, oxidative stress marker like 8‑hydroxy‑2'‑deoxyguanosine (8‑OHdG) effectively down regulated. Henceforth, dropped systolic blood pressure was observed with repressed thrombogenesis in the cerebral veins [117].
However, synthetic AST i.e., CDX-085 pro-drug administration in murine model of thrombosis has shown increased blood flow of the carotid artery up to ~ 20% prior to manifestation of endothelial dysfunction [10, 19].
Anticancer activity
In current scenario, cancer is most perilous cause of death across the globe. It is accounted as second deadliest disease [118]. According to GLOBOCAN reports, an estimated incidences and mortality are constantly growing every year [119]. From many decades, different therapies and strategies are in use to combat against cancer. Among them, natural products based pharmaceuticals are gaining accomplishment in cancer treatments. As per FDA norms, almost 547 natural bioactives and their derivatives have been sanctioned for preclinical, clinical phases I to III, and preregistration studies. They basically originate from plants (47%), bacteria (30%) and fungi (23%) [120, 121].
Oxidative stress and related cellular damages such as DNA mutations and protein anomalies are the root causes of carcinogenesis. Along with other natural products, research scientists have revealed the anticancer role of different carotenoids. Among them, AST was discovered as a strong bioactive showing contribution in prevention and/or treatment of malignant cells [121]. In early 90 s’ its anticancer potential was seen against well-known genotoxic hepatocarcinogen i.e. aflatoxin B1, by studying detoxification mechanisms [122]. Where it showed maximum free radical scavenging activity [123], superoxide reductions and nitric oxide generation properties [124]. In another studies, AST suppressed the DNA damage when exposed to UV-A via its antioxidant cascades [125]. A successive 5 days treatment with 50 μM of AST to the mouse epidermal cell line JB6 P +, it displayed significant anti-neoplastic effects and decreased viability up to 94% [126]. Later, Chen et al. examined anti-proliferative activities of AST (168 μM) on two melanoma cell lines. In both cell lines proliferation inhibitions were observed, up to 50% in A375 and 80% in A2058 cell lines [127]. Similar studies were performed further by another group on rat breast cancer cells (SHZ-88), mouse lewis lung carcinoma cells and human hepatocarcinoma (CBRH-7919). In all the three cell lines, CBRH-7919 was shown higher IC50 value (39 μM) [121, 128]. In addition, AST has induced cell cycle arrest in mice H22 hepatoma cells in-vitro and in-vivo both and showed minor impact for apoptosis induction [129]. Besides, combinatorial effect of AST with β-carotene (shrimp) and lutein (plants) synergistically induced apoptosis by modulating expressions of cyclin D1, BAX, p53 and Bcl-2 in MCF-7 breast cancer cells [130, 131].
Ko et al. have checked AST’s anti-proliferation effect on two human non-small cell lung cancer (NSCLC) cells viz., bronchioloalveolar cell carcinoma (A549) and squamous cell carcinoma (H1703). AST with minimal concentration (20 μM) had shown a reduction in cell proliferation with 50.9% and 39.71% in A549 and H1703 cells respectively [132]. On the other hand 42 μM of the same has inhibited growth of the liver cancer cell line (HepG2) [133]. While in colorectal cancer cells (LS-180), AST induced apoptosis cascade mechanism by triggering BAX and caspase-3 expressions and inhibited growth and proliferation by suppressing Bcl-2 expressions. The activity was correlated to the strong antioxidant activity of AST imparting increase in the levels of superoxide dismutase, catalase, glutathione peroxidase and decreased levels of malondialdehyde [131, 134].
Recently, another review report was produced by a group emphacising an anticancer effects of AST by multiple molecular mechanisms including the role of AST in certain in-vitro anticancer studies, cell cycle arrest in tumor cells, various pro-apoptotic and chemo-sensitizing effects, suppression of cancer diffusion and pre-clinical studies [121]. The earlier literature can easily highlight and qualify the AST as one of the best natural drug combating with cancer.
Respiratory diseases
Respiratory diseases arise due to impaired or disturbed lung functioning. Regular respiratory activities such as breathing and pulmonary functions are majorly get affected. Some of the well-understood causes of lung diseases are bacterial, viral or fungal infections. Other lung diseases are allied to environmental factors and which possibly leads to asthma, acute lung injury (ALI), chronic obstructive pulmonary disease (COPD), emphysema, lung fibrosis, mesothelioma, lung cancer and non-small cell lung cancer (NSCLC) [135].
AST has been found to be a potent detoxifier and oxidative stress reliever in smoker community [136]. Smoking induces more free radicals which lead to an increase in oxidative stress levels and make a person prone to certain secondary health problems such as respiratory diseases, cardiovascular diseases, strokes and many more. Earlier studies have advised that the active smokers do need a higher daily intake of antioxidants than the passive ones to diminish the consequences of extensive and continued exposure to cigarette toxins by them [10].
A successive 3-week administration of AST (5 mg, 20 mg and 40 mg) in persistent smokers was observed to decrease the oxidative damage by suppressing the lipid peroxidation process. The parameters like declined malondialdehyde (MDA) and isoprostane (ISP) levels in blood serum, elevated superoxide dismutase (SOD) and catalase (CAT) activity and overall antioxidant activity have confirmed the antioxidation mechanism induced due to AST administration upto certain threshold concentrations [136]. Beyond the same concentrations, the results were not altered meaningfully. This may suggest that at higher dose the AST might have saturated [10].
Recently, Cheng J. and Eroglu A. have reported the effects of AST on lung diseases such as asthma, acute lung injury (ALI), chronic obstructive pulmonary disease (COPD), emphysema, lung fibrosis, lung cancer and non-small cell lung cancer (NSCLC). Their study has also summarized the molecular mechanisms of those effects. Therefore, the AST has provided an insight on future prospective and direction of using as a potent bioactive nutraceutical in clinical trials with respect to the lung diseases [137].
Anti-diabetic function
Diabetes mellitus (DM) is a chronic hyperglycemic (HG) disorder resulting from an impaired carbohydrate metabolism like insufficient insulin secretion, its action, or both. The DM has three main categories, type 1 (T1DM), type 2 (T2DM) and gestational DM. Among these, T2DM has accounted for about 90% of all the diabetes across the globe. In T2DM, an uncontrolled and long term increased blood glucose level develops life-threatening health issues such as cardiovascular diseases, retinopathy, nephropathy, neuropathy, gangrene and other related complications [138]. The root causes of DM and its complications are linked to oxidative stress and chronic inflammations [139] along with the hampering of cellular homeostasis and its subsequent complications such as vascular dysfunction, etc. [140, 141].
Scientists have reported that there could be a major role of nutritional and pharmacological therapies in the clinical management of diabetes. Wherein, various antioxidants including carotenoids, have been intensively screened for their effects in the prevention of progression in diabetes and its complications [114, 142]. In particular, it has been reported that the AST could significantly reduce the postprandial hyperglycemia in alloxan-induced diabetic mice [143]. Similar observations were pointed out in the pancreatic β-cells, in which oxidative stress induced by hyperglycemic conditions [144, 145].
In another study, a supplementation of the AST (8 mg/day for 8 weeks) in mice has shown that the adiponectin (an adipose-specific protein) can play a protective role in insulin resistance/diabetes. Subsequently it could also reduce the fructosamine and hypo-adiponectinemic conditions, causing increased plasma glucose regulation and fatty acid oxidations [146, 147]. Whereas in T2DM diabetic db/db mice models, AST has protected β-cells of the pancreas from glucose toxicity by reducing oxidative stress and regulating blood glucose levels. In other animal studies, AST demonstrated promising activities in reducing hyperglycemic concentrations via optimizing insulin secretions, regulating glucose metabolism and GLUT4 functions [148, 149].
In type 2 diabetic patients, AST relatively reduced TG, VLDL cholesterol levels, blood pressure, insulin resistance and also shown vasodilation properties [106, 116, 150]. Furthermore, AST demonstrated a slight rise in HDL levels and which was positively correlated to changes in adiponectin concentrations. But still, the mechanism remains unclear [113]. A similar study revealed a likely association between serum adiponectin and HDL cholesterol concentration [151]. Yoshida et al. reported, 12 mg AST supplementation in non-obsess volunteers significantly increased HDL cholesterol concentration and was in with positive correlation to adiponectin levels [106, 114].
The animal model studies with an uptake of AST (35 mg/kg) for almost 12 weeks displayed a correlation between dropped glycemic index and oxidative stress biomarker i.e. malondialdehyde (MDA) content with increased SOD activity in the blood serum [152]. And when AST (1 mg/day) was administered for 18 weeks different observations were recorded which explain the reduction in non-fasting glucose levels with substantial protective action against glucose toxicity in the pancreatic cells which were capable of producing insulin [144]. In another study, AST prohibited impaired functioning of pancreatic β-cell by defending them from the endoplasmic reticulum (ER) stress-induced apoptosis via regulating expressions of c-Jun N-terminal kinases (JNK), PI3K/Akt pathways and ER chaperons [152].
The studies performed in HFFD (high-fat fructose diet) fed mice; daily supplementation of AST (6 mg/kg per day) for about 45 days revealed increased insulin sensitivity and decreased plasma glucose as well as insulin levels [153]. An analogous report in HFFD mice showed augmented glucose metabolism by increased assimilation of glucose in body muscles [154]. Whereas in streptozotocin-induced diabetic rats, AST (0.01% or 0.05%) administration via diet reported declined proportion of activated macrophages (MCP-1) and inflammatory factors such as IL-6 and TNF-α in the plasma [141, 155].
An inclusive data from reviewed literature clearly depicts the potency of AST in the management of diabetes. Prior studies on T1DM and T2DM revealed several mechanisms of action of the same. Hence, AST can be regarded as a safe nutraceutical compound for an effective prevention of DM and DM related complications [141].
Anti-obese function
In the current scenario, obesity is also a globally concerned health issue. It is threatening people to become prospected sufferer from the health hazards such as hyperlipidemia, cardiovascular diseases, hypertension, and type II diabetes. AST supplementation via food has reported less weight gain, reduction of plasma cholesterol and liver triacylglycerol (TAG) in animal models [156]. In other studies, it was also considered to boost up the lipid metabolism during exercise of an individual leading to reduction in the body fat and increment in muscular movements [157]. However, in the actual human studies performed by few researchers, it did not show promising results in reducing body fat or body mass index [158].
Chio et al. demonstrated antioxidant effects of AST in obese individuals. The subjects who had received 5 and 20 mg of AST for successive 3 weeks showed subordinate levels of oxidative stress biomarkers which are linked to lipid peroxidation. When compared with the reduction in malondialdehyde (MDA) and isoprostane (ISP) levels, increased superoxide dismutase (SOD) and total antioxidant activity was observed [159]. Later 12 weeks of supplementation with 20 mg AST was also found promising in reducing the risks of heart attacks. That is due to an overall decrease in lipid profile including LDL concentrations, ApoB and ApoA1/ApoB [160].
In total, multiple studies have concluded that the AST could be helpful in reducing oxidative stresses, in curbing lipid profiles among obese individuals and ultimately in diminishing the emergence of CVDs in corresponding sufferers [10].
Liver diseases
The non-alcoholic fatty liver disease (NAFLD) is one of the key contributors to chronic liver diseases, worldwide. About 10% to 20% of them probably evolve into non-alcoholic steatohepatitis (NASH). Gao et al. have revealed protective effects of AST in NAFLD with different mechanisms of actions such as improvement in mitochondrial oxidative respiration, reduction in oxidative stresses, inflammation, liver fibrosis and liver tumor formation. It has also been proven helpful in enhancement of insulin metabolism, stimulation of M2 macrophage polarization and regulation of lipid metabolism [161].
Wu L. and co-workers have investigated an attenuation effect of AST on hepatocyte damages and mitochondrial functional defects generated due to NAFLD. In-vivo studies on mice showed upregulation of the FGF21/PGC-1α pathway in damaged hepatocytes suggesting a protecting role of AST in NAFLD treatment [162].
In alcoholic liver diseases (ALD), the influence of AST has been checked in binge alcohol feeding animal models by the researchers. The hepatic steatosis and inflammation, as well as certain consequences rising due to heavy ethanol (EtOH) consumption could be appeased by AST intake. Also, the levels of aspartate transaminase and alanine transaminase levels could be dropped. The ethanol triggered expression of cytochrome P-450 2E1 (CYP2E1), pro-inflammatory factors, cytokines, chemokines and ROS levels could also be lowered. Moreover, decreased infiltration of neutrophils was also observed there. In detailed studies, the AST has revealed its protective actions in ethanol-stimulated hepatic damages by blocking the STAT3 pathway and thus constraining oxidative stress and inflammatory reactions [163].
Liu et al. have attempted to reveal the relationship between gut microbiota of AST fed mice and AFLD improvement. In that studies the AST and ethanol-fed C57BL/6J mice were assessed for their AFLD status and gut microbiome composition was assessed. The results were found to be significant as they inflammation was relieved and unnecessary lipid accumulation was decreased. It also lowered the concentration of serum markers of liver injury. In gut microbiome analysis, it has decreased species from the phylum Bacteroidetes and Proteobacteria and also the species from genera Butyricimonas, Bilophila and Parabacteroides, the has increased number of species from phylum Verrucomicrobia and Akkermansia in comparison to EtOH fed group. The study also concluded that the Akkermansia could be a potential source for AST induced management studies of AFLD. AST significantly reversed the action of EtOH altered inflammatory and related metabolic parameters. Furthermore, it transformed 18 and 128 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways involved in lipid metabolism and xenobiotic bio-degradation and metabolism at levels 2 and 3, respectively [164].
In another recent studies, the AST supplementation resulted in the regulation of hepatic cholesterol metabolism via enhanced expressions of certain genes including 3-hydroxy-3- methyl glutaryl CoA reductase, LDL receptor and sterol regulatory element-binding protein 2. Furthermore, two major genes involved in lipid β-oxidation i.e. acyl-CoA oxidase and carnitine palmitoyltransferase-1 were also found to be upregulated, whereas lipogenesis related genes were not significantly affected [165, 166]. In lipid profile meta-analysis (TC, LDL-C, HDL-C and TG) studies, AST showed dropped HDL-C levels but other lipid compositions remained unchanged. Therefore Ursoniu et al. confirmed that AST did not exert any effects on dyslipidemia [158, 167]. Therefore, overall results of many studies have highlighted promising effects of supplementation of AST to the liver disease patients. It could be a new promising area and can avail good hopes in the treatment strategies of liver diseases.
Autoimmune hepatitis
Li et al. investigated the protective role of AST in immunocompromised Balb/C albino mice. Concanavalin A (ConA) (25 mg per kg) was used as an autoimmune hepatitis inducer. Prior to ConA injection, AST was orally given twice a day for 2 weeks. After detecting levels of serum liver enzymes, all histopathological parameters such as, inflammatory cytokines and other marker proteins, it was revealed that AST helps in the reduction of ConA induced hepatic injury. The possible mechanism was deduced to be down-regulation of JNK/p- JNK-mediated apoptosis and autophagy pathways [168]. So the AST which is having multiple potentials could again passed for a best possible candidate in treatment of autoimmune hepatitis.
Immunopathologic and immunomodulatory function
Dhinaut and co-authors recently reported an effect of lifetime dietary supplementation of AST on immunochallenged insect model i.e., mealworm beetle (Tenebrio molitor). The results have shown a reduced immunopathological damages and improved life endurance by immune depressive effects. The in-vitro and short-term in-vivo studies emphasized an immune-stimulating and antioxidant properties of the AST have shown positive effects. But, the reason behind and to what extent the supplements can perform the functions for long term are still unknown [169].
Some researchers have studied the comparative effect of AST and β-carotene on lab animals. The results showed higher immuno-modulating effects for AST than β-carotene [170]. In the case studies on older animals, when AST was given through a regular diet, it showed enhanced antibodies production with a decreased humoral immune response [170, 171]. AST treated human cells displayed boosted immunoglobulin production at the laboratory level [172].
According to a report, an eight-week of dietary supplementation of AST in humans have [173] showed a significant increase in levels as well as the activity of natural killer cells in blood. Also the DNA damage biomarkers were observed to be decreased. The levels of T and B cells were got amplified and the concentration of C-reactive protein (CRP) was significantly lowered in the AST supplemented group [174–177].
Therefore, it can be concluded that the AST supplementation may enhance the health of a human with immuno-alterations.
Anti-aging function
Biological as well as photoageing (UV rays exposure) are the two main reasons for age-related changes in the skins of human beings. Particularly in photoageing, UV light exposure causes degradation of extracellular matrix components such as collagen and elastin. It results in ROS production, pigmentation, wrinkle formation and deterioration of skin texture [178, 179]. In such cases, the role of carotenoid intake especially the supplementation of AST (photo-protective pigment) has proven promising for nurturing the skin physiology [45]. Another study summarizes the positive impact of AST in maintaining skin elasticity, reducing wrinkle formation, preserving epidermal barrier integrity and ultimately controlling the skin ageing-related processes [180–184].
Brain functioning and cognitive disorders
In concern to the brain and nervous system is functioning, an increased influence of oxidative stress and age factors may result in different types of cognitive disorders or diseases. Those can be an Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Autism and Amyotrophic Lateral Sclerosis (ALS), etc. [185–187].
Mental health diseases come under the class of cognitive disorders. Which deals with the brain and mental functioning such as memory, perception, learning and problem-solving abilities. Major cognitive disorders are dementia, amnesia and delirium. Among them, dementia is known for the gradual decline of brain functioning such as memory impairment, loss of concentration and confusion. Whereas amnesia is a memory disorder in which loss of short term memory hampers the daily routine of the affected person. Delirium is a serious confusion state and is characterized by jumbled thinking and a sedentary state. The cancer patients who have gone through chemotherapy are probably induced to chemobrain, a cognitive impairment. Such patient could lose their quality of life due to memory loss and unable to concentrate properly. These impairments were found to be connected to deterioration of neuronal integrity at the hippocampus region and frontal lobe of the brain [188, 189].
Recent studies showed, powerful protective effects of AST on the human brain as it readily clears the border of the blood–brain barrier (BBB) and mitochondrial membrane. Due to its unique property, the brain and nervous system are considered the chief target organs for AST. Consequently, AST could reduce dysfunctions associated with neurons and Central Nervous System (CNS) [189–191].
In current times, AST has been investigated for its protective role particularly in minimizing cognitive impairments ensued by the doxorubicin drug (DOX). In another study, enhanced memory functions and neuro-protective functions by suppressing oxidative stress and several pro-apoptotic stimuli and even bringing optimum acetylcholinesterase activity was seen [192]. In other body parts, it also showed positive effects and cumulatively works on neuro-protective functions and overall brain health. An intake of keto-carotenoid rich diets, particularly AST had shown lowered phospholipid hydroperoxides (PLOOH) levels causing elevated O2 transport to the brain by improving RBCs antioxidant status and diminishing chances of dementia [193–196].
Researchers have also evaluated the combinatorial effect of AST and fish oil on brain health by observing the reduction of harmful effects on polyunsaturated fatty acids (PUFAs) [197–199]. The experimental studies on Wistar rats supplemented with 1 mg/kg of AST and fish oil showed lipid safety deeds at the anterior forebrain. The antioxidant activities (TAEC and FRAP) were appeared to increase in rats fed with AST and fish oil together but unchanged in fish oil alone. In total, AST via dietary supplements could increase antioxidant cascades by reducing oxidative stress and also elevating cognitive behaviors through memory acts and positive mood swings [189, 200, 201].
In the case of cerebral ischemia/reperfusion (IR) and neuropathic pain (NP), multiple in-vivo studies have shown protecting and preventing effects with varied mechanisms of actions elucidating potency of AST as a novel neuropathic drug for treatment [202–207].
Amyotrophic Lateral Sclerosis (ALS) is known as progressive nervous system disease. Particularly affects upper and lower motor neurons in the brain and spinal cord, leading to loss of muscle control. According to prior studies, mutations in the gene encoding Cu/Zu superoxide dismutase 1 (SOD1) is the most common cause of ALS. More than 110 mutations have been studied and described to date, in this regard. The regulatory role of SOD1 is to keep the cellular homeostasis of ROS, but a mutated form of SOD1 lacks its function due to structural instability leading to toxic functional gain in forming ALS [208, 209].
In ALS, increased oxidative stress may collapse cellular functions and ultimately causes cell deaths. The effects of several antioxidants have been checked on SOD1 inhibitor [like diethyldithiocarbamate (DDC)] treated rat spinal neurons. The results have proved that the antioxidants such as L-ascorbic acid, L-histidine, α-tocopherol, β-carotene and AST could inhibited the DDC action and rescued the motor neurons from excessive oxidative stresses and thus surviving the neuronal functions. Among these, AST had shown the most promising effects even at its low concentrations (100 nM) than other antioxidants (1 mM) studied [189, 210].
Inclusively, AST can serve as a potent antioxidant and neuro-protective drug which can combat neuro-degenerative diseases by a different mechanism of actions such as anti-apoptosis lowers ischemia by impelled apoptosis, reduction in cerebral infarction in brain tissue, reduction of glutamate release and reduce free radical damage [185–187].
In this way, the AST has crowned to be one of the most promising prospected drug molecule in treatment of brain functioning and cognitive disorders.
Alzheimer’s disease
Alzheimer’s disease (AD) is measured as a most chronic neuro-degenerative disorder particularly known for memory impairment due to neuronal loss in the hippocampus and neo-cortex regions. Scientists have reported the nutraceutical role of AST in the protection as well as prevention of age-related central nervous system diseases and disorders [184, 211]. In-vivo studies on Wistar rats mimicking Alzheimer’s disease model supplemented with AST powder extracted from shrimp shells (Litopenaeus vannamei) displayed effective improvement in cognitive functions [212]. In another latest research, the effect of AST on cognitive modulations was compared with that of the composite derivative made up of AST with docosahexaenoic acid (DHA). The DHA-acylated AST ester (AST-DHA) was accessed for the neuroprotective actions in APP/PSEN1 (APP/PS1) double transgenic mice. Which has diminished the cognitive malfunctions [207, 213] and hence, it could be considered a potential therapeutic agent for the Alzheimer’s disease.
Several existing studies have elucidated vigorous effects of AST on human brain health, neurogenesis and plasticity generation, particularly in the elder age groups [214, 215]. In amyloid-beta plaques induced oxidative stress, inflammations at the neurofibrillary web junctions cause neuronal death incentives in the brain of AD suffering patients. In such studies, AST supported strong antioxidant, anti-inflammatory, neuro-protective and lipid peroxidation deterrent activities in AD treatments via different gene regulatory/suppressive mechanisms [189, 216].
Parkinson’s disease
Parkinson’s disease (PD), is the second most common progressive multisystem neuro-degenerative disorder and probably occur in aged people. Particularly affects motor and non-motor functions due to loss of dopaminergic neurons. Oxidative stress and neuro-inflammations are the major foundations of PD progressions. Assured treatments using keto-carotenoids especially AST, were found as an effective bioactive for prevention of PD progression and/or restoring the loss of neurons [189].
Successive four weeks supplementation of Haematococcus pluvialis derived AST in PD mice have shown reduced neurotoxicity [217]. Grimmig and collaborators have established an anti-inflammatory effects of AST, showing reduced microglial activation in striatum and substantia nigra regions of basal ganglia. The sustained microglial activation leads to accumulation of pro-inflammatory molecules, which will be harmful to the neural environment dure to their cytotoxic natures [217]. In other studies, dose-dependent antioxidant effects of AST were revealed, where ROS mediated apoptosis was found to be declined in dopaminergic SH-SY5Y cell lines [218]. AST has repressed the incidences of DHA hydroperoxide (DHA-OOH) or 6-hydroxydopamine (6-OHDA) stimulated apoptosis cascade, mitochondrial defects and ROS generations [219, 220]. Ikeda Y. showed AST treatment shutdowns 6-OHDA tempted apoptosis and mitochondrial dysfunction via p38 MAPK phosphorylation blocking and condensing caspase 3/9 and poly (ADP-ribose) polymerase action [189, 219]. Recently AST has been reported for Ca2+ ion transportation in the brain and also in controlling proper signalling activities [185–187].
In a rat model of homocysteine (Hcy) induced hippocampal neurotoxicity and apoptosis, the AST was found to control oxidative stress-induced damages and mitochondrial dysfunctions. It also diminished PI3K/AKT and mitogen-activated protein kinase (MAPK) pathways, which are important in regulating cell growth or cell deaths. That has enabled a blocking of PD and other neurological disorders so far [221]. In catecholamine type of cells such as PC12, AST was found to suppress the oxidative stress induced by MPP+ via SP1/NR1 and HO-1/NOX2 pathways [222, 223].
Ocular diseases
Optic neuropathy is an injury to the optic nerves leading to vision losses. It occurs either due to demyelination, inflammation, ischemic or traumatic causes. The glaucoma is a one of the progressive optic neuropathies causes due to damage in the nerve fiber layer and is the second leading cause of blindness worldwide. Cort and co-authors have observed that the glaucomatous retinal injury in mouse can be recovered after treatment with AST [224]. In nitroglycerin (NTG)-induced migraine mouse models, the AST has shown neuroprotective properties. In case of multifactorial disease i.e., glaucoma; AST can also become a potent remedy for its therapeutic treatment [225].
Lin and co-authors have examined the neuroprotective effects of AST in ischemic optic neuropathy mice model. At molecular level, it reduced the expression of TNFα and IL1β in eye retinas, while oxidative and inflammatory mechanisms have also got down-regulated [226]. In another study, an oxidant effect showed suppression in ischemic caused retinal cell death [225, 227].
The studies of Giannaccare et al. have shown the efficacy of AST in the prevention and treatment of various ophthalmic diseases and disorders including retinal diseases, uveitis, asthenopia (eye fatigue), cataract and ocular surface disorders. Earlier reports on animal and human ocular diseases highlighted that the use of AST retains cellular homeostasis by reducing oxidative stress and controlling different metabolic activities, etc. Several clinical trials needed to be performed for getting an idea about dosage quantities, route and duration of administration of the AST to tackle with various ocular damages, diseases as well as disorders [56].
Carpel tunnel syndrome
Carpel tunnel syndrome (CTS) is broadly characterized as the compression neuropathy [228]. Non-operative management [229, 230] and conventional medications such as injections, non-steroidal anti-inflammatory drugs and recuperation therapies (ultra-sound, stretching, and strengthening) have been evaluated against CTS [231–233].
Some factors like correct nutritional supplementations could help positively in the CTS managements [234–236]. AST was investigated for its effectiveness on the splinting management in CTS. The electromagnetically confirmed CTS experimental group was supplemented with 4 mg AST (12 weeks) and compared to the control (placebo) group. Both the groups were observed to reduce symptoms which are measured through Symptom Severity Scale (SSS). The arm, shoulder and hand disabilities survey didn’t show significant effects. However, impairment measures showed marginal changes in grip, agility and sensations [237]. So there must be a scope for the further studies to understand and optimize probable usage of AST in CTS management.
Stress, fatigue and depression
Oxidative stress, psychological stress and fatigue are interconnected processes in life. Exhaustive mental and physical daily routines induce more oxidative stress. Phosphatidylcholine hydroperoxide (PCOOH), is an oxidative stress marker produced after phospholipid oxidation [193]. AST has the ability to suppress the PCOOH levels, which eventually decreases the chances of people suffering from chronic fatigue syndrome. Also, AST has been reported to heighten the strength [238] and fat burns during workout by CPT-I activation [157]. Imai A. et al. study has shown the anti-fatigue effects of AST and its role in the reduction in oxidative stress markers in healthy people performing their daily mental and physical routine [239].
According to Kim et al. report, AST effectively lowered oxidative stress produced during various pathological environments. It maintains structural and functional activities of mitochondria and also prevents the progression of diseases like cardiovascular, neurodegenerative, liver-related malfunctions such as NAFLD, diabetes, hyperglycemia and other secondary complications. In short, AST rich diet or supplementation possibly provides a prevention against ageing processes and health-related problems [240].
Hayashi et al. investigated the relative effect of nutritional supplement of AST (from Paracoccus carotinifaciens) on stress and sleep behaviors among 20–60 age group of people. After providing daily oral intake of 12 mg AST for successive 8 weeks, the efficacy of treatment was measured in terms of a primary and secondary outcome as POMS (mental stress) and OSA-MA (sleep) respectively. A change in mood swings and depression-dejection states were found to be decreased and quality sleep act was improved in AST ingested group than placebo group with no adverse effects [241].
Fertility
The fertility is one of the major concern of health managements in such hectic times. The problem of infertility is getting worsen day by day. The probable reason behind that is the stressful lifestyles and non-healthy diets. Therefore, it was believed that the dietary supplementation might re-regularize the disturbed fertility cycles.
Male fertility
A dietary supplementation of AST in combination with vitamin E, vitamin C, and calorie-restricted diet in male rats, has shown to increase count and quality of the sperms. It was also reported to improve sperm capacitation and related functions by augmenting redox system balance in sertoli cells [242, 243]. Recently, Wang et al. stated protective action by AST in steroidogenesis from H2O2 induced ROS in mouse Leydig cells [244]. In another study, decreased leptin levels showed increased sperm count and motility and thus elevated events of male fertility [245].
According to Donà et al. in-vitro studies, AST could enter in acrosome membrane of sperm and it assists protein phosphorylation (Tyr-p) in the sperm head without inducing ROS (H2O2) levels. Ultimately enhances the capacitation process by which an easy acrosome reaction (AR) occurs. It also triggers the release of acrosin hydrolytic enzymes which permit sperm and oocyte fertilization [246].
In another reported clinical trial, infertile males with oligoasthenozoospermia were orally supplemented with 4 mg AST and 10 mg vitamin E combination. The semen quality, fertilization and embryo development processes were investigated with their female partners by the assisted reproduction techniques (ART) such as intracytoplasmic sperm injection (ISCI) [247].
Conclusively, we can have a hopeful approach in male fertility problems with an effective supplementation of AST to the patients.
Female fertility
In animal studies, AST feeding in chickens has improved egg quality (shelf life) and the colour of egg yolk. However, in pigs’ carcass characters and meat quality was found to be increased [248]. In case of AST fed minks, the amplified quantity of corpora lutea (CL) along with increased implantation sites and fetus numbers was observed. However, stillborn kits number was observed to be decreased [249].
In another study, the effect of AST during in vitro growth (IVG) was inspected. In this, processes such as oocytes competence development and steroidogenesis of granulosa cells devised from early antral follicles were studied. Due to the antioxidant potential of AST, increased total biomass in blastocysts was found. When supplemented in IVG medium, it amended the overall quality and growth of bovine oocytes, also suppressed granulosa cells’ luteinisation [250].
In postmenopausal women studies, AST promoted vascular endurance and increased antioxidant capacities by reducing elevated levels of oxidative stress. Successive 8-week intake of 12 mg AST in diet significantly reduced systolic and diastolic blood pressure. A drop in vascular resistance in the lower limbs and serum adiponectin, improved physical and mental symptoms were also noted [10, 251].
Recently some researchers have also performed clinical trials concerning the effect of AST on oxidative stress management in women suffering from polycystic ovary syndrome (PCOS) [252]. The AST administration was expected to reduce ROS in FF and induction of antioxidant response elements in PCOS women.
Anti-tuberculosis
Mycobacterial infections and multidrug-resistant (MDR) strains of Mycobacterium tuberculosis (MTB) are one of the foremost causes of mortality and a major reason for socioeconomic burden worldwide [253]. Yearly, about 8 million new tuberculosis cases and 2 million deaths were reported [254]. The number might have increased now. According to a WHO survey, about one-third of the global population is at risk of MTB infections [255].
Recently, the anti-mycobacterial activity of AST and liposome-encapsulated AST were explored. The docking studies of AST with different mycobacterial drug target proteins have provided good interactions than the conventional anti-TB drugs i.e. isoniazid and rifampicin. However, an encapsulated AST displayed better inhibition of mycobacterium (MIC value of 500 μg/mL) against MDR-TB, and H37Rv. Future studies on animal models could provide insight for identifying the exact mechanism of action of AST in TB managements [256].
Anti-viral
Recently Donà et al. appraised the protective effects of AST on isolated and purified human sperm in the presence of human papillomavirus (HPV) 16 capsid protein, L1. AST had shown strong antiviral effects by preventing the binding of HPV L1 to the sperm membrane and thus HPV entry was prohibited [257]. The study may encourage to evaluate, more antiviral capabilities of AST with various viruses.
Anti-COVID 19
In the COVID-19 disease, the inflammations caused by a novel species of SARS CoV-2 and elevated levels of cytokine storms as well as inflammatory factors such as interleukins (IL-1β, IL-6, IL-8), tumor necrosis factor (TNF-α), etc. can be regulated and suppressed by the supplementation of AST. So it can be a promising therapeutic dietary supplement along with primary antiviral medications for the patients suffering from severe pneumonitis, acute lung injury (ALI), Acute Respiratory Distress Syndrome (ARDS), other lungs related infections and also in recovered patients of COVID-19 as a post-treatment care [258–260].
The AST was observed to decrease membrane fluidity and raises the activation of nuclear factor erythroid 2–related factor 2/heme oxygenase-1 pathway. The pathway further stimulates an increased synthesis of antioxidant enzymes viz., peroxidase, catalase, superoxide dismutase, NAD(P)H quinine oxidoreductase-1, glutathione-S-transferase-α1, and thiobarbituric acid reactive substances [261]. AST was also found as a potent inhibitor of superoxide radicals, ROS, cytosolic calcium, myeloperoxidase (MPO), and other oxidative mediators and lipid peroxidation as well. The acceptable dietary intake of AST for COVID-19 is of 2 mg/day, which has been accepted in European Food and Safety Authority and about 24 mg/day in the US [262]. In the US, their FDA has approved a “no objection” certificate for about 20 new dietary components including AST with daily uptake in between 2 mg and 24 mg [263]. In this way the AST and its preparations are getting popular and being acceptable worldwide in the COVID-19 management. However, the effectiveness and exact efficacy of it is yet to be determined at molecular level.
Nutraceutical potential of astaxanthin
In aquaculture and food industries, AST is mainly used as an additive and dietary supplement. Among several carotenoids, β-Carotene and AST are typical components used in chicken and fish feeding, respectively [264]. AST enhances aesthetic qualities as well as the market value of sea-foods like salmons, shrimps, trouts, lobsters, crayfish, fish eggs and also the ornamental fish [38, 43, 101, 265–267]. In farmed Atlantic salmon AST content was reported up to 6 mg/kg–8 mg/kg flesh. In the large trout from European and Japanese markets, 6 mg/kg flesh and 25 mg/kg flesh of AST content was observed respectively [177]. In nature, Haematococcus pluvialis derived all trans 3S,3′S AST is the utmost reported source so ever [43].
In 1987, USFDA authorized AST as a feed supplement in aquaculture industries, and in 1999, AST again approved for its use in food industries too [101]. Globally emerging aquacultures, food industries and their massive demands have fortified AST’s commercial value; Japan is one of the topmost pioneers in AST production and research for those applicatory usages [105].
Several clinical studies have revealed AST’s remarkable anti-oxidative effects, multifunctional health benefits, and its bio-safety properties. Hence, it is now also being used in the cosmetics and nutraceutical industries too. Some globally recognized manufacturing brands producing AST (as a nutraceutical) are listed in Table 2. In some countries, the AST is occasionally used to strengthen the quality of foods and brews [21]. Currently, > 95% of the AST production in the market has been occupied by synthetic AST [38]. However, the natural AST has a great consumer demand and therefore it is used in food, cosmetics and nutraceutical industries. In a global scenario, the AST market is estimated to reach up to 3398.8 million by the year 2027 due to its well-rising alertness in the health and nutrition sector [21]. Assuredly, in emerging decades, valuable AST and its promising bioactivities will endorse its up-surging potency for the betterment of human health.
Table 2.
Astaxanthin (as a nutraceutical) manufacturing global brands (from different biological sources)
| Sr. No | Product Name | Brand Name |
|---|---|---|
| Source: Haematococcus pluvialis | ||
| 1 | ASTAREAL® | BodyFirst® |
| 2 | Astaxanthin | Zenith Nutrition |
| 3 | Astaxanthin | Healthy Hey |
| 4 | Astaxanthin | Himalayan Organics |
| 5 | Astaxanthin | Sports Research |
| 6 | Astaxanthin | Nature’s Velvet |
| 7 | Astaxanthin | Now Foods |
| 8 | BTN Super Antioxidant Astaxanthin | Bionova Targeted Nutrition |
| 9 | Astaxanthin | Truebasics |
| 10 | Astaxanthin | Doctor’s Best |
| 11 |
BioAstin® OmegaAstin™ |
Hawaiian Herbal |
| 12 | Astaxanthin with Phospholipids | Life Extension® |
| 13 | Astaxanthin, AstaLif® Pure Icelandic | California Gold Nutrition |
| 14 | SuperPure® Astaxanthin | Pure Synergy |
| 15 | Astashyn™ | Newtrimed Healthcare Private Limited |
| 16 | Astaxanthin | TRUHEALTHY® |
| 17 | Oriflame Wellness Astaxanthin And Bilberry Extract | Oriflame |
| 18 | Astaxanthin | Source Naturals® |
| 19 | Astaxanthin Plus | Natural Factors® |
| 20 | Natural Astaxanthin | MicrOrganics Green Nutritionals |
| 21 | Natural Astaxanthin Triple Strength | Healthy Origins® |
| 22 | Astaxanthin | Ramini® Bio Nutrition |
| 23 | Astaxanthin | Sam Health® |
| 24 | Natural Astaxanthin | Solgar® |
| 25 | Vegan Astaxanthin | Deva® |
| 26 | Natural Astaxanthin | Vaddmaan |
| 27 | Astaxanthin | Jarrow Formulas® |
| 28 | Astaxanthin | We Like Vitamins |
| 29 | High-Potency Astaxanthin | Swanson® |
| 30 | Astaxanthin | Viva Naturals |
| 31 | Astaxanthin | Simply Potent |
| 32 | Astaxanthin | aSquared Nutrition |
| 33 | Astaxanthin Royal | Vitality Nutritionals |
| 34 | Astaxanthin | ICEHERBS Natural Supplements |
| 35 | Astaxanthin | Webber Naturals |
| 36 | Astaxanthin | Neurapid |
| 37 | Astaxanthin Oil | Horbäach® |
| 38 | Xantox™ VF | Glenmark Pharmaceuticals |
| 39 | Astaxanthin | Cheeky Nutrition |
| 40 | Natural Astaxanthin | MicrOrganics |
| 41 | Astaxanthin | Terranova |
| 42 | AstaFit® | BioLife Science® |
| 43 | AstaXanthin™ with DHA | Good Health Naturally Nutrition™ |
| 44 | Sila Astaxanthin | LJS International Development Co., Ltd |
| 45 | AstaFX® Astaxanthin Super Formula | Purity Products |
| 46 | AstaVibrance Astaxanthin | BioPure.eu |
| 47 | TrueAsta™ | Nature City® |
| 48 | Astaxanthin (New) | NutriGuard™ |
| 49 | Astaxanthin | Design for Health® |
| 50 | Astaxanthin | Prime™ |
| 51 | ASTASHINE™ | Fuji Chemical |
| 52 | Nature Astaxanthin | Newmstar |
| 53 | Astaxanthin Forte | OstroVit® |
| 54 | Astaxanthin | Bio Natura |
| 55 | Astaxanthin Beadlets | Fairvital |
| 56 | Astaxanthin | Biosphere |
| 57 | Astaxanthin | Amazing Nutrition |
| 58 | Astaxanthin | Waka Tani |
| 59 | Astaxanthin | Solaray® |
| 60 | K2-D3 with Astaxanthin | Douglas Labs |
| 61 | Astaxanthin Vegan Max | Barlowe’s Herbal Elixirs |
| 62 | Astaxanthin | California Products |
| 63 | Astaxanthin Extract | HerbaDiet |
| 64 | Astaxanthin | BioBalance® |
| 65 | AstaReal® Astaxanthin | AstaVita® |
| 66 | Astaxanthin | Viridian |
| 67 | Astaxanthin Plus | Prairie Naturals® |
| 68 | Astaxanthin | Vibraxlabs |
| 69 | Astaxanthin | Getilapson™ |
| 70 |
AstaReal® Oil (Oleoresin) AstaReal® Powder AstaReal®Cold Water Soluble Powder AstaReal®Water Soluble Emulsion AstaReal®Bulk softgel capsules AstaMed MYO™ |
AstaReal® AstaMed® |
| 71 | Astaxanthin | ALGORIGIN |
| 72 | Natural Astaxanthin | Vitacost |
| 73 | Astaxanthin | Kala Health |
| 74 | Astaxanthin | BIOMEDTM |
| 75 | Astaxanthin | Health Supplements |
| 76 | GO Astaxanthin Antioxidant High Strength | GO® healthy |
| 77 | Natural Astaxanthin | PipingRock® |
| 78 | AstaZine® Astaxanthin | NutriCology® |
| 79 | Astaxanthin | Healthy Directions |
| 80 | Organic Astaxanthin | EyeScience® |
| 81 | AstaREAL® Astaxanthin | Nature’s Lab |
| 82 | Astaxanthin | Trunature® |
| 83 | Natural Astaxanthin | Puritan’s Pride |
| 84 | Astaxanthin | Dr. Mercola® |
| 85 | Vision Advantage® | Dr. David Williams |
| 86 | Astaxanthin | Dr. Whitaker |
| 87 | Astaxanthin | Lamberts® |
| 88 | AstaZan™ | LifeStream® |
| 89 | Astaxanthin | The Vitamin Shoppe® |
| 90 | Astaxanthin 12,000 | Andrew Lessman’s Procaps Laboratories |
| 91 | Astaxanthin Gold | NutriGold® |
| 92 | Astaxanthin | Time Health |
| 93 | Astaxanthina | Yamamoto® Research |
| 94 | Asta-i-Shield | Vitamode® |
| 95 | Astaxanthin | Regenurex™ |
| 96 | Organic Astaxanthin | Naomi |
| Source: Phaffia rhodozyma | ||
| 97 | Astaxanthin Gummies with Vitamin C (Astaferm™) | Carlson Labs |
| Source: Antarctic Krill (Euphausia superba) | ||
| 98 | High-Potency Krill Oil With Astaxanthin | WholeHealth® |
| 99 | Vestige Krill Oil | Vestige |
| Source: Unknown | ||
| 100 | Astaxanthin Super Antioxidant | Simply Herbal |
| 101 | Astaxanthin Natural | Woohoo Natural® |
| 102 | Eye Health Support | Allbeing |
| 103 | Astaxanthin | Naturalis |
| 104 | Astagold | Sunways (India) Pvt. Ltd |
| 105 | Astaxanthin | Ayurveda Redefined |
| 106 | Astaxanthin | HealthVit |
| 107 | Astaxanthin | Higher Nature® |
| 108 | Avanza® Astaxanthin | Healthcare Pharmaceuticals Limited |
| 109 | Astaxanthin | NUHEALTH® |
In this way, the astaxanthin has been involved and being considered in many important applications as cosmetics, food and feed industries. And now it is getting evolved as the key ingredient of certain nutraceuticals, due to its potent antioxidant activity. Which is > 10 times efficient than the β-carotene and about 1000 times more effective than the vitamin E. The multiple potencies of AST, such as protection against UV-radiation, photo oxidation, inflammation, cancer, ulcer, Helicobacter pylori infection, aging, and age-related ailments, or in the promotion of the immune response, liver functions, heart, eye, joints and prostate health, etc. have elevated its importance as the crucial substance in the formulations and developments of many nutraceutical products [268].
Biosafety and bioavailability of astaxanthin
Earlier studies say that AST is absolutely safe for consumption when taken with food or as a dietary supplement. The studies on rat models revealed that AST accrues in animal tissues without any lethal effects [269–271]. But yes, if excessive AST get consumed it leads to yellow to reddish pigmentation of the skin in animals. For example, AST incorporated into fish feed, causes reddish skin colour in fish. Along with lipid-based formulations, AST’s bioavailability can be amplified [269, 270, 272]. It was found that, super therapeutic concentrations of AST had led no antagonistic effects on platelet, coagulation and fibrinolytic functions [273]. Considerably, no research has reported on significant side effects of AST intake in animals as well as in humans. These results provide backing up for the safety of AST for future clinical studies.
According to some studies, it is advised that AST consumption with omega-3 rich seed oils such as flaxseed, chia, fish, nutella, almonds and walnuts could enhance its bioavailability. The blend of AST (4 mg–8 mg) with foods, capsules, soft gels and skin creams is available in the market. The suggested dose of AST is 2 mg/day–4 mg/day for adult humans. Even no harmful effects were seen at its slightly increased the dose (6 mg/day) [177, 274].
Conclusions and forthcoming directions
The awareness and yearning among the people about ‘wellness industry’ than the earlier ‘illness industry’ is increasing day by day. Which will work as a driving force for development of health supplementary products based on natural healing and nurturing mechanism. The AST, a versatile natural bioactive pigment has been acknowledged by the global scientific society in concern with its health benefits of fulfilling the nutritional gaps of continued increasing populations. This also underlines its importance in the nutraceutical supplementations as well as in the prevention and protection strategies against many pathological problems. The AST is a great antioxidant having about 110 times more anti-oxidation potency than the Vitamin E, 560 times than green tea catechin, 800 times than Coenzyme Q10, 3000 times than resveratrol and about 6000 times more than vitamin C. Also the superiorities of AST such as the potent anti-oxidant, anti-inflammatory, anti-proliferative, anti-cancer, anti-obese, anti-diabetic, anti-ageing, anti-TB, anti-viral, anti-COVID 19, neuro-protective, nephro-protective, and fertility-enhancing properties make it is the most precious thing to be studied for prevention as well as for the treatment of prevalent systematic diseases and/or disorders. Although several reports strongly support the safety profile of AST in human uses, several in-vivo and in-vitro studies have been reported for its nutritional, therapeutic, supportive, regulatory, modulatory, protective and preventive actions. However, a deep investigation is need to be performed in various physiological diseases and disorders. That will clarify its exact mechanism of actions in control and cures at the molecular levels of the cells. While the analysed data in the current review studies suggests that the AST possesses an incredible pharmaceutical, nutraceutical and bio-functional properties. It is safe for human use and has a great bio-acceptability too. But since it cannot be synthesized by animals, it has to be provided them as their dietary supplement to maintain a balanced natural living.
The current review also summarises the primary as well as secondary natural sources of AST, various factors affecting its biosynthesis, followed by its potential applications in pharmaceutical formulations. The superlative and incredible usage of AST in treatment of obesity issues, CVDs and its allied complications, cancer, respiratory as well as liver diseases, stress, fatigue, depressions, aging and fertility issues, neurological and neuro-degenerative diseases, autoimmune disorders, immune-pathogenicity and immune-modulatory functions have been comprehensively discussed and documented in the report. The functional usages of AST in manipulation of tuberculosis, viral infections and in COVID-19 disease have also been highlighted here.
At last, the review report is also detailing the existing as well as the prospective applications of AST in nutraceutical industries. The increments in nutritional as well as aesthetic qualities of sea foods with reference to the European and Japanese market have been discussed here. The review also encompasses the data of biosafety as well as the bioavailability of AST. Its prospected universal market potential and estimated market reach by coming decades is also elaborated further.
In conclusion, the study is going to add some important understandings in the global knowledge base of the field and it may further offer a great chunk of opportunities in coming years. It also provide a promising hope that the AST and its bioactive potential with respect to the pharmaceutical and nutraceutical approaches will uplift the human health and it may further guide the people to battle wisely against many life-threatening health concerns. The review may also encourage few new groups of researchers to explore its futuristic nutraceutical ventures. That is anticipated because the demand and acceptance of the nutraceuticals are going to be one of the prime obligation of human beings in future.
Acknowledgements
The principal author is thankful to the Chhatrapati Shahu Maharaj Research, Training and Human Development Institute (SARTHI), Pune, India, for financial support through the Chief Minister Special Research Fellowship-2019 (CMSRF-2019).
Author contributions
ADP: Investigation, Analysis, Writing—Original Draft. PJK: Investigation, Analysis, Writing—Original Draft, Revisions. PBD: Conceptualization and development of idea, Supervision, Writing—Review and Editing. All authors read and approved the final manuscript.
Declarations
Competing interests
All the three authors declare that they do not have any conflict of interest related to the financial or funding matters.
Footnotes
This article has been updated to amend figures 1 & 3.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Apurva D. Patil, Email: adp.biochem@gmail.com
Pramod J. Kasabe, Email: pjk.biochem@gmail.com
Padma B. Dandge, Email: pbd_biochem@unishivaji.ac.in
References
- 1.Cooper DA, Eldridge AL, Peters JC. Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr Rev. 1999;57:201–214. doi: 10.1111/j.1753-4887.1999.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 2.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Asp Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ye VM, Bhatia SK. Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol Lett. 2012;34:1405–1414. doi: 10.1007/s10529-012-0921-8. [DOI] [PubMed] [Google Scholar]
- 4.Henke NA, Heider SA, Peters-Wendisch P, Wendisch VF. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar Drugs. 2016;14:124. doi: 10.3390/md14070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin TW. Metabolism, nutrition, and function of carotenoids. Annu Rev Nutr. 1986;6:273–297. doi: 10.1146/annurev.nu.06.070186.001421. [DOI] [PubMed] [Google Scholar]
- 6.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. [PubMed] [Google Scholar]
- 7.Johnson EA, An GH. Astaxanthin from microbial sources. Crit Rev Biotechnol. 1991;11:297–326. [Google Scholar]
- 8.Teo CL, Chee ML, Koh KH. COVID-19 awareness, knowledge and perception towards digital health in an urban multi-ethnic Asian population. Sci Rep. 2021;11:10795. doi: 10.1038/s41598-021-90098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olaizola M. The production and health benefits of astaxanthin. Marine nutraceuticals and functional foods. 1. Boca Raton: CRC Press; 2008. pp. 321–343. [Google Scholar]
- 10.Pereira CPM, Souza ACR, Vasconcelos AR, Prado PS, Name JJ. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review) Int J Mol Med. 2021;247:37–48. doi: 10.3892/ijmm.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin HD, Ruck C, Schmidt M, Sell S, Beutner S, Mayer B, Walsh R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl Chem. 1999;71:2253–2262. [Google Scholar]
- 12.Nishida Y, Yamashita E, Miki W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007;11:16–20. [Google Scholar]
- 13.Yamashita E. Extensive bioactivity of astaxanthin from Haematococcus pluvialis in human. Carotenoids: biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 249–260. [DOI] [PubMed] [Google Scholar]
- 14.Brotosudarmo THP, Limantara L, Setiyono E. Heriyanto. Hindawi Int J Food Sci. 2020;2020(2156582):1–16. doi: 10.1155/2020/2156582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Biotechnology Information. PubChem Compound Summary for CID 5281224, Astaxanthin. https://pubchem.ncbi.nlm.nih.gov/compound/Astaxanthin. Accessed 23 Sept 2021.
- 16.Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5:333–342. doi: 10.2217/fca.09.19. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood SF, Gross GJ. Disodium disuccinate astaxanthin (CardaxTM): antioxidant and antiinflammatory cardioprotection. Cardiovasc Drug Rev. 2005;23:199–216. doi: 10.1111/j.1527-3466.2005.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 18.Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules (Basel, Switzerland) 2012;17:2030–2048. doi: 10.3390/molecules17022030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SK, Malinski T, Mason RP, Kubant R, Jacob RF, Fujioka K, Denstaedt SJ, King TJ, Jackson HL, Hieber AD, et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb Res. 2010;126:299–305. doi: 10.1016/j.thromres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Lead Compound CDX-085. https://ir.cardaxpharma.com/press-releases/detail/124/cardax-to-be-granted-chinese-patent-protection-for-lead. Accessed 24 Oct 2021.
- 21.Stachowiak B, Szulc P. Astaxanthin for the food industry. Molecules. 2021;26:2666. doi: 10.3390/molecules26092666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pleissner D, Lin CSK. Valorisation of food waste in biotechnological processes. Sustain Chem Process. 2013;1:21. [Google Scholar]
- 23.Oslan SNH, Shoparwe NF, Yusoff AH, Rahim AA, Chang CS, Tan JS, Oslan SN, Arumugam K, Ariff AB, Sulaiman AZ, Mohamed MS. A review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomolecules. 2021;11:256. doi: 10.3390/biom11020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson EA, Lewis MJ. Astaxanthin Formation by the Yeast Phaffia Rhodozyma. J Gen Microbiol. 1979;115:173–183. [Google Scholar]
- 25.Andersson M, Nieuwerburgh L, Snoeijs P. Pigment transfer from phytoplankton to zooplankton with emphasis on astaxanthin production in the Baltic Sea food web. Mar Ecol Prog Ser. 2003;254:213–224. [Google Scholar]
- 26.Cunningham FX, Gantt EA. Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. Plant Cell. 2011;23:3055–3069. doi: 10.1105/tpc.111.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mawson R. U.S. Patent 5453565A. Accessed 23 Sept 2021.
- 28.Cunningham FX, Gantt EA. Portfolio of plasmids for identification and analysis of carotenoid pathway enzymes: Adonis Aestivalis as a case study. Photosynth Res. 2007;92:245–259. doi: 10.1007/s11120-007-9210-0. [DOI] [PubMed] [Google Scholar]
- 29.Renstrøm B, Berger H, Liaaen-Jensen S. Esterified, optical pure (3S,3’S) astaxanthin from flowers of Adonis annua. Syst Ecol. 1981;9:249–250. [Google Scholar]
- 30.Li Y, Gong F, Guo S, Yu W, Liu J. Adonis amurensis is a promising alternative to Haematococcus as a resource for natural esterified (3S,3'S)- astaxanthin production. Plants. 2021;10:1059. doi: 10.3390/plants10061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czeczuga B. Investigations on carotenoids in Embryophyta. 1. Bryophyta. The Bryologist. 1980;83:21–28. [Google Scholar]
- 32.Takemura M, Misawa N. Carotenoid biosynthesis in liverworts. Carotenoids: biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 115–120. [DOI] [PubMed] [Google Scholar]
- 33.Misawa N, Takemura M, Maoka T. Carotenoid biosynthesis in animals: case of arthropods. Carotenoids: biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 217–220. [DOI] [PubMed] [Google Scholar]
- 34.Eom H, Lee CG, Jin E. Gene expression profile analysis in astaxanthin-induced Haematococcus pluvialis using a c-DNA microarray. Planta. 2006;223:1231–1242. doi: 10.1007/s00425-005-0171-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee YK, Ding SY. Cell cycle and accumulation of astaxanthin in Haematococcus lacustris (Chlorophyta) J Phycol. 1994;30:445–449. [Google Scholar]
- 36.Aflalo C, Meshulam Y, Zarka A, Boussiba S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol Bioeng. 2007;98:300–305. doi: 10.1002/bit.21391. [DOI] [PubMed] [Google Scholar]
- 37.Rao AR, Raghunath Reddy RL, Baskaran V, Sarada R, Ravishankar GA. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J Agric Food Chem. 2010;58:8553–8559. doi: 10.1021/jf101187k. [DOI] [PubMed] [Google Scholar]
- 38.Lim KC, Yusoff FM, Shariff M, Kamarudin MS. Astaxanthin as feed supplement in aquatic animals. Rev Aquac. 2018;10:738–773. [Google Scholar]
- 39.Yuan JP, Chen F. Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcus pluvialis. Food Chem. 2000;68:443–448. [Google Scholar]
- 40.Chekanov K, Lobakova E, Selyakh I, Semenova L, Sidorov R, Solovchenko A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia) Mar Drugs. 2014;12:4504–4520. doi: 10.3390/md12084504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoo KS, Chew KW, Ooi CW, Ong HC, Ling TC, Show PL. Extraction of natural astaxanthin from Haematococcus pluvialis using liquid biphasic flotation system. Bioresour Technol. 2019;290:121794. doi: 10.1016/j.biortech.2019.121794. [DOI] [PubMed] [Google Scholar]
- 42.Jannel S, Caro Y, Bermudes M, Petit T. Novel Insights into the biotechnological production of Haematococcus pluvialis-derived astaxanthin: advances and key challenges to allow its industrial use as novel food ingredient. J Mar Sci Eng. 2020;8:789. [Google Scholar]
- 43.Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/s0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- 44.GRAS Notice (GRN) No 700 (U. S. Food and Drug Administration, 2017). https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm584383.pdf. Accessed 23 Sept 2021.
- 45.Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients. 2018;10:522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sifuentes LR, Marszalek JE, Carbajal GH, Hernández CC. Importance of downstream processing of natural astaxanthin for pharmaceutical application. Front Chem Eng. 2021;2:601483. [Google Scholar]
- 47.Johnson EA. Phaffia rhodozyma: colorful odyssey. Int Microbiol. 2003;6:169–174. doi: 10.1007/s10123-003-0130-3. [DOI] [PubMed] [Google Scholar]
- 48.Kim JH, Kang SW, Kim SW, Chang HI. High-level production of astaxanthin by Xanthophyllomyces dendrorhous mutant JH1 using statistical experimental designs. Biosci Biotechnol Biochem. 2005;69:1743–1748. doi: 10.1271/bbb.69.1743. [DOI] [PubMed] [Google Scholar]
- 49.de la Fuente JL, Rodriguez-Saiz M, Schleissner C, Diez B, Peiro E, Barredo JL. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J Biotechnol. 2010;148:144–146. doi: 10.1016/j.jbiotec.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Kucsera J, Pfeiffer I, Takeo K. Biology of the red yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) Mycoscience. 2000;41:195–199. [Google Scholar]
- 51.Barredo JL, García-Estrada C, Kosalkova K, Barreiro C. Biosynthesis of astaxanthin as a main carotenoid in the heterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J Fungi. 2017;3:44. doi: 10.3390/jof3030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran TN, Tran QV, Huynh HT, Hoang NS, Nguyen HC, Ngo DN. Astaxanthin production by newly isolated Rhodosporidium toruloides: optimization of medium compositions by response surface methodology. Not Bot Horti Agrobot Cluj-Napoca. 2019;47:320–327. [Google Scholar]
- 53.Tsubokura A, Yoneda H, Mizuta H. Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin-producing bacterium. Int J Syst Evol Microbiol. 1999;49:277–282. doi: 10.1099/00207713-49-1-277. [DOI] [PubMed] [Google Scholar]
- 54.Scientific Opinion of the Panel. (European Food Safety Authority, 2007). The EFSA Journal. 2007; 546: 1–30.
- 55.Katsumata T, Ishibashi T, Kyle D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicol Reports. 2014;1:582–588. doi: 10.1016/j.toxrep.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannaccare G, Pellegrini M, Senni C, Bernabei F, Scorcia V, Cicero AFG. Clinical applications of astaxanthin in the treatment of ocular diseases: emerging insights. Mar Drugs. 2020;18:239. doi: 10.3390/md18050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah MM, Liang Y, Cheng JJ, Daroch M. Astaxanthin producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci. 2016;7:531. doi: 10.3389/fpls.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokoyama A, Miki W, Izumida H, Shizuri Y. New trihydroxy-keto-carotenoids isolated from an astaxanthin-producing marine bacterium. Biosci Biotechnol Biochem. 1996;60:200–203. doi: 10.1271/bbb.60.200. [DOI] [PubMed] [Google Scholar]
- 59.Matsuno T. Aquatic animal carotenoids. Fish Sci. 2001;67:771–789. [Google Scholar]
- 60.Maoka T. Carotenoid metabolism in aquatic animals. Carotenoids: biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 29–50. [DOI] [PubMed] [Google Scholar]
- 61.Tsushima M, Katsuyama M, Matsuno T. Metabolism of carotenoids in the apple snail, Pomacea canaliculata. Comp Biochem Physiol. 1997;118B:431–436. [Google Scholar]
- 62.Schiedt K, Bischof S, Glinz E. Recent progress on carotenoid metabolism in animals. Pure Apple Chem. 1991;63:89–100. [Google Scholar]
- 63.Manunta C. Astaxanthin in insects and other terrestrial arthropoda. Nature. 1948;162:298. doi: 10.1038/162298a0. [DOI] [PubMed] [Google Scholar]
- 64.Harashima K, Ohno T, Sawachika T, Hidaka T, Ohnishi E. Carotenoids in orange pupae of the swallowtail, Papilio xuthus. Insect Biochem. 1972;2:29–48. [Google Scholar]
- 65.Maoka T, Kawase N, Ueda T, Nishida R. Carotenoids of dragonflies, from the perspective of comparative biochemical and chemical ecological studies. Biochem Syst Ecol. 2020;89:104001. [Google Scholar]
- 66.Veerman A. Carotenoid metabolism in Tetranychus urticae koch (Acari: Tetranychidae) Comp Biochem Physiol. 1974;47:101–116. doi: 10.1016/0305-0491(74)90095-9. [DOI] [PubMed] [Google Scholar]
- 67.Wybouw N, Kurlos AH, Greenhalgh R, Bryon A, Kosterlitz O, Manabe Y, Osakabe M, et al. Convergent evolution of cytochrome P450s underlies independent origins of keto-carotenoid pigmentation in animals. Proc R Soc B Biol Sci. 2019;286:20191039. doi: 10.1098/rspb.2019.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liaaen-Jensen S. Carotenoids in food chain. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: biosynthesis and metabolism. Basel Switzerland: Birkhäuser; 1998. pp. 359–371. [Google Scholar]
- 69.Maoka T, Akimoto N, Tsushima M, Komemushi S, Mezaki T, Iwase F, Takahashi Y, Sameshima N, Mori M, Sakagami Y. Carotenoids in marine invertebrates living along the Kuroshio current coast. Mar Drugs. 2011;9:1419–1427. doi: 10.3390/md9081419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maoka T, Nakachi S, Kobayashi R, Mori M, Sakagami Y. A new carotenoid, 9Z,9’Z-tetrahydroastaxanthin, from the sea cucumber Plesiocolochirus minutus. Tetrahedron Lett. 2015;56:5954–5955. [Google Scholar]
- 71.McGraw KJ. Colorful songbirds metabolize carotenoids at the integument. J Avian Biol. 2004;35:471–476. [Google Scholar]
- 72.Fox DL. Astaxanthin in the American Flamingo. Nature. 1955;175:942–943. doi: 10.1038/175942a0. [DOI] [PubMed] [Google Scholar]
- 73.LaFountain AM, Pacheco CO, Prum RO, Frank HA. Nuclear magnetic resonance analysis of carotenoids from the burgundy plumage of the Pompadour Cotinga (Xipholena punicea) Arch. Biochem Biophys. 2013;539:133–141. doi: 10.1016/j.abb.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Maoka T. Carotenoid metabolism in terrestrial animals. Carotenoids: Biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 51–66. [DOI] [PubMed] [Google Scholar]
- 75.Yong YYR, Lee YK. Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)? J Phycol. 1991;30:257–261. [Google Scholar]
- 76.Hagen C, Bornman G, Braune W. Reversible lowering of modulated chlorophyll fluorescence after saturating flashes in Haematococcus lacustris (Volvocales) at room temperature. Physiol Plant. 1992;86:593–599. [Google Scholar]
- 77.Zlotnik IS, Sukenik A, Dubinsky Z. Physiological and photosynthetic changes during the formation of red aplanospores in the chlorophyte Haematococcus pluvialis. J Phycol. 1993;29:463–469. [Google Scholar]
- 78.Boussiba S, Bing W, Zarka A, Yuan JP, Chen F. Changes in pigment profiles of Haematococcus pluvialis during exposure to environmental stresses. Biotechnol Lett. 1999;21:601–604. [Google Scholar]
- 79.Lu F, Vonshak A, Boussiba S. Effect of temperature and irradiance on growth of Haematococcus plulialis (Chlorophyceae) J Phycol. 2004;30:829–833. [Google Scholar]
- 80.Tan S, Cunningham FX, Youmans M, Grabowski B, Sun Z, Gantt E. Cytochrome-f loss in astaxanthin accumulating red cells of Haematococcus pluvialis (Chlorophyceae): comparison of photosynthetic activity, photosynthetic enzymes and thylakoid membrane polypeptides in red and green cells. J Phycol. 1995;31:897–905. [Google Scholar]
- 81.Mehler AH. Studies on reaction of illuminated chloroplast. I. Mechanisms of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951;33:65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- 82.Elstner EF. Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol. 1982;33:73–96. [Google Scholar]
- 83.Krinsky NI. Carotenoid protection against oxidation. Pure Appl Chem. 1979;51:649–660. [Google Scholar]
- 84.Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye ZW, Jiang JG, Wu GH. Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnol Adv. 2008;26:352–360. doi: 10.1016/j.biotechadv.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi M, Kakizono T, Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol. 1993;59:867–873. doi: 10.1128/aem.59.3.867-873.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan L, Vonshak A, Zarka A, Boussiba S. Does astaxanthin protect Haematococcus against light damage? Zeitschrift für Naturforschung C. 1998;53:93–100. doi: 10.1515/znc-1998-1-217. [DOI] [PubMed] [Google Scholar]
- 88.Bouvier F, Backhaus RA, Camara B. Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem. 1998;273:30651–30659. doi: 10.1074/jbc.273.46.30651. [DOI] [PubMed] [Google Scholar]
- 89.Rajesh K, Rohit MV, Mohan SV. Chapter-7: microalgae-based carotenoids production. In: Rastogi RP, Madamwar D, Pandey A, editors. Algal green chemistry. Elsevier; 2017. pp. 139–147. [Google Scholar]
- 90.M.I.M. Mosquera, M.J. Galan, D.H. Mendez, Carotenoids extraction using CO2 in supercritical state, from kale (Brassica oleracea, Lin. var. acephala). Proceedings of International Congress on Pigments in Food Technology. (Sevilla, Spain, 1999), pp. 65–69
- 91.Vidhyavathi R, Venkatachalam L, Sarada R, Aswanthanrayana G, Ravishankar GA. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot. 2008;59:1409–1418. doi: 10.1093/jxb/ern048. [DOI] [PubMed] [Google Scholar]
- 92.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 93.Hix LM, Frey DA, Mclaws MD, Osterlie M, Lockwood SF, Bertram JS. Inhibition of chemically-induced neoplastic transformation by a novel tetrasodium diphosphate astaxanthin derivative. Carcinogenesis. 2005;26:1634–1641. doi: 10.1093/carcin/bgi121. [DOI] [PubMed] [Google Scholar]
- 94.Kurihara H, Koda H, Asami S, Kiso Y, Tanaka T. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci. 2002;70:2509–2520. doi: 10.1016/s0024-3205(02)01522-9. [DOI] [PubMed] [Google Scholar]
- 95.Parisi V, Edeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy Italian study. Ophthalmology. 2008;115:324–333.e2. doi: 10.1016/j.ophtha.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 96.Seabra LMJ, Pedrosa LFC. Astaxanthin: structural and functional aspects. Rev Nutr. 2010;23:1041–1050. [Google Scholar]
- 97.WHO Fact sheets (World Health Organization, 2021) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 24 April 2022.
- 98.Puddu P, Puddu GM, Galletti L, Cravero E, Muscari A. Mitochondrial dysfunction as an initiating event in atherogenesis: a plausible hypothesis. Cardiology. 2005;103:137–141. doi: 10.1159/000083440. [DOI] [PubMed] [Google Scholar]
- 99.Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2017;26:445–461. doi: 10.1089/ars.2016.6756. [DOI] [PubMed] [Google Scholar]
- 100.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 102.Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K, Watanabe H. Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Biol Pharm Bull. 2006;29:684–688. doi: 10.1248/bpb.29.684. [DOI] [PubMed] [Google Scholar]
- 103.Maoka T, Etoh H. Some biological functions of carotenoids in Japanese food. Functional Foods of the East. Boca Raton: CRC Press; 2010. pp. 85–97. [Google Scholar]
- 104.Zhang L, Wang H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar Drugs. 2015;13:4310–4330. doi: 10.3390/md13074310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kishimoto Y, Yoshida H, Kondo K. Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. 2016;14:35. doi: 10.3390/md14020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, Tada N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209:520–523. doi: 10.1016/j.atherosclerosis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 107.Miyawaki H, Takahashi J, Tsukahara H, Takehara I. Effects of astaxanthin on human blood rheology. J Clin Biochem Nutr. 2008;43:69–74. doi: 10.3164/jcbn.2008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y, Li S, Guo Y, Yu H, Bao Y, Xin X, Yang H, Ni X, Wu N, Jia D. Astaxanthin attenuates hypertensive vascular remodeling by protecting vascular smooth muscle cells from oxidative stress induced mitochondrial dysfunction. Oxid Med Cell Longev. 2020;2020:4629189. doi: 10.1155/2020/4629189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101:59D–68D. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, Dufossé L. Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorg. 2019;7:186. doi: 10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Preuss HG, Echard B, Yamashita E, Perricone NV. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: Are these effects interdependent? Int J Med Sci. 2011;8:126–138. doi: 10.7150/ijms.8.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, Watanabe H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull. 2005;28:47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- 113.Yanai H, Ito K, Yoshida H, Tada N. Antihypertensive effects of astaxanthin. Integr Blood Press Control. 2008;1:1–3. doi: 10.2147/ibpc.s4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mashhadi NS, Zakerkish M, Mohammadiasl J, Zarei M, Mohammadshahi M, Haghighizadeh MH. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2018;27:341–346. doi: 10.6133/apjcn.052017.11. [DOI] [PubMed] [Google Scholar]
- 115.Becker RC. The role of blood viscosity in the development and progression of coronary artery disease. Cleve Clin J Med. 1993;60:353–358. doi: 10.3949/ccjm.60.5.353. [DOI] [PubMed] [Google Scholar]
- 116.Monroy-Ruiz J, Sevilla MÁ, Carrón R, Montero MJ. Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacol Res. 2011;63:44–50. doi: 10.1016/j.phrs.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Sasaki Y, Kobara N, Higashino S, Giddings JC, Yamamoto J. Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr Res. 2011;31:784–789. doi: 10.1016/j.nutres.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 118.Hu R, Saw CL-L, Yu R, Kong A-NT. Regulation of NF-E2-related factor 2 signaling for cancer chemoprevention: antioxidant coupled with anti-inflammatory. Antioxid Redox Signal. 2010;13:1679–1698. doi: 10.1089/ars.2010.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 120.Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 121.Faraone I, Sinisgalli C, Ostuni A, Armentano MF, Carmosino M, Milella L, Russo D, Labanca F, Khan H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: a systematic review. Pharmacol Res. 2020;155:104689. doi: 10.1016/j.phrs.2020.104689. [DOI] [PubMed] [Google Scholar]
- 122.Gradelet S, Astorg P, Le Bon A-M, Bergès R, Suschetet M. Modulation of aflatoxin B1 carcinogenicity, genotoxicity and metabolism in rat liver by dietary carotenoids: evidence for a protective effect of CYP1A inducers. Cancer Lett. 1997;114:221–223. doi: 10.1016/s0304-3835(97)04668-5. [DOI] [PubMed] [Google Scholar]
- 123.Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997;418:91–97. doi: 10.1016/s0014-5793(97)01355-0. [DOI] [PubMed] [Google Scholar]
- 124.Murakami A, Nakashima M, Koshiba T, Maoka T, Nishino H, Yano M, Sumida T, Kim OK, Koshimizu K, Ohigashi H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leukocytes. Cancer Lett. 2000;149:115–123. doi: 10.1016/s0304-3835(99)00351-1. [DOI] [PubMed] [Google Scholar]
- 125.Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J Photochem Photobiol B Biol. 2006;85:205–215. doi: 10.1016/j.jphotobiol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 126.Yang Y, Yang I, Cao M, Z-y S, Wu R, Guo Y, Fang M, Kong A-N. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018;20:32. doi: 10.1208/s12248-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Y-T, Kao C-J, Huang H-Y, Huang S-Y, Chen C-Y, Lin Y-S, Wen Z-H, Wang H-MD. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of in vitro and in vivo models in melanoma. J Funct Food. 2017;31:20–31. [Google Scholar]
- 128.X-d S, J-j Z, M-r W, W-b L, X-b G, Lv C. Astaxanthin induces mitochondria mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol Pharm Bull. 2011;34:839–844. doi: 10.1248/bpb.34.839. [DOI] [PubMed] [Google Scholar]
- 129.Shao Y, Ni Y, Yang J, Lin X, Li J, Zhang L. Astaxanthin inhibits proliferation and induces apoptosis and cell cycle arrest of mice H22 hepatoma cells. Med Sci Monit. 2016;22:2152–2160. doi: 10.12659/MSM.899419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sowmya PR, Arathi BP, Vijay K, Baskaran V, Lakshminarayana R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem Toxicol. 2017;106(Pt A):58–69. doi: 10.1016/j.fct.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 131.Koklesova L, Liskova A, Samec M, Buhrmann C, Samuel SM, Varghese E, Ashrafizadeh M, Najafi M, Shakibaei M, Büsselberg D, Giordano FA, Golubnitschaja O, Kubatka P. Carotenoids in cancer apoptosis-the road from bench to bedside and back. Cancers. 2020;12:2425. doi: 10.3390/cancers12092425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ko JC, Chen JC, Wang TJ, Zheng HY, Chen WC, Chang PY, Lin YW. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem Pharmacol. 2016;105:91–100. doi: 10.1016/j.bcp.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 133.Nagaraj S, Rajaram MG, Arulmurugan P, Baskaraboopathy A, Karuppasamy K, Jayappriyan KR, Sundararaj R, Rengasamy R. Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis Flotow on human hepatic cancer (HepG2) cell line. Biomed Prev Nutr. 2012;2:149–153. [Google Scholar]
- 134.Hormozi M, Ghoreishi S, Baharvand P. Astaxanthin induces apoptosis and increases activity of antioxidant enzymes in LS-180 cells. Artif Cells Nanomed Biotechnol. 2019;47:891–895. doi: 10.1080/21691401.2019.1580286. [DOI] [PubMed] [Google Scholar]
- 135.Lung Diseases, Environmental Health Topics (National Institute of Environmental Health Sciences, 2022), https://www.niehs.nih.gov/health/topics/conditions/lung-disease/index.cfm. Accessed 24 April 2022.
- 136.Kim JH, Chang MJ, Choi HD, Youn YK, Kim JT, Oh JM, Shin WG. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food. 2011;14:1469–1475. doi: 10.1089/jmf.2011.1626. [DOI] [PubMed] [Google Scholar]
- 137.Cheng J, Eroglu A. The promising effects of astaxanthin on lung diseases. Adv Nutr. 2021;12:850–864. doi: 10.1093/advances/nmaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.IDF Diabetes Atlas, 9th edn. (International Diabetes Federation: Brussels, Belgium, 2019) ISBN 978-2-930229-87-4.
- 139.Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PIH. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 140.Luc K, Schramm-Luc A, Guzik TJ, Mikolajcyk TP, Sangiovanni E, Martinelli G, Bossi L, Carpentier-Maguire E, Tchamgoue AD, Agbor G, et al. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70:809–824. doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 141.Landon R, Gueguen V, Petite H, Letourneur D, Pavon-Djavid G, Anagnostou F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar Drugs. 2020;18:357. doi: 10.3390/md18070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roohbakhsh A, Karimi G, Iranshahi M. Carotenoids in the treatment of diabetes mellitus and its complications: a mechanistic review. Biomed Pharmacother. 2017;91:31–42. doi: 10.1016/j.biopha.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 143.Wang J-J, Chen Z-Q, Lu W-Q. Hypoglycemic effect of astaxanthin from shrimp waste in alloxan-induced diabetic mice. Med Chem Res. 2012;21:2363–2367. [Google Scholar]
- 144.Uchiyama K, Naito Y, Hasegawa G, Nakamura N, Takahashi J, Yoshikawa T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7:290–293. doi: 10.1179/135100002125000811. [DOI] [PubMed] [Google Scholar]
- 145.Sayahi M, Shirali S. The antidiabetic and antioxidant effects of carotenoids: a review. Asian J Pharm Res Health Care. 2017;9:186–191. [Google Scholar]
- 146.Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, Shirahata A, Taniyama M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obesity. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- 147.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 148.Ishiki M, Nishida Y, Ishibashi H, Wada T, Fujisaka S, Takikawa A, et al. Impact of divergent effects of astaxanthin on insulin signaling in L6 cells. Endocrinology. 2013;154:2600–2612. doi: 10.1210/en.2012-2198. [DOI] [PubMed] [Google Scholar]
- 149.Bhuvaneswari S, Anuradha CV. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can J Physiol Pharmacol. 2012;90:1544–1552. doi: 10.1139/y2012-119. [DOI] [PubMed] [Google Scholar]
- 150.Hussein G, Nakagawa T, Goto H, Shimada Y, Matsumoto K, Sankawa U, Watanabe H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007;80:522–529. doi: 10.1016/j.lfs.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 151.Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi- Sunyer FX, Schwenke DC, et al. Adiponectin and the mediation of HDL-cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res. 2012;53:2726–2733. doi: 10.1194/jlr.M030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kitahara A, Takahashi K, Morita N, Murashima T, Onuma H, Sumitani Y, Tanaka T, Kondo T, Hosaka T, Ishida H. The novel mechanisms concerning the inhibitions of palmitate-induced proinflammatory factor releases and endogenous cellular stress with astaxanthin on MIN6 β-cells. Mar Drugs. 2017;15:185. doi: 10.3390/md15060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Arunkumar E, Bhuvaneswari S, Anuradha CV. An intervention study in obese mice with astaxanthin, a marine carotenoid—effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012;3:120–126. doi: 10.1039/c1fo10161g. [DOI] [PubMed] [Google Scholar]
- 154.Nishida Y, Nawaz A, Kado T, Takikawa A, Igarashi Y, Onogi Y, Wada T, Sasaoka T, Yamamoto S, Sasahara M, et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J Cachexia Sarcopenia Muscle. 2020;11:241–258. doi: 10.1002/jcsm.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan K, Pen P-J, Yin M. Anti-coagulatory and anti-inflammatory effects of astaxanthin in diabetic rats. J Food Sci. 2012;77:H76–H80. doi: 10.1111/j.1750-3841.2011.02558.x. [DOI] [PubMed] [Google Scholar]
- 156.Bhuvaneswari S, Arunkumar E, Viswanathan P, Anuradha CV. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010;45:1406–1414. [Google Scholar]
- 157.Aoi W, Naito Y, Takanami Y, Ishii T, Kawai Y, Akagiri S, Kato Y, Osawa T, Yoshikawa T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem Biophys Res Commun. 2008;366:892–897. doi: 10.1016/j.bbrc.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 158.Xia W, Tang N, Kord-Varkaneh H, Low TY, Tan SC, Wu X, Zhu Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: a meta-analysis of randomized controlled trials. Pharmacol Res. 2020;161:105113. doi: 10.1016/j.phrs.2020.105113. [DOI] [PubMed] [Google Scholar]
- 159.Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res. 2011;25:1813–1818. doi: 10.1002/ptr.3494. [DOI] [PubMed] [Google Scholar]
- 160.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. 2011;66:363–369. doi: 10.1007/s11130-011-0258-9. [DOI] [PubMed] [Google Scholar]
- 161.Gao LJ, Zhu YQ, Xu L. Mechanisms of protective effects of astaxanthin in nonalcoholic fatty liver disease. Hepatoma Res. 2021;7:30. [Google Scholar]
- 162.Wu L, Mo W, Feng J, Li J, Yu Q, Li S, Zhang J, Chen K, Ji J, Dai W, Wu J, Xu X, Mao Y, Guo C. Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br J Pharmacol. 2020;177:3760–3777. doi: 10.1111/bph.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han JH, Ju JH, Lee YS, et al. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci Rep. 2018;8:14090. doi: 10.1038/s41598-018-32497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu H, Liu M, Fu X, Zhang Z, Zhu L, Zheng X, Liu J. Astaxanthin prevents alcoholic fatty liver disease by modulating mouse gut microbiota. Nutrients. 2018;10:1298. doi: 10.3390/nu10091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jia Y, Wu C, Kim J, Kim B, Lee SJ. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferatoractivated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J Nutr Biochem. 2016;28:9–18. doi: 10.1016/j.jnutbio.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 166.Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr. 2011;141:1611–1617. doi: 10.3945/jn.111.142109. [DOI] [PubMed] [Google Scholar]
- 167.Ursoniu S, Sahebkar A, Serban MC, Banach M. Lipid profile and glucose changes after supplementation with astaxanthin: a systematic review and meta-analysis of randomized controlled trials. Arch Med Sci. 2015;11:253–266. doi: 10.5114/aoms.2015.50960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li J, Xia Y, Liu T, Wang J, Dai W, Wang F, Zheng Y, Chen K, Li S, et al. Protective effects of astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS ONE. 2015;10:e0120440. doi: 10.1371/journal.pone.0120440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dhinaut J, Balourdet A, Teixeira M, et al. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci Rep. 2017;7:12429. doi: 10.1038/s41598-017-12769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jyonouchi H, Hill R, Tomita Y, Good R. Studies of immunomodulating actions of carotenoids. I. Effects of β-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr Cancer. 1991;16:93–105. doi: 10.1080/01635589109514148. [DOI] [PubMed] [Google Scholar]
- 171.Jyonouchi H, Zhang L, Gross M, Tomita Y. Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer. 1994;21:47–58. doi: 10.1080/01635589409514303. [DOI] [PubMed] [Google Scholar]
- 172.Jyonouchi H, Sun S, Gross M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr Cancer. 1995;23:171–183. doi: 10.1080/01635589509514373. [DOI] [PubMed] [Google Scholar]
- 173.Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab. 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Augusti PR, Quatrin A, Somacal S, Conterato GM, Sobieskim R, Ruviaro AR, Maurer LH, Duarte MM, Roehrs M, Emanuelli T. Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J Clin Biochem Nutr. 2012;51:42–49. doi: 10.3164/jcbn.11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chew BP, Park JS. Carotenoid action on the immune response. J Nutr. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 176.Park JS, Mathison BD, Hayek MG, Massimino S, Reinhart GA, Chew BP. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet Immunol Immunopathol. 2011;144:455–461. doi: 10.1016/j.vetimm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 177.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hwang E, Lee DG, Park SH, Oh MS, Kim SY. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photo-aging of skin by regulation of pro-collagen type I and MMP-1 expression. J Med Food. 2014;17:985–995. doi: 10.1089/jmf.2013.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy- 2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 180.Tominaga K, Hongo N, Karato M, Yamashita E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim Pol. 2012;59:43–47. [PubMed] [Google Scholar]
- 181.Suganuma K, Nakajima H, Ohtsuki M, Imokawa G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci. 2010;58:136–142. doi: 10.1016/j.jdermsci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 182.Yoon HS, Cho HH, Cho S, Lee SR, Shin MH, Chung JH. Supplementing with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: a comparative study with placebo. J Med Food. 2014;17:810–816. doi: 10.1089/jmf.2013.3060. [DOI] [PubMed] [Google Scholar]
- 183.Phetcharat L, Wongsuphasawat K, Winther K. The effectiveness of a standardized rose hip powder, containing seeds and shells of Rosa canina, on cell longevity, skin wrinkles, moisture, and elasticity. Clin Interv Aging. 2015;10:1849–1856. doi: 10.2147/CIA.S90092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sztretye M, Dienes B, Gönczi M, Czirják T, Csernoch L, Dux L, Szentesi P, Keller-Pintér A. Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longev. 2019;2019:3849692. doi: 10.1155/2019/3849692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rzajew J, Radzik T, Rebas E. Calcium-involved action of phytochemicals: carotenoids and monoterpenes in the brain. Int J Mol Sci. 2020;21:1428. doi: 10.3390/ijms21041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kowsalya K, Vidya N, Vijayalakshmi V, Arun M. Super nutritive marine astaxanthin, an effectual dietary carotenoid for neurodegenerative diseases. Int Res J Multidiscipl Tech Maple Tree J. 2019;1:115–124. [Google Scholar]
- 187.Bhatt T, Patel K. Carotenoids: potent to prevent diseases review. Nat Prod Bioprospect. 2020;10:109–117. doi: 10.1007/s13659-020-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 189.Galasso C, Orefice I, Pellone P, Cirino P, Miele R, Ianora A, Brunet C, Sansone C. On the neuroprotective role of astaxanthin: new perspectives? Mar Drugs. 2018;16:247. doi: 10.3390/md16080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Petri D, Lundebye AK. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp Comp Biochem Physiol C. 2007;145:202–209. doi: 10.1016/j.cbpc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 191.Manabe Y, Komatsu T, Seki S, Sugawara T. Dietary astaxanthin can accumulate in the brain of rats. Biosci Biotechnol Biochem. 2018;82:1433–1436. doi: 10.1080/09168451.2018.1459467. [DOI] [PubMed] [Google Scholar]
- 192.El-Agamy SA, Abdel-Aziz AK, Wahdan S, Esmat A, Azab S. Astaxanthin ameliorates doxorubicin-induced cognitive impairment (chemobrain) in experimental rat model: impact on oxidative, inflammatory, and apoptotic machineries. Mol Neurobiol. 2018;55:5727–5740. doi: 10.1007/s12035-017-0797-7. [DOI] [PubMed] [Google Scholar]
- 193.Nakagawa K, Kiko T, Miyazawa T, Kimura GCF, Satoh A, Miyazawa T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr. 2011;105:1563–1571. doi: 10.1017/S0007114510005398. [DOI] [PubMed] [Google Scholar]
- 194.Ajmani RS, Metter EJ, Jaykumar R, Ingram DK, Spangler EL, Abugo OO, Rifkind JM. Hemodynamic changes during aging associated with cerebral blood flow and impaired cognitive function. Neurobiol Aging. 2000;21:257–269. doi: 10.1016/s0197-4580(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 195.Mohanty JG, Eckley DM, Williamson JD, Launer LJ, Rifkind JM. Do red blood cell-b-amyloid interactions alter oxygen delivery in Alzheimer’s disease? Adv Exp Med Biol. 2008;614:29–35. doi: 10.1007/978-0-387-74911-2_4. [DOI] [PubMed] [Google Scholar]
- 196.Leijenaara JF, van Maurik IS, Kuijer JPA, van der Flier WM, Scheltens P, Barkhof F, Prins ND. Lower cerebral blood flow in subjects with Alzheimer’s dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimers Dement. 2017;9:76–83. doi: 10.1016/j.dadm.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cunnane SC, Plourde M, Pifferi F, Bégin M, Féart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. 2009;48:239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 198.Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J, Curi R. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 2009;122:56–64. doi: 10.1016/j.pharmthera.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 199.Mattei R, Polotow TG, Vardaris CV, Guerra BA, Leite JR, Otton R, Barros MP. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: putative anxiolytic effects? Pharmacol Biochem Behav. 2011;99:349–355. doi: 10.1016/j.pbb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 200.Nolan JM, Mulcahy R, Power R, Moran R, Howard AN. Nutritional intervention to prevent Alzheimer’s Disease: potential benefits of xanthophyll carotenoids and Omega-3 Fatty acids combined. J Alzheimers Dis. 2018;64:367–378. doi: 10.3233/JAD-180160. [DOI] [PubMed] [Google Scholar]
- 201.Damodara Gowda KM, Suchetha Kumari N, Ullal H. Role of astaxanthin in the modulation of brain-derived neurotrophic factor and spatial learning behavior in perinatally undernourished Wistar rats. Nutr Neurosci. 2020;23:422–431. doi: 10.1080/1028415X.2018.1515301. [DOI] [PubMed] [Google Scholar]
- 202.Xue Y, Qu Z, Fu J, Zhen J, Wang W, Cai Y, Wang W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res Bull. 2017;131:221–228. doi: 10.1016/j.brainresbull.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 203.Pan L, Zhou Y, Li XF, Wan QJ, Yu LH. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res Bull. 2017;130:211–220. doi: 10.1016/j.brainresbull.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 204.Cakir E, Cakir U, Tayman C, Turkmenoglu TT, Gonel A, Turan IO. Favorable effects of astaxanthin on brain damage due to ischemia- reperfusion injury. Comb Chem High Throughput Screen. 2020;23:214–224. doi: 10.2174/1386207323666200219121600. [DOI] [PubMed] [Google Scholar]
- 205.Sharma K, Sharma D, Sharma M, Sharma N, Bidve P, Prajapati N, Kalia K, Tiwari V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci Lett. 2018;674:162–170. doi: 10.1016/j.neulet.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 206.Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: involvement of ERK and AKT signaling pathway. Eur J Pain. 2019;23:750–764. doi: 10.1002/ejp.1342. [DOI] [PubMed] [Google Scholar]
- 207.Chang MX, Xiong F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Molecules (Basel, Switzerland). 2020;25:5342. doi: 10.3390/molecules25225342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009;10:769–782. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 209.Strong MJ, Kesavapany S, Pant HC. The pathobiology of amyotrophic lateral sclerosis: a proteinopathy? J Neuropathol Exp Neurol. 2005;64:649–664. doi: 10.1097/01.jnen.0000173889.71434.ea. [DOI] [PubMed] [Google Scholar]
- 210.Isonaka R, Hiruma H, Katakura T, Kawakami T. Inhibition of superoxide dismutase selectively suppresses growth of rat spinal motor neurons: comparison with phosphorylated neurofilament-containing spinal neurons. Brain Res. 2011;1425:13–19. doi: 10.1016/j.brainres.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 211.O'Donnell E, Lynch MA. Dietary antioxidant supplementation reverses age-related neuronal changes. Neurobiol Aging. 1998;19:461–467. doi: 10.1016/s0197-4580(98)00082-7. [DOI] [PubMed] [Google Scholar]
- 212.Taksima T, Chonpathompikunlert P, Sroyraya M, Hutamekalin P, Limpawattana M, Klaypradit W. Effects of astaxanthin from shrimp shell on oxidative stress and behavior in animal model of Alzheimer’s disease. Mar Drugs. 2019;17:628. doi: 10.3390/md17110628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Che H, Li Q, Zhang T, Wang D, Yang L, Xu J, Yanagita T, Xue C, Chang Y, Wang Y. Effects of astaxanthin and docosahexaenoic-acid-acylated astaxanthin on Alzheimer’s disease in APP/PS1 double-transgenic mice. J Agric Food Chem. 2018;66:4948–4957. doi: 10.1021/acs.jafc.8b00988. [DOI] [PubMed] [Google Scholar]
- 214.Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yook JS, Okamoto M, Rakwal R, Shibato J, Lee MC, Matsui T, Chang H, Cho JY, Soya H. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol Nutr Food Res. 2016;60:589–599. doi: 10.1002/mnfr.201500634. [DOI] [PubMed] [Google Scholar]
- 216.Galasso C, Corinaldesi C, Sansone C. Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants (Basel, Switzerland) 2017;6:96. doi: 10.3390/antiox6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grimmig B, Daly L, Subbarayan M, Hudson C, Williamson R, Nash K, Bickford PC. Astaxanthin attenuates neurotoxicity in a mouse model of Parkinson’s disease. Funct Foods Health Dis. 2017;7:562–576. [Google Scholar]
- 218.Liu X, Shibata T, Hisaka S, Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 219.Ikeda Y, Tsuji S, Satoh A, Ishikura M, Shirasawa T, Shimizu T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2008;107:1730–1740. doi: 10.1111/j.1471-4159.2008.05743.x. [DOI] [PubMed] [Google Scholar]
- 220.Fakhri S, Aneva IY, Farzaei MH, Sobarzo-Sánchez E. The neuroprotective effects of astaxanthin: therapeutic targets and clinical perspective. Molecules. 2019;24:2640. doi: 10.3390/molecules24142640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang XJ, Chen W, Fu XT, Ma JK, Wang MH, Hou YJ, Tian DC, Fu XY, Fan CD. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Dis. 2019;5:70. doi: 10.1038/s41420-019-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ye Q, Zhang X, Huang B, Zhu Y, Chen X. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar Drugs. 2013;11:1019–1034. doi: 10.3390/md11041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ye Q, Huang B, Zhang X, Zhu Y, Chen X. Astaxanthin protects against MPP+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012;13:156. doi: 10.1186/1471-2202-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cort A, Ozturk N, Akpinar D, et al. Suppressive effect of astaxanthin on retinal injury induced by elevated intraocular pressure. Regul Toxicol Pharmacol. 2010;58:121–130. doi: 10.1016/j.yrtph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 225.Kikuchi K, Dong Z, Shinmei Y, Murata M, Kanda A, Noda K, Harada T, Ishida S. Hindawi, cytoprotective effect of astaxanthin in a model of normal intraocular pressure glaucoma. J Ophthalmol. 2020;2020:9539681. doi: 10.1155/2020/9539681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin WN, Kapupara K, Wen YT, Chen YH, Pan IH, Tsai RK. Haematococcus pluvialis-derived astaxanthin is a potential neuroprotective agent against optic nerve ischemia. Mar Drugs. 2020;18:85. doi: 10.3390/md18020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Otsuka T, Shimazawa M, Inoue Y, et al. Astaxanthin protects against retinal damage: evidence from in vivo and in vitro retinal ischemia and reperfusion models. Curr Eye Res. 2016;41:1465–1472. doi: 10.3109/02713683.2015.1127392. [DOI] [PubMed] [Google Scholar]
- 228.Stevens JC, Sun S, Beard CM, et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134–138. doi: 10.1212/wnl.38.1.134. [DOI] [PubMed] [Google Scholar]
- 229.Falkenburg SA. Choosing hand splints to aid carpal tunnel syndrome recovery. Occup Health Saf. 1987;56:63–64. [PubMed] [Google Scholar]
- 230.Weiss ND, Gordon L, Bloom T, So Y, Rempel DM. Position of the wrist associated with the lowest carpal-tunnel pressure: implications for splint design. J Bone Joint Surg Am. 1995;77:1695–1699. doi: 10.2106/00004623-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 231.O’Connor D, Marshall SC, Massy-Westropp N, Pitt V. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;2003:CD003219. doi: 10.1002/14651858.CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shi Q, Macdermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res. 2011;6:17. doi: 10.1186/1749-799X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wilson JK, Sevier TL. A review of treatment for carpal tunnel syndrome. Disabil Rehabil. 2003;25:113–119. doi: 10.1080/0963828021000007978. [DOI] [PubMed] [Google Scholar]
- 234.Kaplan SJ, Glickel SZ, Eaton RG. Predictive factors in the nonsurgical treatment of carpal tunnel syndrome. J Hand Surg J Br Soc Surg Hand. 1990;15:106–108. doi: 10.1016/0266-7681_90_90061-8. [DOI] [PubMed] [Google Scholar]
- 235.MacDermid JC, Evenhuis W, Louzon M. Inter-instrument reliability of pinch strength scores. J Hand Ther. 2001;14:36–42. doi: 10.1016/s0894-1130(01)80023-5. [DOI] [PubMed] [Google Scholar]
- 236.Phalen GS. The carpal-tunnel syndrome: clinical evaluation of 598 hands. Clin Orthop. 1972;83:29–40. doi: 10.1097/00003086-197203000-00007. [DOI] [PubMed] [Google Scholar]
- 237.Macdermid JC, Vincent JI, Gan BS, Grewal RA. A blinded placebo-controlled randomized trial on the use of astaxanthin as an adjunct to splinting in the treatment of carpal tunnel syndrome. Hand (N Y) 2012;7:1–9. doi: 10.1007/s11552-011-9381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol Pharm Bull. 2006;29:2106–2110. doi: 10.1248/bpb.29.2106. [DOI] [PubMed] [Google Scholar]
- 239.Imai A, Oda Y, Ito N, Seki S, Nakagawa K, Miyazawa T, Ueda F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: a randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients. 2018;10:281. doi: 10.3390/nu10030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kim SH, Kim H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients. 2018;10:1137. doi: 10.3390/nu10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hayashi M, Kawamura M, Kawashima Y, Uemura T, Maoka T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on the status of stress and sleep in adults. J Clin Biochem Nutr. 2020;66:92–102. doi: 10.3164/jcbn.19-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tripathi DN, Jena GB. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology. 2008;248:96–103. doi: 10.1016/j.tox.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 243.Comhaire FH, Mahmoud A. The role of food supplements in the treatment of the infertile man. Reprod Biomed Online. 2003;7:385–391. doi: 10.1016/s1472-6483(10)61881-0. [DOI] [PubMed] [Google Scholar]
- 244.Wang JY, Lee YJ, Chou MC, Chang R, Chiu CH, et al. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse Leydig cells. Mar Drugs. 2015;13:1375–1388. doi: 10.3390/md13031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vahidinia A, Rahbar AR, Shakoori Mahmoodabadi MM. Effect of astaxanthin, vitamin E, and vitamin C in combination with calorie restriction on sperm quality and quantity in male rats. J Diet Suppl. 2017;14:252–263. doi: 10.1080/19390211.2016.1211783. [DOI] [PubMed] [Google Scholar]
- 246.Donà G, Kožuh I, Brunati AM, Andrisani A, Ambrosini G, Bonanni G, Ragazzi E, Armanini D, Clari G, Bordin L. Effect of astaxanthin on human sperm capacitation. Mar Drugs. 2013;11:1909–1919. doi: 10.3390/md11061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clinical Trial Report (National Institute of Health, USA 2019), https://clinicaltrials.gov/ct2/show/NCT02310087. Accessed 24 April 2022.
- 248.Yang YX, Kim YJ, Jin Z, Lohakare JD, Kim CH, Ohh SH, Lee SH, Choi JY, Chae BJ. Effects of dietary supplementation of astaxanthin on production performance, egg quality in layers and meat quality in finishing pigs. Asian-Australas J Anim Sci. 2006;19:1019–1025. [Google Scholar]
- 249.Hansen KB, Tauson AH, Inborr J. Effect of supplementation with the antioxidant astaxanthin on reproduction, pre-weaning growth performance of kits and daily milk intake in mink. J Reprod Fertil Suppl. 2001;57:331–334. [PubMed] [Google Scholar]
- 250.Abdel-Ghani MA, Yanagawa Y, Balboula AZ, Sakaguchi K, Kanno C, Katagiri S, Takahashi M, Nagano M. Astaxanthin improves the developmental competence of in vitro-grown oocytes and modifies the steroidogenesis of granulosa cells derived from bovine early antral follicles. Reprod Fertil Dev. 2019;31:272–281. doi: 10.1071/RD17527. [DOI] [PubMed] [Google Scholar]
- 251.Iwabayashi M, Fujioka N, Nomoto K, Miyazaki R, Takahashi H, Hibino S, Takahashi Y, Nishikawa K, Nishida M, Yonei Y. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti Aging Med. 2009;6:15–21. [Google Scholar]
- 252.Clinical Trial Report (National Institute of Health, USA, 2021), https://clinicaltrials.gov/ct2/show/study/NCT03991286. Accessed 24 April 2022.
- 253.Moravkova M, Slany M, Trcka I, Havelkova M, Svobodova J, Skoric M. Human-to-human and human-to-dog Mycobacterium tuberculosis transmission studied by IS6110 RFLP analysis: a case report. Vet Med. 2011;56:314–317. [Google Scholar]
- 254.Alix E, Godreuil S, Blanc-Potard AB. Identification of a Haarlem genotype-specific single nucleotide polymorphism in the mgt C virulence gene of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:2093–2098. doi: 10.1128/JCM.00278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Global tuberculosis control: WHO report (World Health Organization, 2010). https://apps.who.int/iris/handle/10665/44425. Accessed 24 April 2022.
- 256.Vasudevan S, Venkatraman A, Yahoob SAM, Jojula M, Sundaram R, Boomi P. Biochemical evaluation and molecular docking studies on encapsulated astaxanthin for the growth inhibition of Mycobacterium tuberculosis. J Appl Biol Biotechnol. 2021;9:31–39. [Google Scholar]
- 257.Donà G, Andrisani A, Tibaldi E, Brunati AM, Sabbadin C, Armanini D, Ambrosini G, Ragazzi E, Bordin L. Astaxanthin prevents human papillomavirus L1 protein binding in human sperm membranes. Mar Drugs. 2018;16:427. doi: 10.3390/md16110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ahmadi AR, Nasrabadi RA. Astaxanthin protective barrier and its ability to improve the health in patients with COVID-19. Iran J Microbiol. 2021;13:434–441. doi: 10.18502/ijm.v13i4.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Talukdar J, Bhadra B, Dattaroy T, Nagle V, Dasgupta S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed Pharmacother. 2020;132:110886. doi: 10.1016/j.biopha.2020.110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chia WY, Kok H, Chew KW, Low SS, Show PL. Can algae contribute to the war with COVID-19? Bioengineered. 2021;12:1226–1237. doi: 10.1080/21655979.2021.1910432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fakhri S, Nouri Z, Moradi SZ, Farzaei MH. Astaxanthin, COVID-19 and immune response: focus on oxidative stress, apoptosis and autophagy. Phytother Res. 2020;34:2790–2792. doi: 10.1002/ptr.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Brendler T, Williamson EM. Astaxanthin: how much is too much? A safety review. Phytother Res. 2019;33:3090–3111. doi: 10.1002/ptr.6514. [DOI] [PubMed] [Google Scholar]
- 263.Naidu AS, Pressman P, Roger A, Clemens RA. Coronavirus and nutrition: what is the evidence for dietary supplements usage for COVID-19 control and management? Nutr Today. 2021;56:19–25. [Google Scholar]
- 264.Zhu C, Farré G, Díaz-Gómez J, Capell T, Nogareda C, Sandmann G, Christou P. Engineered maize hybrids with diverse carotenoid profiles and potential applications in animal feeding. Carotenoids: biosynthetic and biofunctional approaches, advances in experimental medicine and biology. Singapore: Springer; 2021. pp. 95–114. [DOI] [PubMed] [Google Scholar]
- 265.Cysewski GR, Lorenz RT. Industrial production of microalgal cell mass and secondary products-species of high potential Haematococcus. Handbook of Microalgal Culture: biotechnology and applied phycology. Oxford: Blackwell Science; 2004. pp. 281–288. [Google Scholar]
- 266.Li J, Zhu D, Niu J, Shen S, Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv. 2011;29:568–574. doi: 10.1016/j.biotechadv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 267.Begum H, Yusoff FM, Banerjee S, Khatoon H, Shariff M. Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr. 2016;56:2209–2222. doi: 10.1080/10408398.2013.764841. [DOI] [PubMed] [Google Scholar]
- 268.Sheikhzadeh N, Tayefi-Nasrabadi H, Oushani AK, Enferadi MH. Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 2012;38:413–419. doi: 10.1007/s10695-011-9519-7. [DOI] [PubMed] [Google Scholar]
- 269.Rao AR, Baskaran V, Sarada R, Ravishankar GA. In vivo bioavailability and antioxidant activity of carotenoids from micro algal biomass—a repeated dose study. Food Res Int. 2013;54:711–717. [Google Scholar]
- 270.Rao AR, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA. Effective inhibition of skin cancer, tyrosinase and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem. 2013;61:3842–3851. doi: 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- 271.Stewart JS, Lignell A, Pettersson A, Elfving E, Soni MG. Safety assessment of astaxanthin rich microalgae biomass: acute and subchronic toxicity studies in rats. Food Chem Toxicol. 2008;46:3030–3036. doi: 10.1016/j.fct.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 272.Odeberg MJ, Lignell A, Pettersson A, Hoglund P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur J Pharm Sci. 2003;19:299–304. doi: 10.1016/s0928-0987(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 273.Serebruany V, Malinin A, Goodin T, Pashkow F. The in vitro effects of xancor, a synthetic astaxanthine derivative, on hemostatic biomarkers in aspirin-naive and aspirin-treated subjects with multiple risk factors for vascular disease. Am J Ther. 2010;17:125–132. doi: 10.1097/MJT.0b013e31819cdbbd. [DOI] [PubMed] [Google Scholar]
- 274.Spiller GA, Dewell A. Safety of an astaxanthin rich Haemaotoccus pluvialis algal extract: a randomized clinical trial. J Med Food. 2003;6:51–56. doi: 10.1089/109662003765184741. [DOI] [PubMed] [Google Scholar]