Abstract
A 73-year-old man receiving hemodialysis and antiplatelets was admitted with a mild case of COVID-19. Heparin was added, and iliopsoas hemorrhage developed. He was successfully treated by interventional radiology. A 76-year-old man receiving hemodialysis and antiplatelets was admitted with mild COVID-19. Heparin was added, and iliacus hemorrhage developed. Despite heparin discontinuation, he died of worsening pneumonia. A 74-year-old man undergoing hemodialysis was admitted with severe COVID-19. Gastrointestinal bleeding developed during continuous hemodiafiltration with heparin. Upon switching to nafamostat and increasing the dose, iliopsoas hemorrhage developed. Despite interventional radiology, he died of infectious complications. Attention to hemorrhagic complications is therefore needed in patients with COVID-19.
Keywords: anticoagulants, antiplatelet therapy, COVID-19, dialysis patients, retroperitoneal hemorrhage
Introduction
Since coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected at the end of 2019, it has rapidly spread worldwide because of its strong infectivity. Because the case fatality rate is high, COVID-19 has had a great impact on medicine, society, and the economy around the world. Underlying diseases such as diabetes, heart disease, chronic kidney disease, obesity, and smoking are risk factors for developing severe COVID-19 (1), and end-stage renal disease has an especially high case fatality rate (2,3). The pathology of COVID-19 involves an infection of alveolar epithelial cells by SARS-CoV-2, followed by an excessive immune response that causes pneumonia and even acute respiratory distress syndrome. Various other organ disorders may also develop (4). The most characteristic complication is coagulopathy, which causes various types of thromboembolism such as pulmonary embolism and ischemic stroke (5). Thrombotic complications are associated with a high rate of fatalities, and prophylactic anticoagulation has thus been proposed to reduce the severity of the disease (6).
Some recent reports have also described hemorrhagic complications of COVID-19 (7). We experienced three patients with COVID-19 undergoing hemodialysis (HD) who developed retroperitoneal hemorrhages during intense antithrombotic therapy. These cases are very informative for clinicians considering the indications for and the types of antithrombotic therapy for patients with COVID-19.
Case Reports
Case 1
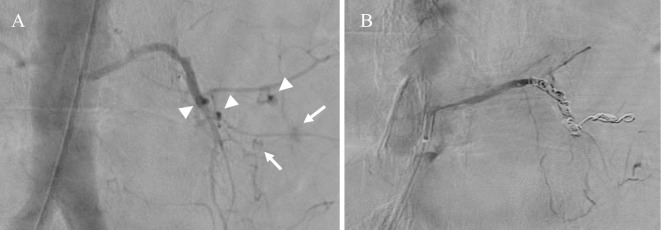
The first patient was a 73-year-old man with a history of diabetes, hypertension, aortic stenosis, heart failure, peripheral arterial disease, and angina. He had begun HD for diabetic nephropathy and undergone coronary stenting for angina 7 years previously. Since then, he had been taking clopidogrel (75 mg) and aspirin (100 mg). He was admitted to our hospital with a diagnosis of COVID-19 based on a positive finding for SARS-CoV-2 polymerase chain reaction, performed to detect COVID-19 among the patients in close contact with other patients with COVID-19. He had developed a sore throat, wet cough, and malaise a few days before admission. At admission, he weighed 51.8 kg and had clear consciousness, a blood pressure of 100/51 mmHg, a pulse rate of 47 beats/min, a respiratory rate of 18 breaths/min, a body temperature of 37.1°C, and oxygen saturation of 99% on room air. Chest computed tomography (CT) showed only one area of a ground-glass opacity in the lower right lobe, and the severity of COVID-19 was rated as mild. The results of blood tests performed on admission are shown in Table 1. Because of the slightly elevated D-dimer concentration and the fact that the patient was undergoing treatment with HD, his risk of thrombotic complications was considered high, and the subcutaneous injection of 5,000 U of heparin calcium twice daily was started on the first day of hospitalization (Day 1). During HD, additional heparin sodium was administered (initial bolus of 1,000 U and continuous infusion of 1,000 U/h; total of 4,000 U/session). On Day 2, he developed a fever of about 38°C, and on Day 4, dexamethasone (DEX) (6 mg) was started. His blood pressure remained within the range of 120 to 140 mmHg. On Day 6, the patient developed low back pain that changed to abdominal pain the following day, and he thereafter developed circulatory shock during HD. Blood tests before HD showed that his hemoglobin concentration had decreased to 7.7 g/dL and that his creatine kinase concentration had increased to 480 U/L, thus suggesting retroperitoneal hemorrhage. At this time, his activated partial thromboplastin time (APTT) was slightly prolonged at 41.1 seconds. We stopped his HD and performed contrast-enhanced CT, which showed a giant left iliopsoas hematoma and the extravasation of contrast medium (Fig. 1). We started blood transfusions, took appropriate infection prevention measures, and performed urgent interventional radiology (IVR) (Fig. 2). The IVR was successful, and complete hemostasis was achieved. A total of 8 U of red blood cells was transfused. For antithrombotic therapy, low-dose aspirin and heparin calcium were discontinued and the anticoagulant during HD was changed from heparin sodium to nafamostat (20 mg/h); only clopidogrel was continued. After the bleeding event, the patient temporarily required a small amount of oxygen as he recovered from COVID-19 pneumonia. The administration of DEX for a total of 10 days improved his clinical condition. Three weeks later, the anticoagulant during HD was changed to dalteparin (an initial bolus of 2,500 U/session), and no rebleeding was observed. On Day 60, he was transferred to another institution for rehabilitation.
Table 1.
Blood Test Findings on Admission.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| White blood cell (/μL) | 4,900 | 4,240 | 6,570 |
| Lymphocyte (/μL) | 872 | 882 | 867 |
| Hemoglobin (g/dL) | 10.8 | 10.8 | 12.3 |
| Platelet (×104/μL) | 10.8 | 11.1 | 9.0 |
| D-dimer (μg/mL) | 1.17 | 1.90 | 7.4 |
| Activated partial thromboplastin time (s) | 41.1 | 30.9 | 41.2 |
| Albumin (g/dL) | 3.2 | 3.3 | 3.0 |
| Total bilirubin (mg/dL) | 0.3 | 0.2 | 0.7 |
| Aspartate aminotransferase (IU/L) | 17 | 7 | 68 |
| Alanine aminotransferase (IU/L) | 9 | 15 | 18 |
| Lactate dehydrogenase (IU/L) | 181 | 152 | 427 |
| Blood urea nitrogen (mg/dL) | 64.5 | 64.9 | 25.3 |
| Creatinine (mg/dL) | 11.62 | 15.05 | 6.58 |
| Ferritin (ng/mL) | 891 | 246 | 988 |
| C-reactive protein (mg/dL) | 2.4 | 0.39 | 23.97 |
For test values that were not measured on admission, the first measured values after admission were adopted.
Figure 1.
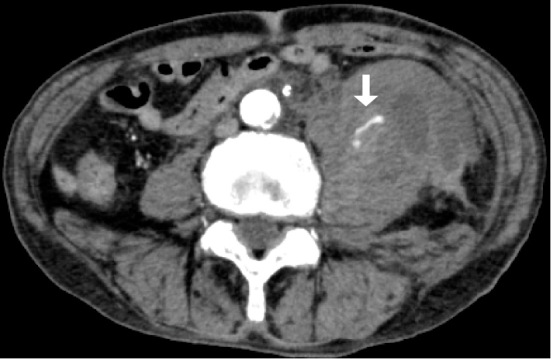
Contrast-enhanced computed tomography in Case 1 shows a left iliopsoas hematoma and extravasation of contrast medium (arrow).
Figure 2.
Angiography in Case 1 shows (A) multiple sites of contrast medium leakage (arrows) and pseudoaneurysms (arrowheads) and (B) coil embolization to the proximal part of the left fourth lumbar artery with no leakage.
Case 2
The second patient was a 76-year-old man with a history of diabetes, hypertension, and ischemic heart disease. He had been receiving HD for diabetic nephropathy and had been taking prasugrel for the ischemic heart disease. He had been diagnosed with COVID-19, but he was asymptomatic and had no pneumonia on admission. The results of blood tests performed on admission are shown in Table 1. On Day 4, because he had developed a fever, fatigue, and an elevated D-dimer concentration, the prophylactic subcutaneous injection of 5,000 U of heparin calcium twice daily was added separately from the administration of heparin sodium during HD (initial bolus of 1,000 U and continuous infusion of 1,000 U/h; total of 4,000 U/session). On Day 6, oxygen administration and DEX were started because of a worsening of the COVID-19 pneumonia symptoms. On Day 9, left thigh pain appeared and plain CT showed a left iliacus hematoma (Fig. 3). We discontinued the heparin calcium and started pain control with opioid analgesics. We also reduced the dose of heparin sodium during HD to 3,000 U/session. On Day 12, the patient's respiratory condition worsened and his D-dimer concentration further increased; thus, steroid pulse therapy (methylprednisolone at 500 mg for 3 days) was started and heparin calcium was restarted at 5,000 U/day. However, his respiratory failure progressed, thus leading to multiple organ failure, and he eventually died on Day 21.
Figure 3.
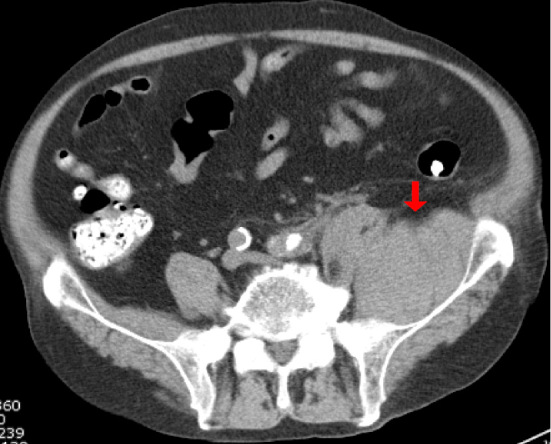
Computed tomography in Case 2 shows a left iliacus hematoma.
Case 3
The third patient was a 74-year-old man with a history of hypertension, resection of gastric cancer, and vertebroplasty for spinal canal stenosis. He had been receiving HD for hypertensive nephrosclerosis. He had undergone surgery for a strangulated ileus 15 days before admission and had been discharged 6 days before admission. Two days before admission, the patient developed fatigue, and his fatigue worsened with the development of respiratory failure after HD on the day of admission; therefore, he visited an emergency hospital. Because he had a positive finding for the SARS-CoV-2 antigen test at that hospital, he was admitted to our hospital. The chest X-ray findings and blood test results on admission are shown in Fig. 4 and Table 1, respectively. Even after the administration of 6 L/min of oxygen, his oxygen saturation remained at 90%, and DEX and favipiravir were started. However, because of a further progression of his respiratory failure, he was transferred to a hospital with an intensive care unit (ICU) for COVID-19 on Day 2. Immediately after the transfer, invasive mechanical ventilation, remdesivir, steroid pulse therapy (methylprednisolone at 1 g for 3 days), and the continuous intravenous infusion of 10,000 U/day of heparin sodium were started. On Day 3, continuous hemodiafiltration (CHDF) with nafamostat (20 mg/h) was started. On Day 4, the patient developed tar-like stool and he was diagnosed with upper gastrointestinal bleeding, and heparin sodium was therefore discontinued. However, because the CHDF circuit life was shortened and frequent circuit replacement was required, the dose of nafamostat was gradually increased to 60 mg/h on Day 9. On Day 12, the patient's anemia progressed, and contrast-enhanced CT showed a right iliopsoas hematoma and the leakage of contrast medium (Fig. 5). Although IVR was urgently performed and hemostasis was achieved, the patient died on Day 54 as a result of several complications including delayed anastomotic leakage following strangulated ileus surgery, cytomegalovirus antigenemia, and an iliopsoas abscess.
Figure 4.
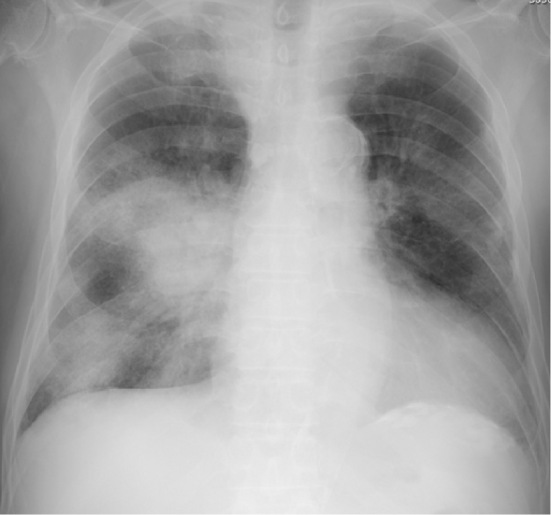
A chest X-ray in Case 3 shows multiple infiltration shadows and ground-glass opacities predominantly in the right lung.
Figure 5.
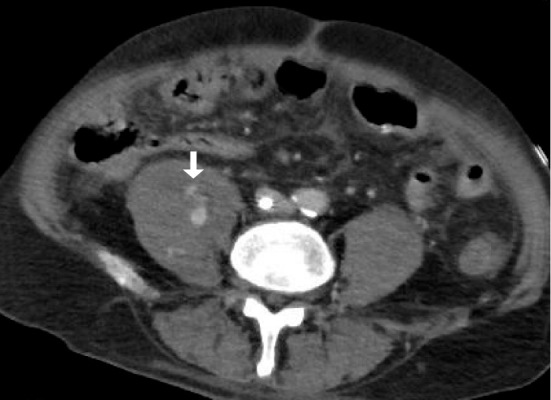
Contrast-enhanced computed tomography in Case 3 shows a right iliopsoas hematoma and extravasation of contrast medium (arrow).
The characteristics of all three patients are shown in Table 2. Each patient's HAS-BLED score (8), which is frequently used as an index to evaluate the risk of serious bleeding complications when deciding whether anticoagulant therapy is indicated for atrial fibrillation, was 3 or 4 out of 9 points, and the risk of bleeding was high.
Table 2.
Characteristics of the Three Patients.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age | 73 | 76 | 74 |
| Sex | Male | Male | Male |
| Comorbidities | DM, HT, IHD, PAD, valvular disease | CHF, DM, IHD | HT, IHD, strangulation ileus (PO), valvular disease |
| Symptoms on admission | dry cough, fatigue, sore throat | None | disorientation, fatigue, hypoxia |
| Antiviral treatment | None | None | Favipiravir→remdesivir |
| Maximum dose of steroid | DEX 6 mg | mPSL 500 mg | mPSL 1 g |
| Antithrombotic therapy | Low dose aspirin, clopidogrel, HC 10,000 U/day, HS 4,000 U/HD |
Prasugrel, HC 10,000 U/day, HS 4,000 U/HD |
HS 10,000 U/day, NM 20 mg/h (CHDF) →NM 60 mg/h (CHDF) |
| HAS-BLED score | 4 | 3 | 3 |
| Bleeding events | Iliopsoas bleeding | Iliacus bleeding | Upper GI bleeding →iliopsoas bleeding |
| Other complications | Pneumonia | ARDS | ARDS, iliopsoas abscess, delayed anastomotic leakage |
| Outcome | Recovery | Death | Death |
ARDS: acute respiratory distress syndrome, CHDF: continuous hemodiafiltration, CHF: chronic heart failure, DEX: dexamethasone, DM: diabetes mellitus, GI: gastrointestinal, HC: heparin calcium, HD: hemodialysis, HS: heparin sodium, HT: hypertension, IHD: ischemic heart disease, mPSL: methylprednisolone, NM: nafamostat mesylate, PAD: peripheral arterial disease, PO: postoperatively, U: units
Discussion
Cases 1 and 2 developed retroperitoneal hemorrhages during combination antiplatelet and anticoagulant therapy, and Case 3 developed upper gastrointestinal bleeding during the combination continuous infusion of heparin sodium and nafamostat and a retroperitoneal hemorrhage during high-dose nafamostat therapy. All three patients had COVID-19, were undergoing HD, and developed hemorrhage during intensive antithrombotic therapy. In Case 2, the patient died of an exacerbation of respiratory failure with elevated D-dimer after reducing the heparin dose, thus bleeding may have affected his prognosis. In Case 3, since the patient had severe respiratory failure at admission, the hemostasis of retroperitoneal bleeding was successful with IVR, and the cause of death was infectious complications, so the effect of bleeding on his prognosis was presumably small.
One of the complications specific to COVID-19 is coagulopathy (5). According to a questionnaire survey of COVID-19-related thrombosis in Japan (9), thrombosis (including arterial thrombosis) occurred in 1.85% (n=105) of all 5,687 patients with COVID-19 and in 13.2% of patients with severe COVID-19. Deep vein thrombosis occurred in 0.72% (n=41) of all patients. In an analysis of 8,271 patients outside Japan, however, the incidence of venous thromboembolism was as high as 21%; the incidence reached 5% in the general ward and 31% in the ICU (10), thus showing a large difference between Japan and other countries. Based on the results of observational studies showing that the mortality rate is low in patients treated with anticoagulants (11), the International Society for Thrombosis and Hemostasis recommends prophylactic anticoagulant therapy using low-molecular-weight heparin when abnormalities are observed in D-dimers and other coagulation-related markers (6). However, original guidelines are considered necessary for Japanese patients in view of the fact that oriental people have a high risk of bleeding when undergoing anticoagulant therapy (12). The National Center for Global Health and Medical Research Hospital proposed an anticoagulant therapy algorithm using unfractionated heparin for Japanese patients with moderate to severe COVID-19 in May 2020 (13), and this algorithm was widely used in practice. In the algorithm, 5,000 U of heparin calcium was subcutaneously injected twice daily for patients with moderate COVID-19, and the continuous intravenous injection of 10,000 U/day of heparin sodium was started for patients with severe COVID-19 with a target APTT of 1.5 to 2.5 times the control value. In all three patients described in the present report, the administration of unfractionated heparin generally followed this algorithm; in addition, anticoagulants during renal replacement therapy were required and the prescribed antiplatelet drugs were continued. Antithrombotic therapy had thus been intensely performed in all cases. Although there are few reports of the risk of thrombosis in COVID-19 patients undergoing dialysis, the above-mentioned questionnaire survey (9) showed that 15% (13 patients) of 86 patients who underwent CHDF or HD developed thrombosis, which suggested that the risk of thrombosis was as high as that of critically ill patients. Hence, it seems appropriate to target dialysis patients for prophylactic anticoagulant therapy regardless of their severity. Since the beginning of 2021, two randomized controlled trials on anticoagulant therapy for COVID-19 have been reported, and a series of results have shown that anticoagulant therapy that is more intense than prophylaxis does not outweigh the prophylaxis at any severity of COVID-19 (14,15). Therefore, intensive anticoagulant therapy is not currently recommended.
As mentioned above, the thrombotic complications of COVID-19 are widely known. However, bleeding complications have also been gradually attracting attention and have been reported in 1.8% of all patients with COVID-19 (7) and in 2.8% of patients with COVID-19 in the ICU (16). A cohort study (17) examining the risk for bleeding in anticoagulant therapy for COVID-19 revealed that use of therapeutic-intensity anticoagulation, critical illness, and elevated D-dimer or ferritin levels at admission were associated with significantly increased risk for major bleeding. In contrast, creatinine clearance and pre-existing chronic kidney disease (CKD) was not found to be associated with bleeding risk. Another cohort study of 4,264 ICU patients in the United States (18) also showed that CKD and dialysis patients were associated with a higher risk of mortality, but they were not significantly associated with major bleeding. Therefore, bleeding complications are not specific to dialysis patients, and the severity and intensity of antithrombotic therapy are considered to be a risk for bleeding.
Some reports have described retroperitoneal hemorrhage as a fatal bleeding complication (19,20), most of which occurred during anticoagulant therapy in patients with background factors such as older age, hypertension, and combination with aspirin. There are three possible mechanisms for retroperitoneal hemorrhage. The first is that the development of microthrombi by microvascular endothelial injury impairs the microcirculation and disrupts the microvessels (21). The second is that atherosclerosis causes either localized weakening or a pseudoaneurysm of the arterial wall, and this is followed by a rupture secondary to increased abdominal pressure due to coughing or vomiting (22). The third is that minor unrecognized trauma ruptures the blood vessels in the muscles of the retroperitoneal space (23). In particular, the latter two mechanisms anatomically support previous findings of the high frequency of retroperitoneal hemorrhage. The reported risk factors for retroperitoneal hemorrhage include anticoagulants, antiplatelet drugs, an older age, coagulopathy, hypertension, arteriosclerosis, liver cirrhosis, and chronic renal failure (24-26). The three patients described in the present report had five to six of these eight risk factors. Furthermore, each patient's HAS-BLED score (8) was 3 to 4 points; the risk of hemorrhagic events was expected to be 3.74 to 8.70 per 100 person-years, which was also considered to be high.
The three patients described in this report were treated at three of the facilities in Ehime prefecture where patients with COVID-19 undergoing HD can be hospitalized. By October 2021, 17 patients with COVID-19 were undergoing HD in Ehime prefecture. The results of surveillance regarding the characteristics of the 17 patients are shown in Table 3. Since 15 of 17 patients had used some antithrombotic drug except during intermittent hemodialysis, no thrombotic complications were observed. No hemorrhagic events occurred in patients without antithrombotic therapy (n=2), with the continuation of antiplatelet drug alone (n=4), or with prophylactic heparin calcium alone (n=7) during COVID-19 treatment. In contrast, retroperitoneal hemorrhage occurred in two of the three patients who received combined antiplatelet and anticoagulant therapy. A comparison of the characteristics and laboratory data of 3 patients with bleeding events with the other 14 patients is shown in Table 4. A comparison of the laboratory data showed a tendency for higher ferritin levels in patients with bleeding events, as shown in the above-mentioned cohort study (17), but no difference in D-dimer. In a comparison of these characteristics, the combination of two or more antithrombotic drugs was significantly more common in the patients with bleeding events (p<0.01). Hence, combination therapy should be strictly discouraged. In addition, to reduce the risk of bleeding in patients undergoing HD, efforts should be made to reduce the amount of heparin sodium given during renal replacement therapy to the minimum necessary amount that can avoid circuit coagulation. The lesson in Case 3 is that the dose of unfractionated heparin at the beginning of hospitalization should have been changed from the therapeutic dose to the prophylactic dose. In addition, regional citrate anticoagulation should have been considered as an option for anticoagulation during CHDF after the onset of upper gastrointestinal bleeding. Although regional citrate anticoagulation is not covered by insurance in Japan, it is recommended by the Kidney Disease: Improving Global Outcomes guidelines (27) since it is associated with fewer bleeding events and a longer circuit life than systemic heparin anticoagulation (28).
Table 3.
Characteristics of Seventeen COVID-19 Patients Undergoing Hemodialysis.
| Case | Wave no. | Age | Sex | Comorbidities | Antithrombotic therapy except during HD | HAS-BLED score | Maximum dose of steroid | Other treatment | Complications | Severity* |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 73 | M | DM, HT, IHD, PAD, valvular disease | Low dose aspirin, clopidogrel, HC 10,000 U/day |
4 | DEX 6 mg | None | Retroperitoneal hemorrhage | Moderate II |
| 2 | 3 | 76 | M | CHF, DM, IHD | Prasugrel, HC 10,000 U/day |
3 | mPSL 0.5 g | None | Retroperitoneal hemorrhage | Severe |
| 3 | 3 | 74 | M | HT, IHD, strangulation ileus (PO), valvular disease | HS 10,000 U/day, NM 20 mg/h (CHDF) →NM 60 mg/h (CHDF) |
3 | mPSL 1 g | FPV →RDV | Upper GI bleeding, retroperitoneal hemorrhage, iliopsoas abscess, delayed anastomotic leakage | Severe |
| 4 | 3 | 79 | M | DM, emphysema, HL, HT | HC 10,000 U/day | 3 | PSL 40 mg | RDV | None | Moderate II |
| 5 | 3 | 69 | F | PKD | None | 2 | 0 | None | None | Mild |
| 6 | 3 | 64 | M | DM, dementia, IHD, old BI, prostatic Ca | Low dose aspirin | 3 | mPSL 0.5 g | None | None | Severe |
| 7 | 3 | 71 | M | Af, CHF, DM, IHD | HC 10,000 U/day | 3 | DEX 6 mg | FPV | Congestive heart failure | Moderate II |
| 8 | 3 | 83 | M | HT, old BI | Low dose aspirin | 5 | 0 | None | Aspiration pneumonia | Mild |
| 9 | 3 | 78 | M | HCC, LC | HC 10,000 U/day | 3 | 0 | FPV | None | Mild |
| 10 | 3 | 71 | M | DM, RTx | Low dose aspirin, HC 10,000 U/day |
3 | DEX 6 mg | None | Bacteremia | Moderate II |
| 11 | 4 | 78 | F | HT, IHD, valvular disease | HC 10,000 U/day | 4 | mPSL 1 g | None | None | Severe |
| 12 | 4 | 70 | M | DM, HT | HC 10,000 U/day | 3 | mPSL 0.25 g | RDV | Aspiration pneumonia, FD, GERD | Moderate II |
| 13 | 4 | 82 | F | Dementia, DM, old BI, PBC, RA | HC 10,000 U/day | 3 | mPSL 0.5 g | RDV, TCZ | None | Severe |
| 14 | 4 | 84 | M | DM | Clopidogrel | 3 | 0 | None | None | Mild |
| 15 | 5 | 51 | M | CAD, DM, HT | HC 10,000 U/day | 2 | mPSL 1 g | TCZ | None | Moderate II |
| 16 | 5 | 55 | M | DM, PAD, obesity | Sarpogrelate | 2 | 0 | None | None | Mild |
| 17 | 5 | 49 | M | DM, HT, obesity | None | 2 | 0 | None | Bronchopneumonia | Moderate I |
Af: atrial fibrillation, BI: brain infarction, Ca: cancer, CAD: coronary artery disease, CHF: chronic heart failure, COVID-19: coronavirus disease 2019, DEX: dexamethasone, DM: diabetes mellitus, FD: functional dyspepsia, FPV: favipiravir, GERD: gastroesophageal reflux disease, GI: gastrointestinal, HC: heparin calcium, HCC: hepatocellular carcinoma, HD: hemodialysis, HS: heparin sodium, HT: hypertension, IHD: ischemic heart disease, LC: liver cirrhosis, mPSL: methylprednisolone, NM: nafamostat mesylate, PAD: peripheral arterial disease, PBC: primary biliary cholangitis, PKD: polycystic kidney disease, PO: postoperatively, RA: rheumatoid arthritis, RDV: remdesivir, RTx: renal transplant, U: units, TCZ: tocilizumab
* SpO2 ≥96% without pneumonia was classified as mild, SpO2 94-95% as moderate I, SpO2 ≤93% as moderate II, and invasive mechanical ventilation or death as severe.
Table 4.
Comparison of Three Patients with Bleeding Events with the Other Patients.
| Valuable | With bleeding events (n=3) |
The other patients (n=14) |
p value |
|---|---|---|---|
| Age (years) | 74 (74-75) | 71 (65-) | 0.75 |
| Gender (male, %) | 3 (100) | 11 (79) | 1 |
| Hypertension (%) | 2 (67) | 6 (43) | 0.58 |
| Diabetes mellitus (%) | 2 (67) | 10 (71) | 1 |
| Atherosclerosis (%)* | 3 (100) | 7 (50) | 0.23 |
| Steroid use (%) | 3 (100) | 8 (57) | 0.52 |
| Combination of ≥2 antithrombotic drugs** | 3 (100) | 1 (7) | <0.01 |
| HAS-BLED score | 3 (3-3.5) | 3 (2.3-3) | 0.32 |
| White blood cell (/μL) | 4,900 (4,570-5,735) | 5,465 (4,203-6,250) | 1 |
| Lymphocytes (/μL) | 872 (870-877) | 708 (559-1,020) | 0.61 |
| Hemoglobin (g/dL) | 10.8 (10.8-11.6) | 10.8 (10.2-11.3) | 0.57 |
| Platelet (×104μL) | 10.8 (9.9-11.0) | 11.6 (10.4-16.3) | 0.31 |
| D-dimer (μg/mL) | 1.9 (1.5-4.7) | 1.7 (1.1-3.1) | 0.53 |
| Albumin (g/dL) | 3.2 (3.1-3.3) | 3.2 (2.8-3.6) | 1 |
| Total bilirubin (mg/dL) | 0.3 (0.3-0.5) | 0.4 (0.2-0.6) | 0.95 |
| Aspartate aminotransferase (IU/L) | 17 (12-43) | 24 (19-27) | 0.49 |
| Alanine aminotransferase (IU/L) | 15 (12-17) | 13 (11-15) | 0.75 |
| Lactate dehydrogenase (IU/L) | 181 (167-304) | 273 (243-321) | 0.36 |
| Blood urea nitrogen (mg/dL) | 64.5 (44.9-64.7) | 68.2 (55.7-73.7) | 0.30 |
| Creatinine (mg/dL) | 11.6 (9.1-13.3) | 11.1 (6.8-13.2) | 0.68 |
| Ferritin (ng/mL) | 891 (569-940) | 191 (96-431) | 0.08 |
| C-reactive protein (mg/dL) | 2.4 (1.4-13.2) | 3.8 (1.7-6.7) | 1 |
Data are expressed as median (interquartile range) or number (percentage) of patients.
Comparison of median between the two groups is tested by the Mann-Whitney U test and ratios by Fisher's exact test.
A two-tailed p value of <0.05 was considered statistically significant.
* Atherosclerosis includes old brain infarction, coronary artery disease, ischemic heart disease, and peripheral arterial disease.
** Antithrombotic drugs include antiplatelet drugs, heparin and nafamostat mesylate.
For your information, Case 1 has already been published in Japanese as one case report in the Journal of Japanese Society for Dialysis Therapy (29).
Conclusion
The frequency of hemorrhagic complications during antithrombotic therapy for COVID-19 is not low. The bleeding risk should be considered in addition to the severity and thrombotic risk of COVID-19 when determining the indications for and the types of antithrombotic therapy. Furthermore, the combined use of antithrombotic drugs should be strictly avoided.
The authors state that they have no Conflict of Interest (COI).
Acknowledgments
We thank Angela Morben, DVM, ELS, for editing a draft of this manuscript. We would also like to thank the infection control nurses working hard to prevent nosocomial infections and the nurses in the COVID-19 ward working at the risk of their own infection.
We also thank Dr. Takeshi Sato, Ozu City Hospital, Dr. Takahide Kato, Saiseikai Imabari Hospital, Dr. Hiroshi Ishii, Saiseikai Saijo Hospital, and Dr. Masahiko Kaneko, Uwajima City Hospital, for providing valuable information about dialysis patients with COVID-19.
References
- 1. Centers for Disease Control and Prevention. Underlying medical conditions associated with high risk for severe COVID-19: information for healthcare providers [Internet]. [cited 2021 Oct 14]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html.
- 2.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 31: 1409-1415, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CM, Weiner DE, Aweh G, et al. COVID-19 infection among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 77: 748-756, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 173: 268-277, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18: 1023-1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palumbo D, Guazzarotti G, De Cobelli F. Spontaneous major hemorrhage in COVID-19 patients: another brick in the wall of SARS-CoV-2-associated coagulation disorders? J Vasc Interv Radiol 31: 1494-1496, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138: 1093-1100, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Horiuchi H, Morishita E, Urano T, et al. COVID-19-related thrombosis in Japan: final report of a questionnaire-based survey in 2020. J Atheroscler Thromb 28: 406-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 29: 100639, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 76: 122-124, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost 111: 789-797, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Sato R, Ishikane M, Kinoshita N, et al. A new challenge of unfractionated heparin anticoagulation treatment for moderate to severe COVID-19 in Japan. Glob Health Med 2: 190-192, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. INSPIRATION Investigators. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 325: 1620-1630, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 397: 2253-2263, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Samkari H, Gupta S, Leaf RK, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med 174: 622-632, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demelo-Rodriguez P, Farfán-Sedano AI, Pedrajas JM, et al. Bleeding risk in hospitalized patients with COVID-19 receiving intermediate- or therapeutic doses of thromboprophylaxis. J Thromb Haemost 19: 1981-1989, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 77: 190-203, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazenberg P, Lechareas S, Vasquez Rios M, Taegtmeyer M, McWilliams R, Dutt T. Rectus sheath and retroperitoneal haematomas in patientswith coronavirus 2019 infection. Br J Haematol 194: 923-927, 2021. [DOI] [PubMed] [Google Scholar]
- 20.Hajian A. A case series of life-threatening hemorrhagic events in patients with COVID-19. Indian J Surg. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 22: 95-97, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppola A, Annunziata A, Gioia MR, Fiorentino G. Bleeding events in COVID-19: the other side of the coin? Monaldi Arch Chest Dis. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 23.McCort JJ. Intraperitoneal and retroperitoneal hemorrhage. Radiol Clin North Am 14: 391-405, 1976. [PubMed] [Google Scholar]
- 24.Sunga KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emerg Med 43: e157-e161, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Sahu KK, Mishra AK, Lal A, George SV, Siddiqui AD. Clinical spectrum, risk factors, management and outcome of patients with retroperitoneal hematoma: a retrospective analysis of 3-year experience. Expert Rev Hematol 13: 545-555, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Kasotakis G. Retroperitoneal and rectus sheath hematomas. Surg Clin North Am 94: 71-76, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1-138, 2012. [Google Scholar]
- 28.Zarbock A, Küllmar M, Kindgen-Milles D, et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically Ill patients with acute kidney injury: a randomized clinical trial. JAMA 324: 1629-1639, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka H, Homma Y, Onji Y, et al. Retroperitoneal hemorrhage in a patient with COVID-19 undergoing hemodialysis: recommendations about antithrombotic therapy based on similar cases. J Jpn Soc Dial Ther 54: 583-589, 2021. [Google Scholar]