Abstract
Wilson disease is an inherited copper metabolism disorder. We herein report a novel endoscopic finding in three men with Wilson disease. These patients underwent upper endoscopy due to gastrointestinal symptoms or during follow-up. In each case, endoscopy revealed lustrous white erosions surrounded by an erythematous mucosa in the greater curvature of the gastric body. A biopsy of the lesions showed orcein-positive tissue, indicating copper deposition, in the interstitial stroma and fundic glands of the mucosa. All patients had been receiving treatment with zinc acetate. These endoscopic findings might have been related to the cytotoxicity of the accumulated copper and zinc acetate.
Keywords: Wilson disease, copper, endoscopy, stomach, zinc acetate
Introduction
Wilson disease is an inherited disorder of copper metabolism that leads to impaired copper homeostasis and copper overload in the liver, brain, and other organs (1). About 50% of patients with Wilson disease have gastrointestinal symptoms, such as abdominal pain, nausea, or vomiting (2). To our knowledge, there have only been two reports on the endoscopic findings of the stomach in patients with Wilson disease (3,4).
We herein report three cases of Wilson disease wherein we identified a novel endoscopic finding of lustrous white erosions surrounded by an erythematous mucosa in the stomach.
Case Reports
Case 1
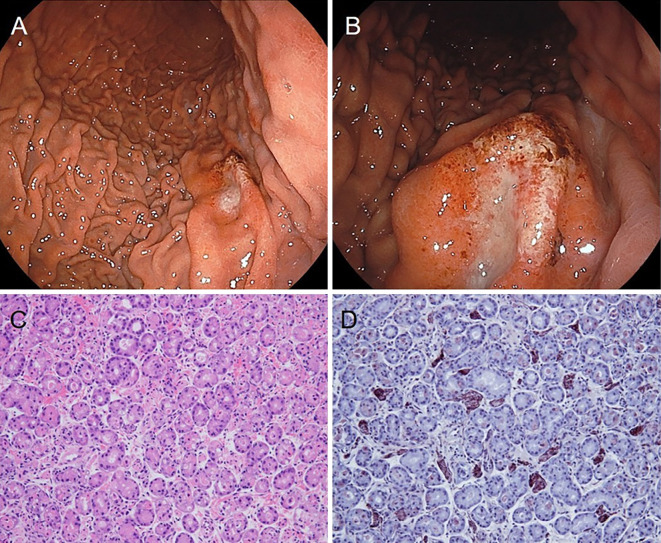
A 20-year-old man who had been diagnosed with Wilson disease at 18 years old complained of epigastric pain. He had cirrhosis of the liver and had undergone balloon-occluded retrograde transvenous obliteration for gastric varices at 19 years old. He did not have a history of Helicobacter pylori infection. He had no family history of Wilson disease. Furthermore, the genetic mutations in ATPase copper-transporting beta (ATP7B) could not be investigated. His medications included trientine hydrochloride (1,500 mg/day), zinc acetate hydrate (150 mg/day), and propranolol hydrochloride (30 mg/day). Laboratory data revealed pancytopenia, mildly elevated hepatobiliary enzymes, and decreased ceruloplasmin and copper levels. A urinary examination revealed an increased amount of copper excretion (Table). Endoscopy revealed white erosions with central hematin deposits surrounded by erythema in the greater curvature of the upper corpus of the stomach (Fig. 1A, B). Pathological examination of the biopsy specimens obtained from the lesion revealed orcein-positive granules in the interstitial stroma (Fig. 1C, D). An 8-week treatment with esomeprazole (20 mg/day) improved his epigastric pain and the white erosions (Fig. 2).
Table.
The Laboratory Data, Urinary Findings, and Genetic Mutations Observed in Each Case.
| Case 1 | Case 2 | Case 3 | ||
|---|---|---|---|---|
| WBC | 2,800 | 3,400 | 1,800 | /μL |
| RBC | 465 | 411 | 362 | 104/μL |
| Hb | 11.7 | 12.9 | 10.5 | g/dL |
| Ht | 36.8 | 36.9 | 32.4 | % |
| Plt | 5.1×104 | 15.5×104 | 3.0×104 | /μL |
| AST | 32 | 30 | 46 | U/L |
| ALT | 48 | 51 | 39 | U/L |
| γ-GTP | 406 | 64 | 36 | U/L |
| T-Bil | 0.5 | 1.2 | 1.8 | mg/dL |
| BUN | 15 | 10 | 14 | mg/dL |
| Cre | 0.75 | 0.59 | 0.91 | mg/dL |
| Alb | 4.4 | 4.4 | 3.7 | g/dL |
| PT% | 75 | 97 | 49 | % |
| Serum ceruloplasmin | <2.0 | 3.0 | <2.0 | mg/dL |
| Serum Cu | 13 | 21 | 12 | μg/dL |
| Urine Cu | 103 | 358 | 61 | μg/day |
| ATP7B mutations | Not applied |
Missense mutations R919 G and G1186S |
Not applied |
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelets, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: gamma-glutamyl transpeptidase, T-Bil: total bilirubin, BUN: blood urea nitrogen, Cre: creatinine, Alb: albumin, PT%: prothrombin activity, Cu: Copper, ATP7B: ATPase copper-transporting beta
Figure 1.
Endoscopic images and pathological examination findings of biopsy specimens obtained from the lesion in case 1. (A, B) Endoscopy reveals white erosions with hematin deposits surrounded by an erythematous mucosa in the greater curvature of the upper corpus of the stomach. (C) Inflammatory cell infiltration is observed in the interstitial stroma (Hematoxylin and Eosin staining, ×100). (D) Copper deposition is observed as granules in the interstitial stroma (Orcein stain, ×100).
Figure 2.
Endoscopic images of case 1 after eight-week treatment with esomeprazole (20 mg/day). (A, B) The white erosions in the greater curvature of the upper corpus of the stomach have improved.
Case 2
A 27-year-old man who had been diagnosed with Wilson disease at 5 years old complained of epigastric pain, nausea, and vomiting. He had a history of reflux esophagitis, a hiatal hernia, and depression, and he had also undergone H. pylori eradication. He had no family history of Wilson disease. However, a concrete genetic mutation in ATP7B, which is a compound heterozygote bearing the missense mutations R919G and G1186S, was found. He was on trientine hydrochloride (1,500 mg/day), zinc acetate hydrate (150 mg/day), and several antipsychotic drugs. Laboratory data revealed mildly elevated hepatobiliary enzyme levels and decreased ceruloplasmin and copper levels. A urinary examination revealed an increased amount of copper excretion (Table). Endoscopy revealed linear white erosions surrounded by an erythematous mucosa in the greater curvature of the middle corpus of the stomach (Fig. 3A, B). A pathological examination of the biopsy specimens obtained from the lesion showed orcein-positive granules in the fundic glands and interstitial stroma (Fig. 3C, D). His symptoms improved after he was started on esomeprazole (20 mg/day).
Figure 3.
Endoscopic images and pathological examination findings of the biopsy specimens obtained from the lesion in Case 2. (A, B) Endoscopy reveals a linear white erosion on an erythematous mucosa in the greater curvature of the middle corpus of the stomach. (C) Mild inflammatory cell infiltration is observed in the interstitial stroma (Hematoxylin and Eosin staining, ×100). (D) Copper deposition is observed as granules in the fundic glands and interstitial stroma (Orcein stain, ×100).
Case 3
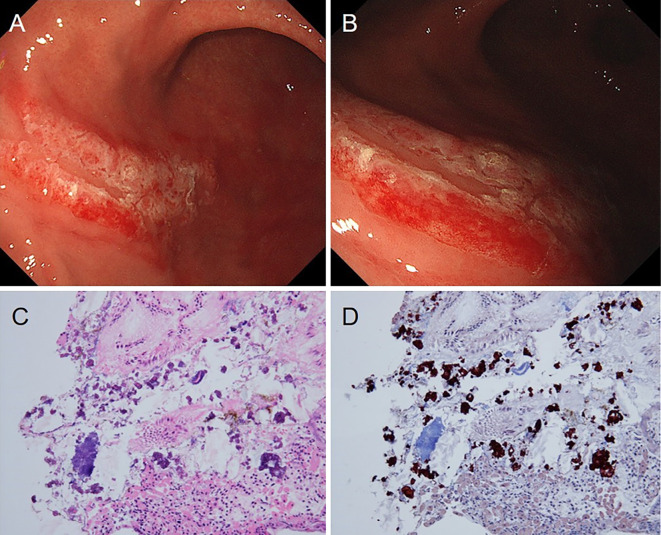
A 42-year-old man who had been diagnosed with Wilson disease at 30 years old presented to our hospital for a regular examination. He had no upper abdominal symptoms. He had a history of liver cirrhosis, rheumatoid arthritis, and autism. He had undergone endoscopic injection sclerotherapy for esophageal varices at 32 years old. He did not have a history of an H. pylori infection. He had no family history of Wilson disease. Moreover, the genetic mutations in ATP7B could not be investigated. His medications included zinc acetate hydrate (150 mg/day), spironolactone (50 mg/day), methotrexate (10 mg/day), celecoxib (400 mg/day), rebamipide (200 mg/day), and rabeprazole (10 mg/day). Laboratory data revealed pancytopenia, mildly elevated hepatobiliary enzymes, a decreased prothrombin activity, and decreased ceruloplasmin and copper levels. A urinary examination revealed an increased amount of copper excretion (Table). Endoscopy revealed mottled white erosions on the erythematous mucosa in the greater curvature of the fornix of the stomach (Fig. 4A, B). A pathological examination of the biopsy specimens obtained from the lesion revealed interstitial and stromal fibrosis, which stained positively with orcein stain (Fig. 4C, D). He had undergone endoscopy 10 times over 10 years, and similar lesions were noted to have appeared and disappeared at different sites (such as the antrum, body, or fornix of the stomach) without any symptoms (Fig. 5); throughout this period, he continued to take celecoxib for pain due to rheumatoid arthritis.
Figure 4.
Endoscopic images and pathological examination findings of biopsy specimens obtained from the lesion in case 3. (A, B) Endoscopy reveals mottled white erosions on an erythematous mucosa in the greater curvature of the fornix of the stomach. (C) Inflammatory cell infiltration is observed in the interstitial stroma (Hematoxylin and Eosin staining, ×100). (D) Copper deposition is observed as interstitial stroma with fibrosis (Orcein stain, ×100).
Figure 5.
Endoscopic images of case 3 taken four years ago. (A, B) Similar lesions were observed at various sites, such as the antrum and body of the stomach.
Discussion
This is the first report on the endoscopic findings of a gastric lesion in patients with Wilson disease receiving treatment with zinc acetate; in all three cases, endoscopic findings revealed a similar, unusual lesion with lustrous white erosions surrounded by an erythematous mucosa.
A pathological examination of biopsy specimens obtained from the lesions in all three patients revealed orcein-positive tissue, indicating copper deposition. This is also the first report on a biopsy-based identification of copper deposition in the stomach of patients with Wilson disease. Tchounwou et al. reported that copper toxicity may stem from transitions between Cu (II) and Cu (I), which can result in the generation of superoxide and hydroxyl radicals (5). Gotteland et al. reported that the gastric mucosal barrier capacity was reduced after exposure to large amounts of copper (6). Therefore, the cytotoxicity of copper deposited in the stomach may cause mucosal injury. Although the possibility of regular oral administration of celecoxib being the cause of gastric mucosal injury cannot be ruled out in Case 3, copper was noted to be deposited in the biopsy specimen of the lesion, which is considered to be an endoscopic finding related to Wilson disease.
Zinc therapy is a treatment for Wilson disease and is used as both front-line and maintenance therapy. Zinc acetate was administered in the three cases that we have described in this report. The patients in Cases 1, 2, and 3 had consumed zinc acetate for two years, five months, and nine years, respectively. Zinc acetate is mainly used in Japan; it is not used commonly in some countries due to high costs, and zinc sulphate is used instead (3). Many studies have reported a varying frequency of gastrointestinal side effects of zinc therapy in patients with Wilson disease (3,4,7-10). Wiggelinkhuizen et al. reported that gastrointestinal side effects appeared in 12.5% of the patients treated by zinc therapy (7). Conversely, Wiernicka et al. reported that gastrointestinal side effects appeared in 40% of pediatric patients treated by zinc sulphate (3); in their report, 21 out of 53 children complained of gastrointestinal side effects, such as abdominal pain, nausea, or vomiting. Due to persistent and severe abdominal pain, endoscopy was performed in 7 of these 21 pediatric patients, and gastritis with ulcerations or erosions was observed in all patients (3). Furthermore, Antczak-Kowalska et al. reported that gastrointestinal side effects appeared in 65.2% of all patients with Wilson disease, including 81.3% of those treated by zinc sulphate. They also reported that endoscopy revealed hyperemia and erosion of the gastric mucosa in 32.2% and 28.7% of all patients with Wilson disease, respectively, and in 31.3% and 46.9% of patients treated by zinc sulphate, respectively (4). However, they did not provide clear endoscopic images of the hyperemia or erosion or any biopsy findings of the lesions. The mucosal injury effect of zinc sulphate is believed to be related to the corrosiveness of zinc chloride, which is formed by the action of gastric hydrochloric acid on zinc sulphate (3,11). In the present cases, zinc acetate might have caused mucosal injury through a similar mechanism. Although zinc acetate is thought to be safer and better tolerated than zinc sulphate, the present cases demonstrate that zinc acetate also causes gastric mucosal injury (7).
The pathogenesis of the gastric lesions found in the present cases should be investigated. We believe that the erythematous mucosa was a result of the cytotoxicity of the accumulated copper, as a pathological examination of the biopsy specimens obtained from the mucosa revealed copper deposition. In addition, because the white lustrous erosions improved with suppression of the gastric acid by proton pump inhibitor (PPI) therapy, we believe that these lesions were formed on the mucosa after the administration of zinc acetate due to an increase in the amount of corrosive zinc chloride resulting from gastric acid hypersecretion. Our hypothesis concerning the mechanism underlying the gastric mucosal injuries in the present cases is shown in Fig. 6. The gastric lesions in our cases were located at the greater curvature and localized, but the mechanism underlying their formation remains unclear. Prospective studies are therefore needed to determine the pathogenesis of these lesions.
Figure 6.
Schematic illustration explaining our hypothesis concerning the mechanism underlying gastric mucosal injury in Wilson disease. Erythematous mucosa is formed due to the cytotoxicity of the accumulated copper in the stomach. Lustrous white erosions are formed on the erythematous mucosa after the administration of zinc acetate due to an increase in the amount of corrosive zinc chloride (formed as a result of the reaction between zinc acetate and the excessive gastric acid secreted). This leads to the appearance of lustrous white erosions surrounded by erythematous mucosa, a novel endoscopic finding of Wilson disease.
We were unable to confirm the relationship between gastrointestinal symptoms and the endoscopic findings based on only a few cases. If patients with Wilson disease receiving zinc treatment develop new gastrointestinal symptoms, gastric mucosal injury (such as that observed in the present cases) can be suspected. When a mucosal injury is detected, a higher dose of PPIs or potassium competitive acid blockers (P-CAB) might be effective for improving the symptoms or the injury. Furthermore, based on the reported mechanism of gastric injury (3,11), PPI seems to be a better alternative to P-CAB or mucosal protective agents in patients with Wilson disease treated by zinc salts. Patients with Wilson disease undergo upper endoscopy regularly for the evaluation of esophageal or gastric varices secondary to cirrhosis of the liver; however, patients who develop gastrointestinal symptoms should also undergo endoscopy to distinguish between these lustrous white erosions surrounded by an erythematous mucosa and other gastrointestinal diseases.
In conclusion, we herein report our novel endoscopic finding of a lustrous white gastric erosion surrounded by an erythematous mucosa in patients with Wilson disease. This endoscopic finding might be related to the cytotoxicity of the accumulated copper and zinc acetate.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This project by the F-Study group was aimed at identifying novel endoscopic findings of the gastrointestinal tract at the Department of Gastroenterology, Osaka City University Graduate School of Medicine.
References
- 1.Scheinberg IH. Wilson's disease. J Rheumatol Suppl 7: 90-93, 1981. [PubMed] [Google Scholar]
- 2.Stremmel W, Meyerrose KW, Niederau C, Hefter H, Kreuzpaintner G, Strohmeyer G. Wilson disease: clinical presentation, treatment, and survival. Ann Intern Med 115: 720-726, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Wiernicka A, Janczyk W, Dadalski M, Avsar Y, Schmidt H, Socha P. Gastrointestinal side effects in children with Wilson's disease treated with zinc sulphate. World J Gastroenterol 19: 4356-4362, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antczak-Kowalska M, Czlonkowska A, Litwin T, Nehring P, Niewada M, Przybylkowski A. Gastropathy in patients with Wilson disease. Scand J Gastroenterol 55: 14-17, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Tchounwou PB, Newsome C, Williams J, Glass K. Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG(2)) cells. Met Ions Biol Med 10: 285-290, 2008. [PMC free article] [PubMed] [Google Scholar]
- 6.Gotteland M, Araya M, Pizarro F, Olivares M. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig Dis Sci 46: 1909-1914, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Wiggelinkhuizen M, Tilanus ME, Bollen CW, Houwen RH. Systematic review: clinical efficacy of chelator agents and zinc in the initial treatment of Wilson disease. Aliment Pharmacol Ther 29: 947-958, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Bruha R, Marecek Z, Pospisilova L, et al. Long-term follow-up of Wilson disease: natural history, treatment, mutations analysis and phenotypic correlation. Liver Int 31: 83-91, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu N, Fujiwara J, Ohnishi S, et al. Effects of long-term zinc treatment in Japanese patients with Wilson disease: efficacy, stability, and copper metabolism. Transl Res 156: 350-357, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Brewer GJ, Dick RD, Johnson VD, Brunberg JA, Kluin KJ, Fink JK. Treatment of Wilson's disease with zinc: XV long-term follow-up studies. J Lab Clin Med 132: 264-278, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Molokhia MM, Portnoy B. Bleeding gastric erosion after oral zinc sulphate. Br Med J 1: 1145, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]