Abstract
While human immunodeficiency virus (HIV)-associated wasting has declined with significant advances in antiretroviral therapy (ART), weight gain and metabolic syndrome (MetS) are now becoming a problem for people living with HIV (PLWH) worldwide. The development of a new and more effective ART regimen has increased viral suppression and improved immunologic function recovery, leading to the extension of the lifespan of PLWH. It has recently been reported as one of the significant factors associated with weight gain, obesity, and long-term metabolic consequences in PLWH. This article reviewed the epidemiology of overweight and MetS among PLWH and the known risk factors for weight gain and its major comorbidities, such as dyslipidemia, diabetes mellitus, cardiovascular diseases, neurocognitive disorders, and liver diseases, in PLWH. In addition, reports on the pharmacological and surgical management of overweight and obesity in PLWH has been briefly summarized.
Keywords: HIV; Metabolic syndrome; Weight gain; Obesity; Antiretroviral therapy, Highly active
Introduction
Initiation of antiretroviral therapy (ART) often leads to weight gain. Although some of this weight gain is thought to be appropriate and considered part of the normal return to health following the successful suppression of human immunodeficiency virus (HIV), excessive weight gain may lead to obesity and other negative metabolic consequences [1]. Metabolic syndrome (MetS) is a cluster of symptoms that are risk factors for cardiovascular diseases (CVDs) and type 2 diabetes mellitus, primarily including abdominal obesity, atherogenic dyslipidemia, hypertension, hyperglycemia, insulin resistance, a proinflammatory state, and a prothrombotic state [2,3]. The presence of any three of the following five risk factors indicates MetS: (1) elevated waist circumference by population- or country-specific definition (≥90 cm in men and ≥85 cm in women in Korea) [4]; (2) elevated triglycerides ≥150 mg/dL or pharmacological treatment of elevated triglycerides; (3) reduced high-density lipoprotein cholesterol (HDL-C, <40 mg/dL in men and <50 mg/dL in women), or pharmacological treatment of reduced HDL-C; (4) elevated blood pressure (systolic ≥130 and/or diastolic ≥85 mmHg) or antihypertensive treatment in a patient with a history of hypertension; and (5) elevated fasting glucose ≥100 mg/dL or pharmacological treatment of hyperglycemia [3].
The global prevalence of this MetS among people living with HIV (PLWH) is 16.7 - 31.3% according to a meta-analysis, possibly reflecting the extended lifespan of PLWH [5]. Understanding the burden of MetS and obesity to improve quality of life in PLWH is important. However, the lack of a single definition for MetS and the complex relationship between its risk factors has been a great barrier and source of complication in studies comparing MetS in PLWH versus the general population [3,6].
Weight gain and obesity are important causal factors of MetS in the general population. Obesity in PLWH results in increased inflammation, increased ectopic lipid disposition, and alterations in lipid and glucose metabolism, contributing to the development of diabetes mellitus, neurocognitive impairments, hepatic diseases, and CVDs [7]. With the introduction of highly active ART (HAART), the lifespan of PLWH has increased dramatically, and the proportion of overweight and obese PLWH have been reported to also increase [8,9,10]. This insidiously progressing long-term metabolic complication could become a new challenge in our clinics and perhaps has received less attention than other acute complications of HAART or HIV infection.
This article reviewed the current epidemiology of overweight, obesity, and dyslipidemia as MetS among PLWH and risk factors for weight gain and its major comorbidities associated with obesity in PLWH. The Korean Society for the Study of Obesity (KSSO) defines overweight and obesity as a body mass index (BMI) of 23.0 – 24.9 and ≥25 kg/m2, respectively [4]. However, studies from other countries often define overweight and obesity as BMI 25.0 – 29.9 and ≥30 kg/m2, respectively. Therefore, this review also described each study according to the definition of each one. In addition, a brief review of reports on the pharmacological and surgical management of overweight and obesity in PLWH is described.
Prevalence of overweight, obesity, and MetS in PLWH
There have been several recent reports of an increasing proportion of overweight and obesity among PLWH globally [8,9,10,11,12,13,14,15,16]. Among these, one study reported trends in BMI change and weight gain with ART among PLWH in the United States and Canada [10]. This cohort study compared 14,084 PLWH from 17 cohorts with controls matched by sex, race, and age between 1998 and 2010. After 3 years of ART, obesity (BMI ≥30 kg/m2) increased from 9.0% (at baseline) to 18%, whereas 22% of individuals with a normal BMI (18.5 – 24.9 kg/m2) at baseline became overweight (BMI 25.0 – 29.9 kg/m2), and 18% of those overweight at baseline became obese. Similarly, other studies have confirmed the high prevalence of overweight, obesity, and weight gain during ART [8,9,11,12,13,14,15,16].
Women with HIV type 1 (HIV-1) experience a significantly greater increase in BMI following ART initiation than men [10,17]. Pooled BMI data from three randomized clinical trials of ART initiation in PLWH in the United States compared 760 women and 3,041 men to test whether BMI changes in the first 96 weeks following ART initiation differed by sex. After 96 weeks, adjusting for pre-ART initiation age, CD4+ T cell count, HIV-1 viral load, race/ethnicity, study, and ART regimen, the mean BMI change for women was 0.59 kg/m2 (95% confidential interval [CI]: 0.37 – 0.81) more than that for men (P <0.001). Sex was associated with pre-ART CD4+ T cell counts and HIV-1 viral load, suggesting that for subgroups with higher viral loads and lower CD4+ T cell counts at baseline, the estimated BMI changes in women are larger than the average estimated difference [17].
Interestingly, Phalane et al. reported that the prevalence of MetS in sub-Saharan Africa was lower in PLWH than in the HIV-negative control group (28.1% vs. 43.9%, P = 0.013) [18]. In contrast, a Polish study reported that the prevalence of MetS (22.2%) in PLWH was higher than in the general population [19]. Data from 1,861 in-care PLWH in four southern United States from the 2013 – 2014 Medical Monitoring Project survey showed overall MetS prevalence was 33.5%, and women were 2.24 times more likely to have MetS than men (95% CI: 1.69 – 2.97) [20]. This study showed that age and female sex were significant predictors of MetS and emphasized the importance of regular screening for MetS risk factors among aging PLWH during clinical follow-up [20].
To date, there have been few studies on weight and MetS in Korean PLWH. The report on obesity among Korean PLWH was a cross-sectional study investigating the prevalence of and risk factors for metabolic abnormalities, not MetS, by definition [21]. Among 1,091 HIV-infected patients in the Korea HIV/acquired immune deficiency syndrome (AIDS) cohort study enrolled across 19 hospitals between 2006 and 2013, the prevalence of obesity (BMI ≥25 kg/m2) was reported as high as 16.4% (140/856), and there was no difference between treatment-naïve and treatment-experienced patients (17.6% vs. 15.6%, P = 0.452). This study also reported that 5.5% of the patients had abdominal obesity, and increased levels of fasting glucose, total cholesterol, LDL-C, and triglycerides were present in 10.4%, 6.0%, 5.5%, and 32.1% of PLWH. Decreased HDL-C levels were observed in 44.2% of the patients, and high systolic blood pressure in 14.3% of PLWH [21].
A recent Asia-Pacific regional cohort study enrolling 4,931 PLWH from the Therapeutics Research, Education, and AIDS Training in Asia HIV Observational Database (TAHOD) cohort reported that at ART initiation, the median weight was 55 (interquartile range [IQR]: 48 – 63) kg and BMI was 20.5 kg/m2 (IQR: 18.4 – 22.9). At 1, 2, and 3 years of ART, the overall mean weight gain was 2.2, 3.0, and 3.7 kg, respectively. Weight gain after ART initiation was significantly higher among those initiating ART with a lower CD4+ T cell count, higher HIV RNA, and an integrase strand transfer inhibitor (INSTI)-based regimen after controlling for baseline BMI. This study also reported that 295/3,503 had incident MetS [1.18 (95% CI: 1.05 – 1.32)/100 person-years], and greater weight gain over 3 years of ART was associated with the development of MetS components such as hypertension, impaired glucose, and dyslipidemia, irrespective of baseline BMI levels [15].
Dyslipidemia and ART
Dyslipidemia is characterized by one or more of the following: increased triglyceride (≥150 mg/dL), total cholesterol (≥200 mg/dL), LDL-C levels (≥130 mg/dL), and/or decreased HDL-C levels (≤40 mg/dL). Before the introduction of HAART, Grunfeld et al. reported that patients with HIV infection showed decreased total cholesterol and HDL-C levels, but patients with AIDS had elevated plasma triglyceride and free fatty acid levels [22]. HIV is associated with chronic inflammation and immune activation, leading to decreased levels of total cholesterol and HDL-C and increased levels of LDL-C and triglycerides [23,24]. Dyslipidemia has been suggested to be associated with age, sex, genetic factors, and lifestyle, including diet [25].
A comprehensive review of lipid levels as a risk factor for CVDs in PLWH in Asian regions (including Korea) reported that dyslipidemia was less prevalent in patients who first presented with HIV compared with in HIV-negative controls. In addition, total cholesterol levels were on average lower in HIV-positive patients, whereas findings were inconsistent regarding triglyceride levels [26]. This review also showed that ART had an unfavorable effect on blood lipid levels in Asian PLWH [26].
HIV infection and HAART are associated with abnormalities in lipid metabolism [27]. ART regimens containing tenofovir disproxil fumarate (TDF) are associated with lower lipid levels than those containing didanosine, zidovudine, stavudine, or abacavir [28,29]. Recently, TDF was replaced with tenofovir alafenamide (TAF) because of the renal and bone toxicity of TDF, resulting in a significant increase in triglyceride, total cholesterol, LDL-C, and HDL-C levels [30,31]. A prospective study investigating the incidence of dyslipidemia in PLWH reported that older non-nucleoside reverse transcriptase inhibitors (NNRTIs), such as efavirenz and ritonavir-boosted protease inhibitors (PIs) containing ARTs, were associated with a greater risk of dyslipidemia than newer agents from the same class of ART. In addition, dyslipidemia was less common with INSTIs than with boosted PIs [32]. This report also found that, compared with dolutegravir, dyslipidemia was more common with elvitegravir/cobicistat and raltegravir but less common with rilpivirine. [32]. Raltegravir, dolutegravir, and bictegravir had superior lipid profiles than boosted PI, efavirenz, and elvitegravir/cobicistat, in studies conducted on naïve participants, and are associated with a clinically significant decrease in lipoproteins by switching studies [33].
Weight gain and ART
In the 1990s, lower lean and fat mass were observed in ART-naïve PLWH than in people without HIV before the onset of severe CD4+ T cell depletion [1,34,35]. During the first year of ART in PLWH, weight gain was universally reported, regardless of the type of the regimen [36,37,38]. Some older antiretroviral agents, such as nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) and PIs, redistributed fat from the subcutaneous tissue on the face and extremities to excess adipose tissue on the abdomen, known as lipodystrophy [39]. After the avoidance of older ARTs, especially thymidine analogue NRTIs (zidovudine and stavudine) and indinavir, the incidence of lipodystrophy among PLWH has dramatically declined [1]. However, HAART containing INSTIs plus TAF has been reported to be associated with greater weight gain than other regimens [40,41,42,43]. Weight gain was greater in more recent trials and with the use of newer ART regimens. Through the pooled analysis of eight randomized controlled clinical trials of treatment-naïve PLWH initiating ART between 2003 and 2015, comprising >5,000 participants and 10,000 person-years of follow-up, weight gain after initiation of HAART could be due to partial return to a healthy condition with the restoration of lean body mass, but some composition of ART was found to contribute to greater weight gain with demographic factors and HIV-related factors [43]. INSTI use was associated with greater weight gain than PIs or NNRTIs, with dolutegravir and bictegravir associated with more weight gain than elvitegravir/cobicistat. Among the NNRTIs, rilpivirine was associated with greater weight gain than efavirenz. Among nucleoside/NRTIs, TAF is associated with greater weight gain than TDF, abacavir, or zidovudine [43].
In the Women’s Interagency HIV Study from 2006 to 2017, 234 women living with HIV who switched to, or were added an INSTI to ART (SWAD group), were compared with 884 women on non-INSTI ART (STAY group). On average, compared with the STAY group, the SWAD group experienced mean greater increases of 2.1 kg in weight and 0.8 kg/m2 in BMI, and these changes were all significant [40]. The randomized multicenter trial with 613 PLWH in Cameroon compared weight gain between dolutegravir/lamivudine/TDF and efavirenz/lamivudine/TDF regimen groups and reported a greater weight gain (median weight gain, 5.0 vs. 3.0 kg, P <0.001) and higher incidence of obesity (22% vs. 16%, P = 0.043) in dolutegravir/lamivudine/TDF treatment [41].
A Swiss cohort study conducted between 2016 and 2019 showed that TAF affected weight. A total of 4,375 PLWH who received TDF-containing ART for ≥6 months were enrolled, and 3,484 (79.6%) switched to TAF while 891 (20.4%) continued TDF. After 18 months, switching to TAF was associated with an adjusted mean weight increase of 1.7 kg (95% CI: 1.5 – 2.0), compared with 0.7 kg (95% CI: 0.4 – 1.0) with the continued use of TDF (between-group difference, 1.1 kg [95% CI: 0.7 – 1.4]). Among individuals with a normal BMI, 13.8% who switched to TAF became overweight/obese compared with 8.4% of those continuing TDF (difference, 5.4% points [95% CI: 2.1 – 8.8]). TAF-ART has become the first-line treatment in all major HIV treatment guidelines, and new concerns remain that replacing TDF with TAF is associated with adverse metabolic changes, including weight increase and obesity development [44].
The 96-week data from the Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE) trial, including 1,053 PLWH, identified that mean weight gain was highest (7.1 kg) in the TAF/emtricitabine/dolutegravir group, followed by 4.3 kg in the TDF/emtricitabine/dolutegravir group and 2.3 kg in the TDF/emtricitabine/efavirenz group, and was greater among women than among men [42]. Differences in weight gain were significant between each regimen, possibly due to the additive effects of dolutegravir and TAF on weight gain [42,45].
There are few reports on the effects of ART on weight gain in Asian PLWH [46,47]. A Japanese retrospective observational study evaluated ART-associated weight gain in adult treatment-naïve Asian PLWH for 5 years following treatment initiation [47]. Five years after treatment initiation, the average weight gain among PLWH who were started on dolutegravir-, darunavir-, and elvitegravir-based treatment was 5.3, 4.1, and 4.6 kg, respectively, whereas those started on raltegravir-, lopinavir-, and atazanavir-based treatment gained an average of 1.9, 2.1, and 2.3 kg, respectively. Average weight gain in PLWH who started treatment with the backbone drugs, TAF, abacavir, and TDF, was 4.1, 3.0, and 3.0 kg, respectively, and those treated with dolutegravir plus TAF/emtricitabine gained an average of 6.7 kg [47]. The regimen, including DTG and TAF, was associated with the greatest weight gain, similar to previous reports. Kuo et al. reported weight gain among 693 PLWH in Taiwan, who had good viral suppression, up to 48 weeks after switching from a PI- or NNRTI-based regimen to TAF/emtricitabine/elvitegravir/cobicistat [46]. Significant weight gain occurred after switching from non-INSTI-based ART to co-formulated elvitegravir/cobicistat/emtricitabine/TAF and was associated with dyslipidemia and an increase in hemoglobin A1c levels [46].
Few studies have examined the relationship between ART and weight gain in Korean HIV patients. The longitudinal analysis study of PLWH enrolled in TAHOD cohort including 20 Korean HIV patients showed that Asian PLWH starting with INSTI-based ART had greater weight gain than those with NNRTI (difference = 2.1 kg; 95% CI: 0.7 – 3.5), weight gain after ART initiation was significantly higher among those initiating ART and INSTI-based regimens after controlling for baseline BMI [15].
Several factors have been found to be associated with weight gain during ART. Several studies reported sex differences in weight gain associated with ART: women have been consistently found to experience greater weight gain [41,42,43,44]. The New Antiretroviral and Monitoring Strategies in HIV-infected Adults in Low-Income Countries (NAMSAL) trial showed that women and those with high viral loads (≥100,000 copies/mL) at baseline were at higher risk of weight gain (by at least 10.0% from baseline) following dolutegravir-based treatment initiation [41]. Similarly, the ADVANCE trial showed that women gained more weight than men in all treatment regimens [42]. Recently, Mulenga et al. presented 144-week data from the VISEND (Virological Impact of Switching from Efavirenz and Nevirapine based first line ART Regimens to Dolutegravir) study in Zambia, an open-label non-inferiority study that compared second-line dolutegravir- and boosted PI-based regimens with TDF/lamivudine or TAF/emtricitabine vs. zidovudine/lamivudine as the backbone [48]. Consistent with other trials, weight gain was more likely in the TAF arms, particularly among women. As weight gain was more pronounced in the TAF group, especially in women, therapy should be individualized considering the risk of obesity and other comorbidities. Sax et al. also reported that baseline demographic factors associated with weight gain included a lower CD4 cell count, higher HIV-1 RNA titer, no injection drug use, female sex, and black race [43]. A baseline CD4+ T cell count of <50 cell/mm3 was also observed as a factor associated with a greater increase in BMI from a previous report [9]. Some studies showed racial differences in INSTI-related weight gain; black or African PLWH were more likely to gain weight on ART [43,44]. Therefore, updated DHHS guidelines recommend that clinicians consider the possibility of weight gain in women, particularly black women, when initiating or changing ART regimens [49].
Weight gain after ART initiation, higher HIV-1 RNA titer and lower CD4+ T cell count at baseline were the factors associated with increased weight gain during ART [41]. Sax et al. hypothesized that PLWH taking a newer ART regimen have a lower possibility of adverse gastrointestinal effects than those taking older ART, which might result in greater weight gain among PLWH taking newer ART [43]. The mechanism of this weight gain associated with specific ART is incompletely understood but adipose tissue dysregulation or dysfunction may be associated with INSTI-related weight gain [50,51]. Moreover, in vitro studies have shown that INSTIs may affect appetite through the levels of leptin and adiponectin in adipose tissue exposed to dolutegravir [52]. Therefore, further laboratory-based studies may be needed to understand the mechanism of the association between adipose tissue and ART. Moreover, long-term clinical outcome data on whether ART-associated weight gain could be reversible by switching from INSTIs and/or TAF are needed. Although weight gain and obesity could be potential problems for future clinical outcomes associated with newer ARTs, current guidelines do not advise restricting the use of these ARTs, even in people who may be more vulnerable to weight gain and the development of obesity after ART initiation. This is because the available data continue to support the benefits of HAART in extending life expectancy by effective and rapid suppression and control of HIV infection.
Whether ART-associated weight gain leads to the development of CVD and diabetes mellitus remains unknown. Many studies have reported changes in the levels of lipid profiles or serum glucose as the parameter of MetS, but long-term follow-up data are rare regarding disease occurrences, such as diabetes mellitus and cardiovascular accidents, as a consequence of these changes. Taramasso et al. suggested that despite increasing weight and BMI, PLWH had a significantly improved metabolic profile compared with previously reported data [14]. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) cohort did not show a correlation between BMI change and CVD risk in overweight and obese PLWH [53] nor a correlation between ART-related weight gain and increased incidence of diabetes mellitus [54]. The veterans aging cohort study included 4,184 men and 127 women from 2000 to 2008, followed up for an additional 5 years to assess mortality, and observed that weight gain after ART was associated with lower mortality for those who were not initially overweight [55]. The proportion of women enrolled in this study was too small (2.9% of the whole cohort) and it was difficult to apply the findings to newer ARTs. Hence, it was possible that the effects of weight gain on mortality, especially among women, were not examined properly [25]. Therefore, further long-term research is needed to clarify the clinical impact of ART-associated weight gain on the development of CVDs, diabetes mellitus, and mortality, especially among Korean PLWH.
Comorbidities associated with weight gain and obesity
1. Diabetes mellitus
Risk factors associated with diabetes mellitus in the general population are also common among PLWH, including older age, high BMI, central adiposity, family history of diabetes, and Hispanic or African American ethnicity [56,57,58,59,60]. Low CD4+ T cell counts and treatment regimens with older NRTIs and PIs are additional risk factors for diabetes among PLWH [58,61]. The risk of diabetes mellitus according to weight gain among PLWH from the Veterans Aging Cohort Study is greater than that in the general population [54]. PLWH had a lower prevalence of diabetes mellitus at baseline (12% in PLWH vs. 23% in those HIV-negative), however, the association between weight gain and the risk of diabetes mellitus was linear for PLWH and uninfected people, but the slope of the association was steeper for PLWH. For each 5 pounds (~2.27 kg) of weight gained, PLWH were at a higher increased risk (14.0%) of developing diabetes mellitus than HIV-negative individuals (8.0%, P <0.01) [54]. Similar to HIV-negative people, PLWH with diabetes have a higher risk of CVD, chronic kidney disease, and mortality than those without diabetes [60].
A study using the Korean National Health Insurance Service (NHIS) claims database including 14,134 PLWH and 282,039 HIV-negative general population from 2004 to 2016 reported that the incidence of diabetes mellitus was higher in PLWH than in the general population (10.4% in PLWH vs. 6.6%, P <0.001). Moreover, the incidence of diabetes mellitus was found to be higher in PLWH than in controls at younger ages [62]. However, no robust study has defined the relationship between the weight gain effect during ART and the incidence of diabetes mellitus among Korean PLWH; however, further long-term follow-up cohort studies are needed to clarify the effect of weight gain during ART on the development of diabetes mellitus among Korean PLWH.
2. CVDs
A recent systematic review of five databases reported that the risk of CVDs development in PLWH was twice that of the general population, and the global burden of HIV-associated CVDs has tripled over the past two decades [63]. Triant et al. reported that the difference in acute myocardial infarction (AMI) rates between PLWH and non-HIV patients was significant, with a relative risk (RR) of 1.75 (95% CI: 1.51 – 2.02; P <0.0001), and the RRs (for HIV vs. non-HIV) were 2.98 (95% CI: 2.33 – 3.75; P <0.0001) for women and 1.40 (95% CI: 1.16 – 1.67; P = 0.0003) for men, adjusting for age, sex, race, hypertension, diabetes, and dyslipidemia [64]. Therefore, the authors suggested that AMI rates and cardiovascular risk factors increased in HIV patients compared with that in non-HIV patients, particularly among women, and that cardiac risk modification strategies might be important for the long-term care of PLWH [64].
However, the contribution of obesity to the development of CVD in PLWH is not well understood. The D:A:D study reported the contribution of obesity to increase CVD risk, showing that the RR of CVD for obese PLWH (BMI ≥30 kg/m2) compared with that for normal weight PLWH (BMI of 23 – 25 kg/m2) was 1.31 (95% CI: 1.03 – 1.67) [56]. However, a study using the Korean NHIS claims database including 14,134 PLWH and 282,039 HIV-negative general population from 2004 to 2016 reported that the incidence of CVD among PLWH was lower than that in the general population in all age groups (1.4% vs. 3.1%, P <0.001), while adjusted incidence ratios for CVD among PLWH are increasing [62]. Therefore, further long-term follow-up studies are necessary to clarify the relationship between CVD and HIV infection among Korean PLWH.
3. Neurocognitive impairment
Inflammation linked to abdominal obesity has been suggested to contribute to neurocognitive impairment (NCI) among PLWH [65,66,67]. A cross-sectional substudy of the CNS HIV Anti-Retroviral Therapy Effects Research cohort showed that central obesity was associated with higher prevalence of NCI in PLWH, whereas diabetes appeared to be associated with NCI only in older patients [67]. Sattler et al. reported that neurocognitive function was significantly linked to abdominal obesity, systemic inflammation (high IL-6 levels), and immune activation in plasma (high sCD14 levels) and cerebrospinal fluid (high sCD40L), suggesting that abdominal obesity, inflammation, and central nervous system immune activation may be potential therapeutic targets for NCI in HIV-positive patients [65]. Yu et al. also reported that MetS in PLWH was most strongly associated with the neurocognitive domains of learning, fine motor skills, and executive function, and diabetes and elevated triglycerides were the MetS components most strongly linked to increased global neurocognitive deficits in PLWH [66].
4. Liver diseases
Non-alcoholic fatty liver disease (NAFLD), ranging from simple steatosis (also known as non-alcoholic fatty liver, NAFL) to non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis, which may ultimately progress to hepatocellular carcinoma, is associated with metabolic abnormalities, such as obesity, diabetes, hypertension, and dyslipidemia [68]. The prevalence of NAFL (13 - 65%) and NASH (10%) in PLWH was reported as higher than that in the global HIV-negative population (25% and 3 - 5%, respectively) [69,70,71]. One meta-analysis study including 10 studies from the United States (n = 4), Canada (n = 1), France (n = 2), Italy (n = 1), Japan (n = 1), and China (n = 1) showed that the prevalence of NAFLD (by imaging studies), NASH, and fibrosis (by biopsied populations) among PLWH was 35.3% (95% CI: 28.9 – 42.5), 41.7% (95% CI: 22.3 – 64.0), and 21.7% (95% CI: 13.3 – 33.7), respectively [72]. The risk factors for NAFLD in PLWH were high BMI, waist circumference, type 2 diabetes, hypertension, triglycerides, and high CD4+ T cell count. However, HIV viral load, duration of HIV infection, duration of ART, and CD4+ T cell count nadir were not associated with NAFLD [72].
Management of weight to control MetS
Therapeutic weight loss is an important factor in reducing the complications of obesity in the general population. The degree of weight loss required for therapeutic benefit was known as 3 - 10% for preventing diabetes mellitus, 5 - 15% for hypertension, 3 - 15% for dyslipidemia, and 10% for NAFLD [73]. Studies analyzing the effects of weight loss on MetS parameters in PLWH showed mixed results [74,75]. Engelson et al. reported that moderate weight loss achieved by a short-term program of diet and exercise in obese HIV-positive women appeared safe and induced loss of adiposity in both subcutaneous adipose tissue and visceral adipose tissue regions [74]. Despite reduced food intake, weight, and fat loss, as well as improvements in strength, fitness, and quality of life, the lack of improvement in metabolic parameters suggests that additional interventions are necessary to reduce the risk of diabetes mellitus and CVDs in this population [74]. However, Fitch et al. reported that intensive lifestyle modification significantly improved important cardiovascular risk indices in PLWH with MetS and suggested that lifestyle modification might be a useful strategy to decrease cardiovascular risk in PLWH [75]. A prospective clinical trial evaluated the effect of moderate diet-induced weight loss in obese HIV-positive women and showed that moderate diet-induced weight loss improves multi-organ insulin sensitivity in HIV-positive women to the same extent as in HIV-negative women [76].
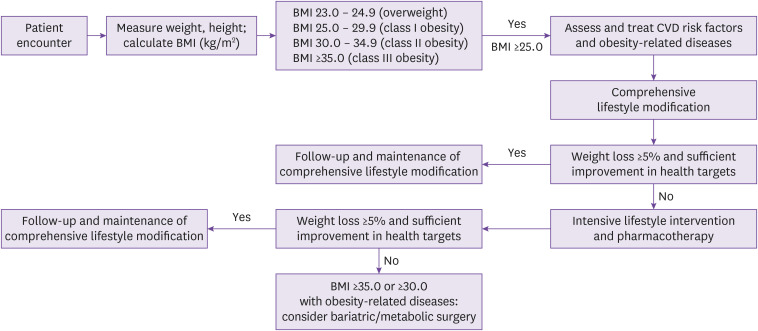
Exercise and physical activity with medical nutritional therapy (for example, low-calorie diets) are important factors in lifestyle interventions for weight loss. Behavior therapy can be applied not only as a programmed behavior intervention for weight control but also to change behaviors related to food intake and physical activity [4]. According to the KSSO guidelines, pharmacological intervention is recommended when non-pharmacotherapy-treated patients with BMI of ≥25 kg/m2 fail to lose more than 5% weight (Fig. 1) [4]. As of 2020, four types of anti-obesity agents have been approved for long-term administration in Korea: orlistat (Xenical®, Roche S.p.A, Milano, Italy, pancreatic and gastric lipase inhibitor), naltrexone-bupropion (Contrave®, Orexigen Therapeutics, La Jolla, CA, USA, reuptake inhibitor of dopamine and morepinephrine/opoid antagonist), liraglutide (Saxenda®, Novo Nordisk, Kalundborg, Denmark, glucagon-like peptide-1 [GLP-1] agonists), and phentermine/topiramate (Qsymia®, Alvogen Korea, Seoul, Korea, norepinephrine-relieving agent/GABA receptor modulator) [4]. The placebo-subtracted mean weight loss at ≥1 year of pharmacotherapy in the general population was approximately 9.8 kg in phentermine/topiramate, 5.3 – 5.9 kg in liraglutide, 6.6 – 15.8 kg in semaglutide (GLP-1 agonist), 4.4 kg in naltrexone/bupropion, and 3.1 kg in orlistat, but all of them have respective adverse effects and drug-drug interactions, so prescription to patients must be individualized considering underlying medical comorbidities and concomitant medications [77].
Figure 1. Treatment algorithm for the primary care of patients with obesity suggested by the 2020 Korean Society for the Study of Obesity Guidelines. (J Obes Metab Syndr 2021;30:81–92 [4]).
BMI, body mass index; CVD, cardiovascular disease.
Orlistat was recommended to avoid concomitant use with most available ART drugs, except injectable entry inhibitors, and naltrexone/bupropion also has serious drug-drug interactions with NNRTIs, PIs, and INSTIs [78]. Liraglutide has no clinically relevant interactions with NRTIs, INSTIs, and entry inhibitors, but it has a drug-drug interaction with ritonavir and PIs. Therefore, it is not contraindicated but clinical monitoring is recommended. Atazanavir and rilpivirine should be administered 4 h before liraglutide due to the potential for inhibition of gastric secretion, resulting in decreased concentrations of atazanavir and rilpivirine [78]. Pentermine/topiramate also has some drug interactions with antiretroviral agents, such as ritonavir or cobicistat (moderate CYP2D6 inhibitor) and efavirenz (CNS depression effect); therefore, it should be administered under close clinical monitoring because of the potential for increased concentrations of phentermine (http://www.uptodate.com. accessed April 21, 2022) [78]. Furthermore, if phentermine/topiramate is co-administered with TDF or TAF, it is recommended to monitor for increased risk of renal toxicity, but there is no known interaction between NNRTIs and entry inhibitors [78]. There have been no well-designed studies to evaluate the effects of these anti-obesity agents on weight reduction in PLWH receiving HAART globally; therefore, further long-term, large-scale prospective cohort studies are needed to clarify the effect of anti-obesity pharmacotherapy and weight loss on MetS in obese and overweight PLWH in the future.
In Korea, since January 2019, the national health insurance plan has covered bariatric surgery for the treatment of patients with severe obesity (BMI ≥35 kg/m2 or BMI ≥30 kg/m2 plus obesity-related comorbidities) [4]. Evidence of the efficacy of PLWH is limited because only a small number of case series or retrospective studies of PLWH exist; however, most studies suggest that bariatric surgery is a safe and effective method for reducing weight in morbidly obese PLWH [79,80,81,82,83,84]. Only one study assessed the pharmacokinetics of ART regimens in HIV-positive patients after bariatric surgery. It reported that four PLWH displayed detectable viral load along with a significant decrease in raltegravir and atazanavir treatment, leading to ART change with subsequent undetectable viral load, and one had persistent detectable viral load despite ART change. Therefore, the authors suggested that ART should be monitored after post-sleeve gastrectomy to control HIV infection [84].
Conclusion
Weight gain and obesity are increasing in PLWH worldwide, and the prevalence of obesity is high in Korea (16.4% from 2006 to 2013). In particular, the newly introduced ARTs of INSTIs and TAF, are well known to be related to weight gain during HAART. Patient characteristics, such as female sex, are risk factors; therefore, it is necessary to consider these when prescribing HAART to vulnerable PLWH. There have been no well-designed studies defining the relationship between weight gain during treatment and the incidence of complications, such as diabetes mellitus, CVDs, neurocognitive disorders, and NAFLD. Further follow-up cohort studies are needed to clarify these long-term outcomes among Korean PLWH. Furthermore, large-scale, long-term follow-up studies are needed to determine whether weight reduction in overweight or obese PLWH can reduce the morbidity of chronic MetS and mortality.
ACKNOWLEDGMENTS
I would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Funding: None.
Conflict of Interest: No conflict of interest.
References
- 1.Koethe JR, Lagathu C, Lake JE, Domingo P, Calmy A, Falutz J, Brown TT, Capeau J. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020;6:48. doi: 10.1038/s41572-020-0181-1. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity International Diabetes Federation Task Force on Epidemiology and Prevention. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, Kim B, Kim SJ, Kim YH, Kim JH, Kim JH, Kim EM, Nam GE, Park JY, Son JW, Shin YA, Shin HJ, Oh TJ, Lee H, Jeon EJ, Chung S, Hong YH, Kim CH Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO) 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr. 2021;30:81–92. doi: 10.7570/jomes21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One. 2016;11:e0150970. doi: 10.1371/journal.pone.0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todowede OO, Sartorius B. Prevalence of metabolic syndrome, discrete or comorbid diabetes and hypertension in sub-Saharan Africa among people living with HIV versus HIV-negative populations: a systematic review and meta-analysis protocol. BMJ Open. 2017;7:e016602. doi: 10.1136/bmjopen-2017-016602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailin SS, Gabriel CL, Wanjalla CN, Koethe JR. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep. 2020;17:138–150. doi: 10.1007/s11904-020-00483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, Weintrob A, Barthel RV, Fraser S, Agan BK Infectious disease clinical research program HIV working group. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf MC, Saag MS, Mugavero MJ. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17:1281–1289. doi: 10.3851/IMP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, Buchacz K, Napravnik S, Mayor AM, Horberg MA, Blashill AJ, Willig A, Wester CW, Silverberg MJ, Gill J, Thorne JE, Klein M, Eron JJ, Kitahata MM, Sterling TR, Moore RD North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32:50–58. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasse B, Iff M, Ledergerber B, Calmy A, Schmid P, Hauser C, Cavassini M, Bernasconi E, Marzolini C, Tarr PE, Aubert V, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Gorgievski M, Günthard H, Haerry D, Hasse B, Hirsch HH, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos R, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, Rauch A, Regenass S, Rickenbach M, Rudin C, Schöni-Affolter F, Schmid P, Schultze D, Schüpbach J, Speck R, Staehelin C, Tarr P, Telenti A, Trkola A, Vernazza P, Weber R, Yerly S Swiss HIV Cohort Study. Obesity trends and body mass index changes after starting antiretroviral treatment: The Swiss HIV cohort study. Open Forum Infect Dis. 2014;1:ofu040. doi: 10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandson KM, Taejaroenkul S, Smeaton L, Gupta A, Singini IL, Lama JR, Mngqibisa R, Firnhaber C, Cardoso SW, Kanyama C, Machado da Silva AL, Hakim JG, Kumarasamy N, Campbell TB, Hughes MD. A randomized comparison of anthropomorphic changes with preferred and alternative efavirenz-based antiretroviral regimens in diverse multinational settings. Open Forum Infect Dis. 2015;2:ofv095. doi: 10.1093/ofid/ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilozue C, Howe B, Shaw S, Haigh K, Hussey J, Price DA, Chadwick DR. Obesity in the HIV-infected population in Northeast England: a particular issue in Black-African women. Int J STD AIDS. 2017;28:284–289. doi: 10.1177/0956462416649131. [DOI] [PubMed] [Google Scholar]
- 14.Taramasso L, Bonfanti P, Ricci E, Maggi P, Orofino G, Squillace N, Menzaghi B, Madeddu G, Molteni C, Vichi F, Riguccini E, Saracino A, Santoro C, Guastavigna M, Francisci D, Di Biagio A, De Socio GV CISAI study group. Metabolic syndrome and body weight in people living with HIV infection: analysis of differences observed in three different cohort studies over a decade. HIV Med. 2022;23:70–79. doi: 10.1111/hiv.13165. [DOI] [PubMed] [Google Scholar]
- 15.Han WM, Law MG, Choi JY, Ditangco R, Kumarasamy N, Chaiwarith R, Ly PS, Khusuwan S, Merati TP, Do CD, Yunihastuti E, Azwa I, Lee MP, Pham TN, Chan YJ, Kiertiburanakul S, Ng OT, Tanuma J, Pujari S, Zhang F, Gani Y, Mave V, Ross J TREAT Asia HIV Observational Database of IeDEA Asia-Pacific. Weight changes, metabolic syndrome and all-cause mortality among Asian adults living with HIV. HIV Med. 2022;23:274–286. doi: 10.1111/hiv.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakal DR, Coelho LE, Luz PM, Clark JL, De Boni RB, Cardoso SW, Veloso VG, Lake JE, Grinsztejn B. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 2018;73:2177–2185. doi: 10.1093/jac/dky145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Womens Health (Larchmt) 2018;27:1162–1169. doi: 10.1089/jwh.2017.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phalane E, Fourie CMT, Schutte AE. The metabolic syndrome and renal function in an African cohort infected with human immunodeficiency virus. South Afr J HIV Med. 2018;19:813. doi: 10.4102/sajhivmed.v19i1.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogalska-Płońska M, Grzeszczuk A, Rogalski P, Łucejko M, Flisiak R. Metabolic syndrome in HIV infected adults in Poland. Kardiol Pol. 2018;76:548–553. doi: 10.5603/KP.a2017.0249. [DOI] [PubMed] [Google Scholar]
- 20.Sears S, Buendia JR, Odem S, Qobadi M, Wortley P, Mgbere O, Sanders J, Spencer EC, Barnes A. Metabolic syndrome among people living with HIV receiving medical care in Southern United States: Prevalence and risk factors. AIDS Behav. 2019;23:2916–2925. doi: 10.1007/s10461-019-02487-8. [DOI] [PubMed] [Google Scholar]
- 21.Oh DH, Ahn JY, Kim SI, Kim MJ, Woo JH, Kim WJ, Baek JH, Kim SW, Choi BY, Lee MH, Choi JY, Han MG, Kang C, Kim JM, Choi JY Korea HIV/AIDS Cohort Study. Metabolic complications among Korean patients with HIV infection: The Korea HIV/AIDS Cohort Study. J Korean Med Sci. 2017;32:1268–1274. doi: 10.3346/jkms.2017.32.8.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 23.El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, Grunfeld C, Raghavan SS. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 24.Funderburg NT, Mehta NN. Lipid abnormalities and inflammation in HIV inflection. Curr HIV/AIDS Rep. 2016;13:218–225. doi: 10.1007/s11904-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapuła M, Suchacz M, Załęski A, Wiercińska-Drapało A. Impact of combined antiretroviral therapy on metabolic syndrome components in adult people living with HIV: A literature review. Viruses. 2022;14:122. doi: 10.3390/v14010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bijker R, Choi JY, Ditangco R, Kiertiburanakul S, Lee MP, Siwamogsatham S, Pujari S, Ross J, Wong CY, Wong WW, Yunihastuti E, Law M. Cardiovascular disease and cardiovascular disease risk in HIV-positive populations in the Asian region. Open AIDS J. 2017;11:52–66. doi: 10.2174/1874613601711010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo J. Dyslipidemia and lipid management in HIV-infected patients. Curr Opin Endocrinol Diabetes Obes. 2011;18:144–147. doi: 10.1097/MED.0b013e328344556e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crane HM, Grunfeld C, Willig JH, Mugavero MJ, Van Rompaey S, Moore R, Rodriguez B, Feldman BJ, Lederman MM, Saag MS, Kitahata MM. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011;25:185–195. doi: 10.1097/QAD.0b013e328341f925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valantin MA, Bittar R, de Truchis P, Bollens D, Slama L, Giral P, Bonnefont-Rousselot D, Pétour P, Aubron-Olivier C, Costagliola D, Katlama C TOTEM trial group. Switching the nucleoside reverse transcriptase inhibitor backbone to tenofovir disoproxil fumarate + emtricitabine promptly improves triglycerides and low-density lipoprotein cholesterol in dyslipidaemic patients. J Antimicrob Chemother. 2010;65:556–561. doi: 10.1093/jac/dkp462. [DOI] [PubMed] [Google Scholar]
- 30.Plum PE, Maes N, Sauvage AS, Frippiat F, Meuris C, Uurlings F, Lecomte M, Léonard P, Paquot N, Fombellida K, Vaira D, Moutschen M, Darcis G. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis. 2021;21:910. doi: 10.1186/s12879-021-06479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagoutte-Renosi J, Flammang M, Chirouze C, Beck-Wirth G, Bozon F, Brunel AS, Drobacheff-Thiebaut MC, Foltzer A, Hustache-Mathieu L, Kowalczyk J, Michel C, Davani S, Muret P. Real-life impact on lipid profile of a switch from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-infected patients. Curr HIV Res. 2021;19:84–89. doi: 10.2174/1570162X18666200824101838. [DOI] [PubMed] [Google Scholar]
- 32.The RESPOND Study Group. Incidence of dyslipidemia in people with HIV who are treated with integrase inhibitors versus other antiretroviral agents. AIDS. 2021;35:869–882. doi: 10.1097/QAD.0000000000002811. [DOI] [PubMed] [Google Scholar]
- 33.Saumoy M, Sanchez-Quesada JL, Ordoñez-Llanos J, Podzamczer D. Do all integrase strand transfer inhibitors have the same lipid profile? review of randomised controlled trials in naïve and switch scenarios in HIV-infected patients. J Clin Med. 2021;10:3456. doi: 10.3390/jcm10163456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macallan DC, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA, Sawyer MB, McManus TJ, Griffin GE. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995;333:83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 35.Macallan DC. Wasting in HIV infection and AIDS. J Nutr. 1999;129(1S Suppl):238S–42S. doi: 10.1093/jn/129.1.238S. [DOI] [PubMed] [Google Scholar]
- 36.Guaraldi G, Stentarelli C, Zona S, Santoro A, Beghetto B, Carli F, Orlando G, Franceschetto A, Casolo A, Mussini C. The natural history of HIV-associated lipodystrophy in the changing scenario of HIV infection. HIV Med. 2014;15:587–594. doi: 10.1111/hiv.12159. [DOI] [PubMed] [Google Scholar]
- 37.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, Myers L, Melbourne K, Ha B, Daar ES. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–196. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dubé MP, Kelesidis T, Rothenberg J, Stein JH, Brown TT. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62:853–862. doi: 10.1093/cid/ciw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–4856. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 40.Kerchberger AM, Sheth AN, Angert CD, Mehta CC, Summers NA, Ofotokun I, Gustafson D, Weiser SD, Sharma A, Adimora AA, French AL, Augenbraun M, Cocohoba J, Kassaye S, Bolivar H, Govindarajulu U, Konkle-Parker D, Golub ET, Lahiri CD. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis. 2020;71:593–600. doi: 10.1093/cid/ciz853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, Lantche Wandji M, Tetsa Tata D, Omgba Bassega P, Abong Bwenda T, Varloteaux M, Tongo M, Mpoudi-Ngolé E, Montoyo A, Mercier N, LeMoing V, Peeters M, Reynes J, Delaporte E New Antiretroviral and Monitoring Strategies in HIV-infected Adults in Low-Income Countries (NAMSAL) ANRS 12313 Study Group. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020;7:e677–e687. doi: 10.1016/S2352-3018(20)30238-1. [DOI] [PubMed] [Google Scholar]
- 42.Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, Serenata C, Akpomiemie G, Masenya M, Qavi A, Chandiwana N, McCann K, Norris S, Chersich M, Maartens G, Lalla-Edward S, Vos A, Clayden P, Abrams E, Arulappan N, Hill A. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7:e666–e676. doi: 10.1016/S2352-3018(20)30241-1. [DOI] [PubMed] [Google Scholar]
- 43.Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, Brown TT, Rockstroh JK, Wei X, Carter CC, Zhong L, Brainard DM, Melbourne K, Das M, Stellbrink HJ, Post FA, Waters L, Koethe JR. Weight gain following initiation of antiretroviral therapy: Risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surial B, Mugglin C, Calmy A, Cavassini M, Günthard HF, Stöckle M, Bernasconi E, Schmid P, Tarr PE, Furrer H, Ledergerber B, Wandeler G, Rauch A Swiss HIV cohort study. Swiss HIV cohort study. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: A cohort study. Ann Intern Med. 2021;174:758–767. doi: 10.7326/M20-4853. [DOI] [PubMed] [Google Scholar]
- 45.Diggins CE, Russo SC, Lo J. Metabolic consequences of antiretroviral therapy. Curr HIV/AIDS Rep. 2022;19:141–153. doi: 10.1007/s11904-022-00600-6. [DOI] [PubMed] [Google Scholar]
- 46.Kuo PH, Sun HY, Chuang YC, Wu PY, Liu WC, Hung CC. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis. 2020;92:71–77. doi: 10.1016/j.ijid.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Ando N, Nishijima T, Mizushima D, Inaba Y, Kawasaki Y, Kikuchi Y, Oka S, Gatanaga H. Long-term weight gain after initiating combination antiretroviral therapy in treatment-naïve Asian people living with human immunodeficiency virus. Int J Infect Dis. 2021;110:21–28. doi: 10.1016/j.ijid.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Mulenga LB, Fwoloshi S, Mweemba A, Siwingwa M, Sivile S, Kampamba D, Engamba DC, Mbewe N, Phiri H, Shibemba A, Simons B, Wester CW, Chirwa L, Hill A. Dolutegravir with recycled nRTIs is noninferior to PI-based ART: VISEND trial. CROI Abstract 135; Conference on Retroviruses and Opportunistic Infections; 2022. [Google Scholar]
- 49.CLINICAL INFO. A working group of the office of AIDS research advisory council (OARAC). Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. [Accessed 5 April 2022]. Available at: https://clinicalinfo.hiv.gov.
- 50.Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33:10–19. doi: 10.1097/QCO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorwood J, Bourgeois C, Pourcher V, Pourcher G, Charlotte F, Mantecon M, Rose C, Morichon R, Atlan M, Le Grand R, Desjardins D, Katlama C, Fève B, Lambotte O, Capeau J, Béréziat V, Lagathu C. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in Human/Simian adipose tissue and human adipocytes. Clin Infect Dis. 2020;71:e549–e560. doi: 10.1093/cid/ciaa259. [DOI] [PubMed] [Google Scholar]
- 52.Pickering R, Asundi A, Lin N. In vitro model to assess antiretroviral therapy on adipocyte biology. CROI Abstract 514; Conference on Retroviruses and Opportunistic Infections; 2021. [Google Scholar]
- 53.Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, Smith CJ, d’Arminio Monforte A, Phillips A, Weber R, Lundgren J, Law MG D:A:D Study Group. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17:255–268. doi: 10.1111/hiv.12294. [DOI] [PubMed] [Google Scholar]
- 54.Herrin M, Tate JP, Akgün KM, Butt AA, Crothers K, Freiberg MS, Gibert CL, Leaf DA, Rimland D, Rodriguez-Barradas MC, Ruser CB, Herold KC, Justice AC. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr. 2016;73:228–236. doi: 10.1097/QAI.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, Logeais M, Rimland D, Rodriguez-Barradas MC, Ruser C, Justice AC. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60:1852–1859. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achhra AC, Sabin C, Ryom L, Hatleberg C, Antonella d'Aminio M, de Wit S, Phillips A, Pradier C, Weber R, Reiss P, El-Sadr W, Bonnet F, Mocroft A, Lundgren J, Law MG D:A:D Study Group. Body mass index and the risk of serious non-AIDS events and all-cause mortality in treated HIV-positive individuals: D: A: D cohort analysis. J Acquir Immune Defic Syndr. 2018;78:579–588. doi: 10.1097/QAI.0000000000001722. [DOI] [PubMed] [Google Scholar]
- 57.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: A systematic review and meta-analysis. Epidemiology. 2018;29:431–441. doi: 10.1097/EDE.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 58.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, Leaf D, Justice AC Veterans Aging Cohort Study. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One. 2018;13:e0194199. doi: 10.1371/journal.pone.0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Putcharoen O, Wattanachanya L, Sophonphan J, Siwamogsatham S, Sapsirisavat V, Gatechompol S, Phonphithak S, Kerr SJ, Chattranukulchai P, Avihingsanon Y, Ruxrungtham K, Avihingsanon A HIV-NAT 006 team. New-onset diabetes in HIV-treated adults: predictors, long-term renal and cardiovascular outcomes. AIDS. 2017;31:1535–1543. doi: 10.1097/QAD.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 61.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 62.Kim JH, Noh J, Kim W, Seong H, Kim JH, Lee WJ, Baek Y, Hyun J, Sohn Y, Cho Y, Kim MH, Ahn S, Lee Y, Ahn JY, Jeong SJ, Ku NS, Yeom JS, Kim C, Choi JY. Trends of age-related non-communicable diseases in people living with HIV and comparison with uninfected controls: A nationwide population-based study in South Korea. HIV Med. 2021;22:824–833. doi: 10.1111/hiv.13139. [DOI] [PubMed] [Google Scholar]
- 63.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, Rajagopalan S, Kottilil S, Nair H, Newby DE, McAllister DA, Mills NL. Global burden of atherosclerotic cardiovascular disease in people living with HIV: Systematic review and meta-analysis. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sattler FR, He J, Letendre S, Wilson C, Sanders C, Heaton R, Ellis R, Franklin D, Aldrovandi G, Marra CM, Clifford D, Morgello S, Grant I, McCutchan JA CHARTER Group. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68:281–288. doi: 10.1097/QAI.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu B, Pasipanodya E, Montoya JL, Moore RC, Gianella S, McCutchan A, Ellis R, Heaton RK, Jeste DV, Moore DJ, Marquine MJ. Metabolic syndrome and neurocognitive deficits in HIV infection. J Acquir Immune Defic Syndr. 2019;81:95–101. doi: 10.1097/QAI.0000000000001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I CHARTER Group. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demir M, Lang S, Steffen HM. Nonalcoholic fatty liver disease - current status and future directions. J Dig Dis. 2015;16:541–557. doi: 10.1111/1751-2980.12291. [DOI] [PubMed] [Google Scholar]
- 69.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 70.Cervo A, Shengir M, Patel K, Sebastiani G. NASH in HIV. Curr HIV/AIDS Rep. 2020;17:601–614. doi: 10.1007/s11904-020-00531-0. [DOI] [PubMed] [Google Scholar]
- 71.Benmassaoud A, Ghali P, Cox J, Wong P, Szabo J, Deschenes M, Osikowicz M, Lebouche B, Klein MB, Sebastiani G. Screening for nonalcoholic steatohepatitis by using cytokeratin 18 and transient elastography in HIV mono-infection. PLoS One. 2018;13:e0191985. doi: 10.1371/journal.pone.0191985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31:1621–1632. doi: 10.1097/QAD.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 73.Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, Hu FB, Raz I, Van Gaal L, Wolfe BM, Ryan DH. Advances in the science, treatment, and prevention of the disease of obesity: Reflections from a diabetes care editors’ expert forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, Kotler DP. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55:1327–1336. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 75.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, Grinspoon SK. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006;20:1843–1850. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 76.Reeds DN, Pietka TA, Yarasheski KE, Cade WT, Patterson BW, Okunade A, Abumrad NA, Klein S. HIV infection does not prevent the metabolic benefits of diet-induced weight loss in women with obesity. Obesity (Silver Spring) 2017;25:682–688. doi: 10.1002/oby.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gjermeni E, Kirstein AS, Kolbig F, Kirchhof M, Bundalian L, Katzmann JL, Laufs U, Blüher M, Garten A, Le Duc D. Obesity-an update on the basic pathophysiology and review of recent therapeutic advances. Biomolecules. 2021;11:1426. doi: 10.3390/biom11101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cope RJ, Fischetti BS, Kavanagh RK, Lepa TM, Sorbera MA. Safety and efficacy of weight-loss pharmacotherapy in persons living with HIV: A review of the literature and potential drug-drug interactions with antiretroviral therapy. Pharmacotherapy. 2019;39:1204–1215. doi: 10.1002/phar.2342. [DOI] [PubMed] [Google Scholar]
- 79.Akbari K, Som R, Sampson M, Abbas SH, Ramus J, Jones G. The effect of bariatric surgery on patients with HIV infection: a literature review. Obes Surg. 2018;28:2550–2559. doi: 10.1007/s11695-018-3319-4. [DOI] [PubMed] [Google Scholar]
- 80.Selke H, Norris S, Osterholzer D, Fife KH, DeRose B, Gupta SK. Bariatric surgery outcomes in HIV-infected subjects: a case series. AIDS Patient Care STDS. 2010;24:545–550. doi: 10.1089/apc.2010.0132. [DOI] [PubMed] [Google Scholar]
- 81.Pourcher G, Peytavin G, Schneider L, Gallien S, Force G, Pourcher V. Bariatric surgery in HIV patients: experience of an obesity reference center in France. Surg Obes Relat Dis. 2017;13:1990–1996. doi: 10.1016/j.soard.2017.09.514. [DOI] [PubMed] [Google Scholar]
- 82.Sharma G, Strong AT, Boules M, Tu C, Szomstein S, Rosenthal R, Rodriguez J, Taege AJ, Kroh M. Comparative outcomes of bariatric surgery in patients with and without human immunodeficiency virus. Obes Surg. 2018;28:1070–1079. doi: 10.1007/s11695-017-2996-8. [DOI] [PubMed] [Google Scholar]
- 83.Sharma P, McCarty TR, Ngu JN, O’Donnell M, Njei B. Impact of bariatric surgery in patients with HIV infection: a nationwide inpatient sample analysis, 2004-2014. AIDS. 2018;32:1959–1965. doi: 10.1097/QAD.0000000000001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amouyal C, Buyse M, Lucas-Martini L, Hirt D, Genser L, Torcivia A, Bouillot JL, Oppert JM, Aron-Wisnewsky J. Sleeve gastrectomy in morbidly obese HIV patients: Focus on anti-retroviral treatment absorption after surgery. Obes Surg. 2018;28:2886–2893. doi: 10.1007/s11695-018-3308-7. [DOI] [PubMed] [Google Scholar]