Abstract
Purpose
This study aimed to evaluate the rates of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67 heterogeneity in multifocal or multicentric breast cancer (MMBC) and its association with treatment pattern and disease outcomes.
Methods
MMBC patients with ER, PR, HER2, and Ki67 results for each tumor focus were retrospectively analyzed using Kappa test and categorized into the homogeneous group (Homo group) and the heterogeneous group (Hetero group). Chi-square tests were performed to compare the clinical features and treatment options between the groups. Disease-free survival (DFS) and overall survival (OS) rates were estimated from Kaplan–Meier curves and compared between two groups.
Results
A total of 387 patients were included, and 93 (24.0%) were classified into the Hetero group. Adjuvant endocrine therapy was more frequently assigned for patients in the Hetero group than in the Homo group (84.9% vs. 71.7%, p = 0.046). There was no difference in terms of adjuvant anti-HER2 therapy (28.3% vs. 19.6%, p = 0.196) and chemotherapy (69.9% vs. 69.8%, p = 0.987) usage between the two groups. At a median follow-up of 36 months, DFS rates were 81.2% for the Hetero group and 96.5% for the Homo group (p = 0.041; adjusted HR, 2.95; 95% CI, 1.04–8.37). The estimated 3-year OS rates for the groups were 95.8% and 99.5%, respectively (p = 0.059; adjusted HR, 5.36; 95% CI, 0.97–29.69).
Conclusion
Heterogeneity of ER, PR, HER2, or Ki67 was present in 24.0% patients with MMBC. Biomarkers heterogeneity influenced adjuvant endocrine therapy usage and was associated with worse disease outcomes, indicating further clinical evaluation.
Keywords: biomarkers, breast neoplasms, intertumoral heterogeneity, multifocal, multicentric, prognosis
Introduction
Breast cancer is a heterogeneous group of diseases in which individual patient differs in morphological features, molecular profiles, therapeutic responses, and prognosis (1). Morphological variability such as pathological type and histological grade has been well documented for decades and forms the basis for histological classification of breast cancer. More recently, different molecular phenotypes of breast cancer have been defined by genetic or immunohistochemistry testing. For example, the well-defined 2013 St Gallen subtypes of breast cancer were based on the expressions of estrogen (ER) and progesterone (PR) receptors, human epidermal growth factor receptor 2 (HER2), and Ki67 proliferative index, which provide prognostic information and can be used to tailor systemic adjuvant therapy (2).
The molecular heterogeneity can occur either between different tumors within the same patient (intertumoral heterogeneity) or within the same tumor (intratumoral heterogeneity) (1). Heterogeneous expressions of ER, PR, HER2, and Ki67 have been widely reported between core needle biopsy and surgical samples, between different regions of a primary tumor, between a primary tumor and a matched metastatic lesion, or between metastatic lesions (3–9). Beyond spatial heterogeneity, heterogeneity can be observed as the natural evolution of a tumor or as consequences of anticancer treatments (10–12).
Multifocal/multicentric breast cancer (MMBC) has become more frequently diagnosed with the popular breast cancer screening program and the advancement of imaging methods (13, 14). In a previous study that evaluated the heterogeneity of ER, PR, HER2, and Ki67 between different foci in MMBC, the heterogeneity of these molecular markers was present in 4.4%, 15.9%, 9.7%, and 15.0% cases (13). MMBC with biomarkers heterogeneity represents a situation in breast cancer treatment where there are few guidelines to direct care. However, there are few studies investigating the therapeutic and prognostic impact of such heterogeneity. Herein, we performed this retrospective study to evaluate the rates of ER, PR, HER2, and Ki67 heterogeneity in patients with MMBC and its impacts on systemic adjuvant therapy decision-making and disease outcomes.
Methods
Study Population
Patients who received surgery and were diagnosed with multifocal or multicentric breast cancer at Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine from January 2009 to December 2018 were retrospectively analyzed. Clinicopathological characteristics, adjuvant treatment, and follow-up data were retrieved from Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB). The eligibility criteria were as follows: (1) at least one invasive tumor focus; (2) no distant metastasis at diagnosis; and (3) ER, PR, HER2, and Ki67 both tested between different tumor foci. Those who received neo-adjuvant therapy and those with only in situ tumor foci were excluded from the present study. Patients who did not have all samples tested for biomarkers were also exploratorily evaluated for disease outcomes.
Histopathology Assessments
Histopathology analysis for different tumor foci on surgical specimens were independently performed and reviewed by two pathologists at the Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (15, 16). In this study, multifocality was defined as the presence of more than one focus of carcinoma in one breast quadrant (MFBC), and multicentricity was defined as the presence of a focus in a different breast quadrant from the main lesion (MCBC) (13). Immunohistochemistry (IHC) of ER, PR, Ki67, and HER2 were performed on 4-µm slices of formalin-fixed paraffin-embedded (FFPE) specimens with primary antibodies against ER (SP1, 1:100, Dako, Denmark), PR (PgR 636, 1:100, Dako, Denmark), HER2 (4B5, Roche, Switzerland), Ki67 (MIB-1, 1:100, Dako, Denmark) by Ventana autostain system, BenchMark XT as previously described (15). In brief, the tissue sections were incubated with primary antibody of ER, PR, and Ki67 for 32 min at 42°C and of HER2 for 16 min at 42°C, which were then counterstained with hematoxylin. ER/PR was considered positive if there were ≥1% of the tumor cells with nuclear staining (16). HER2 was scored as 0 to 3+ by IHC, and those with IHC 2+ were further examined with fluorescence in situ hybridization (FISH) according to the ASCO/CAP guidelines, where HER2 positivity was defined as either IHC 3+ or IHC 2+ with FISH amplification (17–19). The Ki67 index was scored as the percentage of positively nuclear staining cells among at least 500–2,000 uniformly distributed cells or 2,000 cells from the hotspot and negative areas (20). Molecular subtypes were determined based on 2013 St Gallen system: luminal A-like (ER+/PR ≥ 20%/HER2-/Ki67 < 20%), luminal B-like (HER2−) (ER+/HER2−/Ki67 ≥ 20% or ER+/PR < 20%/HER2 or ER−/PR+/HER2−), luminal B-like (HER2+) (ER+ or PR+/HER2+), HER2+ (ER−/PR−/HER2+), and triple negative (ER−/PR−/HER2−) (2). Patients with concordant status of ER, PR, HER2, and Ki67 among all tumor foci were categorized into the homogeneous group (Homo group), while the heterogeneous group (Hetero group) was defined as the existence of at least one discordance for ER, PR, HER2, or Ki67 between different foci. The main focus referred to the largest tumor focus, and the other foci were named minor foci. Distance between the main and minor foci was assessed on pathological specimens, which was defined as the shortest distance between the edges of two tumor foci.
Treatment and Follow-Up
Adjuvant treatment decisions were made through multidisciplinary team (MDT) meetings attended by surgical oncologists, medical oncologists, radiation oncologists, and pathologists (21). Decision was tailored according to the tumor biological features, stage at diagnosis, patient medical complications, and preferences. The patients were followed up every 3 months during the first 2 years after surgery, every 6 months from the third to the fifth year and once per year hereafter till death. DFS was defined as the period from the date of surgery to first local-regional relapse, contralateral breast cancer, secondary new malignant tumor, distant relapse, or death. OS was calculated from the date of surgery to the date of death. For patients who were free from DFS/OS events at the time of last follow-up, DFS/OS were calculated as the period from the date of surgery to the date of last follow-up.
Statistics
Kappa tests were performed to evaluate the concordance rates of pathological type, histological grade, ER, PR, HER2, Ki67, and molecular subtype between the larger tumor focus and the smaller focus. For tumors with three or four foci, the results were considered concordant only when the biomarkers status of all tumor foci were concordant. The clinical features and adjuvant therapy options were compared between the Homo group and the Hetero group using chi-square test or Fisher’s exact test. DFS and OS rates were estimated from Kaplan–Meier curves and compared between the two groups via log-rank test. Cox proportional hazard model was used to calculate the hazard ratios for relapse and death. Clinical features and disease outcomes were also compared between MFBC and MCBC. Two-side p < 0.05 was considered statistically significant. All the statistical procedures were performed on SPSS (version 26.0).
Results
Baseline Clinicopathological Characteristics
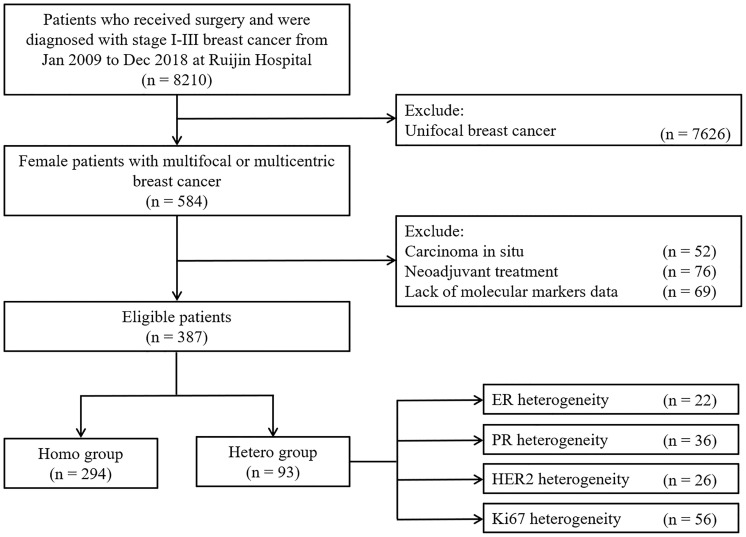
There were 8,210 stage II–III breast cancer patients who received surgery from January 2009 to December 2018 at Ruijin Hospital, among which 584 (7.11%) women were diagnosed with multifocal or multicentric breast cancers and 387 were included in the study (Figure 1). There were 52, 76, and 69 cases who were excluded from the study, as they had in situ foci only, received neo-adjuvant therapy, or lacked molecular markers data, respectively. Physical examination, sonography, mammography, and MRI identified 16.9%, 66.8%, 33.4%, and 77.2% of these patients, respectively (Supplementary Figure S1). As shown in the Supplementary Figure S2, the median distance between the main and minor foci was 12.6 [interquartile range (IQR), 7.2–20.0] mm, which showed no significant difference between the two groups (12.4 mm vs. 15.0 mm, p = 0.082).
Figure 1.
Flow chart of 387 patients in the study.
The demographic and clinicopathological characteristics for the cohort are summarized in Table 1. The median age for the patients was 55 (IQR, 46–64) years, and 41.5% patients were pre/peri-menopausal at diagnosis. Patients with two foci accounted for 91.5% and 65.4% had multifocal diseases. Comparisons of MCBC and MFBC are summarized in Supplementary Table S1. Thirty-four (8.8%) patients received breast-conserving surgery, and sentinel lymph node biopsy was performed in 115 (31.3%) patients. There were 151 (39.0%) patients whose main tumor foci were larger than 2.0 cm, and 144 (37.2%) patients had positive axillary lymph nodes (ALN). A total of 77 (19.9%) and 140 (36.2%) were diagnosed with non-IDC in the main and minor tumor foci, respectively (Supplementary Figure S3). Luminal A-like, luminal B-like (HER2-), luminal B-like (HER2+), HER2+, and triple negative breast cancers were present in 117 (30.2%), 135 (34.9%), 50 (12.9%), 47 (12.1%), and 38 (9.9%) patients, respectively. There were significant differences in terms of pathological type of the minor tumor focus (p < 0.001) and molecular subtype of the main tumor focus (p < 0.001) between the Hetero group and the Homo group.
Table 1.
Baseline clinical and pathological characteristics.
| Characteristics | TotalN = 387 (%) | HomoN = 294 (%) | HeteroN = 93 (%) | p-value |
|---|---|---|---|---|
| Age (y/o) | 55 (46–64) | 55 (46–64) | 55 (47–65) | 0.619 |
| Menstrual status | 0.538 | |||
| Pre/Peri- | 160 (41.5) | 124 (42.3) | 36 (38.7) | |
| Post- | 226 (58.5) | 169 (57.7) | 57 (51.3) | |
| Number of foci | 0.378 | |||
| 2 | 354 (91.5) | 271 (92.2) | 83 (89.2) | |
| 3/4 | 33 (8.5) | 23 (7.8) | 10 (10.8) | |
| Location of foci | 0.842 | |||
| Multifocal | 253 (65.4) | 193 (65.6) | 60 (64.5) | |
| Multicentric | 134 (34.6) | 101 (34.4) | 33 (35.5) | |
| Breast surgery | 0.727 | |||
| BCS | 34 (8.8) | 25 (8.5) | 9 (9.7) | |
| Mastectomy | 353 (91.2) | 269 (91.5) | 84 (90.3) | |
| Axillary surgery | 0.733 | |||
| SLNB | 115 (31.3) | 89 (30.9) | 26 (32.9) | |
| ALND | 252 (68.7) | 199 (69.1) | 53 (67.1) | |
| Pathological type a | 0.656 | |||
| IDC | 310 (80.1) | 237 (80.6) | 73 (78.5) | |
| Non-IDC | 77 (19.9) | 57 (19.4) | 20 (21.5) | |
| Pathological type b | <0.001 | |||
| IDC | 247 (63.8) | 211 (71.8) | 36 (38.7) | |
| Non-IDC | 140 (36.2) | 83 (28.2) | 57 (61.3) | |
| Tumor size a | 0.754 | |||
| ≤2.0 cm | 236 (61.0) | 178 (60.5) | 58 (62.4) | |
| >2.0 cm | 151 (39.0) | 116 (39.5) | 35 (37.6) | |
| ALN status | 0.521 | |||
| Negative | 243 (62.8) | 182 (61.9) | 61 (65.5) | |
| Positive | 144 (37.2) | 112 (38.1) | 32 (34.4) | |
| Histological grade a | 0.739 | |||
| I | 24 (6.2) | 20 (6.8) | 4 (4.3) | |
| II | 183 (47.3) | 141 (48.0) | 42 (45.2) | |
| III | 96 (24.8) | 71 (24.1) | 25 (26.9) | |
| NA | 84 (21.7) | 62 (21.1) | 22 (23.6) | |
| Molecular subtype a | <0.001 | |||
| LA | 117 (30.2) | 103 (35.0) | 14 (15.1) | |
| LB (HER2−) | 135 (34.9) | 87 (29.6) | 48 (51.6) | |
| LB (HER2+) | 50 (12.9) | 34 (11.6) | 16 (17.2) | |
| HER2+ | 47 (12.1) | 39 (13.3) | 8 (8.6) | |
| TNBC | 38 (9.9) | 31 (10.5) | 7 (7.5) |
Main focus.
Minor focus.
ALN, axillary lymph node; ALND, axillary lymph node dissection; BCS, breast-conserving surgery; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; LA, Luminal A-like; LB, Luminal B-like; NA, not available; SLNB, sentinel lymph node biopsy; TNBC, triple negative breast cancer; y/o, years old.
Rates of Molecular Markers Heterogeneity
As shown in Table 2, concordance rates of ER, PR, HER2, and Ki67 among different tumor foci were 94.3%, 90.7%, 93.3%, and 87.1%, respectively (all p values <0.001). Among the whole cohort, a total of 93 (24.0%) patients showed intertumoral heterogeneity of molecular markers, and the remaining 294 (76.0%) were homogeneous. There were 60 (23.7%) patients with MFBC and 33 (24.0%) with MCBC who were classified to the Hetero group (p = 0.842, Supplementary Tables S1–3).
Table 2.
Concordance rates of pathological type, histological grade, ER, PR, HER2, and Ki67 status.
| Main focus | Minor focus | Concordancerate (%) | Kappa | p-value | ||
|---|---|---|---|---|---|---|
| Pathological type | IDC | Non-IDC | 78.0 | 0.473 | <0.001 | |
| IDC | 236 | 74 | ||||
| Non-IDC | 11 | 66 | ||||
| Histological grade | I | II | III | 88.4 | 0.772 | <0.001 |
| I | 15 | 3 | 0 | |||
| II | 3 | 139 | 5 | |||
| III | 1 | 16 | 59 | |||
| ER | Negative | Positive | 94.3 | 0.841 | <0.001 | |
| Negative | 79 | 8 | ||||
| Positive | 14 | 286 | ||||
| PR | Negative | Positive | 90.7 | 0.798 | <0.001 | |
| Negative | 121 | 18 | ||||
| Positive | 18 | 230 | ||||
| HER2 | Negative | Positive | 93.3 | 0.819 | <0.001 | |
| Negative | 279 | 11 | ||||
| Positive | 15 | 82 | ||||
| Ki67 | < 20% | ≥ 20% | 87.1 | 0.743 | <0.001 | |
| < 20% | 163 | 8 | ||||
| ≥ 20% | 42 | 174 | ||||
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
The molecular subtypes were identical within the same patient in 310 (80.1%) of the 387 cases using the 2013 St Gallen standard, with 104 luminal A-like tumors, 93 luminal B-like (HER2−) tumors, 42 luminal B-like (HER2+) tumors, 39 HER2-enriched tumors, and 32 triple negative breast cancers (Table 3). Molecular subtypes differed among different tumor foci in 77 (19.9%) patients, including 46 (18.2%) and 31 (23.1%) with MFBC and MCBC, respectively (p = 0.336, Supplementary Tables S1, 4, 5).
Table 3.
Concordance rates of molecular subtypesa.
| Main focus | Minor focus | Concordance rate (%) | Kappa | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| LA | LB (HER2−) | LB (HER2+) | HER2+ | TNBC | 80.1 | 0.830 | <0.001 | |
| LA | 104 | 6 | 1 | 3 | 3 | |||
| LB (HER2−) | 32 | 93 | 4 | 1 | 5 | |||
| LB (HER2+) | 4 | 4 | 42 | 0 | 0 | |||
| HER2+ | 0 | 0 | 2 | 39 | 6 | |||
| TNBC | 3 | 1 | 1 | 1 | 32 | |||
The cutoff value of Ki67 was 20% for differentiating luminal A-like and luminal B-like (HER2−).
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; LA, luminal A-like; LB, luminal B-like; TNBC, triple negative breast cancer.
Heterogeneity of Molecular Markers and Adjuvant Therapy
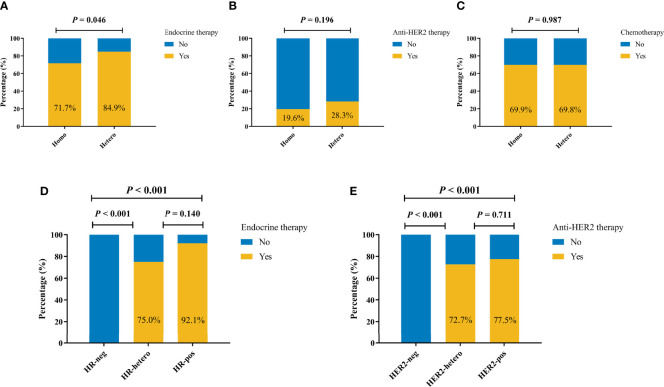
There were 150 patients with recorded MDT-recommended adjuvant therapies, and the compliance was 95.3%, 96.0%, and 97.3% to chemotherapy, endocrine therapy, and anti-HER2 therapy, respectively. A total of 45 (84.9%) out of 330 patients with at least two invasive tumor foci in the Hetero group received adjuvant endocrine therapy, which was significantly higher than that of patients in the Homo group (71.7%, p = 0.046, Figure 2A). There were no significant differences in the usage rates of adjuvant anti-HER2 therapy (28.3% vs. 19.6%, p = 0.196, Figure 2B) and chemotherapy (69.9% vs. 69.8%, p = 0.987, Figure 2C) between the two groups.
Figure 2.
Adjuvant systemic therapy by molecular markers status among 330 patients with at least two invasive tumor foci. Adjuvant endocrine therapy (A), anti-HER2 therapy (B), and chemotherapy (C) by molecular markers status. (D) Adjuvant endocrine therapy by HR status. (E) Adjuvant anti-HER2 therapy by HER2 status.
As shown in Figure 2D, endocrine therapy was more frequently utilized among patients with HR heterogeneity than HR-negative patients (75.0% vs. 0.0%, p < 0.001), while the rates were comparable among patients with at least one HR+ tumor foci (75.0% vs. 92.1%, p = 0.140). Similarly, HER2 heterogeneity was associated with higher rate of anti-HER2 therapy compared with HER2-negative patients (72.7% vs. 0.0%, p < 0.001), and once again, no significant difference was observed among patients with at least one HER2+ tumor focus (72.7% vs. 77.5%, p = 0.711, Figure 2E).
Heterogeneity of Molecular Markers and Disease Outcomes
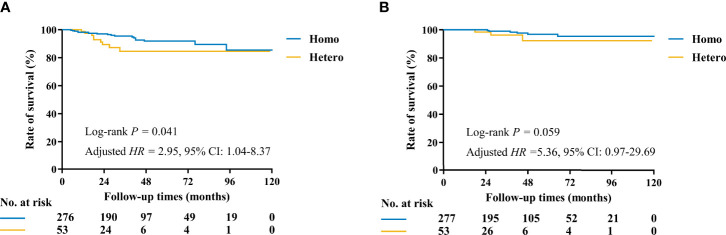
At a median follow-up of 35 (IQR, 19–57) months, 21 DFS events and 5 deaths were recorded (Table 4). Patients in the Hetero group had significantly worse DFS (81.2% vs. 96.5%, p = 0.041) and comparable OS (95.8% vs. 99.5%, p = 0.059) than those in the Homo group (Table 5 and Figure 3). After adjusting age, tumor size, ALN status, molecular subtype, and systemic treatments in multivariate models, patients in the Hetero group had significantly worse DFS (adjusted HR, 2.95; 95% CI, 1.04–8.37) and comparable OS (adjusted HR, 5.36; 95% CI, 0.97–29.69) than those in the Homo group (Supplementary Table S1).
Table 4.
Details of DFS and OS events by status of molecular markers among 330 patients with at least two invasive tumor foci.
| TotalN = 330 (%) | HomoN = 277 (%) | HeteroN = 53 (%) | |
|---|---|---|---|
| DFS events | |||
| No recurrence | 309 (93.6) | 261 (94.2) | 48 (90.6) |
| Local-regional recurrence | 5 (1.5) | 4 (1.4) | 1 (1.9) |
| Contralateral breast cancer | 4 (1.2) | 4 (1.4) | 0 (0.0) |
| Second non-breast malignancy | 2 (0.6) | 1 (0.4) | 1 (1.9) |
| Distant recurrence | 8 (2.4) | 6 (2.2) | 2 (3.8) |
| Death without recurrence | 2 (0.6) | 1 (0.4) | 1 (1.9) |
| OS events | |||
| Alive | 323 (97.9) | 272 (98.2) | 51 (96.2) |
| Death of any cause | 7 (2.1) | 5 (1.8) | 2 (3.9) |
| Death with recurrence | 5 (1.5) | 4 (1.4) | 1 (1.9) |
| Death without recurrence | 2 (0.6) | 1 (0.4) | 1 (1.9) |
DFS, disease-free survival; OS, overall survival.
Table 5.
Univariate analysis of prognostic factors affecting DFS and OS among 330 patients with at least two invasive tumor foci.
| Characteristics | p-value | |
|---|---|---|
| DFS | OS | |
| Age (<50 y/o vs. ≥50 y/o) | 0.741 | 0.277 |
| Menstrual status (Pre/Peri- vs. Post-) | 0.966 | 0.969 |
| Number of foci (2 vs. 3/4) | 0.264 | 0.557 |
| Location of foci (Multifocal vs. Multicentric) | 0.571 | 0.703 |
| Histology type (IDC vs. non-IDC) a | 0.802 | 0.789 |
| Tumor size (≤2.0 vs. >2.0 cm) a | 0.786 | 0.292 |
| ALN status (Negative vs. Positive) | 0.572 | 0.841 |
| Histological grade (I vs. II vs. III vs. NA)a | 0.637 | 0.892 |
| Molecular subtypea | 0.309 | 0.460 |
| Chemotherapy (No vs. Yes) | 0.698 | 1.000 |
| Endocrine therapy (No vs. Yes) | 0.102 | 0.458 |
| Anti-HER2 therapy (No vs. Yes) | 0.379 | 0.352 |
| Group (Homo vs. Hetero) | 0.041 | 0.059 |
Main focus.
ALN, axillary lymph node; IDC, invasive ductal carcinoma; DFS, disease-free survival; NA, not available; OS, overall survival; y/o, years old.
Figure 3.
Kaplan–Meier curves of DFS and OS by molecular markers status among 330 patients with at least two invasive tumor foci. (A) The estimated 3-year DFS rates for the Homo and Hetero groups were 96.5% and 81.2%, respectively (p = 0.041). (B) The estimated 3-year OS rates for the Homo and Hetero groups were 99.5% and 95.8%, respectively (p = 0.059).
Discussion
The study was designed to evaluate the rates of molecular markers heterogeneity and its associations with systemic adjuvant therapy and disease outcomes in MMBC. Molecular markers showed good concordance among different tumor foci. Heterogeneity of ER, PR, HER2, and Ki67 were present in 24.0% MMBC, which was associated with more adjuvant endocrine therapy usage (p = 0.046) and shorter DFS (p = 0.041), indicating the necessity of molecular assessments for different tumor foci in patients with MMBC.
There were some published literatures that reported the rates of intertumoral biomarkers heterogeneity among different foci in MMBC (13, 22–25). For example, Buggi and colleagues enrolled 113 invasive multiple breast cancers, and they reported mismatches on ER, PR, HER2, and Ki67 in 4.4%, 15.9%, 9.7%, and 15.0% cases (13). Similarly, the rate of ER, PR, HER2, and Ki67 heterogeneity in our cohort was 5.7%, 9.3%, 6.7%, and 22.9%, respectively. Moreover, molecular subtypes differed in 77 (19.9%) patients as classified by 2013 St Gallen system, comparable to the results of Pekar et al. (12.7%) (14). There might be some causes of molecular heterogeneity. First, heterogeneity of molecular markers was more frequent among patients whose minor tumor focus was invasive carcinoma of special type or carcinoma in situ in our study, making pathological differences a potential explanation. This finding will guide us to select patients for a second molecular evaluation in the present clinical practice. However, there would be other innate tumor properties beyond histopathology but crucial to tumor tumorigenesis and evolution, which control tumor heterogeneity. Recent studies have revealed that extensive genetic diversity caused by genome instability and mutation will affect key cancer pathways, eventually driving phenotypic variation (10–12, 26). In light of this, intratumor heterogeneity can lead to underestimation of the tumor genomics landscape from pathology only and may present major challenges to personalized medicine, which should be further evaluated by emerging technologies such as next-generation and single-cell sequencing (12). Moreover, non-mutational epigenetic reprogramming and cellular plasticity can also contribute to tumor heterogeneity (27, 28). Last but not the least, technical issue and analytical artifact may also affect accuracy of molecular evaluation, which could be avoided by standardization.
Accurate biomolecular analysis is of great significance to make treatment options and to evaluate functional outcomes and quality of life of breast cancer patients in the era of precise medicine (2, 29–31). However, with limited knowledge on the consequences of molecular heterogeneity for therapeutic decision-making, it has been accepted that biomarkers can be assessed only in the largest individual tumor focus (32). This is based on the observations that molecular markers in MMBC are usually homogeneous. However, if the tumor foci demonstrate different pathological or histological features, biomarkers evaluation of the smaller focus will be necessary. According to Buggi et al., 14 out of the 113 (12.4%) patients received additional systemic treatments with the biomarkers analysis for the smaller tumor focus (13). In the present study, heterogeneity of biomarkers was also found to be significantly associated with the usage of adjuvant endocrine therapy among patients with at least two invasive tumor foci. Additional biomarkers evaluation of the other foci would potentially change the adjuvant treatment decisions, especially for those whose main tumor focus lacked HR or HER2. This would further impact breast cancer patient’s survival.
To the best of the authors’ knowledge, this was the largest study to evaluate the prognostic significance of intertumoral biomarkers heterogeneity. Our cohort revealed that patients in the Hetero group had clinically worse DFS and OS compared those in the Homo group, although the differences were not statistically significant. However, the results should be taken as exploratory only and interpreted with caution, particularly given the relatively small number of DFS and OS events and short follow-up. Consistent with our results, Pekar et al. concluded that patients with phenotypically heterogeneous MMBC had a significantly shorter breast-cancer-specific survival (HR = 2.87; 95% CI, 1.08–7.64, p = 0.034) and OS (HR = 2.80; 95% CI, 1.05–7.44, p = 0.039) (14). The inferior disease outcomes in the Hetero group were thought to be associated with the biology behavior itself and possible undertreatment or low treatment sensitivity. Taken together, these results suggested the necessity of evaluating molecular markers for different tumor foci in patients with MMBC (33). However, we acknowledge that it is not a routine to test all tumor foci in the present clinical practice. Possibilities could be attributed to lack of evidence or economic reasons. In addition, it is important to point out that patients who have MMBC but do not have all foci tested may be more likely to have homogeneous tumors, since the finding of MMBC with discordant grades may prompt biomarker evaluation. Therefore, it could be useful to evaluate the clinical features and disease outcomes among patients who do not have all samples tested for biomarkers. However, this was a small group in our cohort, and only 3 out of 30 patients with multiple diseases who lacked biomarkers data experienced DFS events, indicating that a large cohort with more patients are needed to validate this recommendation.
The present study enrolled both multifocal and multicentric diseases, which were heterogeneous and could be further differentiated into MFBC and MFBC. Patients with MCBC were different to those with MFBC in terms of number of tumor foci, tumor size, and molecular subtype (Supplementary Table S1). However, the biomarker and subtype heterogeneity rates were comparable between the two subgroups (Supplementary Table S1–S5), while for DFS and OS, no significant differences were observed between the two groups (Supplementary Figure S3). To date, relatively few reports have directly compared the clinical–pathological features, treatment patterns, and survivals of patients with MFBC and MCBC, which warrant further research (34–36).
The incidence of MMBC increases with the advancement of preoperative imaging, and intertumoral molecular heterogeneity has attracted the attention of clinicians. For example, MRI can identify 74.6%, 54.2%, and 67.3% of MMBC that were not identified by physical examination, sonography, or mammography, respectively (Supplementary Figure S1). We performed the study for the first time to evaluate the rates of biomarkers heterogeneity in MMBC and its impacts on adjuvant therapy and survival. However, there were several limitations in the present study. First, this was a single-institutional retrospective study, so there might be selection bias and limited applicability. Further validation in other cohorts will provide us more insights to the therapeutic and prognostic role of biomarkers heterogeneity. Second, the number of DFS and OS events was very small, and survival did not differ significantly between the groups. Therefore, the evaluation of outcomes was exploratory and hypothesis generating and warranted larger study. Third, the present study enrolled patients over a 9-year period from January 2009 through December 2018, which might exert an influence to disease outcomes.
In conclusion, heterogeneity of ER, PR, HER2, or Ki67 was present in 24.0% patients with MMBC. Biomarkers heterogeneity was associated with more adjuvant endocrine therapy usage and worse disease outcomes, indicating the necessity of molecular assessments for different tumor foci in patients with MMBC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
XC conceived and designed the study. SL analyzed and interpreted the data for presentation and was a main contributor in writing the manuscript. XC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number: 81772797); Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007); and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine-”Guangci Excellent Youth Training Program” (GCQN-2017-A18). All these financial sponsors had no role in the study design, data collection, analysis, or interpretation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.833093/full#supplementary-material
References
- 1. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical Management of Breast Cancer Heterogeneity. Nat Rev Clin Oncol (2015) 12:381–94. doi: 10.1007/s10555-017-9684-y [DOI] [PubMed] [Google Scholar]
- 2. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for Subtypes-Dealing With the Diversity of Breast Cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol (2011) 22:1736–47. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, et al. A Meta-Analysis of Oestrogen Receptor, Progesterone Receptor and Human Epidermal Growth Factor Receptor 2 Discordance Between Primary Breast Cancer and Metastases. Eur J Cancer (2014) 50:277–89. doi: 10.1016/j.ejca.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 4. Yates LR. Intratumoral Heterogeneity and Subclonal Diversification of Early Breast Cancer. Breast (2017) 34 (Suppl 1):S36–42. doi: 10.1016/j.breast.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 5. Yeo SK, Guan JL. Breast Cancer: Multiple Subtypes Within a Tumor? Trends Cancer (2017) 3:753–60. doi: 10.1016/j.trecan.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falck AK, Bendahl PO, Chebil G, Olsson H, Ferno M, Ryden L. Biomarker Expression and St Gallen Molecular Subtype Classification in Primary Tumours, Synchronous Lymph Node Metastases and Asynchronous Relapses in Primary Breast Cancer Patients With 10 Years' Follow-Up. Breast Cancer Res Treat (2013) 140:93–104. doi: 10.1007/s10549-013-2617-8 [DOI] [PubMed] [Google Scholar]
- 7. Corso G, Magnoni F, Provenzano E, Girardi A, Iorfida M, De Scalzi AM, et al. Multicentric Breast Cancer With Heterogeneous Histopathology: A Multidisciplinary Review. Future Oncol (2020) 16:395–412. doi: 10.2217/fon-2019-0540 [DOI] [PubMed] [Google Scholar]
- 8. Ng CK, Martelotto LG, Gauthier A, Wen HC, Piscuoglio S, Lim RS, et al. Intra-Tumor Genetic Heterogeneity and Alternative Driver Genetic Alterations in Breast Cancers With Heterogeneous HER2 Gene Amplification. Genome Biol (2015) 16:107. doi: 10.1186/s13059-015-0657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller KE, Marotti JD, Tafe LJ. Pathologic Features and Clinical Implications of Breast Cancer With HER2 Intratumoral Genetic Heterogeneity. Am J Clin Pathol (2019) 152:7–16. doi: 10.1093/ajcp/aqz010 [DOI] [PubMed] [Google Scholar]
- 10. Burrell RA, McGranahan N, Bartek J, Swanton C. The Causes and Consequences of Genetic Heterogeneity in Cancer Evolution. Nature (2013) 501:338–45. doi: 10.1038/nature12625 [DOI] [PubMed] [Google Scholar]
- 11. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumoral Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med (2012) 366:883–92. doi: 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dagogo-Jack I, Shaw AT. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat Rev Clin Oncol (2018) 15:81–94. doi: 10.1038/nrclinonc.2017.166 [DOI] [PubMed] [Google Scholar]
- 13. Buggi F, Folli S, Curcio A, Casadei-Giunchi D, Rocca A, Pietri E, et al. Multicentric/multifocal Breast Cancer With a Single Histotype: Is the Biological Characterization of All Individual Foci Justified? Ann Oncol (2012) 23:2042–6. doi: 10.1093/annonc/mdr570 [DOI] [PubMed] [Google Scholar]
- 14. Pekar G, Gere M, Tarjan M, Hellberg D, Tot T. Molecular Phenotype of the Foci in Multifocal Invasive Breast Carcinomas: Intertumoral Heterogeneity is Related to Shorter Survival and may Influence the Choice of Therapy. Cancer (2014) 120:26–34. doi: 10.1002/cncr.28375 [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Sun L, Mao Y, Zhu S, Wu J, Huang O, et al. Preoperative Core Needle Biopsy is Accurate in Determining Molecular Subtypes in Invasive Breast Cancer. BMC Cancer (2013) 13:390. doi: 10.1186/1471-2407-13-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol (2010) 28:2784–95. doi: 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J Clin Oncol (2007) 25:118–45. doi: 10.1200/JCO.2006.09.2775 [DOI] [PubMed] [Google Scholar]
- 18. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol (2013) 31:3997–4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 19. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol (2018) 36:2105–22. doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 20. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in Breast Cancer: Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst (2011) 103:1656–64. doi: 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tong Y, Chen X, Fei X, Lin L, Wu J, Huang O, et al. Can Breast Cancer Patients With HER2 Dual-Equivocal Tumours be Managed as HER2-Negative Disease? Eur J Cancer (2018) 89:9–18. doi: 10.1016/j.ejca.2017.10.033 [DOI] [PubMed] [Google Scholar]
- 22. Garimella V, Long ED, O'Kane SL, Drew PJ, Cawkwell L. Oestrogen and Progesterone Receptor Status of Individual Foci in Multifocal Invasive Ductal Breast Cancer. Acta Oncol (2007) 46:204–7. doi: 10.1080/02841860600897884 [DOI] [PubMed] [Google Scholar]
- 23. Choi Y, Kim EJ, Seol H, Lee HE, Jang MJ, Kim SM, et al. The Hormone Receptor, Human Epidermal Growth Factor Receptor 2, and Molecular Subtype Status of Individual Tumor Foci in Multifocal/Multicentric Invasive Ductal Carcinoma of Breast. Hum Pathol (2012) 43:48–55. doi: 10.1016/j.humpath.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 24. Boros M, Ilyes A, Nechifor Boila A, Moldovan C, Eniu A, Stolnicu S. Morphologic and Molecular Subtype Status of Individual Tumor Foci in Multiple Breast Carcinoma. A Study of 155 Cases With Analysis of 463 Tumor Foci. Hum Pathol (2014) 45:409–16. doi: 10.1016/j.humpath.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 25. Singh K, Wang Y, Marketkar S, Kalife ET, Steinhoff MM. Comparison of Estrogen Receptor, Progesterone Receptor and HER2 Results in Concurrent Ipsilateral Samples With Invasive Breast Carcinoma; A Retrospective Study of 246 Biopsies From 119 Patients. Hum Pathol (2017) 65:123–32. doi: 10.1016/j.humpath.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 26. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 27. Yuan S, Norgard RJ, Stanger BZ. Cellular Plasticity in Cancer. Cancer Discovery (2019) 9:837–51. doi: 10.1158/2159-8290.CD-19-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discovery (2022) 12:31–46. doi: 10.1158/2159-8290.CD-21-1059 [DOI] [PubMed] [Google Scholar]
- 29. Venetis K, Invernizzi M, Sajjadi E, Curigliano G, Fusco N. Cellular Immunotherapy in Breast Cancer: The Quest for Consistent Biomarkers. Cancer Treat Rev (2020) 90:102089. doi: 10.1016/j.ctrv.2020.102089 [DOI] [PubMed] [Google Scholar]
- 30. Zemlin C, Stuhlert C, Schleicher JT, Wörmann C, Altmayer L, Lang M, et al. Longitudinal Assessment of Physical Activity, Fitness, Body Composition, Immunological Biomarkers, and Psychological Parameters During the First Year After Diagnosis in Women With Non-Metastatic Breast Cancer: The BEGYN Study Protocol. Front Oncol (2021) 11:762709. doi: 10.3389/fonc.2021.762709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Criscitiello C, Guerini-Rocco E, Viale G, Fumagalli C, Sajjadi E, Venetis K, et al. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anticancer Agents Med Chem (2022) 22:787–800. doi: 10.2174/1871520621666210706144112 [DOI] [PubMed] [Google Scholar]
- 32. Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, et al. Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast. Arch Pathol Lab Med (2009) 133:1515–38. doi: 10.5858/133.10.1515 [DOI] [PubMed] [Google Scholar]
- 33. Navale P, Bleiweiss IJ, Jaffer S, Nayak A. Evaluation of Biomarkers in Multiple Ipsilateral Synchronous Invasive Breast Carcinomas. Arch Patho Lab Med (2019) 143:190–6. doi: 10.5858/arpa.2017-0494-OA [DOI] [PubMed] [Google Scholar]
- 34. Lynch SP, Lei X, Chavez-MacGregor M, Hsu L, Meric-Bernstam F, Buchholz TA, et al. Multifocality and Multicentricity in Breast Cancer and Survival Outcomes. Ann Oncol (2012) 23:3063–9. doi: 10.1093/annonc/mds136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kadioğlu H, Özbaş S, Akcan A, Soyder A, Soylu L, Koçak S, et al. Comparison of the Histopathology and Prognosis of Bilateral Versus Unilateral Multifocal Multicentric Breast Cancers. World J Surg Oncol (2014) 12(1):266–74. doi: 10.1186/1477-7819-12-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ataseven B, Lederer B, Blohmer JU, Denkert C, Gerber B, Heil J, et al. Impact of Multifocal or Multicentric Disease on Surgery and Locoregional, Distant and Overall Survival of 6,134 Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Ann Surg Oncol (2015) 22:1118–27. doi: 10.1245/s10434-014-4122-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.