Abstract
Background
Reproductive‐age women occasionally face the pathological condition of adenomyosis, which is often concurrent with endometriosis. It is believed that endometriosis and adenomyosis increases the risk of obstetric complications. Although new insights into the mechanism of obstetric complications due to endometriosis are emerging, there is little information on the etiology of adverse pregnancy outcomes in pregnant women with adenomyosis.
Methods
We performed a literature review focusing on the pathophysiological pathways of obstetric complications in women with adenomyosis using currently available basic and clinical studies. We used the internet search engines PubMed and Google Scholar to search for studies published between January 2000 and June 2021. We carefully read pertinent sections within each document to ensure relevancy.
Main findings
The prevalence of adverse pregnancy outcomes in women with adenomyosis is increased after adjusting for conceiving by assisted reproductive technology. Thus, adenomyosis emerges as a relevant factor associated with several obstetric complications such as preterm birth, preterm premature rupture of membranes, placental abruption, small for gestational age/fetal growth restriction, and preeclampsia.
Conclusion
It is plausible that the impact of adenomyosis on pregnancy outcomes is not always the same; rather it is dependent on the degree of uterine involvement and subtypes.
Keywords: adenomyosis, endometriosis, labor complication, placental abruption, preterm birth
1. INTRODUCTION
Adenomyosis is a gynecological disorder characterized by a pathologic condition in which endometrium or endometrial‐like structures are present and function in the myometrium. Adenomyosis is symptomatic in two‐thirds of cases; the most frequent symptoms are menorrhagia, dysmenorrhea, and metrorrhagia. It is generally estimated that adenomyosis is present in 20–35% of women. 1 Women with adenomyosis are aware of various degrees of clinical symptoms and exhibit diverse diagnostic imaging findings.
Adenomyosis often coexists with other benign pelvic diseases, uterine leiomyomas, and endometriosis. According to a study that diagnosed thickening of the junctional zone (JZ) on MRI as adenomyosis, the coexistence of endometriosis with adenomyosis was 80.6% in women with dysmenorrhea. 2 Endometriosis is characterized by the presence of endometrial‐like tissue outside the uterus, and the main clinical manifestations are chronic pelvic pain and infertility. Generally, adenomyosis and endometriosis share various clinical symptoms and biological and molecular features, but recent structural and molecular research has shown that they are a single disease representing different clinical characteristics. 3 Many studies conducted in recent decades show the association of endometriosis with adverse pregnancy outcomes for the mother and fetus, such as preterm birth, small for gestational age (SGA) infants, fetal growth restriction (FGR), preeclampsia, and placental abnormalities. The mechanisms of obstetric complications in women exhibiting endometriosis histologically after laparoscopic surgery have already been proposed. Published studies have demonstrated that surgical excision of endometriosis before conception does not reduce the risk of adverse pregnancy outcomes. 4 Recent studies suggested that adenomyosis has a negative impact on female infertility. 5 A systematic review from Denmark demonstrated that adenomyosis may impair fertility by altering endometrial function and receptivity and cause miscarriage. 6 The high risk of obstetric complications in women with adenomyosis has been elucidated based on limited available evidence intended for pregnant women conceiving using assisted reproductive technology (ART) therapy, which is one of the factors increasing poor pregnancy outcomes. The reasons why obstetric complications caused by adenomyosis have not been clarified in detail are that women with adenomyosis become pregnant very infrequently, and their diagnostic criteria have not been established. Thus, the mechanism of obstetric complications in patients with adenomyosis still remains unclear.
Clarifying the relationship between adenomyosis and adverse pregnancy outcomes is necessary to improve maternal and fetal mortality and morbidity. The present review aimed to demonstrate the clinical evidence of the relationship between adenomyosis and adverse pregnancy outcomes. Furthermore, based on our findings, we presented the pathophysiological pathways of increasing obstetric complications in pregnant women with adenomyosis.
2. CURRENT STUDIES INVESTIGATING OBSTETRIC COMPLICATIONS CAUSED BY ADENOMYOSIS
To generate and identify the most relevant literature for our review, we isolated and used the following terms as a search criterion: "adenomyosis," "obstetric complication," "preterm delivery," "fetal growth restriction," "placental abruption," and "preeclampsia." We used internet search engines such as PubMed and Google Scholar to search for studies published between January 2000 and June 2021. PubMed and Google Scholar generated 2,299 and 21,400 articles, respectively, based on the set criteria. The number of articles generated by PubMed for the keywords "adenomyosis" AND "obstetric complication" was 372. The number of reports generated by Google Scholar for the keywords "adenomyosis" AND "obstetric complication" was 2,280. Table 1 shows the search results for all the combined keywords including "adenomyosis." Pertinent sections within each document were carefully read to ensure relevancy to our topic, after which several articles were excluded and eight relevant reports were selected for more detailed evaluation. Three co‐authors examined the content of the selected articles. After reading the full text of the reports, we included them in our study. In addition, publications available for readers and explaining the physiological mechanisms of obstetric complications in women with adenomyosis were adopted as fit‐for‐purpose papers. Eligible studies were retrieved from an electronic search engine at the Tottori University library, were published in English, and explained the mechanisms of obstetric complications caused by adenomyosis.
TABLE 1.
Number of articles hit by searching for each keyword alone or in combination
| Search engines | Key words | No. of ref. | |
|---|---|---|---|
| PubMed | |||
| Adenomyosis | Obstetric complication | 372 | |
| Adenomyosis | Preterm delivery | 34 | |
| Adenomyosis | Fetal growth restriction | 10 | |
| Adenomyosis | Placental abruption | 5 | |
| Adenomyosis | Preeclampsia | 19 | |
| Google Scholar | |||
| Adenomyosis | Obstetric complication | 2,280 | |
| Adenomyosis | Preterm delivery | 83 | |
| Adenomyosis | Fetal growth restriction | 530 | |
| Adenomyosis | Placental abruption | 502 | |
| Adenomyosis | Preeclampsia | 1,410 |
This paper is not a systematic review and attempts to explain the mechanisms of obstetric complications caused by adenomyosis. Therefore, the validity of individual documents' results using statistical methods has not been considered.
3. PROPOSED DIAGNOSTIC IMAGING CRITERIA FOR ADENOMYOSIS
The direct invasion of endometrial tissue into the myometrium has been accepted as the pathogenetic cause of adenomyosis. Histological examination of adenomyosis specimens showed ectopic endometrial tissue characteristics such as endometrial stroma and glands within the myometrium. However, the gold standard is histological examination after hysterectomy that verifies the diagnostic criterion of adenomyosis. The methodology for diagnosing adenomyosis has changed from histopathological to image‐based techniques due to diagnostic equipment becoming more widely available within gynecological practices. Non‐invasive imaging equipment such as transvaginal sonography and MRI could access adenomyosis correctly. Proposed signs of adenomyosis on both imaging modalities contain “heterogenetic myometrial profiles,” “myometrial cysts,” “poor definition of the endo‐myometrial junction,” and “linear striations radiating out from the endometrium”. 7 Ultrasonography has the advantages of affordability and repeatability, but the disadvantage of poor accuracy. The Morphological Uterus Sonographic Assessment consensus (MUSA) published in 2015 delineates ultrasonographic imaging of adenomyosis. 8 This protocol includes seven detailed items that present the imaging features of adenomyosis and practical techniques to develop untrained sonographers. MRI is purely an objective examination, but not an optional first choice to diagnose adenomyosis. Direct findings indicating the presence of adenomyosis are the low‐intensity area on T2‐weighted images, representing the smooth muscle hyperplasia and the heterotropic endometrial tissue. Furthermore, small high‐signal‐intensity areas consist of small hemorrhages due to ectopic endometrium. Thickening of the junctional zone due to the invasion of endometrial‐like tissue is one of the diagnostic criteria for adenomyosis. 2 The criterion of a difference between junctional zone maximum thickness and junctional zone minimum thickness > 5 mm is one of the reliable aspects among the three features correlating with thickening JZ and adenomyosis. 9 However, there are no internationally approved MRI‐based geographic criteria for adenomyosis.
Based on MRI findings, Kishi et al. categorized adenomyosis into four subtypes. 10 Intrinsic type adenomyosis develops in the inner uterine layer without affecting the outer structures and originates from the invagination of the basalis of the endometrial tissue into the myometrium. Extrinsic type adenomyosis arises in the outer myometrium of the uterus, disrupting the serosa but not affecting the inner components. An appropriate suggestion about the leading cause of extrinsic type is that pelvic endometriosis first creates utero‐rectal adhesion and then invades anteriorly into the uterus. In this type of adenomyosis, concomitant endometriosis and obliteration of the cul‐de‐sac have been observed in almost all cases. 10 The presence of intramural type adenomyosis in the myometrium keeps the JZ and the serosa intact. Subtype IV adenomyosis, recognized as a diffuse type, has a distinct morphological presentation from the other three subtypes. Women with adenomyosis are aware of various degrees of clinical symptoms and exhibit diverse diagnostic imaging findings. However, the unresolved globally recognized and fixed criteria for diagnostic imaging characteristics of adenomyosis may cause different outcomes in studies of pregnancy outcomes in women with adenomyosis.
4. CLINICAL EVIDENCE OF CORRELATION BETWEEN ADENOMYOSIS AND OBSTETRIC COMPLICATIONS
The increasing awareness of obstetric complications in adenomyosis is an important opportunity to detect adenomyosis before conception because the diagnostic approach changed from pathological examination to imaging modalities.
4.1. Preterm delivery, preterm premature rupture of membranes
Table 2 shows the clinical evidence of the correlation between adenomyosis and obstetric complications, reported in the past two decades. Eight studies presented data on this outcome. The inclusion diagnostic criteria for adenomyosis were apparent uterine enlargement and having already known features on MRI or ultrasound examination before or after conception. However, two prospective studies used the responses to a self‐reported questionnaire by pregnant women for the presence or history of adenomyosis. A case–control study was the first to report that adenomyosis was associated with a significantly increased risk of spontaneous preterm delivery and preterm premature rupture of membranes (PROM) (odds ratio [OR] 1.84 and 1.98, 95% confidence interval [CI] 1.32–4.31 and 1.39‐3.15, respectively). 11 A Japanese retrospective case–control study clarified that the adenomyosis group was more likely to have preterm delivery than the control group matched to adenomyosis cases. 12 Furthermore, the same study by Hashimoto et al. demonstrated an increased risk of preterm birth attributed to adenomyosis by matched analysis adjusted for age and ART therapy (OR: 3.1, 95% CI: 1.2–7.2). Mochimaru et al. found higher rates of extreme preterm delivery (OR 4.3, 95% CI 1.0–18.4) and preterm PROM (OR 5.5, 95% CI 1.7–17.7) compared with the control group. 13 Multiple logistic regression analyses showed that the risk of preterm births in women with adenomyosis was higher at less than 37 weeks (OR 2.49, 95% CI 1.81–3.41) and less than 34 weeks of pregnancy (OR 1.91, 95% CI 1.02–3.55). 14 Shinohara et al. classified uterine adenomyosis into focal and diffuse types and examined the risk of preterm birth. The risk of preterm birth (OR 5.24, 95% CI 2.15–12.8) and preterm PROM (OR 5.56, 95% CI, 1.42–21.7) in diffuse‐type adenomyosis was significantly increased compared with the control group. 15 Regarding obstetric complications, the risk of preterm birth in the focal‐type group was not found to be higher than that in the control group.
TABLE 2.
Studies on the frequency of obstetric complications in patients with adenomyosis
| Study | Year | Type of study and cases | Outcome |
|---|---|---|---|
| Juang | 2007 | Retrospective case–control | Preterm delivery (OR 1.84, 95% CI 1.32–4.31) |
| Mochimaru | 2015 | Retrospective case–control |
Preterm delivery (OR 5.0, 95% CI 2.2–11.4) SGA (OR 4.3, 95% CI 1.8–10.3) |
| Hashimoto | 2018 | Retrospective case–control |
Preterm delivery (OR 3.1, 95% CI 1.2–7.2) Placental malposition (OR 4.9, 95% CI 1.4–16.3) SGA (OR 3.5, 95% CI 1.2–9.0) Preeclampsia (OR 21.0, 95% CI 4.8–124.5) |
| Shin | 2018 | Retrospective case–control | Preterm delivery (OR 3.36, 95% CI 1.66–6.82) |
| Scala | 2018 | Retrospective study | SGA (OR 3.74, 95% CI 1.16–12.1) |
| Yamaguchi | 2019 | Prospective cohort |
Preterm delivery (OR 2.49, 95% CI 1.81–3.41) SGA (OR 1.68, 95% CI 1.13–2.51) |
| Harada | 2019 | Prospective cohort |
Preterm delivery (OR 2.95, 95% CI 2.14–4.09) Placental abruption (OR 3.29, 95% CI 1.22–8.89) FGR (OR 2.88, 95% CI 1.70–4.86) Preeclampsia (OR 1.86, 95% CI 1.11–3.14) |
| Shinohara | 2020 | Retrospective case–control |
Preterm delivery (OR 2.60, 95% CI 1.23–5.50) HDP (OR 2.68, 95% CI 1.06–6.80) |
Abbreviations: CI, confidence interval; FGR, fetal growth restriction; HDP, hypertensive disorders of pregnancy; HR, hazard ratio; OR, odds ratio; SGA, small for gestational age.
In vitro fertilization is a likely risk factor for preterm birth. 16 However, only two studies have stratified the effects of infertility treatment and analyzed the impact of adenomyosis on preterm delivery. Shin et al. showed that patients with adenomyosis had higher preterm birth rates than those without adenomyosis (OR 3.36, 95% CI, 1.66–6.82). 17 The authors stated that the prevalence of preterm birth was significantly higher in pregnant women with adenomyosis after ART therapy than in those with natural conception (OR, 8.75; 95% CI, 1.66–46.19). In addition, the risk of preterm birth before 32 weeks was much higher in the adenomyosis group than in the non‐adenomyosis group (OR 24.53, 95% CI, 9.12–66.02). We have earlier shown that women with a history of adenomyosis had an increased risk of adverse pregnancy outcomes than those without adenomyosis (OR 1.72, 95% CI 1.37–2.16). 18 The odds ratio of preterm birth, preterm PROM, and extremely preterm birth significantly increased in women with adenomyosis (OR 2.95, OR 3.74, and OR 3.63, respectively). We used logistic regression analysis to adjust for the influence of adenomyosis from the effects of ART therapy. Women experiencing adenomyosis, who conceived naturally or after infertility treatment (except for ART therapy), had higher frequencies of preterm birth (OR, 2.57 95% CI 1.77–3.75), preterm PROM (OR 2.80, 95% CI 1.43–5.46), and extremely preterm birth (OR 4.76, 95% CI 1.75–12.91).
4.2. Small for gestational age infant/Fetal growth restriction
Two retrospective case–control studies and one Japanese prospective cohort study demonstrated that the birth of small for gestational age (SGA) infants or fetal growth restriction (FGR) was significantly more likely to occur in the adenomyosis group than in the control group: OR = 4.3 (95% CI 1.8–10.3), 3.5 (95% CI 1.2–9.0), 1.68 (95% CI 1.13–2.51), respectively. 12 , 13 , 14 Scala et al. reported a higher odds ratio of SGA infants in pregnant women with endometriosis complicated by diffuse adenomyosis. 19 The prevalence of SGA was significantly different between women with endometriosis and those with diffuse adenomyosis. In addition, logistic regression analysis demonstrated that the presence of diffuse adenomyosis was the only contributing factor associated independently with the birth rate of an SGA infant in pregnant women with endometriosis (OR 3.74, 95% CI 1.16–12.1). We also found that the risk of FGR in women with adenomyosis conceived by spontaneous or infertility treatment without ART therapy was higher than that in women without adenomyosis (OR 2.88, 95% CI 1.70–4.86). 18
4.3. Placenta‐related abnormalities
Only two studies presented data on placental abnormalities, showing a significantly higher risk in the adenomyosis group. The prevalence of placenta previa and low‐lying placenta was significantly higher in the adenomyosis group than in the control group (OR, 4.9; 95% CI: 1.4–16.3). 12 Of note, ART was used in more than 45% of cases. A Japanese prospective cohort study on women who conceived using ART therapy revealed that the risk of placenta previa in women with a history of adenomyosis was higher than that in women without adenomyosis (OR 4.31, 95% CI 1.05–17.77). 18 On the contrary, the risk of placenta previa in women who experienced adenomyosis was not higher than in women who did not receive ART therapy. Women who successfully undergo in vitro fertilization have a higher risk of placenta previa than women who conceive naturally. Pregnant women with a history of adenomyosis who conceived naturally or after infertility treatment without ART therapy had higher frequencies of placental abruption (OR 3.29, 95% CI 1.22–8.89). 18
4.4. Hypertensive disorders of pregnancy, preeclampsia
In women of reproductive age, the prevalence of concurrent high blood pressure and adenomyosis increases with advanced age. 20 The definitions for hypertensive disorders of pregnancy (HDP) include preexisting hypertension before pregnancy. To assess the contributing factors in the development of preeclampsia by adenomyosis, two studies excluded chronic hypertension from HDP. They found that the prevalence of preeclampsia in the adenomyosis group was much higher than that in the control group (OR: 21.0, 95% CI: 4.8–124.5). 12 Hashimoto et al. also found that the higher risk of preeclampsia in primiparous women with adenomyosis was independent of maternal age (OR, 16.2; 95% CI 3.6–97.8). Pregnant women with adenomyosis, but not endometriosis, had a higher risk of mild preeclampsia than those without adenomyosis (OR 1.86, 95% CI 1.11–3.14). 18 Mild preeclampsia refers to a pregnant woman characterized by new onset of hypertension and proteinuria, which does not conform to severe features. HDP accompanying preexisting adenomyosis developed in 8 of 61 (13.1%) subjects, and the prevalence of HDP in the control group was 5.3% (OR 2.68, 95% CI 1.06–6.80). 15 In addition, diffuse‐type adenomyosis was more likely to concur with HDP (OR 3.54, 95% CI 1.23–10.2) and severe HDP (OR 7.45, 95% CI 1.70–32.6). However, the risk of developing HDP was not significantly different between the focal‐type and control groups.
5. PROPOSED MECHANISMS OF OBSTETRIC COMPLICATION IN PATIENTS WITH ADENOMYOSIS
Endometriosis is observed in the pelvis other than the uterus and also in the extra genitalia organs, while adenomyosis is defined as endometrial‐like foci that exist within the uterine myometrium. Therefore, the adverse effect of adenomyosis on pregnancy outcomes has been considered more intense than that of endometriosis. The exact mechanisms of obstetric complications caused by adenomyosis remain unknown, because information from biopsy of the uterus collected immediately pre‐ and post‐delivery was not available. Few studies have accounted for the pathophysiology of adenomyosis‐complicated pregnancies; however, many reports have only investigated the effects of endometriosis on pregnancy outcomes.
The etiology of infertility and miscarriages in reproductive‐age women with adenomyosis undergoing embryo transfer could potentially explain how adenomyosis alters the course of pregnancy. Although it is uncertain whether the potential proposed mechanisms that impede embryo implantation and placenta formation in infertile patients with adenomyosis give rise to obstetric complications beyond mid‐pregnancy, we presume the process of adverse pregnancy outcomes attributed to adenomyosis using aforementioned mechanisms.
The eutopic endometrium in women with adenomyosis has aberrant immunological changes contributing to implantation failure. Bourdon et al. noted in their systematic review that numerous immunological cells and soluble factors are present in eutopic and ectopic endometrial‐like tissues. 21 Macrophages, uterine natural killer (uNK) cells, HLA molecules, and T lymphocytes are potentially involved in immune‐inflammatory pathways. A potential mechanism of implantation failure resulting in obstetric complications can be the immunological changes that collaborate with steroid‐hormonal aberrations, known as progesterone resistance.
5.1. Preterm delivery, preterm premature rupture of membrane
Spontaneous preterm birth is a multifactorial condition and likely results from specific interactions between the environment and genetic factors. 22 , 23 Thus, a specific cause is difficult to discern in most cases of preterm births. Inflammation can be proposed as one of the reasons for the association between adenomyosis and preterm birth. A study by Tamura et al. revealed that the rate of uterine infection in patients with diffuse‐type adenomyosis was higher than that in patients with focal‐type adenomyosis. 24 However, we often encounter many clinical cases of preterm births without apparent pathogen infection. Sterile inflammation can induce a common inflammatory pathway leading to labor and contribute to the onset of parturition and preterm birth. 25 The pathogenic processes from microbial or sterile inflammations can induce a common pathway, so‐called progesterone resistance, resulting in preterm birth. Pathogenic organisms bind to toll‐like receptors in decidual and induce the transcription factor NF‐kappaB, which triggers an inflammatory response and activates various pro‐inflammatory mediators (e.g., interleukin [IL] 1, 6, and 8; tumor necrosis factor [TNF]). 26 The IL‐1beta binds to its receptor to activate the ERK1/2 MAP kinase signaling cascade and inhibits decidual cell progesterone receptor expression. 27 Like microbial inflammations, thrombin inhibits progesterone receptor protein and mRNA expression. In addition, the critical mediators of this response are IL‐1beta and TNF, which enhance prostaglandin production by inducing COX‐2 expression in the amnion and decidua. 26 A synopsis of mechanisms of preterm labor caused by abnormal inflammatory response mimics immune changes seen in epithelial to mesenchymal transition in the uterus affected with adenomyosis (Figure 1). 21 , 28
FIGURE 1.
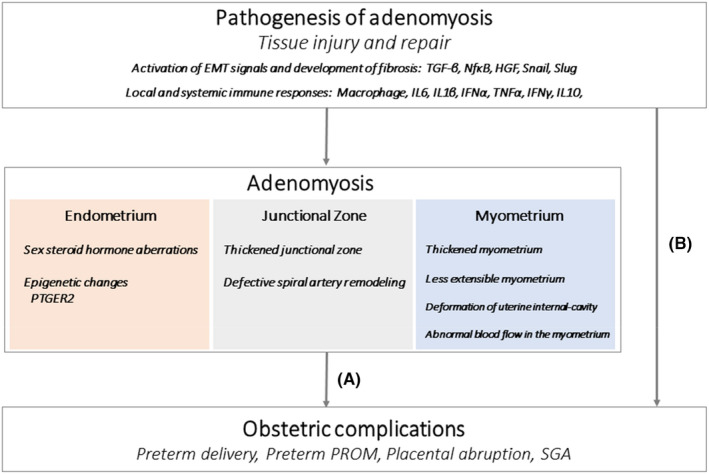
Proposed mechanisms of obstetric complication in patient with adenomyosis (A) alterations in the uterus affected with adenomyosis lead to obstetric complications; (B) obstetric complications arise in the pathogenesis of adenomyosis
Other studies have reported increased intrauterine pressure in patients with adenomyosis, leading to preterm birth. Increased contractility of the myometrium and intrauterine pressure often precede preterm birth. Regarding the high risk of preterm PROM in patients with adenomyosis, Juang et al. believed that increased prostaglandin expression may play a role in the irritable uterine contraction and fragile fetal membranes in patients with adenomyosis associated with preterm PROM. 11 A study of myometrium thickness by ultrasonography showed that the uterine wall was thicker and less stretched during the second trimester of pregnancy in the preterm delivery group than in the term delivery group. 29 More frequent premature births were noted in women who had little change in the thickness of the uterine myometrium from the first to the second trimester. These results suggest a positive correlation between the rate of preterm birth and uterine wall thickness and stiffness. A recent study reported that women with deep infiltrating endometriosis have pathological conditions of endometriotic‐like lesions in the decidua in close contact with fetal membranes at term pregnancy. 30 The authors observed that the fetal membranes in puerperium women with deep infiltrating endometriosis were thicker than that of controls and microscopically noticed that the trophoblastic layer was altered by extracellular material and inflammatory infiltrates. These data support the hypothesis that maternal deep infiltrating endometriosis persists during pregnancy and affects the decidual side of the chorion, possibly involved in preterm PROM.
Studies to date have demonstrated that surgical excision of endometriosis before conception did not decrease the prevalence of adverse pregnancy outcomes in women with a history of endometriosis. 4 , 31 These findings suggest that women with or without treatment for endometriosis are more likely to have irreversible epigenetic changes in the eutopic endometrium. These are endometrial abnormalities that subsequently lead to preterm birth. In addition, because epigenetic changes are reversible and sensitive to environmental changes, alterations in endometrial gene methylation and gene expression affect adverse pregnancy outcomes by altering endometrial function. One study hypothesized that the alterations of an imprinted gene in endometriosis, the hypomethylation of prostaglandin E receptor 2 (PTGER2), could be associated with preterm birth. 32
5.2. Small for gestational age infant/Fetal growth restriction
Numerous causes have been suggested for FGR attributed to adenomyosis, among which placental dysfunction is predominant. Research for identifying the distance between uterine adenomyosis and the placenta‐attachment position by MRI indicates that placental localization close to adenomyosis increases the risk of FGR. 33 The risk of FGR in pregnant women whose placenta overlapped with adenomyosis was more frequent than that in the placenta detached with adenomyosis. In particular, the pregnant women with more than 1/2 placental area covering adenomyosis gave birth to offspring of smaller birth weight. A Japanese radiologist conducted a clinical study to assess the blood flow in the gravid uterus and fibroids using a non‐contrast magnetic resonance angiography technique. The blood flow within the adenomyosis lesions was increased, while the placenta had declined blood flow in women with adenomyosis and severe FGR. 34 The larger the placental contact with adenomyosis, the greater the risk of reduced blood circulation within the intervillous space. One hypothesis is that the insufficient feature of placental perfusion results in a small placenta, leading to fetal growth restrictions.
5.3. Placenta‐related abnormalities
A recent cohort study comparing pregnant women with deep infiltrating endometriosis associated with disruption of the uterine serosa to a control group found that the rate of placental abruption was significantly higher among those with rectovaginal deep endometriosis. 35 Uterine structural deformation due to leiomyoma, as seen in adenomyosis, was proposed to be a modest risk factor for abruption. 36 The submucosal location of uterine fibroids and the large volume of leiomyoma increased the risk of placental abruption. These mechanically and biologically unstable sites alter placental implantation after inadequate decidualization or increase the shear stress of placenta attachment. In such cases, abnormalities in the early maldevelopment of the spiral arteries lead to vascular disruption and bleeding, resulting in decidual necrosis and placental inflammation. Thrombin formation after bleeding plays a critical role in the pathogenesis of placental abruption. Chronic retroplacental hemorrhage can lead to the rapid development of potentially life‐threatening clinical manifestations of abruption. In addition, thrombin is a potent, direct uterotonic agent that can lead to uterine hypertonus and contractions. The increased incidence of placental abruption in women with preterm PROM was reported in a retrospective study. 37 We also demonstrated that the risk of preterm PROM in women with adenomyosis was much higher than that in women with endometriosis. 18 Thus, one hypothesis is that, in women with adenomyosis, the high incidence of preterm PROM may be a causative factor for placental abruption.
5.4. Hypertensive disorders of pregnancy, preeclampsia
Defective remodeling of spiral arteries in the myometrial segment was first described in patients with preeclampsia, alone or in combination with FGR. 38 A major feature of the pathophysiology of preeclampsia is the failure of fetal trophoblasts to invade the spiral arteries, resulting in reduced placental perfusion and hypoxia. Brosens et al. coined the term “great obstetrical syndromes” to describe obstetrical complications that may contribute to preterm delivery, fetal growth restriction, and preeclampsia caused by abnormal placental remodeling and early incomplete placentation. 39 Brosens accounted for the limited range of spiral artery remodeling in the JZ in pregnancy complicated by severe preeclampsia compared with the physiological remodeling in normal pregnancy. 40 Histopathologic studies have revealed that the rate of non‐transformed spiral arteries in the uterine myometrium is more frequent in women with preeclampsia than in women with preterm labor. 41 Obstetric complications in women with adenomyosis may vary depending on the degree of remodeling failure of the spiral artery in the uterine junctional zone. Increased sterile inflammatory status during pregnancy is known to facilitate the development of preeclampsia. 42 Sterile inflammation potentially develops as a consequence of chronic hemorrhage caused by abnormal placental malformation in early gestation and chronic inflammation in the myometrium, concurrent with adenomyosis. The uNK cells are a crucial component of immune response and play an essential role during implantation, which precedes accumulation around spiral arteries before trophoblast invasion. The dysfunction of uNK cells plays a crucial role in the impaired implantation, an event described in women with adenomyosis. 21 Therefore, it is plausible that sterile inflammation caused by adenomyosis might interfere with deep placentation, contributing to the onset of preeclampsia.
6. THE DIFFERENT SUBTYPES OF ADENOMYOSIS EXERT A POSSSIBLE INFLUENCE ON PREGNANCY OUTCOMES
We present a feasible explanation for the risk of obstetric complications in women with adenomyosis that may vary depending on the conditions of the adenomyosis, such as location subtype. Adenomyosis has been conventionally categorized into focal and diffuse types according to the expansion of mimic endometrium‐like tissue within the uterine muscle. A Japanese multicenter retrospective survey revealed that women diagnosed with diffuse adenomyosis had a higher risk of preeclampsia and uterine infection than women diagnosed with focal type. 24 According to the systematic review that analyzed women with adenomyosis receiving uterus‐sparing surgery, women with diffuse adenomyosis had reduced pregnancy rates compared with women with focal type. 43 Diffuse adenomyosis involves alterations in the entire uterine muscle layer, particularly sub‐endometrial constituents, responsible for adverse obstetric complications. A Japanese group recently proposed categorizing adenomyosis into four subtype groups adjacent to the pathophysiological mechanism of adenomyosis. 10 Iwasawa et al. investigated ART therapy outcomes by classifying the adenomyosis based on the location subtype from imaging of MRI. 44 They reported that women with intrinsic adenomyosis (classical type adenomyosis), demonstrated higher miscarriage rates and lower live birth rates than women with extrinsic adenomyosis (located in extrinsic sites). From the aforementioned, the alterations of the inner uterine layer are integral components of normal decidualization and placentation and contribute to adverse obstetric complications. In particular, the intactness of the JZ or endometrial‐myometrial border layer may significantly impact the establishment of pregnancy and have negative consequences for pregnancy outcomes. Further prospective studies, which explore the divergent obstetric complications according to the subtype of adenomyosis, are needed to expand on current findings.
7. CONCLUSIONS
Adenomyosis is a heterogenic disorder with varying extent of lesions, ranging from multiple lesions with diffuse myometrial hypertrophy to more discrete focal lesions. It has emerged as one of the relevant factors associated with several late‐term pregnancy complications based on literature analysis. Therefore, it is plausible that the impact of adenomyosis on pregnancy outcomes is not always the same; rather it is dependent on the degree of uterine involvement and subtypes. The increased risk of obstetric complications caused by thickening of the myometrium, aberrant uterine distensibility, and deformation of the uterine cavity attributed to adenomyosis is different from the risk of obstetric complications in women with endometriosis. The pathophysiological explanation of adverse pregnancy outcomes in women with adenomyosis includes many mechanisms, and complex biochemical pathways remain unclear.
In order to prevent impaired inadequate placentation, trophoblast invasion, and uteroplacental perfusion with subsequent higher risk for preterm birth, placental abruption, FGR, and preeclampsia, there are no treatments before and after conception in women with adenomyosis. Ultra‐long gonadotropin‐releasing hormone (GnRH) analog protocols for women with adenomyosis conceived with ART therapy may increase the pregnancy rate. 45 However, there is no evidence that ART therapy after GnRH analog treatment reduces obstetric complications. In addition, obstetricians do not recommend adenomyomectomy before conception to reduce the risk of obstetric complications due to the high risk of uterine rupture. Further research in the field should aim at developing infertility treatments for women with adenomyosis that can reduce the risk of obstetric complications.
Further molecular research is needed to clarify the pathophysiological pathways responsible for modifying the impact of adenomyosis on pregnancy outcomes. Thus, future studies targeting pregnant women categorized according to the different subtypes of adenomyosis are needed to confirm the preceding theories.
CONFLICT OF INTEREST
Takashi Harada, Fuminori Taniguchi, and Tasuku Harada declare that they have no conflict of interest.
DISCLOSURES
Human/Animal rights: No human and animal experiments were performed by any of the authors for the purpose of this article.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Harada T, Taniguchi F, Harada T. Increased risk of obstetric complications in patients with adenomyosis: A narrative literature review. Reprod Med Biol. 2022;21:e12473. doi: 10.1002/rmb2.12473
References
- 1. Vercellini P, Viganò P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465‐477. [DOI] [PubMed] [Google Scholar]
- 2. Leyendecker G, Bilgicyildirim A, Inacker M, et al. Adenomyosis and endometriosis. Re‐visiting their association and further insights into the mechanisms of auto‐traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291:917‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H. Relationship between adenomyosis and endometriosis; Different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol. 2020;253:191‐197. [DOI] [PubMed] [Google Scholar]
- 4. Saraswat L, Ayansina DT, Cooper KG, et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG. 2017;124:444‐452. [DOI] [PubMed] [Google Scholar]
- 5. Harada T, Khine YM, Kaponis A, Nikellis T, Decavalas G, Taniguchi F. The Impact of Adenomyosis on Women's Fertility. Obstet Gynecol Surv. 2016;71:557‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dueholm M. Uterine adenomyosis and infertility, review of reproductive outcome after in vitro fertilization and surgery. Acta Obstet Gynecol Scand. 2017;96:715‐726. [DOI] [PubMed] [Google Scholar]
- 7. Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147‐S160. [DOI] [PubMed] [Google Scholar]
- 8. Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284‐298. [DOI] [PubMed] [Google Scholar]
- 9. Exacoustos C, Manganaro L, Zupi E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2014;28:655‐681. [DOI] [PubMed] [Google Scholar]
- 10. Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, Taniguchi F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207(114):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 11. Juang CM, Chou P, Yen MS, Twu NF, Horng HC, Hsu WL. Adenomyosis and risk of preterm delivery. BJOG. 2007;114:165‐169. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto A, Iriyama T, Sayama S, et al. Adenomyosis and adverse perinatal outcomes: increased risk of second trimester miscarriage, preeclampsia, and placental malposition. J Matern Fetal Neonatal Med. 2018;31:364‐369. [DOI] [PubMed] [Google Scholar]
- 13. Mochimaru A, Aoki S, Oba MS, Kurasawa K, Takahashi T, Hirahara F. Adverse pregnancy outcomes associated with adenomyosis with uterine enlargement. J Obstet Gynaecol Res. 2015;41:529‐533. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi A, Kyozuka H, Fujimori K, et al. Risk of preterm birth, low birthweight and small‐for‐gestational‐age infants in pregnancies with adenomyosis: A cohort study of the Japan Environment and Children's Study. Acta Obstet Gynecol Scand. 2019;98:359‐364. [DOI] [PubMed] [Google Scholar]
- 15. Shinohara S, Okuda Y, Hirata S, Suzuki K. Adenomyosis as a potential risk factor for adverse pregnancy outcomes: a multicenter case‐control study. Tohoku J Exp Med. 2020;251:231‐239. [DOI] [PubMed] [Google Scholar]
- 16. Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy‐related complications and adverse pregnancy outcomes in singleton pregnancies: a meta‐analysis of cohort studies. Fertil Steril. 2016;105:73‐85. [DOI] [PubMed] [Google Scholar]
- 17. Shin YJ, Kwak DW, Chung JH, Kim MY, Lee SW, Han YJ. The risk of preterm births among pregnant women with adenomyosis. J Ultrasound Med. 2018;37:1937‐1943. [DOI] [PubMed] [Google Scholar]
- 18. Harada T, Taniguchi F, Amano H, et al. Adverse obstetrical outcomes for women with endometriosis and adenomyosis: A large cohort of the Japan Environment and Children's Study. PLoS One. 2019;14:e0220256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scala C, Leone Roberti Maggiore U, Racca A, et al. Influence of adenomyosis on pregnancy and perinatal outcomes in women with endometriosis. Ultrasound Obstet Gynecol. 2018;52:666‐671. [DOI] [PubMed] [Google Scholar]
- 20. Schimmel MS, Bromiker R, Hammerman C, et al. The effects of maternal age and parity on maternal and neonatal outcome. Arch Gynecol Obstet. 2015;291:793‐798. [DOI] [PubMed] [Google Scholar]
- 21. Bourdon M, Santulli P, Jeljeli M, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;27:108‐129. [DOI] [PubMed] [Google Scholar]
- 22. Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(487):e1‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esplin MS, Manuck TA, Varner MW, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol. 2015;213(429):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura H, Kishi H, Kitade M, et al. Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol. 2017;16:330‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Negishi Y, Shima Y, Takeshita T, Morita R. Harmful and beneficial effects of inflammatory response on reproduction: sterile and pathogen‐associated inflammation. Immunol Med. 2021;44:98‐115. [DOI] [PubMed] [Google Scholar]
- 26. Schatz F, Guzeloglu‐Kayisli O, Arlier S, Kayisli UA, Lockwood CJ. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update. 2016;22:497‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guzeloglu‐Kayisli O, Kayisli UA, Semerci N, et al. Mechanisms of chorioamnionitis‐associated preterm birth: interleukin‐1β inhibits progesterone receptor expression in decidual cells. J Pathol. 2015;237:423‐434. [DOI] [PubMed] [Google Scholar]
- 28. Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592‐601. [DOI] [PubMed] [Google Scholar]
- 29. Kim YM, Kim SH, Kim JH, et al. Uterine wall thickness at the second trimester can predict subsequent preterm delivery in pregnancies with adenomyosis. Taiwan J Obstet Gynecol. 2019;58:598‐603. [DOI] [PubMed] [Google Scholar]
- 30. Marcellin L, Méhats C, Gogusev J. Histopathological alterations in fetal membranes of women with endometriosis. Reprod Sci. 2018;25:782‐787. [DOI] [PubMed] [Google Scholar]
- 31. Lalani S, Choudhry AJ, Firth B, et al. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta‐analysis. Hum Reprod. 2018;33:1854‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi H, Kawahara N, Ogawa K, Yoshimoto C. Shared molecular features linking endometriosis and obstetric complications. Reprod Sci. 2020;27:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 33. Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. [DOI] [PubMed] [Google Scholar]
- 34. Yorifuji T, Makino S, Yamamoto Y, Sugimura M, Kuwatsuru R, Takeda S. Time spatial labeling inversion pulse magnetic resonance angiography in pregnancy with adenomyosis. J Obstet Gynaecol Res. 2013;39:1480‐1483. [DOI] [PubMed] [Google Scholar]
- 35. Exacoustos C, Lauriola I, Lazzeri L, De Felice G, Zupi E. Complications during pregnancy and delivery in women with untreated rectovaginal deep infiltrating endometriosis. Fertil Steril. 2016;106:1129‐1135. [DOI] [PubMed] [Google Scholar]
- 36. Jenabi E, Ebrahimzadeh ZS. The association between uterine leiomyoma and placenta abruption: A meta‐analysis. J Matern Fetal Neonatal Med. 2017;30:2742‐2746. [DOI] [PubMed] [Google Scholar]
- 37. Hnat MD, Mercer BM, Thurnau G, et al. Perinatal outcomes in women with preterm rupture of membranes between 24 and 32 weeks of gestation and a history of vaginal bleeding. Am J Obstet Gynecol. 2005;193:164‐168. [DOI] [PubMed] [Google Scholar]
- 38. Brosens A. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177‐191. [PubMed] [Google Scholar]
- 39. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416‐1423. [DOI] [PubMed] [Google Scholar]
- 41. Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063‐1069. [DOI] [PubMed] [Google Scholar]
- 42. Nadeau‐Vallée M, Obari D, Palacios J, et al. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152:R277‐R292. [DOI] [PubMed] [Google Scholar]
- 43. Tan J, Moriarty S, Taskin O, et al. Reproductive outcomes after fertility‐sparing surgery for focal and diffuse adenomyosis: a systematic review. J Minim Invasive Gynecol. 2018;25:608‐621. [DOI] [PubMed] [Google Scholar]
- 44. Iwasawa T, Takahashi T, Maeda E, et al. Effects of localisation of uterine adenomyosis on outcome of in vitro fertilisation/intracytoplasmic sperm injection fresh and frozen‐thawed embryo transfer cycles: a multicentre retrospective cohort study. Reprod Biol Endocrinol. 2021;19:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol. 2011;51:280‐283. [DOI] [PubMed] [Google Scholar]


