Abstract
Background
Northeast India shares its international border with Southeast Asia and has a number of malaria endemic zones. Monitoring genetic diversity of malaria parasites is important in this area as drug resistance and increasing genetic diversity form a vicious cycle in which one favours the development of the other. This retrospective study was done to evaluate the genetic diversity patterns in Plasmodium falciparum strains circulating in North Lakhimpur area of Assam in the pre-artemisinin era and compare the findings with current diversity patterns.
Methods
Genomic DNA extraction was done from archived blood spot samples collected in 2006 from malaria-positive cases in Lakhimpur district of Assam, Northeast India. Three antigenic markers of genetic diversity were studied – msp-1 (block-2), msp-2 (block-3) and the glurp RII region of P. falciparum using nested PCR.
Results
Allelic diversity was examined in 71 isolates and high polymorphism was observed. In msp-1, eight genotypes were detected; K1 (single allele), MAD20 (six different alleles) and RO33 (single allele) allelic families were noted. Among msp-2 genotypes, 22 distinct alleles were observed out of which FC27 had six alleles and IC/3D7 had 16 alleles. In RII region of glurp, nine genotypes were obtained. Expected heterozygosity (H E) values of the three antigenic markers were 0.72, 0.81 and 0.88, respectively. Multiplicity of infection (MOI) values noted were 1.28, 1.84 and 1.04 for msp-1, msp-2 and glurp, respectively.
Conclusion
Results suggest a high level of genetic diversity in P. falciparum msp (block-2 of msp-1 and block-3 of msp-2) and the glurp RII region in Northeast India in the pre-artemisinin era when chloroqunine was the primary drug used for uncomplicated falciparum malaria. Comparison with current studies have revealed that the genetic diversity in these genes is still high in this region, complicating malaria vaccine research.
Keywords: Plasmodium falciparum, vaccines, drug resistance, Merozoite surface protein, India, glutamate-rich protein
Introduction
Malaria is caused by the genus Plasmodium, five species of which are known to cause primary human infection. In tropical and subtropical regions, malaria has become a major health problem. The majority of mortality occurs in young children below 5 years of age residing in tropical regions. In 2020, the estimated global malaria case burden was 241 million with 627 000 deaths [1]. The WHO African region contributes 95 % of malaria cases whereas Southeast Asia contributes 2 % [1]. In humans, Plasmodium falciparum causes a severe form of malaria with high morbidity and mortality and there are reports of dissemination of drug-resistant strains from northeast India to other parts of the country [2].
A number of vaccines have been tried against the erythrocytic stage of the malaria parasite using different types of surface proteins like merozoite surface protein (MSP), apical membrane antigen-1 (AMA-1) and erythrocytic binding antigen-175 (EBA-175). The most abundant surface antigen in P. falciparum is MSP [3, 4]. PfMSP-1/2/4/5/10 family consists of glycosyl phosphatidyl inositol (GPI) anchored proteins and PfMSP-3/6/7/9 family members are soluble MSP proteins but they belong to other anchored GPI proteins [5]. MSP is amongst the forefront antigens for producing an effective vaccine against the blood stage of malaria parasites; these proteins are exposed to the immune system and they are present in the merozoites. GPI anchored MSPs play a vital role in the entire life cycle of the parasite and cannot be detached or knocked out. MSP is involved in binding of erythrocyte and also in complex formation with other MSP parasite proteins [6]. The most important function of MSP is invasion of mature erythrocytes via the sialic acid (SA) dependent and independent pathways [7]. Msp-1(block-2), msp-2 (block-3) and glurp (RII-region) genes have highly polymorphic variations throughout the world and are expressed throughout the life cycle of P. falciparum [8, 9]. Msp-1 contains 17 blocks of which block-2 has K1, MAD20 and RO33 allelic families [10]. Block-3 of msp-2 contains two alleles viz. FC27 and IC/3D7 [11]. Msp-based vaccines are difficult to achieve because of the presence of several polymorphic genes and high allelic diversity [12]. The most successful pre-erythrocytic stage vaccine RTS,S/AS01 underwent multiple phases of clinical trials and has shown protection rates of only 39 % for malaria and 29 % for severe malaria [13]. Multiple clones of parasite infecting the host might be a major contributing factor for these low efficacy numbers [14]. Currently, the vaccine has been utilized in a pilot programme at Malawi in children aged up to 2 years [15].
In India, chloroquine (CQ)-resistant P. falciparum was first reported from Karbi-Anglong district in Assam in 1973 and in 1979, sulfadoxine/pyrimethamine (SP) resistance in P. falciparum was also reported in the same area [16, 17]. In late 1990s, SP-resistant P. falciparum was reported from Changlang, Arunachal Pradesh district (NMEP 1997). With widespread resistance, the anti-malarial drug policy was changed for Northeast India in 2008, heralding the beginning of artemisinin based combination therapies (ACT) [18]. It is possible that Northeast India may act as a gateway for the entry and dissemination of drug-resistant P. falciparum to the rest of India [19]. Increasing genetic diversity does not bode well for the possible future implementation and effectiveness of malaria vaccine candidates in this region. Selective antibiotic pressure and also changes of drug policy leads to a gradual increase in parasite diversity patterns with resistant genotypes [20]. Drug resistance and increasing genetic diversity form a vicious cycle in which one favours the development of the other. This retrospective study evaluated the genetic diversity patterns in parasite strains circulating in the North Lakhimpur area of Assam in the pre-artemisinin era to observe any differences with current diversity patterns. A nested PCR-based protocol was adopted to study the allelic variations in P. falciparum glurp, msp-1 and msp-2 antigens in isolates from Lakhimpur district, Assam.
Methods
Study site and population
The samples utilized in the present study were archived samples collected in 2006 from Nowboicha Primary Health Centre area, Lakhimpur, Assam [21]. It is situated approximately between longitude 94.1514˚ E and latitude 27.2064˚ N with an area of 2277 Km2 and population of 10,42,137 as per 2011 census data. This district receives high rainfall (annually 3215 mm) with an average temperature of 23.8 °C. The region also contains many tea gardens and dense forests. This region was selected as the study site since it is one of the malaria endemic district of Assam and shares its northern border with Arunachal Pradesh, which is again a malaria endemic state of Northeast India.
Sample collection
Patients having primary malaria like clinical symptoms such as intermediate fever, chill, vomiting and body ache were screened by rapid diagnostic test (RDT) kit (Falcivax/Xephyr biomedical, India) for P. falciparum (HRP2) and P. vivax (LDH) followed by blood smear preparation for microscopy. Finger-prick blood samples from RDT positive, mono-infective, uncomplicated (as per WHO classification) P. falciparum cases were collected in Whatman filter paper (FTA card) after obtaining written consent from the participants. A total of 100 FTA card blood samples were collected, air-dried and preserved at −20 °C. The archived samples from 2006 were maintained in our department and 71 of these isolates were used for the current study. The remaining 29 FTA card blood samples could not be restored; they were spoiled and fragmented due to the storage problem in the freezer. The samples used in the current study were completely anonymised and no identifying features, names or other patient characteristics (age, sex, etc.) have been incorporated in the current study findings.
DNA extraction from dry blood spots
Genomic DNA was isolated from each sample using six dried blood spots, 3 mm in diameter by Harris Uni-Core puncher. Between each sample, the Harris Uni-Core puncher was cleaned properly using 70 % ethanol followed by autoclaved double distilled water. DNA was isolated with the help of commercial kits (QIAamp DNA blood mini kit, Qiagen) and stored at −20 °C for further processing.
PCR-based identification of malaria parasite
Plasmodium species identification was done using nested PCR targeting the 18S small subunit of ribosomal RNA gene (rRNA) specific for Plasmodium species as described earlier [22]. In the primary PCR, 20 µl reaction volume containing 1 µl of genomic DNA, 0.25 µM primer (IDT-integrated DNA technologies, Bangalore, India) concentration and 1X readymade Master Mix (Promega, Madison, WI, USA) was used. In the secondary PCR, a 2 µl template from the primary PCR was used in 20 µl reaction mixture containing 1X readymade PCR Master Mix (Promega) and 0.5 µM primer (IDT) concentration. Both primary and secondary PCR were done in a Biorad C1000 system. Initial denaturation was done for 5 min at 94 °C followed by 35 cycles of denaturation for 30 s at 94 °C. Annealing was done for 1 min at 55 °C, extension for 1 min at 72 °C followed by a final extension at 72 °C for 5 min. In the Plasmodium species-specific PCR, amplification bands of 120 bp indicate P. vivax and 205 bp indicates P. falciparum. Laboratory-adapted 3D7 and DD2 strains were used as positive controls.
Genotyping of vaccine candidate antigens
Nested PCR was performed to amplify the msp-1 (block 2), msp-2 (block 3) and region 2 (RII) of glurp and their allelic variants. K1, MAD20 and RO33 families of msp-1, FC27 and 3D7/IC families of msp-2 and region 2 (RII) of glurp were amplified using allele-specific primers as previously described [23]. For detection of allelic variants of Pfmsp-1, nPCR was undertaken according to the protocol by Soulama et al. [24]. For detection of Pfmsp-1, Pfmsp-2 and glurp allelic variants, primary PCR was performed in a 20 µl reaction volume containing 1 µL of DNA, 0.125 mM (for Pfmsp-1), 0.25 mM (for Pfmsp-2 and glurp) of primer (IDT) and 1X readymade PCR Master Mix (Promega). Primary PCR cycling conditions for all three genes were: initial denaturation for 5 min at 95 °C followed by 25 cycles of denaturation for 1 min at 94 °C, annealing for 2 min at 58 °C and extension for 2 min at 72 °C. This was followed by a final extension at 75 °C for 5 min. Secondary PCR reactions were run on a Biorad C1000 Thermal cycler using 2 µl of primary PCR product as template, 0.25 mM primer (IDT) and 1X readymade PCR Master Mix (Promega). Secondary cycling conditions of PCR were as follows: one step denaturation for 5 min at 95 °C followed by 25 cycles of denaturation for 1 min at 94 °C, annealing at 61 °C for 2 min (for Pfmsp-1 and Pfmsp-2), 58 °C for 2 min (for glurp) and extension for 2 min at 72 °C. A final extension step was carried out at 75 °C for 5 min.
In allele-specific nested PCRs, the amplified DNA fragments were grouped according to their sizes: for Pfmsp-1 and Pfmsp-2 the alleles were considered identical if the fragment sizes were within 10 bp; a larger interval of 50 bp was considered for glurp as previously described [8, 25].
Multiplicity of infection (MOI) and heterozygosity (H E)
To identify MOI obtained in msp1, msp2 and glurp genes, PCR fragments were grouped as previously described [25]. Monoclonal infection was identified by a single fragment in the loci whereas multiple PCR fragments in the loci indicated a polyclonal infection. The ratio of alleles derived for each gene to the PCR positive samples for the same gene was defined as MOI [26]. Expected heterozygosity (H E) was estimated as described previously elsewhere [8].
The distribution pattern of Plasmodium species varies among the eight Northeastern states of India. In Assam, predominant species is the P. falciparum. Therefore, the study was restricted to this Plasmodium species only. Apart from P. falciparum and P. vivax, other human malaria parasites reported from Assam are P. ovale and P. malariae. The first and only P. ovale case reported was from Jorhat district of Assam [27]. One P. malariae case was reported from Assam and from two other states of Northeast India, viz: Arunachal Pradesh and Tripura, the same species was also reported [28–30]. So far, no study has been carried out in this region of India for other species of Plasmodium based on their genetic diversity pattern. Although P. vivax is co-endemic in northeastern states of India, it still remains neglected.
Results
Genotyping of msp-1, msp-2 and glurp antigens
Dried blood spots collected from 71 P. falciparum positive cases were included in this study. All these samples were mono-infection cases and were positive for P. falciparum by genus specific PCR. Out of these samples, Pfmsp1, Pfmsp2 and Pfglurp genes could be successfully amplified individually in 64 (90.14 %), 63 (88.73 %) and 65 (91.54 %) samples, respectively. A total of eight different allelic variants of msp-1, 22 allelic variants of msp-2 and eight allelic variants of glurp were detected. The MOI detected for msp-1, msp-2 and glurp were 1.28, 1.84 and 1.04, respectively.
In Pfmsp-1 locus, the dominant allelic family was MAD20 (68.75 %, n=44) with six different allele fragments in the range of 180–280 bp; the 220 bp fragment size was predominant (40.91 %, n=18). Among the nine allelic families of MAD20, the following allelic combinations were observed: 180 and 220 bp (n=1), 190 and 200 bp (n=1), 190 and 220 bp (n=4), 200 and 250 bp (n=2) and 220 and 280 bp (n=2). Both K1 (9.38 %, n=6) and RO33 (34.38 %, n=22) allelic families showed only one allelic variant of 200 and 190 bp, respectively. The frequency of monoclonal infection and polyclonal infection was 87.5 % (n=56) and 12.5 % (n=8), respectively. The detected MOI value and H E were 1.28 and 0.81 (Table 1). PCR-based allele frequencies of Pfmsp-1 are shown in Fig. 1.
Table 1.
Pfmsp-1 (block II region) allelic variants of P. falciparum detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam
|
Genotypes |
Frequency (%) |
Allele size (bp) |
No. of alleles |
Monoclonal infection |
Polyclonal infection |
MOI |
H E |
|---|---|---|---|---|---|---|---|
|
(n=64) |
(n=64) |
||||||
|
K1 |
6 (9.3 %) |
200 |
1 |
87.50 % |
12.50 % |
1.28 |
0.81 |
|
MAD20 |
44 (68.7 %) |
180–280 |
6 |
||||
|
RO33 |
22 (34 %) |
190 |
1 |
bp – base pair.
Fig. 1.
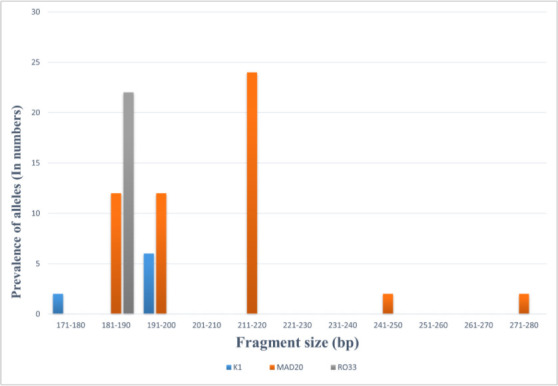
Allele frequencies of P. falciparum msp-1 detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam.
In Pfmsp-2 locus, the dominant and highly polymorphic allelic family was IC/3d7 (80.95 %, n=51) with 16 different fragments (490–700 bp) and the predominant fragment size was 510 bp (7.84 %, n=4). In the IC/3d7 allelic family, among the 16 alleles, multiple bands were observed in nine allele families. Out of these, the 510 and 610 bp combination was predominant (39.22 %, n=20), followed by 520 and 620 bp (n=5), 520 and 610 bp (n=2), 710 and 620 bp (n=2), 490 and 510 bp (n=2), 500 and 610 bp (n=1), 610 and 710 bp (n=1), 700 and 810 bp (n=1), 490 and 520 bp (n=1). FC27 allelic family (47.62 %, n=30) showed six different allele fragments (250–400 bp) and 310 bp (43.33 %, n=13) fragment size was predominant. The frequency of monoclonal infection and polyclonal infection were 71.43 % (n=45) and 28.57 % (n=18), respectively. The detected MOI value for Pfmsp-2 was 1.84 and expected heterozygosity was 0.88 (Table 2). PCR-based allele frequencies of Pfmsp-2 are shown in Fig. 2.
Table 2.
Pfmsp-2 block III allelic variants of P. falciparum detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam
|
Genotypes |
Frequency (%) |
Allele size (bp) |
No. of alleles |
Monoclonal infection |
Polyclonal infection |
MOI |
H E |
|---|---|---|---|---|---|---|---|
|
(n=63) |
(n=63) |
||||||
|
FC27 |
30 (47.6 %) |
250–400 |
6 |
71.42 % |
26.98 % |
1.84 |
0.88 |
|
IC/3d7 |
51 (80.9 %) |
490–700 |
16 |
(n=45) |
(n=18) |
bp – base pair; H E – expected heterozygosity.
Fig. 2.
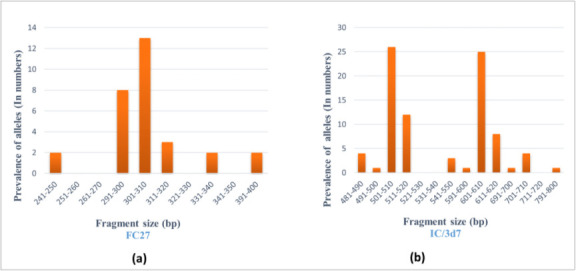
Allele frequencies of P. falciparum msp-2 detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam: (a) frequency of FC27; (b) frequency of IC/3D7.
In the RII region of Pfglurp, nine different allelic fragments (genotype I–IX) were observed. The most commonly detected allelic variant was the 800 bp fragment, which was denoted as genotype VI (47 %, n=32) followed by 900–950 bp genotype IX (23.52 %, n=16). In both the genotypes IV and V, the frequency of allelic variants was 7.35 % (n=5). Genotype VII was detected in four samples (5.88 %) and genotype I and II were determined in only one sample each (1.47 %). Among the nine different allele fragments, multiple infections were found in three cases (Table 3). The frequencies of both monoclonal and polyclonal infections were 95.38 % (n=62) and 4.61 % (n=3), respectively. The detected MOI value and expected heterozygosity were 1.04 and 0.72, respectively (Table 3). PCR based allele frequencies of Pfglurp are shown in Fig. 3. A comparison of the m.o.i. over the years in India and other countries from South-East Asia is shown in Table 4.
Table 3.
Pfglurp RII region allelic variants of P. falciparum detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam
|
Genotypes |
Frequency (%) |
Allele size |
Monoclonal infection |
Polyclonal infection |
MOI |
H E |
|---|---|---|---|---|---|---|
|
(n=65) |
(50 bp bin) |
(n=65) |
||||
|
I |
1 (1.47 %) |
400–450 |
95.38 % (n=62) |
4.61 % (n=3) |
1.04 |
0.72 |
|
II |
1 (1.47 %) |
451–500 |
||||
|
III |
2 (2.94 %) |
551–600 |
||||
|
IV |
5 (7.35 %) |
601–650 |
||||
|
V |
5 (7.35 %) |
701–750 |
||||
|
VI |
32 (47 %) |
751–800 |
||||
|
VII |
4 (5.88 %) |
801–850 |
||||
|
VIII |
2 (2.94 %) |
851–900 |
||||
|
IX |
16 (23.52 %) |
901–950 |
bp – base pair; H E – expected heterozygosity.
Fig. 3.
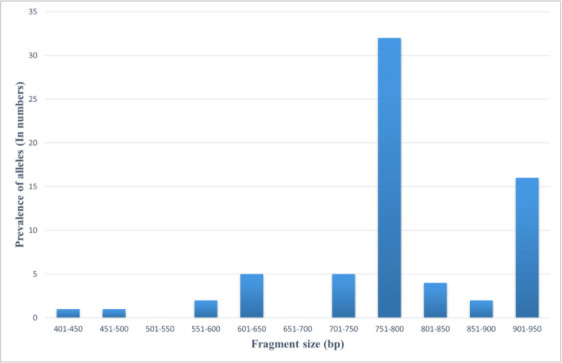
Allele frequencies of P. falciparum glurp detected by nPCR from archived (2006) samples collected in Lakhimpur, Assam.
Table 4.
Comparison of MOI in India and other South-East Asian countries over the years
|
Sl. No. |
Reference |
Study period |
Study area |
MOI |
H E |
||||
|---|---|---|---|---|---|---|---|---|---|
|
msp-1 |
msp-2 |
glurp |
msp-1 |
msp-2 |
glurp |
||||
|
1 |
Present study |
2005–2006 |
Lakhimpur, Assam, NE India |
1.28 |
1.84 |
1.04 |
0.81 |
0.88 |
0.72 |
|
2 |
Joshi et al. 2007 [32] |
na |
Karbi Anglong, Assam |
2.1 |
1.2 |
– |
– |
– |
– |
|
Kamrup, Assam |
1.4 |
1.1 |
– |
– |
– |
– |
|||
|
Keonjhar, Orissa |
1.1 |
1.3 |
– |
– |
– |
– |
|||
|
Sundergarh, Orissa |
1.2 |
1.6 |
– |
– |
– |
– |
|||
|
Darjeeling, West Bengal |
1.1 |
1 |
– |
– |
– |
– |
|||
|
3 |
Bharti et al. 2012 [42] |
2005 |
Madhya Pradesh, Central India |
1.27 |
– |
– |
0.94 |
– |
– |
|
2009 |
1.34 |
– |
– |
0.91 |
– |
– |
|||
|
4 |
Kumar et al. 2014 [61] |
2005 2011 |
Assam, NE India |
– |
– |
1.21 1.12 |
– – |
– – |
0.87 0.85 |
|
5 |
Akter et al. 2012 [70] |
2004, 2005 and 2008 |
Bangladesh |
2.7 |
– |
1.2 |
– |
– |
– |
|
6 |
Mohd Abd Razak et al. 2016 [73] |
2008–2009 |
Kalabakan, East Malaysia |
1.65 |
1.2 |
– |
0.17 |
0.37 |
0.70 |
|
2011 and 2014 |
Kota Marudu, East Malaysia |
1.05 |
1.05 |
– |
0.24 |
0.25 |
0.69 |
||
|
7 |
Patel et al. 2017 [20] |
2013–2015 |
Chhattisgarh, Central India |
1.67 |
1.28 |
– |
– |
– |
– |
|
8 |
Patgiri et al. 2019 [51] |
2015 |
Tripura, NE India |
1.56 |
1.31 |
1.06 |
0.89 |
0.81 |
0.85 |
|
9 |
Hussain et al. 2011 [35] |
2008 |
Jharkhand, India |
1.38 |
1.39 |
– |
– |
– |
– |
|
10 |
Atroosh et al. 2011 [74] |
na |
Malaysia |
1.37 |
1.2 |
– |
0.57 |
0.55 |
– |
|
11 |
Congpuong et al. 2014 [71] |
2009, 2012–2013 |
Thai-Myanmar Border |
2.15 |
2.93 |
1.26 |
– |
– |
– |
|
12 |
Soe et al. 2017 [72] |
2009–2010 |
Myanmar |
1.94 |
– |
– |
– |
– |
– |
|
13 |
Yuan et al. 2012 [75] |
2007 2008 2009 2010 |
China-Myanmar Border |
1.34 |
– |
– |
– |
– |
– |
|
1.22 |
– |
– |
– |
– |
– |
||||
|
1.62 |
– |
– |
– |
– |
– |
||||
|
1.46 |
– |
– |
– |
– |
– |
||||
H E – expected heterozygosity.
Discussion
The present study describes the genetic diversity patterns of P. falciparum potential vaccine candidate antigenic genes msp-1, msp-2 and glurp in archived samples collected in 2006 from a malaria endemic region of Assam, Northeast India. These P. falciparum isolates belong to the pre-artemisinin era when chloroquine was the mainstay of treatment for falciparum malaria. The genetic diversity patterns in terms of allelic variants, MOI and H E observed in that period, when compared to those observed in published current and historical studies conducted in geographically similar areas, have the potential to reveal temporal variation in diversity patterns. This information adds to the existing knowledge of P. falciparum genetic diversity in field-collected samples and can provide important leads for vaccine research.
Various parts of Northeast India are highly malaria endemic where persistent malaria transmission is reported [31]. Numerous studies have reported that the allele frequency of each gene is associated with the degree of endemicity. In high endemicity areas such as Asia and Africa, a greater number of alleles were reported whereas in low endemicity areas, the number of alleles detected was lower [32–36]. In our study, all the reported allelic families of msp-1 (K1, RO33 and MAD20), msp-2 (FC27 and IC/3d7) and RII region of glurp were observed.
MSP-1 has a molecular weight of 195 KDa and is present in both merozoites and mature schizonts; msp-1 is dimorphic and contains three genotypes (K1, MAD20, and RO33) and sequence analysis has revealed that it consists of 5300 bases which code for more than 1700 amino acids [37]. In our study, we have observed that the 220 bp MAD20 (n=44) allele of msp-1 is predominant followed by RO33 (n=22) and K1 (n=6) which is in contrast to reports from North-West Colombia [38], Indonesia [39], Ethiopia [9], Central India [20] and previous studies performed in different regions of Northeast India [32, 40–42]. Myanmar reported MAD20 as dominant allele type [43, 44]. Tripura reported predominance of msp-1 K1 (38) allele and Arunachal Pradesh reported that the msp1-RO33 allele was dominant and both these findings are in contrast to our study findings [19]. A recent study from Africa also demonstrated that the distribution of msp-1 alleles had changed in high transmission seasons and one dominant allele was replaced by another allele type [45].
Our samples were collected before ACT drug policy was implemented in Northeast India when CQ was the first-line malaria treatment and our results are similar to that observed in North-West Colombia (1997) where CQ was the first line therapy for malaria [38]. The change in anti-malarial drug policy in 2008 from chloroquine to ACT might have had some impact on the genetic diversity patterns observed. It was documented that change in local anti-malarial drug policies could cause dynamic changes in genetic diversity of a parasite population [46]. In a study carried out in Africa, it was observed that parasite diversity was progressively decreased after the introduction of ACT, since the disease transmission intensity was reduced [47]. A study from Bioko island also reported that after implementing several anti-malarial drug policies, genetic variations of msp-1 and msp-2 alleles were higher than before [48].
MSP-2 is a small molecular protein (45–52 KDa) located in chromosome-2 and is expressed in merozoites. It is mainly associated with high anti-immunogenicity and increased disease morbidity [49]. The current study showed that the IC/3d7 allele with 16 allelic types was dominant over FC27, which corroborates with previous studies performed in Myanmar [43, 44], Cameroon [50], Central India [20] and Tripura, Northeast India [51]. In our study, nine types of msp-2 allelic combinations were observed; the 510 bp with 610 bp combination was dominant. These results are in contrast with a previous Indian study carried out in Orissa, Madhya Pradesh and Rajasthan [52]. In an earlier study carried out in India with a very small number of P. falciparum isolates, only the FC-27 allele type was reported [53, 54]. From many parts of the world, viz. Senegal [55], Gambia [56], Colombia [57], Honduras [34] and Papua New Guinea [58], the FC-27 family has been reported as the predominant allele type over IC/3d7. As in msp-1 alleles, this discrepancy could be due to the pattern of disease transmission intensity, change in drug policy, anti-malarial drug pressure and higher level of intragenic recombination in a parasite population [44, 45].
GLURP (glutamate-rich protein) is exo-antigenic in nature and has a molecular weight of 220 KDa. PfGLURP antigen induces protective immunity and thereby has a vital role in malaria vaccine development [59, 60]. In Northeast India, only a few studies have been reported on polymorphism of Pfglurp RII region. The first study in Assam, Northeast India reported ten different types of glurp alleles based on their base pair sizes [61]. Similarly, in Nigeria, 12 alleles of glurp were reported before and after ACT implementation [62]. Later, nine allele types of glurp were reported from Northeast Indian states of Arunachal Pradesh [40] and Tripura [51]. Our study found nine different types of glurp alleles in Assam, which is similar to previous reports from Northeast India. In low endemic areas like Central South America [63], Honduras [34], Colombia [64] and Brazil [65], the frequency of glurp allele has been reported to range from two to four; while in endemic regions like India [66], Thailand [67] and Sudan [68], the glurp allele frequency is higher.
MOI and H E are indirect measures of parasite genetic diversity in a particular region. The degree of transmission intensity usually regulates the rate of the MOI but the relationship of parasite prevalence and degree of endemicity with MOI is still inconclusive [69]. In this study, the detected MOI for the polymorphic genes msp-1, msp-2 and glurp were found to range from 1.04 to 1.84. MOI of the polymorphic genes was similar to those reported earlier from Indian studies [20, 32, 51, 61]. Considering the differences in pre-artemisinin era vs. artemisinin era MOI values (Table 4), it can be seen that the MOI values for Pfmsp-1 in the current study were marginally lower than those observed in Chhattisgarh [20] and Tripura [51]. Some areas like Bangladesh and Thai-Myanmar border are more endemic for malaria and have reported higher Pfmsp-1 MOI values than Indian studies [70–72]. Pfmsp-2 MOI values observed in the present study were, however, higher as compared to most Indian studies [20, 32, 35, 51]; but lower than that observed in the Thai-Myanmar border area [71]. Pfglurp MOI values did not show much variation in the pre-artemisinin vs. artemisinin era studies from Southeast Asia including India [51, 61, 70, 71]. Heterozygosity (H E) values observed in our study for Pfmsp-1 and Pfmsp-2 were slightly higher than observed in Malaysia [73, 74] but comparable to Indian studies [42, 51] whereas, for Pfglurp, it was slightly lower than those reported earlier from Tripura and Assam, Northeast India [51, 61]. Slight changes in MOI and H E values over the years may be due to differences in geographical locations, sampling intervals and subsequent changes in the malaria drug policy in Northeast India.
Conclusion
The present study evaluated the antigenic diversity of P. falciparum clinical isolates from 2006 in Assam, Northeast India before the implementation of ACT. While the malaria burden has reduced in the study area and entire Northeast India drastically over the years, a similar trend in reduction of P. falciparum genetic diversity parameters was not observed in comparison with newer studies. Information from wide-scale studies on genetic diversity of the malaria parasite may help in a better understanding of the population dynamics of the parasite and how this contributes towards the development of an efficient vaccine for elimination of malaria over time.
Funding information
The work presented in this study was carried out using funds received from ICMR- Regional Medical Research Centre, N. E. Region, Dibrugarh, Assam, India.
Acknowledgements
The authors thank ICMR-Regional Medical Research Centre, N.E. Region (Indian Council of Medical Research), Dibrugarh, India, for supporting this work. The authors would like to acknowledge the efforts of the entire staff of the Malaria section of the institute for their valuable insights.
Author contributions
V.S., T.N., S.J.P. and D.R.B. contributed in conceptualization of the study. V.S., T.N., S.J.P. and D.R.B. contributed equally in conducting the study, analysis and validation of the results, drafting and critically reviewing the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
The original study was approved by the Institutional Ethics Committee (Human) of ICMR-Regional Medical Research Centre, North East Region, Dibrugarh, Assam. The archived samples were used in this study after complete anonymization and no additional consent was obtained from the patients for the current study.
Footnotes
Abbreviations: ACT, artemisinin-based combination therapy; GLURP, glutamate-rich protein; HE, expected heterozygosity; MOI, multiplicity of infection; MSP, merozoite surface protein; RDT, rapid diagnostic test.
References
- 1.WHO World Malaria Report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 2.Mohapatra PK, Prakash A, Taison K, Negmu K, Gohain AC, et al. Evaluation of chloroquine (CQ) and sulphadoxine/pyrimethamine (SP) therapy in uncomplicated falciparum malaria in Indo-Myanmar border areas. Trop Med Int Health. 2005;10:478–483. doi: 10.1111/j.1365-3156.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- 3.Berzins K. Merozoite antigens involved in invasion. Chem Immunol. 2002;80:125–143. doi: 10.1159/000058843. [DOI] [PubMed] [Google Scholar]
- 4.Genton B, Reed ZH. Asexual blood-stage malaria vaccine development: facing the challenges. Curr Opin Infect Dis. 2007;20:467–475. doi: 10.1097/QCO.0b013e3282dd7a29. [DOI] [PubMed] [Google Scholar]
- 5.Gaur D, Mayer DCG, Miller LH. Parasite ligand–host receptor interactions during invasion of erythrocytes by Plasmodium merozoites . Int J Parasitol. 2004;34:1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Sanders PR, Kats LM, Drew DR, O’Donnell RA, O’Neill M, et al. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun. 2006;74:4330–4338. doi: 10.1128/IAI.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin M, Yamodo I, Ranjan R, Li X, Mines G, et al. Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum. Role of the RhopH3–MSP1 complex. Biochim Biophys Acta. 2014;1843:2855–2870. doi: 10.1016/j.bbamcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mwingira F, Nkwengulila G, Schoepflin S, Sumari D, Beck H-P, et al. Plasmodium falciparum msp1, msp2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J. 2011;10:79. doi: 10.1186/1475-2875-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed H, Kassa M, Mekete K, Assefa A, Taye G, et al. Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates in Northwest Ethiopia. Malar J. 2018;17:386. doi: 10.1186/s12936-018-2540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takala SL, Smith DL, Stine OC, Coulibaly D, Thera MA, et al. A high-throughput method for quantifying alleles and haplotypes of the malaria vaccine candidate Plasmodium falciparum merozoite surface protein-1 19 kDa. Malar J. 2006;5:31. doi: 10.1186/1475-2875-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira MU, Hartl DL. Plasmodium falciparum: worldwide sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-2 (MSP-2) Exp Parasitol. 2007;115:32–40. doi: 10.1016/j.exppara.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Barrera SM, Pérez MA, Knudson A, Nicholls RS, Guerra AP. Genotypic survery of Plasmodium falciparum based on the msp1, msp2 and glurp genes by multiplex PCR. Biomedica. 2010;30:530–538. doi: 10.7705/biomedica.v30i4.291. [DOI] [PubMed] [Google Scholar]
- 13.RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen CM, Vekemans J, Lievens M, Kester KE, Regules JA, et al. S malaria vaccine efficacy and immunogenicity during Plasmodium falciparum challenge is associated with HLA genotype. Vaccine. 2018;36:1637–1642. doi: 10.1016/j.vaccine.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Geneva: The World Health Organization (WHO); 2021. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk.https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk [Google Scholar]
- 16.Sehgal PN, Sharma MID, Sharma SL. Resistance to chloroquine in falciparum malaria in Assam state. India. J Commun Dis. 1973;5:175–180. [Google Scholar]
- 17.Das S, Barkakaty BN. Pyrimethamine in combination with sulfadoxine or sulfalene in P. falciparum infected cases in India. Indian J Malariol. 1981;18:109–116. [Google Scholar]
- 18.WHO National Framework for Malaria Elimination in India (2016–2030). Directorate of National Vector Borne Disease Control Programme (NVBDCP). Directorate General of Health Services (DGHS) Ministry Of Health & Family Welfare, Government of India. 2016 [Google Scholar]
- 19.Sarma DK, Mohapatra PK, Bhattacharyya DR, Chellappan S, Karuppusamy B, et al. Malaria in North-East India: Importance and Implications in the Era of Elimination. Microorganisms. 2019;7:12. doi: 10.3390/microorganisms7120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel P, Bharti PK, Bansal D, Raman RK, Mohapatra PK, et al. Genetic diversity and antibody responses against Plasmodium falciparum vaccine candidate genes from Chhattisgarh, Central India: implication for vaccine development. PLoS One. 2017;12:e0182674. doi: 10.1371/journal.pone.0182674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohapatra PK, Sarma DK, Prakash A, Bora K, Ahmed MA, et al. Molecular evidence of increased resistance to anti-folate drugs in Plasmodium falciparum in North-East India: a signal for potential failure of artemisinin plus sulphadoxine-pyrimethamine combination therapy. PLoS One. 2014;9:e105562. doi: 10.1371/journal.pone.0105562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 23.Snounou G. Malaria Methods and Protocols. Springer; 2002. Genotyping of Plasmodium spp; pp. 103–116. [DOI] [PubMed] [Google Scholar]
- 24.Soulama I, Nébié I, Ouédraogo A, Gansane A, Diarra A, et al. Plasmodium falciparum genotypes diversity in symptomatic malaria of children living in an urban and a rural setting in Burkina Faso. Malar J. 2009;8:135. doi: 10.1186/1475-2875-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayengue PI, Ndounga M, Malonga FV, Bitemo M, Ntoumi F. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum isolates from Brazzaville, Republic of Congo. Malar J. 2011;10:276. doi: 10.1186/1475-2875-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiwuwa MS, Ribacke U, Moll K, Byarugaba J, Lundblom K, et al. Genetic diversity of Plasmodium falciparum infections in mild and severe malaria of children from Kampala, Uganda. Parasitol Res. 2013;112:1691–1700. doi: 10.1007/s00436-013-3325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash A, Mohapatra P, Bhattacharyya D, Bk G, Mahanta J. Plasmodium ovale: First case report from Assam, India. Curr Sci. 2003;84:1187–1188. [Google Scholar]
- 28.Dev V. Plasmodium malariae--a case of quartan malaria in Assam. J Commun Dis. 2000;32:149–151. [PubMed] [Google Scholar]
- 29.Mohapatra PK, Prakash A, Bhattacharyya DR, Goswami BK, Ahmed A, et al. Detection & molecular confirmation of a focus of Plasmodium malariae in Arunachal Pradesh, India. Indian J Med Res. 2008;128:52–56. [PubMed] [Google Scholar]
- 30.Das CS, Dutta P, Kalita MC. First phylogenetic evidence of Plasmodium malariae from northeast region of India. International Journal of Parasitology Research. 2015;7:136–139. [Google Scholar]
- 31.Dev V, Hira CR, Rajkhowa MK. Malaria-attributable morbidity in Assam, north-eastern India. Ann Trop Med Parasitol. 2001;95:789–796. doi: 10.1080/00034980120111136. [DOI] [PubMed] [Google Scholar]
- 32.Joshi H, Valecha N, Verma A, Kaul A, Mallick PK, et al. Genetic structure of Plasmodium falciparum field isolates in eastern and north-eastern India. Malar J. 2007;6:60. doi: 10.1186/1475-2875-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahajan RC, Farooq U, Dubey ML, Malla N. Genetic polymorphism in Plasmodium falciparum vaccine candidate antigens. Indian J Pathol Microbiol. 2005;48:429–438. [PubMed] [Google Scholar]
- 34.Haddad D, Snounou G, Mattei D, Enamorado IG, Figueroa J, et al. Limited genetic diversity of Plasmodium falciparum in field isolates from Honduras. Am J Trop Med Hyg. 1999;60:30–34. doi: 10.4269/ajtmh.1999.60.30. [DOI] [PubMed] [Google Scholar]
- 35.Hussain MM, Sohail M, Kumar R, Branch OH, Adak T, et al. Genetic diversity in merozoite surface protein-1 and 2 among Plasmodium falciparum isolates from malarious districts of tribal dominant state of Jharkhand, India. Ann Trop Med Parasitol. 2011;105:579–592. doi: 10.1179/2047773211Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulenge FM, Hunja CW, Magiri E, Culleton R, Kaneko A, et al. Genetic diversity and population structure of Plasmodium falciparum in Lake Victoria Islands, a region of intense transmission. Am J Trop Med Hyg. 2016;95:1077–1085. doi: 10.4269/ajtmh.16-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe K, Sakihama N, Walliker D, Babiker H, Abdel-Muhsin A-MA, et al. Allelic dimorphism-associated restriction of recombination in Plasmodium falciparum msp1. Gene. 2007;397:153–160. doi: 10.1016/j.gene.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Gómez D, Chaparro J, Rubiano C, Rojas MO, Wasserman M. Genetic diversity of Plasmodium falciparum field samples from an isolated Colombian village. Am J Trop Med Hyg. 2002;67:611–616. doi: 10.4269/ajtmh.2002.67.611. [DOI] [PubMed] [Google Scholar]
- 39.Sorontou Y, Pakpahan A. Genetic diversity in MSP-1 gene of Plasmodium falciparum and its association with malaria severity, parasite density, and host factors of asymptomatic and symptomatic patients in Papua, Indonesia. Int J Med Sci Public Health. 2015;4:1584. doi: 10.5455/ijmsph.2015.05062015327. [DOI] [Google Scholar]
- 40.Sarmah NP, Sarma K, Bhattacharyya DR, Sultan A, Bansal D, et al. Molecular characterization of Plasmodium falciparum in Arunachal Pradesh from Northeast India based on merozoite surface protein 1 & glutamate-rich protein. Indian J Med Res. 2017;146:375–380. doi: 10.4103/ijmr.IJMR_291_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur H, Sehgal R, Goyal K, Makkar N, Yadav R, et al. Genetic diversity of Plasmodium falciparum merozoite surface protein‐1 (block 2), glutamate‐rich protein and sexual stage antigen Pfs25 from Chandigarh. Trop Med Int Health. 2017;22:1590–1598. doi: 10.1111/tmi.12990. [DOI] [PubMed] [Google Scholar]
- 42.Bharti PK, Shukla MM, Sharma YD, Singh N. Genetic diversity in the block 2 region of the merozoite surface protein-1 of Plasmodium falciparum in central India. Malar J. 2012;11:78. doi: 10.1186/1475-2875-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang J-M, Moon S-U, Kim J-Y, Cho S-H, Lin K, et al. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum field isolates from Myanmar. Malar J. 2010;9:131. doi: 10.1186/1475-2875-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lê HG, Kang J-M, Jun H, Lee J, Thái TL, et al. Changing pattern of the genetic diversities of Plasmodium falciparum merozoite surface protein-1 and merozoite surface protein-2 in Myanmar isolates. Malar J. 2019;18:241. doi: 10.1186/s12936-019-2879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y-A, Shiu T-J, Tseng L-F, Cheng C-F, Shih W-L, et al. Dynamic changes in genetic diversity, drug resistance mutations, and treatment outcomes of falciparum malaria from the low-transmission to the pre-elimination phase on the islands of São Tomé and Príncipe. Malar J. 2021;20:467. doi: 10.1186/s12936-021-04007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salgueiro P, Vicente JL, Figueiredo RC, Pinto J. Genetic diversity and population structure of Plasmodium falciparum over space and time in an African archipelago. Infect Genet Evol. 2016;43:252–260. doi: 10.1016/j.meegid.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Huang B, Tuo F, Liang Y, Wu W, Wu G, et al. Temporal changes in genetic diversity of msp-1, msp-2, and msp-3 in Plasmodium falciparum isolates from Grande Comore Island after introduction of ACT. Malar J. 2018;17:83. doi: 10.1186/s12936-018-2227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J-T, Li J, Zha G-C, Huang G, Huang Z-X, et al. Genetic diversity and allele frequencies of Plasmodium falciparum msp1 and msp2 in parasite isolates from Bioko Island, Equatorial Guinea. Malar J. 2018;17:458. doi: 10.1186/s12936-018-2611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.al-Yaman F, Genton B, Anders RF, Falk M, Triglia T, et al. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1994;51:593–602. doi: 10.4269/ajtmh.1994.51.593. [DOI] [PubMed] [Google Scholar]
- 50.Basco LK, Tahar R, Escalante A. Molecular epidemiology of malaria in Cameroon. XVIII. Polymorphisms of the Plasmodium falciparum merozoite surface antigen-2 gene in isolates from symptomatic patients. Am J Trop Med Hyg. 2004;70:238–244. [PubMed] [Google Scholar]
- 51.Patgiri SJ, Sarma K, Sarmah N, Bhattacharyya N, Sarma DK, et al. Characterization of drug resistance and genetic diversity of Plasmodium falciparum parasites from Tripura, Northeast India. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-50152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ranjit MR, Sharma YD. Genetic polymorphism of falciparum malaria vaccine candidate antigen genes among field isolates in India. Am J Trop Med Hyg. 1999;61:103–108. doi: 10.4269/ajtmh.1999.61.103. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharya P, Malhotra P, Sharma P, Okenu DM, Chauhan VS. Merozoite surface antigen 2 (MSA-2) gene of Plasmodium falciparum strains from India. Mol Biochem Parasitol. 1995;74:125–127. doi: 10.1016/0166-6851(96)83010-6. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharya PR, Kumar M, Das RH. Surprisingly little polymorphism in the merozoite-surface-protein-2 (MSP-2) gene of Indian Plasmodium falciparum . Ann Trop Med Parasitol. 1999;93:561–564. doi: 10.1080/00034989958069. [DOI] [PubMed] [Google Scholar]
- 55.Zwetyenga J, Rogier C, Tall A, Fontenille D, Snounou G, et al. No influence of age on infection complexity and allelic distribution in Plasmodium falciparum infections in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Am J Trop Med Hyg. 1998;59:726–735. doi: 10.4269/ajtmh.1998.59.726. [DOI] [PubMed] [Google Scholar]
- 56.Conway DJ, Greenwood BM, McBride JS. The epidemiology of multiple-clone Plasmodium falciparum infections in Gambian patients. Parasitology. 1991;103:1–6. doi: 10.1017/s0031182000059217. [DOI] [PubMed] [Google Scholar]
- 57.Ntoumi F, Contamin H, Rogier C, Bonnefoy S, Trape JF, et al. Age-dependent carriage of multiple Plasmodium falciparum merozoite surface antigen-2 alleles in asymptomatic malaria infections. Am J Trop Med Hyg. 1995;52:81–88. doi: 10.4269/ajtmh.1995.52.81. [DOI] [PubMed] [Google Scholar]
- 58.Felger I, Tavul L, Kabintik S, Marshall V, Genton B, et al. Plasmodium falciparum: extensive polymorphism in merozoite surface antigen 2 alleles in an area with endemic malaria in Papua New Guinea. Exp Parasitol. 1994;79:106–116. doi: 10.1006/expr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 59.Holder AA. Developments with anti‐malarial vaccines. Ann N Y Acad Sci. 1993;700:7–21. doi: 10.1111/j.1749-6632.1993.tb26301.x. [DOI] [PubMed] [Google Scholar]
- 60.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, et al. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–2620. doi: 10.1128/IAI.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar D, Dhiman S, Rabha B, Goswami D, Deka M, et al. Genetic polymorphism and amino acid sequence variation in Plasmodium falciparum GLURP R2 repeat region in Assam, India, at an interval of five years. Malar J. 2014;13:450. doi: 10.1186/1475-2875-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguetse CN, Ojo JA, Nchotebah C, Ikegbunam MN, Meyer CG, et al. Genetic diversity of the Plasmodium falciparum glutamate-rich protein R2 region before and twelve years after introduction of artemisinin combination therapies among febrile children in Nigeria. Am J Trop Med Hyg. 2018;98:667–676. doi: 10.4269/ajtmh.17-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ariey F, Chalvet W, Hommel D, Peneau C, Hulin A, et al. Plasmodium falciparum parasites in French Guiana: limited genetic diversity and high selfing rate. Am J Trop Med Hyg. 1999;61:978–985. doi: 10.4269/ajtmh.1999.61.978. [DOI] [PubMed] [Google Scholar]
- 64.Montoya L, Maestre A, Carmona J, Lopes D, Do Rosario V, et al. Plasmodium falciparum: diversity studies of isolates from two Colombian regions with different endemicity. Exp Parasitol. 2003;104:14–19. doi: 10.1016/s0014-4894(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 65.Pratt-Riccio LR, Perce-da-Silva D de S, Lima-Junior J da C, Theisen M, Santos F, et al. Genetic polymorphisms in the glutamate-rich protein of Plasmodium falciparum field isolates from a malaria-endemic area of Brazil. Mem Inst Oswaldo Cruz. 2013;108:523–528. doi: 10.1590/S0074-02762013000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranjit MR, Das A, Das BP, Das BN, Dash BP, et al. Distribution of Plasmodium falciparum genotypes in clinically mild and severe malaria cases in Orissa, India. Trans R Soc Trop Med Hyg. 2005;99:389–395. doi: 10.1016/j.trstmh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, et al. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999;93:369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 68.A-Elbasit IE, A-Elgadir TME, Elghazali G, Elbashir MI, Giha HA. Genetic fingerprints of parasites causing severe malaria in a setting of low transmission in Sudan. J Mol Microbiol Biotechnol. 2007;13:89–95. doi: 10.1159/000103600. [DOI] [PubMed] [Google Scholar]
- 69.Zhong D, Koepfli C, Cui L, Yan G. Molecular approaches to determine the multiplicity of Plasmodium infections. Malar J. 2018;17:172. doi: 10.1186/s12936-018-2322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akter J, Thriemer K, Khan WA, Sullivan DJ Jr, Noedl H, et al. Genotyping of Plasmodium falciparum using antigenic polymorphic markers and to study anti-malarial drug resistance markers in malaria endemic areas of Bangladesh. Malar J. 2012;11:386. doi: 10.1186/1475-2875-11-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Congpuong K, Sukaram R, Prompan Y, Dornae A. Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders. Asian Pac J Trop Biomed. 2014;4:598–602. doi: 10.12980/APJTB.4.2014APJTB-2014-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soe TN, Wu Y, Tun MW, Xu X, Hu Y, et al. Genetic diversity of Plasmodium falciparum populations in southeast and western Myanmar. Parasit Vectors. 2017;10:322. doi: 10.1186/s13071-017-2254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohd Abd Razak MR, Sastu UR, Norahmad NA, Abdul-Karim A, Muhammad A, et al. Genetic diversity of Plasmodium falciparum populations in malaria declining areas of Sabah, East Malaysia. PLOS ONE. 2016;11:e0152415. doi: 10.1371/journal.pone.0152415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atroosh WM, Al-Mekhlafi HM, Mahdy MA, Saif-Ali R, Al-Mekhlafi AM, et al. Genetic diversity of Plasmodium falciparum isolates from Pahang, Malaysia based on MSP-1 and MSP-2 genes. Parasit Vectors. 2011;4:233. doi: 10.1186/1756-3305-4-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan L, Zhao H, Wu L, Li X, Parker D, et al. Plasmodium falciparum populations from northeastern Myanmar display high levels of genetic diversity at multiple antigenic loci. Acta Trop. 2013;125:53–59. doi: 10.1016/j.actatropica.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


