Abstract
In Asia, Clitoria ternatea flowers are commonly used as a traditional medicinal herb and as a food colourant. Their bioactive compounds have anti-inflammatory, anti-microbial and anti-biofilm activities. Pseudomonas aeruginosa is one of the major pathogens that cause biofilm-associated infections resulting in an increase in antimicrobial resistance. Hence, the aim of this study was to investigate if the anti-biofilm properties of the anthocyanin-rich fraction of C. ternatea flowers were effective against P. aeruginosa . The effect of the anthocyanin-rich fraction of C. ternatea flowers on P. aeruginosa biofilms formed on a polystyrene surface was determined using the crystal violet assay and scanning electron microscopy (SEM). The anthocyanin-rich fraction reduced biofilm formation by four P. aeruginosa strains with a minimum biofilm inhibitory concentration value ranging between 0.625 and 5.0 mg ml−1. We further show that the biofilm-inhibiting activity of C. ternatea flowers is not due to the flavonols but is instead attributed to the anthocyanins, which had significant biofilm inhibitory activity (64.0±1.1 %) at 24 h in a time–response study. The anthocyanin-rich fraction also significantly reduced bacterial attachment on the polystyrene by 1.1 log c.f.u. cm−2 surface based on SEM analysis. Hence, anthocyanins from C. ternatea flowers have potential as an agent to decrease the risk of biofilm-associated infections.
Keywords: anthocyanin, antimicrobial, biofilm-inhibiting activity, blue pea, crystal violet, flavonol
Introduction
Antibiotics have been used for the treatment of bacterial diseases since the early 20th century [1]. Antibiotics are incorporated in animal feed to promote growth, and to treat and prevent disease while antibiotic-plant-based sprays and solutions are used to combat plant infections [2]. An increase in the use of antibiotics in clinical practice as well as in agriculture and poultry farming has not only led to the emergence of antibiotic resistance but also to environmental antibiotic pollution (e.g. antibiotics released from human waste streams and waste from livestock farming into water, manure and soil). This calls for stricter management of antibiotic usage to contain the development of resistant microorganisms [3]. Antibiotic resistance limits the effectiveness of current drugs and, significantly, causes treatment failure of bacterial infections [4–6]. Treatment of infections becomes a challenge when resistance at both the cellular and community levels occurs in parallel [7]. Biofilms are aggregates of microbial cells (single or multiple bacterial species) encased in a self-produced matrix mainly composed of polysaccharides, proteins, nucleic acids, lipids, extracellular DNA and water [8, 9] that are attached to biotic (living tissues) or abiotic surfaces (e.g. medical implants) [10]. Biofilm infections can be divided into device- and non-device-related infections. Non-device-related infections occur in human tissues, causing various chronic diseases such as cystic fibrosis pneumonia, periodontitis, endocarditis and chronic wound infections [11, 12]. Device-related infections involve medical implants and catheters such as intravenous and urinary catheters, joint prostheses, cardiovascular and biliary stents, contact lenses, pacemakers and breast implants [11, 12].
Pseudomonas aeruginosa is an opportunistic pathogen responsible for severe nosocomial infections and has developed resistance to multiple antimicrobial agents. P. aeruginosa infections pose a high risk to immunocompromised patients where infections can cause severe respiratory problems [13, 14]. P. aeruginosa has been associated with patients with chronic or inherited lung diseases such as bronchiectasis and cystic fibrosis. These individuals are highly susceptible to persistent pulmonary infection, ventilator-associated pneumonia, catheter-related bloodstream infections as well as chronic wounds where biofilm formation benefits P. aeruginosa in terms of maintaining persistent infections, ability to evade the immune system and development of antimicrobial resistance [13]. In a clinical situation, infections caused by biofilm-forming pathogens require aggressive treatment with a combination of antibiotics, which is challenging as infections may recur or persist even after the recommended treatment protocol due to the presence of biofilms [15, 16]. When the problem is device-related, there is a requirement to remove and replace the device, which requires surgery, increased costs, risks and complications [16]. Quorum sensing is an important regulator of biofilm formation. Bacteria use this intercellular signalling system in the communication and regulation of gene expression through the release of autoinducers into the environment. These autoinducers may serve as important targets for the repression of biofilm formation [14]. The las and rhl quorum sensing systems have been linked to biofilm formation, maturation and virulence of P. aeruginosa [17, 18].
Numerous studies have focused on identifying antibiofilm agents from natural products and synthetic agents that are non-biocidal, as such molecules should not lead to the development of drug resistance [19]. Clitoria ternatea flowers, commonly known as butterfly pea flowers, have been traditionally used to treat various ailments and as a food colourant. C. ternatea flowers, being rich in anthocyanins and flavonols, have been reported to possess various bioactive properties including antioxidant, antibacterial, anti-diabetic and anti-inflammatory activities [20–22]. The extract has antibacterial activity against, for example, Escherichia coli , Klebsiella pneumoniae and P. aeruginosa [20, 23]. However, the mode of action of the extract for antibacterial and anti-biofilm activities remains unclear. Thus, this study was undertaken to explore the anti-biofilm properties of the anthocyanin-rich fraction of C. ternatea flowers towards P. aeruginosa at non-biocidal concentrations.
Methods
Preparation and extraction of samples
Freshly harvested C. ternatea cv. Double Blue flowers were obtained from a horticulture nursery in Subang Jaya, Malaysia. Only the petals of fresh C. ternatea flowers were used in the extraction process. The petals were cut into small pieces (approximately 0.5×0.3 cm) before usage. Ethanol extracts of the fresh flower material (10 g) were prepared by immersing the flowers in 200 ml of 50 % ethanol with constant shaking for 3 h at room temperature (25 °C) while aqueous extracts were prepared from flowers in 200 ml distilled water subjected to constant shaking in a 50 °C water bath for 1 h. Extracts were then vacuum-filtered. The solution was concentrated under vacuum at 45 °C using a rotary evaporator followed by freeze-drying at −80 °C. The freeze-dried extracts were kept at −80 °C until further analysis.
Semi-purification of crude extract by Amberlite XAD-16 column chromatography
The anthocyanin-rich fraction was prepared according to a previous study [24]. The freeze-dried extract of C. ternatea flowers (5 g) was dissolved in distilled water (100 ml), adjusted to pH 2 and partitioned with ethyl acetate (100 ml) to facilitate the removal of flavonols. The aqueous fraction (containing anthocyanins) were collected and again partitioned with ethyl acetate twice more. The ethyl acetate fractions (containing flavonols) were pooled together and kept separately. The aqueous and ethyl acetate fractions were concentrated under vacuum at 37 °C in a rotary evaporator. The anthocyanin fraction was subjected to further purification using Amberlite XAD-16 column chromatography. Briefly, one litre of purified water was used to rinse the column followed by activation with 0.5 l of 2 % aqueous sodium hydroxide solution. Acidified water (one litre) was used to wash the column to pH 3. The concentrated sample (10 ml) of the anthocyanin fraction was loaded onto the column, which was then rinsed with 0.3 l of acidified water (pH 3) at a flow rate of 10 ml min−1 to remove phenolic acids. Anthocyanins were then eluted with acidified methanol [95 : 5, methanol–acidified water (pH 2), v/v]. The methanol fraction (containing anthocyanins) and ethyl acetate fraction (containing flavonols) were subjected to freeze-drying at −80 °C. The freeze-dried extracts were kept at −80 °C until further analysis.
Antibiofilm and biofilm disruption assay
The antibiofilm activity of the C. ternatea flower anthocyanin-rich fraction obtained from partial purification of the crude ethanol extract was tested against laboratory control strains obtained from the American Type Culture Collection (ATCC) of P. aeruginosa (ATCC 9027, ATCC 27853, ATCC 10145 and ATCC BAA-47). Biofilm inhibitory activity was evaluated using a microtitre plate assay [25]. Bacteria were grown in 10 ml of Brain Heart Infusion (BHI) broth at 37 °C for 18 h. The overnight bacterial suspension was adjusted to 0.5 McFarland standard to obtain an optical density reading at 625 nm (OD625) of 0.1, which is about 1×108 c.f.u. ml–1. The suspension was then diluted 1 : 100 in BHI broth containing 1 % glucose. The anthocyanin-rich fraction was sterilized using a polyethersulfone Millipore filter (0.22 µm). The diluted bacterial suspension (100 µl) was added to a 96-well plate after which 100 µl of the anthocyanin-rich fraction was added to obtain a final concentration of 0.313–5.0 mg ml−1. The plate was incubated at 37 °C for 24 h. After incubation, the contents of the wells were aspirated and wells were rinsed with water gently, three times. The wells were heat fixed at 60 °C for 1 h followed by staining with 0.2 ml of 0.1 % crystal violet for 10 min. The wells were rinsed with water and air-dried. Lastly, 200 µl of 95 % ethanol was added and absorbance was measured at 570 nm. The negative control was a bacterial suspension with BHI broth (no treatment) while the positive control was a bacterial suspension with the addition of 100 % sodium hypochlorite, a disinfectant with effective biofilm-eliminating properties [26]. The strains were classified into the following groups based on biofilm formation: OD570 <2=weak biofilm producer, 2≤OD570≤4=moderate biofilm producer, OD570 >4=strong biofilm producer [27]. As for the biofilm disruption assay, the wells were inoculated with bacterial suspension alone and incubated for 24 h at 37 °C. The wells were washed three times with PBS before the addition of extracts with the same concentration range as above, and further incubated for 24 h. After 24 h, the solution in the wells was removed and wells were rinsed with water three times before being subjected to the crystal violet assay as described above.
Growth curve (pour-plate method)
The effect of the anthocyanin-rich fraction (5 mg ml−1) on P. aeruginosa ATCC 9027 growth was evaluated using the standard pour-plate method [28]. Serial dilutions (1 :10) at 103 to 106 of treated bacteria culture was performed and 100 µl of individual diluted bacteria culture was dispensed onto the bottom of Petri dishes. Then 18 ml of molten Mueller-Hinton agar (45 °C) was poured and mixed with the sample by gently swirling the plate. The agar was allowed to thoroughly solidify before inverting the plates for incubation at 37 °C for 24 h. The number of P. aeruginosa (ATCC 9027) colonies was recorded from plates with 30–300 colonies as colony-forming units (c.f.u.). The microbial data were then transformed to logarithm of the number of c.f.u. (log c.f.u. ml–1) [29].
Attachment assay
The attachment assay was carried out on P. aeruginosa ATCC 9027 to determine if the anthocyanin-rich fraction (5.0 mg ml−1) could reduce or inhibit bacterial attachment onto hydrophobic (polystyrene) and hydrophilic (glass) surfaces [25]. Polystyrene surfaces were obtained by cutting Petri dishes into 7 cm in length by 2 cm in width. The polystyrene strips were sterilized in 10 % sodium hypochlorite and then immersed in 70 % ethanol followed by distilled water. Glass slides were sterilized by immersion in 100 % acetone followed by 70 % ethanol and rinsed with distilled water. Bacteria were grown in BHI broth at 37 °C for 18 h. The bacterial cell culture was centrifuged at 4000 g (10 min at 4 °C) and the pellet was washed gently with PBS and resuspended to achieve an optical density reading of 1±0.2 at 550 nm. The bacterial suspension (15 ml) was added to 15 ml of he anthocyanin-rich fraction (5 mg ml−1) or 15 ml of BHI broth (negative control) in a Petri dish with a glass or polystyrene strip and incubated at 37 °C for 1 h. After incubation, the glass or polystyrene strips were gently rinsed three times with PBS followed by staining with 0.1 % (w/v) crystal violet. The attached cells were counted using a light microscope (BX51) equipped with NIS-Elements software (Olympus) at 1000× magnification with immersion oil and a total of 30 fields of view. The number of attached cells was expressed as log c.f.u. cm–2.
Scanning electron microscopy (SEM) analysis
The biofilms of P. aeruginosa ATCC 9027 were allowed to grow on polystyrene squares (1×1 cm) placed in 24-well polystyrene plates supplemented with or without anthocyanin-rich fraction of C. ternatea flowers (5 mg ml−1) and incubated for 24 h at 37 °C. The cells were fixed for 2 h using 2.5 % glutaraldehyde before washing with PBS. The samples were dehydrated with increasing concentrations (50, 60, 70, 80, 90 and 100 %) of ethanol sequentially for 10 min each time. The samples were kept in a desiccator containing silica gel. The surface of the samples was gold-sputtered using a sputter coater (Q150RS; Quorum) for 10 min prior to SEM examination. The effects of the extract on bacterial biofilm formation on a polystyrene surface was examined using an Hitachi S3400N-II scanning electron microscope by observing bacterial cell morphology as well as cell numbers and biofilm cell density.
Quantitative real-rime PCR (RT-qPCR)
P. aeruginosa ATCC 9027 (1×106 c.f.u. ml−1) was treated with and without anthocyanin-rich fraction of C. ternatea flowers (5 mg ml−1) in a 24-well polystyrene plate and incubated for 24 h at 37 °C. After incubation, cells were washed with sterile PBS three times and collected after 10 min of centrifugation (4000 g at 4 °C). The extraction of total RNA from treated and untreated groups was done using TRIzol Reagent (Invitrogen) followed by cDNA synthesis using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific) according to the protocol stated by the manufacturer. RT-qPCR was performed with the 2×Brilliant III Ultra-fast SYBR Green qPCR Kit (Agilent) using the primer sets described in Table 1. RT-qPCR (Quant Studio5 Real-Time PCR system; Applied Biosystems) was performed under the following set of temperatures: 50 °C for 2 min, followed by initial denaturation for 3 min at 95 °C. Consecutively, 40 PCR cycles were performed at 95 °C for 5 s, 64 °C for 10 s and 72 °C for 10 s. The genes proC and rpoD were used as internal controls. The critical threshold cycle (CT) value was determined followed by calculation and determination of the fold change. The change-in-threshold was determined using the 2−ΔΔCT method to calculate the relative expression of the treated group compared to the control (untreated) group [30].
Table 1.
List of primers used for real-time PCR analysis
|
Gene |
Forward primer (5′–3′) |
Reverse primer (5′–3′) |
|---|---|---|
|
lasI |
AGGTGTTCTTGAGCATGTAGG |
CGAGATGGAGATCGATGGTTATG |
|
lasR |
GATGAAGGCGTTCTCGTAGTC |
GCTCAAGTGGGAAATTGGAATG |
|
rhlI |
TTCATCGAGAAGCTGGGTTG |
GCTCCTGGCGATGATGTAG |
|
rhlR |
GGCTTCGATTACTACGCCTATG |
TTCTGCATCTGGTAGCGTTC |
|
algC |
CAACTGGTCCAGCAGTTCTC |
GGTGTAGTTCAGCTTCCAGTTC |
|
pelC |
GATCTGCAACTGGTCGATGA |
GTTCTTGTACTGCCACTCCTC |
|
lasB |
GATCGGCTACGACATCAAGAA |
GCACGTCGATACCGTTGTA |
|
pslD |
CGATCGTTGTTGACGGTGTA |
TTCGTGCCCAAGTCGATG |
|
lecB |
ACGGCGTCGTTGTAATCAT |
AAAGTGCAGATCCAGGTCAG |
|
proC |
CAGGCCGGGCAGTTGCTGTC |
GGTCAGGCGCGAGGCTGTCT |
|
rpoD |
ACTTCTTGGCGATGGAGATG |
TCGCCGAGATCAAGGAAATC |
Statistical analysis
All experiments were carried out in triplicate. The results were expressed as the mean value ±sd. The data obtained were analysed using either an independent t-test, one-way ANOVA followed by post-hoc Tukey’s test or two-way ANOVA followed by post-hoc Bonferroni test and significance was set at P<0.05 using the SPSS 23 software (IBM).
Results and discussion
Antibiofilm activity of C. ternatea flower water extract, ethanol extract and anthocyanin-rich fraction
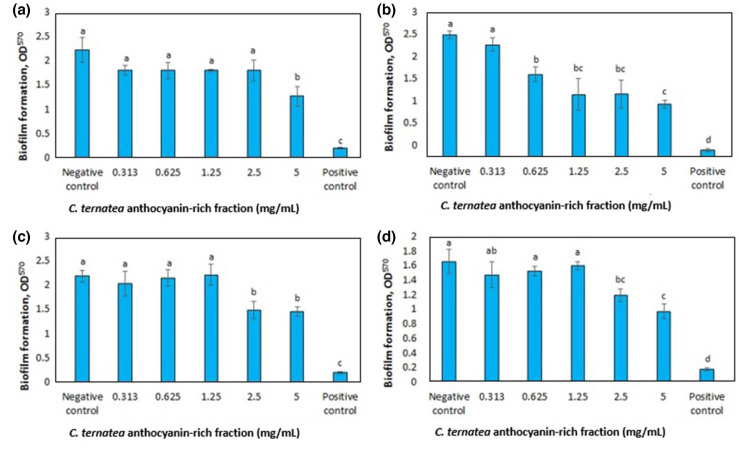
The biofilm inhibitory activity of C. ternatea flower crude water and ethanol extracts and anthocyanin-rich fraction was investigated by quantifying biofilm formation by four P. aeruginosa strains when treated with extracts at a concentration range of 0.313–5 mg ml−1. The crude water and solvent extracts did not display inhibition of biofilm formation over the range of concentrations tested (data not shown). The anthocyanin-rich fraction inhibited biofilm formation with increasing concentrations (Fig. 1) as observed in the reduction of absorbance readings. A lower absorbance reading (OD570nm) indicates lower biomass of biofilm as stained by crystal violet. The minimum biofilm inhibitory concentration (MBIC) was determined when there was a significant difference between the treated group at the lowest concentration and the negative control.
Fig. 1.
Effect of the C. ternatea anthocyanin-rich fraction on biofilm formation at 24 h of P. aeruginosa (a) ATCC 10145, (b) ATCC 9027, (c) ATCC 27853 and (d) ATCC BAA-47. Different letters indicate significant differences in biofilm formation at P<0.05, as analysed by one-way ANOVA. Bacterial suspension without treatment was the negative control while bacterial suspension treated with sodium hypochlorite was used as the positive control.
The MBIC of the C. ternatea anthocyanin-rich fraction towards the P. aeruginosa strains is presented in Table 2. The strains were classified as strong, moderate or weak biofilm producers based on the OD values according to an established protocol, which also enables investigation of the influence of treatment on the strains [27]. The MBIC values among the four strains were compared, with P. aeruginosa ATCC 9027 having the lowest value (0.625 mg ml−1) and P. aeruginosa ATCC 10145 the highest (5 mg ml−1). As the MBIC for P. aeruginosa ATCC 10145 was 5 mg ml−1, biofilm inhibition was assessed for all strains treated with 5 mg ml−1 C. ternatea anthocyanin-rich fraction. The difference in OD at 5 mg ml−1 divided by the OD of the negative control was used to calculate the percentage of inhibition of biofilm. At 5 mg ml−1, the biofilm inhibition of the anthocyanin-rich fraction against P. aeruginosa ATCC 9027 (57.0 %) was higher compared to P. aeruginosa ATCC 27853 (33.6 %) but not higher than the biofilm inhibition of P. aeruginosa ATCC 10145 and P. aeruginosa ATCC BAA-47. The MBIC value for P. aeruginosa ATCC 9027 treated with the anthocyanin-rich fraction was lower compared to P. aeruginosa ATCC 10145 and P. aeruginosa ATCC BAA-47, but there were no significant differences in biofilm inhibition at 5 mg ml−1 among the strains. The MBIC value of the anthocyanin-rich fraction against P. aeruginosa strains was comparable to other natural compounds such as cinnamon leaf essential oil which caused cellular shrinkage, cell wall damage and decreased biofilm density [31], and betacyanins from red pitahaya and red spinach which had antibiofilm activity through reduction of bacterial hydrophobicity and attachment to the surface without affecting bacterial cell viability [25].
Table 2.
Minimum biofilm inhibitory concentration (MBIC) of C. ternatea anthocyanin-rich fraction against P. aeruginosa strains
|
Bacterial strain |
Biofilm production* |
MBIC (mg ml−1) |
Biofilm inhibition (%) at 5 mg ml−1 |
|---|---|---|---|
|
P. aeruginosa ATCC 10145 |
Moderate |
5.0 |
42.8±7.4ab |
|
P. aeruginosa ATCC 9027 |
Moderate |
0.625 |
57.0±3.3b |
|
P. aeruginosa ATCC 27853 |
Moderate |
2.5 |
33.6 ± 4.2a |
|
P. aeruginosa ATCC BAA-47 |
Weak |
2.5 |
41.4±6.4ab |
MBIC values were means of three biological replicates. Values for biofilm inhibition (%) are expressed as mean±sd. Different letters indicate significant difference in biofilm inhibition among different bacterial strains at P<0.05, as analysed by one-way ANOVA.
*Weak: OD570 <2; Moderate: 2≤OD570≤4; Strong: OD570 >4 [25].
The biofilm inhibition activity of C. ternatea flowers against P. aeruginosa is probably due to the anthocyanins since the effect was not observed in crude water or solvent extracts. The lack of antibiofilm activity in the crude extracts was probably due to the lower concentration of anthocyanins. A previous study reported that flower juice of C. ternatea had anti-biofilm activity against P. gingivalis [32]. However, the effective concentration was not reported as the fresh juice was tested at different percentages (v/v) of the juice. The flower juice inhibited biofilm formation at all concentrations tested compared to the control (untreated group), in which 100 % C. ternatea flower juice was found to have the best biofilm inhibitory effect (89 %) at 24 h. Another study found that C. ternatea flower extract at 0.06 mg ml−1 significantly prevented S. mutans biofilm formation and eradicated the established biofilm compared to S. mutans treated with tetracycyline [23]. However, the mechanism of action in eliciting this activity against S. mutans is not known. The study was extended and reported that the extract inhibited violacein produced by Chromobacterium violaceum bacteria, routinely used as a model for quorum sensing [33]. To the best of our knowledge, this is the first report on the anti-biofilm activity of the C. ternatea flower anthocyanin-rich fraction towards P. aeruginosa strains. P. aeruginosa has been listed as one of the ESKAPE pathogens responsible for a majority of nosocomial infections and capable of ‘escaping’ the biocidal action of antimicrobial agents [34, 35].
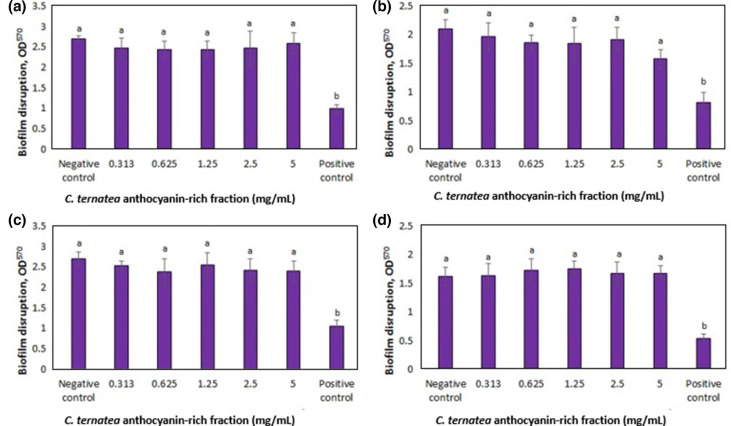
The ability of the C. ternatea anthocyanin-rich fraction to disrupt pre-formed biofilms was also determined (Fig. 2). The anthocyanin-rich fraction was not able to disrupt pre-formed biofilms against all P. aeruginosa strains tested. This suggests that the C. ternatea anthocyanins may inhibit bacterial cell attachment to the surface but are unable to act on pre-formed biofilms. Further investigations are needed to understand the possible mechanism of action for the antibiofilm activity of the anthocyanin-rich fraction as attachment is the initial step required for biofilm formation followed by bacterial cell proliferation, matrix production and detachment [36].
Fig. 2.
Effect of the C. ternatea anthocyanin-rich fraction on biofilm disruption at 24 h of P. aeruginosa (a) ATCC 10145, (b) ATCC 9027, (c) ATCC 27853 and (d) ATCC BAA-47. Different letters indicate significant differences in biofilm disruption at P<0.05, as analysed by one-way ANOVA. Bacterial suspension without treatment was the negative control while bacterial suspension treated with sodium hypochlorite was used as the positive control.
Effect of the anthocyanin-rich fraction on biofilm formation and bacterial viability
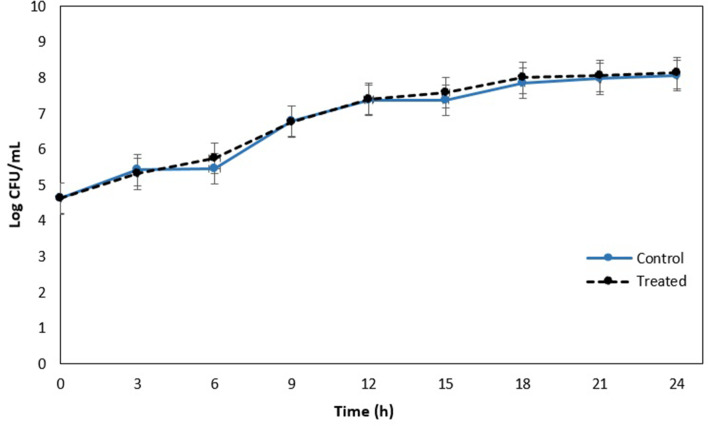
P. aeruginosa ATCC 9027 was selected for further studies as it had the lowest MBIC value compared to the other strains. A concentration of the anthocyanin-rich fraction of 5 mg ml−1 was chosen for further investigation as this produced the highest inhibitory effect on biofilm formation. To determine if the reduction in biofilm formation was a true effect of the anthocyanin-rich fraction and not a result of toxic activity towards bacterial viability, P. aeruginosa (ATCC 9027) growth in the presence of 5 mg ml−1 anthocyanin-rich fraction was determined every 3 h over a 24 h period. We observed that viability was not affected relative to the negative control (Fig. 3). Bacteria will develop drug resistance towards drugs that affect microbial cell viability, thus limiting the application of a drug [25]. Subsequent experiments on the antibiofilm potential and mechanism of action were carried out with the anthocyanin-rich fraction at 5 mg ml−1 since this concentration did not affect bacterial viability.
Fig. 3.
Impact of the C. ternatea anthocyanin-rich fraction (5 mg ml−1) on bacterial growth of P. aeruginosa ATCC 9027 over 24 h. There was no significant difference in bacterial growth over the respective time points (P>0.05) as analysed by an independent t-test.
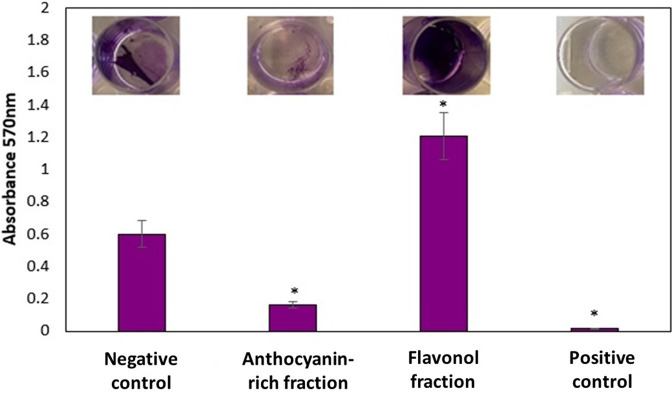
The antibiofilm activity of the C. ternatea flowers may be attributed to the presence of flavonols and or anthocyanins. The crude extracts of C. ternatea flowers did not demonstrate any antibiofilm activity over the same concentration range tested with the anthocyanin-rich fraction (0.313–5 mg ml−1). Therefore, the potential antibiofilm activity of the flavonol fraction was determined. Prior to column chromatography to obtain the anthocyanin fraction, the crude solvent extract of C. ternatea flowers was partitioned with ethyl acetate to obtain the flavonol fraction. The antibiofilm activity of the flavonol fraction was compared to the anthocyanin-rich fraction at 5 mg ml−1 at 24 h. The flavonol fraction did not possess antibiofilm activity, but enhanced biofilm formation compared to the negative control seen as an increase in absorbance readings and biofilm biomass was observed by crystal violet staining (Fig. 4). Many studies have reported on the antibiofilm activity of plant-derived anthocyanin or flavonol fractions individually, towards various bacterial strains. However, the effect or the potency of these fractions may differ based on the content, composition or structural variation of the compounds. One study found the flavonoid-rich fraction (obtained through ethyl acetate partitioning) of Centella asiatica leaves had antibiofilm activity against P. aeruginosa (PAO1) [37]. The fraction was mainly composed of kaempferol, quercetin, apigenin, rutin and naringin [38] and it is believed that all the constituents acted synergistically in inhibiting biofilm formation. Although the flavonols of C. ternatea flowers consist mostly of various derivatives of kaempferol and quercetin [21], they differ in the structural/functional groups that, together, may have acted antagonistically to enhance biofilm formation. Thus, we believe that the antibiofilm effect of C. ternatea flowers is due to the anthocyanins. Further isolation of individual anthocyanins from the anthocyanin-rich fraction could identify the active molecule responsible for the antibiofilm activity.
Fig. 4.
Biofilm formation by P. aeruginosa ATCC 9027 treated with the C. ternatea anthocyanin-rich fraction and flavonol fraction at 5 mg ml−1 (24 h) and the corresponding biofilm biomass stained with crystal violet (0.1%). Significant differences relative to the negative control are marked (*P<0.05), as analysed by one-way ANOVA. The negative control was bacterial suspension with BHI broth (no treatment) while the positive control was bacterial suspension with the addition of sodium hypochlorite.
Effect of the anthocyanin-rich fraction on bacterial attachment to hydrophilic and hydrophobic surfaces
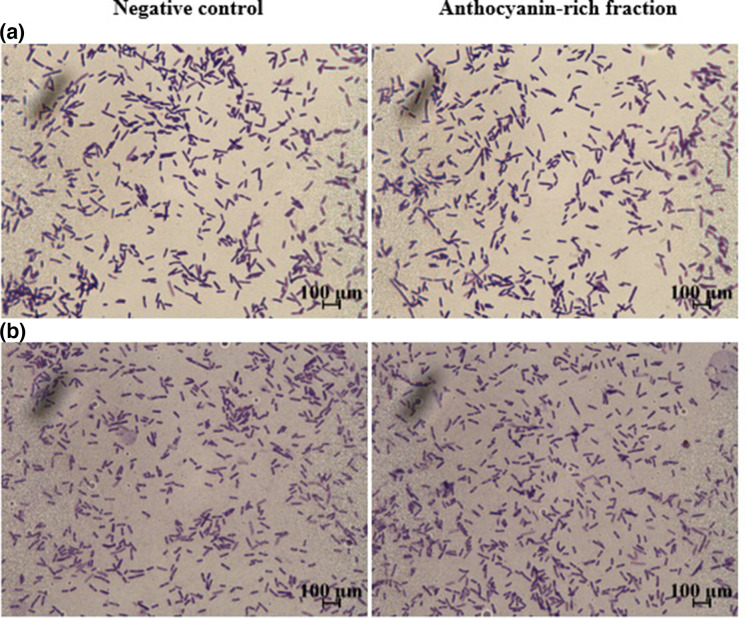
Bacterial cell attachment to the surface is the initial step required for biofilm formation followed by bacterial cell proliferation, matrix production and detachment [36]. Hydrophobic bacteria would have a greater ability to attach to hydrophobic surfaces such as polystyrene, compared to hydrophilic surfaces [25]. Various outer membrane proteins are involved in the cell surface hydrophobicity and adhesion of P. aeruginosa [39]. P. aeruginosa (ATCC 9027) was evaluated for its ability to adhere to a hydrophobic (polystyrene) and hydrophilic (glass) surface treated with C. ternatea anthocyanin-rich fraction for 1 h (Table 3 and Fig. 5). Prior to the application of the anthocyanin-rich fraction, the number of P. aeruginosa attached to the polystyrene and glass surface was 7.03 and 7.25 log c.f.u. cm–2, respectively (Table 3). The anthocyanin-rich fraction did not reduce the attachment of bacteria to the polystyrene or glass surface when compared to the negative control.
Table 3.
Effect of C. ternatea anthocyanin-rich fraction (5 mg ml−1) on the attachment of P. aeruginosa (ATCC 9027) to polystyrene and glass surfaces
|
Bacterial strain |
No. of P. aeruginosa cells attached |
|||
|---|---|---|---|---|
|
Polystyrene (log c.f.u. cm –2 ) |
Glass (log c.f.u. cm –2 ) |
|||
|
Negative control |
Anthocyanin-rich fraction |
Negative control |
Anthocyanin-rich fraction |
|
|
P. aeruginosa (ATCC 9027) |
7.03 ± 0.17* |
7.12 ± 0.13* |
7.25 ± 0.09* |
7.19 ± 0.11* |
*Same letters indicate no significant difference of the bacterial strain within the same surface at P>0.05, as analysed by two-way ANOVA.
Fig. 5.
Micrographs (1000× magnification; bars, 100 µm) of crystal violet- (0.1%) stained P. aeruginosa (ATCC 9027) attached to (a) polystyrene and (b) glass surfaces after 1 h in the presence or absence of C. ternatea anthocyanin-rich fraction (5 mg ml−1).
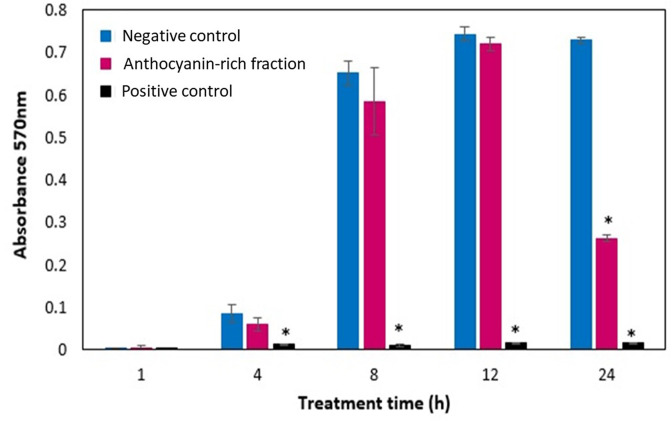
Biofilm inhibitory activity of the C. ternatea flower anthocyanin-rich fraction was evaluated against P. aeruginosa (ATCC 9027) in a time–response manner at 1, 4, 8, 12 and 24 h at 5 mg ml−1 (Fig. 6), helping to understand biofilm formation and maturation as well as the effect of the fraction at different stages. The biofilm of P. aeruginosa (ATCC 9027) reached maturation around 8–12 h. The anthocyanin-rich fraction was found to have no effect against the biofilm during the maturation phase. However, the anti-biofilm effect of the fraction was observed at 24 h, which is during the stationary phase of the bacteria (Figs. 3 and 6). The results obtained suggest the anthocyanin-rich fraction does not exert its anti-biofilm effect through inhibition of the initial phase of bacterial cell attachment but possibly through a different mode of action that occurs later during the stationary phase of bacterial growth which may directly or indirectly affect biofilm formation.
Fig. 6.
Biofilm formation of P. aeruginosa (ATCC 9027) treated with C. ternatea anthocyanin-rich fraction (5 mg ml−1) at 1, 4, 8, 12 and 24 h. Significant differences from the negative control group at respective time intervals are marked (*P<0.05), as analysed by one-way ANOVA. The negative control was bacterial suspension with BHI broth (no treatment) while the positive control was bacterial suspension with the addition of sodium hypochlorite.
P. aeruginosa (ATCC 9027) was treated with C. ternatea anthocyanin-rich fraction and visualized by SEM (Fig. 7). The reduction in biofilm formation of P. aeruginosa (ATCC 9027) observed using the crystal violet assay after 24 h (Fig. 6) was supported by the SEM images (Fig. 7). The SEM images demonstrate that the bacterial cells treated with the anthocyanin-rich fraction appear to be intact and have a lower cell density compared to the negative (untreated) control. The number of P. aeruginosa (ATCC 9027) bacterial cells was significantly reduced (P<0.05) after treatment with the C. ternatea anthocyanin-rich fraction (Table 4). P. aeruginosa (ATCC 9027) showed a 1.01 log reduction after treatment with the C. ternatea anthocyanin-rich fraction compared to the negative control. In the industry setting, effective cleaning should achieve at least a 1 log order of microorganism removal from surfaces [40]. The reduction of bacterial cell number (log c.f.u. cm−2) using the anthocyanin-rich fraction is similar to treatment with betacyanin, a natural biofilm-inhibiting agent. Betacyanin possessed biofilm inhibition activity against S. aureus and P. aeruginosa , and was able to reduce 1.1–1.2 log c.f.u. cm−2 of bacteria attached to a surface [25].
Fig. 7.
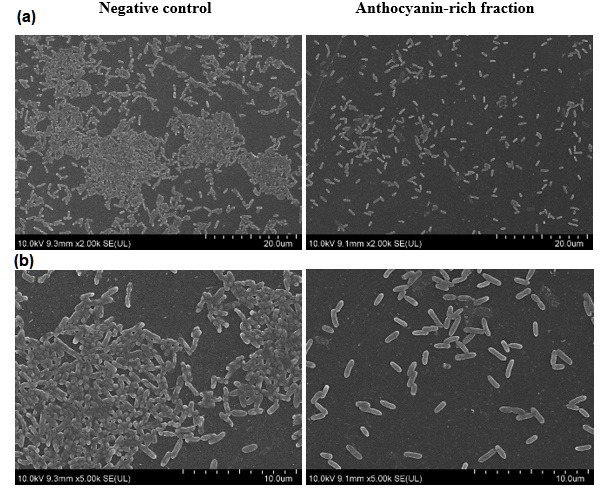
SEM images of biofilm formation. Biofilm formation by P. aeruginosa (ATCC 9027) treated with and without C. ternatea anthocyanin-rich fraction (5 mg ml−1) at (a) 2000× (bar, 20 µm) and (b) 5000× (bar, 10 µm) magnification at 24 h.
Table 4.
Effect of C. ternatea anthocyanin-rich fraction on the number of bacterial cells on SEM images of P. aeruginosa (ATCC 9027)
|
Bacterial strain |
Bacterial cell no. (log c.f.u. cm–2) |
|
|---|---|---|
|
Negative control |
Anthocyanin-rich fraction |
|
|
P. aeruginosa (ATCC 9027) |
6.14±0.06a |
5.13±0.07b |
Different letters indicate significant difference in bacterial cell number at P<0.05, as analysed by an independent t-test.
Effect of anthocyanin-rich fraction on P. aeruginosa (ATCC 9027) gene expression
A substance may have exerted its antibiofilm activity either through alterations in gene expression, interference in quorum sensing pathways, inhibition of adhesion or alteration of metabolic pathways [40, 41]. The antibiofilm effect of the anthocyanin-rich fraction against P. aeruginosa (ATCC 9027) was therefore investigated by using gene expression studies. P. aeruginosa biofilm formation is known to be regulated by the quorum sensing system, two-component regulatory system GacS/GacA and RetS/LadS pathway, and c-di-GMP-dependent polysaccharide biosynthesis [42]. Real-time PCR-based quantification of the lasR lasI, rhlR and rhlI genes was performed to assess the effect of the anthocyanin-rich fraction on gene regulation in the quorum-sensing pathway as well as on the algC, lecB, pslD and pelC genes involved in biofilm formation, structure and stability and lasB gene involved in the cytotoxicity and virulence of P. aeruginosa . The housekeeping genes were proC and rpoD (Table 1).
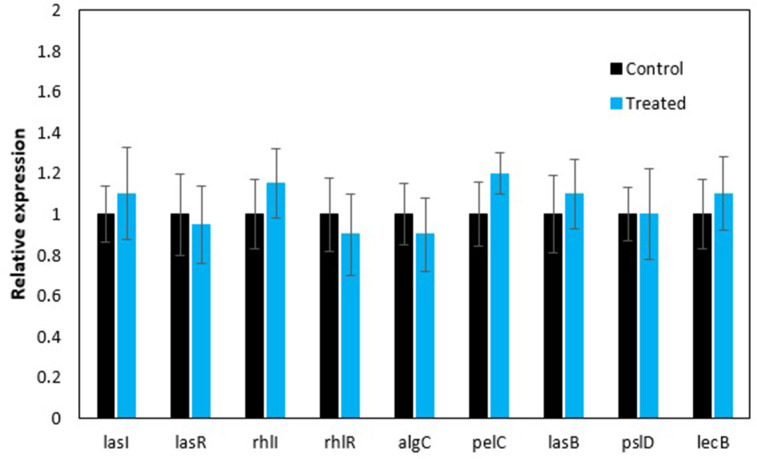
There was no significant reduction in fold change of gene expression at the mRNA level in bacteria treated with the anthocyanin-rich fraction (Fig. 8). There are many other genes which could have been regulated that resulted in the observed inhibition of biofilm formation. One study found plant-derived ursolic acid with antibiofilm activity against Escherichia coli at non-toxic concentrations and inhibited biofilms without interfering with quorum sensing-related genes. Results from DNA microarrays found that ursolic acid induced genes involved in chemotaxis and mobility (cheA, tap, tar and motAB), heat shock response (hslSTV and mopAB), cysteine synthesis (cysK), sulphur metabolism (cysD), as well as several genes with unknown functions [43].
Fig. 8.
RT-qPCR results of gene expression of P. aeruginosa (ATCC 9027) treated with C. ternatea anthocyanin-rich fraction for 24 h. Expression of genes in both the control and experimental groups was normalized to proC and rpoD as the reference genes. The control group expression level is designated as 1, so values >1 indicate up-regulation and values <1 indicate down-regulation. There was no significant difference between the control and treated group at P<0.05, as analysed by an independent t-test.
The findings obtained in the current study suggest the possibility of other genes or pathways are likely to be responsible for the antibiofilm activity. The samples for gene expression studies were collected at 24 h because the anti-biofilm effect was not observed at earlier time points tested. It is also possible that the gene expression may have occurred before 24 h, and thus the effect was not seen at 24 h. Further studies would be needed and are recommended such as utilization of DNA microarrays to measure the expression levels of large numbers of genes simultaneously, which may assist in the elucidation of the genes that may responsible for the antibiofilm effect.
In conclusion, the anthocyanin-rich fraction of C. ternatea flowers has significant biofilm inhibitory activity towards P. aeruginosa and also reduces bacterial attachment to polystyrene surfaces. The compound can be potentially developed as an anti-biofilm agent to treat antibiofilm-related infections of P. aeruginosa or used in a clinical setting to prevent biofilm formation on surgical and non-surgical apparatuses. Determining the mode of action of the bioactive fraction together with in vivo studies would assist in improving the effectiveness of this anthocyanin-rich fraction in biofilm inhibition. In addition, the study could be extended to investigate the anti-biofilm activity of the C. ternatea flower anthocyanin-rich fraction towards Gram-positive bacteria such as Staphylococcus aureus .
Funding information
This work was funded and supported by the Tropical Medicine and Biology platform and School of Science, Monash University Malaysia.
Author contributions
E.J.J. contributed to data curation, formal analysis, investigation and writing – original draft preparation. S.N. contributed to supervision and writing – review and editing. Y.Y.L. contributed to supervision and writing – review and editing. W.S.C. contributed to conceptualization, funding acquisition, project administration, resources, supervision and writing – review and editing.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ATCC, American Type Culture Collection; BHI, brain heart Infusion; MBIC, minimum biofilm inhibitory concentration; RT-qPCR, quantitative real-time PCR; SEM, scanning electron microscopy.
References
- 1.Penesyan A, Gillings M, Paulsen IT. Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules. 2015;20:5286–5298. doi: 10.3390/molecules20045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave Commun. 2018;4:1–13. doi: 10.1057/s41599-018-0152-2. [DOI] [Google Scholar]
- 3.Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23:23. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma VK, Johnson N, Cizmas L, McDonald TJ, Kim H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere. 2016;150:702–714. doi: 10.1016/j.chemosphere.2015.12.084. [DOI] [PubMed] [Google Scholar]
- 5.Scheffler RJ, Colmer S, Tynan H, Demain AL, Gullo VP. Antimicrobials, drug discovery, and genome mining. Appl Microbiol Biotechnol. 2013;97:969–978. doi: 10.1007/s00253-012-4609-8. [DOI] [PubMed] [Google Scholar]
- 6.Hancock RE. Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infect Dis. 2005;5:209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro SM, Felício MR, Boas EV, Gonçalves S, Costa FF, et al. New frontiers for anti-biofilm drug development. Pharmacol Ther. 2016;160:133–144. doi: 10.1016/j.pharmthera.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan R. Biofilms: microbial cities of scientific significance. J Microbiol Exp. 2014;1:00014. doi: 10.15406/jmen.2014.01.00014. [DOI] [Google Scholar]
- 11.Mihai MM, Holban AM, Giurcaneanu C, Popa LG, Oanea RM, et al. Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr Top Med Chem. 2015;15:1552–1576. doi: 10.2174/1568026615666150414123800. [DOI] [PubMed] [Google Scholar]
- 12.Worthington RJ, Richards JJ, Melander C. Small molecule control of bacterial biofilms. Org Biomol Chem. 2012;10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33:e00181–e00189. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma G, Rao S, Bansal A, Dang S, Gupta S, et al. Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals. 2014;42:1–7. doi: 10.1016/j.biologicals.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 16.Høiby N, Ciofu O, Johansen HK, Song Z, Moser C, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3:55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambello MJ, Kaye S, Iglewski BH. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun. 1993;61:1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman DE. Molecular genetics of exopolysaccharide production by mucoid Pseudomonas aeruginosa . Eur J Clin Microbiol. 1986;5:6–10. doi: 10.1007/BF02013452. [DOI] [PubMed] [Google Scholar]
- 19.Yong YY, Dykes GA, Choo WS. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol. 2019;45:201–222. doi: 10.1080/1040841X.2019.1573802. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaraj EJ, Lim YY, Choo WS. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J Food Sci Technol. 2021;58:2054–2067. doi: 10.1007/s13197-020-04745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyaraj EJ, Lim YY, Choo WS. Effect of organic solvents and water extraction on the phytochemical profile and antioxidant activity of Clitoria ternatea flowers. ACS Food Sci Technol. 2021;1:1567–1577. doi: 10.1021/acsfoodscitech.1c00168. [DOI] [Google Scholar]
- 22.Vidana Gamage GC, Lim YY, Choo WS. Anthocyanins from Clitoria ternatea flower: biosynthesis, extraction, stability, antioxidant activity, and applications. Front Plant Sci. 2021;12:792303. doi: 10.3389/fpls.2021.792303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanti Y, Setiawan T, Lay BW. Antibacterial, antibiofilm and quorum sensing inhibitory activities of Clitoria ternatea anthocyanin against Streptococcus mutans . Int J Infect Dis. 2018;73:143–144. doi: 10.1016/j.ijid.2018.04.3739. [DOI] [Google Scholar]
- 24.Chang YJ, Pong LY, Hassan SS, Choo WS. Antiviral activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against dengue virus type 2 (GenBank accession no. MH488959) Access Microbiol. 2020;2:000073. doi: 10.1099/acmi.0.000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong YY, Ong MWK, Dykes G, Choo WS. Betacyanin-inhibited biofilm formation of co-culture of Staphylococcus aureus and Pseudomonas aeruginosa on different polymer surfaces. FEMS Microbiol Lett. 2021;368:fnaa214. doi: 10.1093/femsle/fnaa214. [DOI] [PubMed] [Google Scholar]
- 26.Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ, et al. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob Resist Infect Control. 2018;7:1–7. doi: 10.1186/s13756-018-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 28.Benson HJ. Bensons Microbiological Applications: Laboratory Manual in General Microbiology. Boston: McGraw-Hill Higher Education; 2005. [Google Scholar]
- 29.Brown AE. In: Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. Reidy PE, Fornango JS, editors. New York, USA: McGraw-Hill. Inc; 2005. [Google Scholar]
- 30.Sarabhai S, Harjai K, Sharma P, Capalash N. Ellagic acid derivatives from Terminalia chebula Retz. increase the susceptibility of Pseudomonas aeruginosa to stress by inhibiting polyphosphate kinase. J Appl Microbiol. 2015;118:817–825. doi: 10.1111/jam.12733. [DOI] [PubMed] [Google Scholar]
- 31.Wijesinghe GK, Feiria SB, Maia FC, Oliveira TR, Joia F, et al. In-vitro antibacterial and antibiofilm activity of Cinnamomum verum leaf oil against Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae . An Acad Bras Cienc. 2021;93:e20201507. doi: 10.1590/0001-3765202120201507. [DOI] [PubMed] [Google Scholar]
- 32.Widyarman AS, Sumadi S, Agustin TP. Antibiofilm effect of Clitoria ternatea flower juice on Porphyromonas gingivalis in vitro. J Indones Dent Assoc. 2018;1:7–11. doi: 10.32793/jida.v1i1.288. [DOI] [Google Scholar]
- 33.Yanti Y, Setiawan T, Lay BW. Clitoria ternatea ethanolic extract prevents dental caries via inhibiting Streptococcus mutans growth and quorum sensing. Food Res. 2021;5:492–497. doi: 10.26656/fr.2017.5(2).508. [DOI] [Google Scholar]
- 34.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 35.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, de Camargo ER, et al. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009;34:103–110. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Vasavi HS, Arun AB, Rekha PD. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J Microbiol Immunol Infect. 2016;49:8–15. doi: 10.1016/j.jmii.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Ondeko DA, Juma BF, Baraza LD, Nyongesa PK. LC-ESI/MS and GC-MS methanol extract analysis, phytochemical and antimicrobial activity studies of Centella asiatica . Asian J Chem Sci. 2020;32–51:32–51. doi: 10.9734/ajocs/2020/v8i319046. [DOI] [Google Scholar]
- 39.Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, et al. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J Biol Chem. 2004;279:52816–52819. doi: 10.1074/jbc.C400445200. [DOI] [PubMed] [Google Scholar]
- 40.Gibson H, Taylor JH, Hall KE, Holah JT. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J Appl Microbiol. 1999;87:41–48. doi: 10.1046/j.1365-2672.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 41.Shen F, Ge C, Yuan P. Metabolomics study reveals inhibition and metabolic dysregulation in Staphylococcus aureus planktonic cells and biofilms induced by carnosol. Front Microbiol. 2020;11:2315. doi: 10.3389/fmicb.2020.538572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. 2015;2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren D, Zuo R, González Barrios AF, Bedzyk LA, Eldridge GR, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol. 2005;71:4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]