Abstract
We report the molecular evidence of dengue virus (DENV) and chikungunya virus (CHIKV) infection in symptomatic individuals in Cameroon and Gabon, respectively. Arthropod-borne viruses (arboviruses) are distributed in the tropical or subtropical regions, with DENV having the highest burden. The morbidity and mortality related to arboviral diseases raise the concern of timely and efficient surveillance and care. Our aim was to assess the circulation of arboviruses [DENV, CHIKV, Zika virus (ZIKV)] among febrile patients in Dschang (West Cameroon) and Kyé-ossi (South Cameroon, border with Gabon and Equatorial Guinea). Dried blood spots were collected from 601 consenting febrile patients, and 194 Plasmodium spp.-negative samples were tested for the molecular detection of cases of DENV, CHIKV and ZIKV infection. Overall, no case of ZIKV infection was found, whereas one case of DENV infection and one case of CHIKV infection were detected in Dschang and Kyé-ossi, respectively, with the CHIKV-infected patient being resident in Gabon. Our findings suggest the need to establish an active surveillance of arbovirus transmission in Cameroon and bordering countries.
Keywords: Dengue virus, Chikungunya virus, febrile patients, real-time PCR, Cameroon, Gabon
Impact Statement
Previous evidence of the circulation of the arboviruses DENV and CHIKV in Cameroon was mainly derived through serological studies. This study shows molecular evidence of DENV and CHIKV infection in two febrile patients from Dschang-Cameroon and Kyé-ossi – a case imported from Gabon – respectively. These findings confirm the circulation of arthropod-borne viruses in these areas, which could be misdiagnosed as malaria.
Introduction
The spread of arboviral diseases is associated with wider dispersal of competent arthropod vectors, deforestation and closer contact of humans with sylvatic animals, movement of people and increasing human population densities, and transportation of goods, animals and agricultural products [1]. All of these factors apply to Cameroon, where several reports of dengue virus (DENV) and chikungunya virus (CHIKV) circulation and outbreaks exist [2–7].
In the last two decades, DENV and CHIKV circulation have been reported in Cameroon and neighbouring countries such as Gabon, the Democratic Republic of Congo, Equatorial Guinea, Nigeria, the Central African Republic and the Republic of Congo [3, 8]. No case of Zika virus (ZIKV) infection has ever been reported from Cameroon. ZIKV is known to circulate mainly in Asia and to have spread to the Pacific and the Americas [9]. African strains of ZIKV were found in countries that neighbour Cameroon, such as the Central African Republic and Nigeria, and they appeared to be more transmissible and pathogenic to foetus than Asian strains [10].
The climatic conditions and entomological features of Cameroon are suitable for arbovirus circulation [11]. A recent survey reiterates the presence of Aedes aegypti and Ae. albopictus across the country in towns such as Bafoussam and Ebolowa, which are close to Dschang and Kyé-ossi, respectively [12].
Fever is a common symptom of infectious diseases [13]. In malaria-endemic countries, fever is perceived as malaria by both caretakers and care-seekers [14–16]. Therefore, it is through passive surveillance that arboviral diseases are identified as causes of fever [17]. Awareness is raised concerning these emerging and re-emerging viruses, which cause outbreaks worldwide, as was the case in Cameroon [2, 5].
Our study aimed at the molecular identification of DENV, CHIKV and ZIKV among febrile patients not infected by Plasmodium spp., coming for consultation at healthcare facilities in Dschang (West Region) and Kyé-ossi (South Region).
Methods
We carried out a cross-sectional descriptive study in two healthcare facilities (HCFs). We collected data and samples during the rainy season in Dschang and Kyé-ossi (May–September 2017).
Dschang is a semi-urban town of the West region of Cameroon, with more than 250 000 inhabitants. Dschang District Hospital is the leading public health centre, serving the surrounding health areas. Kyé-ossi is a small semi-urban town of the South region, with more than 20 000 inhabitants. The medicalized health centre of Kyé-ossi is the leading public health centre, serving the nearest towns of Gabon and Equatorial Guinea (Fig. 1)
Fig. 1.
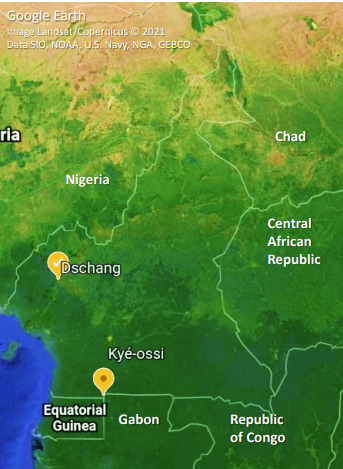
Cameroon map with study sites.
We recruited consecutive consenting febrile patients and collected drops of blood on filter papers (Whatman 1). We air-dried the filter papers and kept each of them separately in plastic bags at room temperature. We extracted parasite and human DNA from these samples using an Invitrogen kit (PureLink Genomic DNA Mini kit; Life Technologies, 2012, USA) for a final elution of 100 µl. We performed Plasmodium spp. detection following a nested PCR protocol specific for 18S rRNA gene fragments as previously described [18]. See Table S1 for a list of primers used.
We administered questionnaires to each participant or guardian of children before collecting blood samples. It was mainly to collect general information such as age, living area, gender, profession, signs and symptoms experienced prior to the consultation and possible treatment initiated.
On samples not infected with Plasmodium spp., we performed molecular tests for the detection of DENV/CHIKV/ZIKV on blood spot-eluted samples. We cut blood spots on filter papers and eluted them in 850 µl AVL lysis buffer (QIAmp viral RNA Mini kit, Qiagen, Inc., Valencia, CA, USA) for 2 h at room temperature on a thermal shaker. We extracted viral RNA from 400 µl of eluted blood samples using the QIAmp viral RNA Mini kit, according to the manufacturer’s instructions, in 45 µl final volume of PCR grade water, and then stored it at −80 °C until further processing. Viral RNA was amplified by multiplex real-time PCR for CHIKV, DENV and ZIKV by CDC trioplex real-time assay [19]. We further confirmed positive samples using a virus-specific nested real-time assay [20–24]. For all real-time PCR assays, we used the SensiFAST Probe No-ROX One-Step kit (Bioline), according to the manufacturer’s protocol, and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad). See Table S2 for the primers and probe sequences.
We analysed data using Microsoft Excel 2013 and Epi Info version 7.2.
Results
Overall, 601 febrile patients were recruited in the two healthcare facilities, and 194 of them not infected with Plasmodium spp. were included in this survey. Sixty-six (34.02 %) were from Kyé-ossi and 128 (65.97 %) from Dschang. The female gender was predominant (58.76 %, 114/194) and the mean age was 23.08±18.44 years, ranging from 3 weeks to 84 years old, with 20.62 % (40/194) being <5 years old. All of them complained about fever with a mean duration of 3.57±3.29 days (range 0 to 19 days) before coming for consultation.
One case of DENV infection was detected among the samples collected from Dschang. It was from a 20-year-old university student who reported 3 days of fever, headache and asthenia. They started a symptomatic treatment at home but did not remember the name of the medicine taken.
One case of CHIKV infection was detected among the samples collected from Kyé-ossi. It was from a 7-year-old child brought from the closest town of Gabon (Bitam) to seek care. They reported having fever, headache, cough, abdominal pain and anorexia for 2 days. They started a treatment at home based on antipyretics and an antimalarial drug (artemether–lumefantrine).
Both patients recovered fully without clinical complications. No case of ZIKV infection was detected.
Discussion
In 2002, DENV-1 antibodies were first identified in a 49-year-old Caucasian male and his 19-year-old daughter living in Yaoundé, Cameroon. The clinical features of the disease in the Caucasian male were similar to those of the young man of Dschang (fever and headache) [25]. Several studies have reported symptomatic cases of DENV in Africa [3] and more specifically in Cameroon, detected indirectly (antibodies) [26] or directly (viral RNA detection) [2], as in the present study. The widespread distribution of Ae. aegypti and the imported Ae. albopictus in Cameroon [12, 27] could explain the presence of DENV in Dschang, as previously described by Tchuandom et al. [26].
CHIKV is known to be endemic in Africa [8], especially in Gabon, where the present case originated [28]. RNA of CHIKV was identified in Kribi, South region of Cameroon, close to Kyé-ossi [7]. A few studies recognized other regions of Cameroon as being endemic for CHIKV [5, 7, 8, 29], but mainly through serological analyses. The clinical features of the boy were typical of CHIKV in children [30].
Given the extensive trade and cross-border travel between Cameroon and its neighbouring countries, the conditions for the spread of arboviral diseases across the region are certainly favourable, as mentioned by Russo et al. [8]
In the context of malaria endemicity, with no active surveillance system in place for arboviral diseases [no rapid diagnostic tests (RDT), no symptom-based detection], cases of fever will be treated with antimalarial drugs and antibiotics, in spite of malaria RDT and thick film negativity [25]. This highlights the need for an active arboviral diseases surveillance and response plan for Africa and country-specific plans for early diagnosis and treatment of cases, and efficient response to outbreaks.
One limitation of this study is related to the elimination of malaria-positive cases before arbovirus screening. Two studies reported cases of DENV/malaria co-infected children in Yaoundé, Cameroon [31, 32]. Moreover, several studies around the world described cases of DENV/malaria, malaria/DENV/CHIKV and malaria/CHIKV co-infections [33, 34]. This suggests an underestimation of the cases of DENV and CHIKV infections among our study population.
Funding information
The non-profit organisations Mingha Africa Onlus (Rome, Italy) and Projet Intégré pour la Promotion de l’Auto-Développement (PIPAD, Dschang, Cameroon) provided funding for sample collection in Cameroon and shipment to Italy. G.B.D.D. was supported by PhD student mobility grant 2017, Sapienza University of Rome (disp no 280/2017, prot. no. 0006599, 31 January 2017).
Acknowledgements
We thank the participants and the health personnel of the Dschang District hospital and the medicalized health centre of Kyé-ossi for their collaboration. Special thanks go to Yola Canaan Foundation for their technical support in Kyé-ossi.
Author contributions
G.B.D.D.: conceptualization, investigation, project administration, data curation, writing – original draft. G.V.: methodology, formal analysis, writing – original draft. C.F.: methodology, formal analysis, writing – original draft. G.M.P.: validation, writing – review. and editing. C.S.: validation, writing – review. and editing. M.L.: data curation, writing – review and editing. A.T.T.: data curation, writing – review and editing. E.B.: methodology, formal analysis. G.M.: methodology, formal analysis. A.A.: methodology, formal analysis. G.R.: funding acquisition, writing – review and editing, supervision. M.S.S.: funding acquisition, resources, writing – review and editing, supervision. G.R.: conceptualization, funding acquisition, writing – review and editing, supervision.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
The Ethics Review and Consultancy Committee (ERCC) of the Cameroon Bioethics Initiative (CAMBIN) gave approval for this study through ethical clearance CBI/427/ERCC/CAMBIN. All participants provided written informed consent before their enrolment in the study.
Footnotes
Abbreviations: CDC, centers for disease control and prevention; CHIKV, Chikungunya virus; DENV, Dengue virus; HCF, health care facility; PCR, polymerase chain reaction; ZIKV, Zika virus.
References
- 1.Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 2.Nemg Simo FB, Sado Yousseu FB, Evouna Mbarga A, Bigna JJ, Melong A, et al. Investigation of an outbreak of dengue virus serotype 1 in a rural area of Kribi, South Cameroon: a cross-sectional study. Intervirology. 2018;61:265–271. doi: 10.1159/000499465. [DOI] [PubMed] [Google Scholar]
- 3.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9:13626. doi: 10.1038/s41598-019-50135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demanou M, Pouillot R, Grandadam M, Boisier P, Kamgang B, et al. Evidence of dengue virus transmission and factors associated with the presence of anti-dengue virus antibodies in humans in three major towns in Cameroon. PLoS Negl Trop Dis. 2014;8:e2950. doi: 10.1371/journal.pntd.0002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demanou M, Antonio-Nkondjio C, Ngapana E, Rousset D, Paupy C, et al. Chikungunya outbreak in a rural area of Western Cameroon in 2006: A retrospective serological and entomological survey. BMC Res Notes. 2010;3:128. doi: 10.1186/1756-0500-3-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousseu FBS, Nemg FBS, Ngouanet SA, Mekanda FMO, Demanou M. Association between dengue virus infection and, socio-demographic characteristics, population behavior and disease symptoms. Figshare. 2018 [Google Scholar]
- 7.Maurice D, Alain S-MS, Christophe V, Rene N, Irene KT, et al. Molecular characterization of chikungunya virus from three regions of Cameroon. Virol Sin. 2015;30:470–473. doi: 10.1007/s12250-015-3663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo G, Subissi L, Rezza G. Chikungunya fever in Africa: a systematic review. Pathog Glob Health. 2020;114:136–144. doi: 10.1080/20477724.2020.1748965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu ZY, Shi WF, Qin CF. The evolution of Zika virus from Asia to the Americas. Nat Rev Microbiol. 2019;17:131–139. doi: 10.1038/s41579-018-0134-9. [DOI] [PubMed] [Google Scholar]
- 10.Aubry F, Jacobs S, Darmuzey M, Lequime S, Delang L, et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat Commun. 2021;12:916. doi: 10.1038/s41467-021-21199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuill TM. The ecology of tropical arthropod-borne viruses. Annu Rev Ecol Syst. 1986;17:189–219. doi: 10.1146/annurev.es.17.110186.001201. [DOI] [Google Scholar]
- 12.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2019;13:e0007137. doi: 10.1371/journal.pntd.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbara MAT, Liliane MT, Olivia A-A, Olivier TDS, Jean CM, et al. The assessment of fever in under-five children in the Ekounou Health Area of Yaounde, Cameroon: Usefulness of rapid diagnostic tests. Int J Med Med Sci. 2017;9:33–40. doi: 10.5897/IJMMS2016.1259. [DOI] [Google Scholar]
- 14.Birhanu Z, Abebe L, Sudhakar M, Dissanayake G, Yihdego YY-E, et al. Malaria related perceptions, care seeking after onset of fever and anti-malarial drug use in malaria endemic settings of Southwest Ethiopia. PLoS One. 2016;11:1–20. doi: 10.1371/journal.pone.0160234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertz JT, Munishi OM, Sharp JP, Reddy EA, Crump JA. Comparing actual and perceived causes of fever among community members in a low malaria transmission setting in northern Tanzania. Trop Med Int Health. 2013;18:1406–1415. doi: 10.1111/tmi.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassile T, Lokina R, Mujinja P, Mmbando BP. Determinants of delay in care seeking among children under five with fever in Dodoma region, central Tanzania: a cross-sectional study. Malar J. 2014;13:1–10. doi: 10.1186/1475-2875-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousseu FBS, Nemg FBS, Ngouanet SA, Mekanda FMO, Demanou M. Detection and serotyping of dengue viruses in febrile patients consulting at the New-Bell District Hospital in Douala, Cameroon. PLoS One. 2018;13:e0204143. doi: 10.1371/journal.pone.0204143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djeunang Dongho GB, Gunalan K, L’Episcopia M, Paganotti GM, Menegon M, et al. Plasmodium vivax infections detected in a large number of febrile duffy-negative Africans in Dschang, Cameroon. Am J Trop Med Hyg. 2021;104:987–992. doi: 10.4269/ajtmh.20-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago GA, Vázquez J, Courtney S, Matías KY, Andersen LE, et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun. 2018;9:1391. doi: 10.1038/s41467-018-03772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drosten C, Göttig S, Schilling S, Asper M, Panning M, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol. 2002;40:2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 22.Edwards CJ, Welch SR, Chamberlain J, Hewson R, Tolley H, et al. Molecular diagnosis and analysis of Chikungunya virus. J Clin Virol. 2007;39:271–275. doi: 10.1016/j.jcv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14:416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yenchitsomanus PT, Sricharoen P, Jaruthasana I, Pattanakitsakul SN, Nitayaphan S, et al. Rapid detection and identification of dengue viruses by polymerase chain reaction (PCR) Southeast Asian J Trop Med Public Health. 1996;27:228–236. [PubMed] [Google Scholar]
- 25.Krippner R, von Laer G. First confirmed dengue-1 fever cases reported from Cameroon. J Travel Med. 2002;9:273–274. doi: 10.2310/7060.2002.24119. [DOI] [PubMed] [Google Scholar]
- 26.Tchuandom SB, Tchouangueu TF, Antonio-Nkondjio C, Lissom A, Djang JON, et al. Seroprevalence of dengue virus among children presenting with febrile illness in some public health facilities in Cameroon. Pan Afr Med J. 2018;31:177. doi: 10.11604/pamj.2018.31.177.16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, et al. Aedes mosquitoes and aedes-borne arboviruses in africa: current and future threats. Int J Environ Res Public Health. 2018;15:E220. doi: 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyrefitte CN, Bessaud M, Pastorino BAM, Gravier P, Plumet S, et al. Circulation of Chikungunya virus in Gabon, 2006-2007. J Med Virol. 2008;80:430–433. doi: 10.1002/jmv.21090. [DOI] [PubMed] [Google Scholar]
- 29.Peyrefitte CN, Rousset D, Pastorino BAM, Pouillot R, Bessaud M, et al. Chikungunya virus, Cameroon, 2006. Emerg Infect Dis. 2007;13:768–771. doi: 10.3201/eid1305.061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohan A, Kiran DHN, Manohar IC, Kumar DP. Epidemiology, clinical manifestations, and diagnosis of Chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol. 2010;55:54–63. doi: 10.4103/0019-5154.60355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monamele GC, Demanou M. First documented evidence of dengue and malaria co-infection in children attending two health centers in Yaoundé, Cameroon. Pan Afr Med J. 2018;29:227. doi: 10.11604/pamj.2018.29.227.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nkenfou CN, Fainguem N, Dongmo-Nguefack F, Yatchou LG, Kameni JJK, et al. Enhanced passive surveillance dengue infection among febrile children: Prevalence, co-infections and associated factors in Cameroon. PLoS Negl Trop Dis. 2021;15:e0009316. doi: 10.1371/journal.pntd.0009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salam N, Mustafa S, Hafiz A, Chaudhary AA, Deeba F, et al. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: a systematic review. BMC Public Health. 2018;18:710. doi: 10.1186/s12889-018-5626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raut CG, Rao NM, Sinha DP, Hanumaiah H, Manjunatha MJ. Chikungunya, dengue, and malaria co-infection after travel to Nigeria, India. Emerg Infect Dis. 2015;21:908–909. doi: 10.3201/eid2105.141804. [DOI] [PMC free article] [PubMed] [Google Scholar]


