Abstract
Purpose
Until 2018, National Cancer Comprehensive Network guidelines recommended androgen deprivation therapy (ADT) for all men with intermediate-risk prostate cancer who had undergone radiation therapy. Intermediate risk was stratified as favorable and unfavorable in 2018, and ADT recommendation was limited to men with unfavorable intermediate-risk prostate cancer. Data suggesting this stratification and treatment deintensification were first published in December 2013. This study characterizes US national trends for demographic, clinical, and socioeconomic factors associated with ADT use in men with intermediate-risk prostate cancer who have undergone definitive radiation therapy.
Methods and Materials
This retrospective cohort study examined 108,185 men in the National Cancer Database who were diagnosed with intermediate-risk prostate cancer from 2004 to 2016. Temporal trends in demographic, clinical, and socioeconomic factors among men with intermediate-risk prostate cancer and associations with the use of ADT were characterized.
Results
In total, 108,185 men diagnosed with intermediate-risk prostate cancer underwent radiation therapy from 2004 to 2016. Of these men, 41.09% received ADT. Among the 60,705 men with favorable intermediate-risk prostate cancer, 32.06% received ADT. Among the 47,480 men with unfavorable intermediate-risk prostate cancer, 52.64% received ADT. On multivariate analysis, use of ADT was associated with age and year of diagnosis; being a race other than White; having government-based insurance; having a higher prostate-specific antigen level, tumor stage, and Gleason score; receiving treatment at a nonacademic center; and receiving external beam radiation therapy alone.
Conclusions
The findings highlight that ADT use is variable in men undergoing definitive radiation therapy for intermediate-risk prostate cancer, with the data suggesting that several clinical and socioeconomic disparities influence its use. The findings suggest that a significant proportion of men with favorable intermediate-risk prostate cancer receive ADT and remain candidates for treatment de-escalation, whereas a significant proportion of men with unfavorable intermediate-risk prostate cancer may be undertreated when ADT is omitted.
Introduction
Prostate cancer is a significant health burden, with 191,930 new cases estimated and 33,330 deaths in the United States in 2020.1 Until 2018, National Cancer Comprehensive Network guidelines recommended androgen deprivation therapy (ADT) for all men with intermediate-risk prostate cancer who had undergone radiation therapy. Intermediate risk was stratified as favorable and unfavorable in 2018, and ADT recommendation was limited to men with unfavorable intermediate-risk prostate cancer. Data suggesting this stratification and treatment de-intensification were initially published in December 2013.2 A recent secondary analysis of the Radiation Therapy Oncology Group (RTOG) 9408 randomized trial confirmed no improvement in the rate of distant metastasis or prostate cancer–specific mortality with the use of 4 months of ADT compared with radiation therapy alone for patients with favorable intermediate-risk prostate cancer.3 In contrast, the rate of distant metastasis or prostate cancer–specific mortality was reduced with ADT in patients with unfavorable intermediate-risk prostate cancer. The present study aimed to examine the use of ADT in men with intermediate-risk prostate cancer and characterize national trends.
Methods
Data source
The National Cancer Database (NCDB) is an oncology-focused national database established by the American College of Surgeons and the Commission on Cancer of the American College of Surgeons. The NCDB tabulates longitudinal data from more than 70% of all new cancer diagnoses annually, encompassing more than 1500 hospitals across all 50 US states. The collected data include cancer characteristics, primary and adjuvant management, and long-term outcomes, as well as patient demographic information such as age, sex, race, educational level, income, and insurance status. The present study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. Because the study used deidentified data from the NCDB database, the requirement for formal institutional review and the need for informed patient consent were waived, consistent with the policies of Weill Cornell Medicine.
Study population
Men diagnosed with intermediate-risk prostate cancer who underwent radiation therapy from 2004 to 2016 were included in this study. Intermediate-risk prostate cancer was defined as either a Gleason score of 7, a prostate-specific antigen (PSA) level of 10 to 20 ng/mL, or a clinical stage of T2b-T2c. A subgroup analysis of men with favorable and unfavorable intermediate-risk prostate cancer was based on National Comprehensive Cancer Network criteria. Favorable intermediate risk was defined as the presence of 1 intermediate risk factor. Unfavorable intermediate risk was defined as a Gleason score of 4 + 3 = 7 or more than 1 intermediate risk factor. Intermediate risk factors were defined as a Gleason score of 7, a PSA level of 10 to 20 ng/mL, or a clinical stage of T2b-T2c. Men who had contraindications to or refused ADT were excluded from the analysis.
Statistical analysis
Demographic characteristics and clinical characteristics were compared for all patients who were treated with ADT and who were not in bivariate analysis. The Pearson χ2 test was performed for categorical variables, with frequencies and percentages reported, and the Wilcoxon sum rank test was performed for continuous variables, with medians and interquartile ranges reported. The Cochran-Armitage test was used to identify significant trends in the use of ADT with time and by therapy treatment. Multivariate logistic regression analyses were used to examine demographic and clinical factors associated with ADT use, with adjusted odds ratios reported. All tests were 2-sided and were considered significant at an α level of .05. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
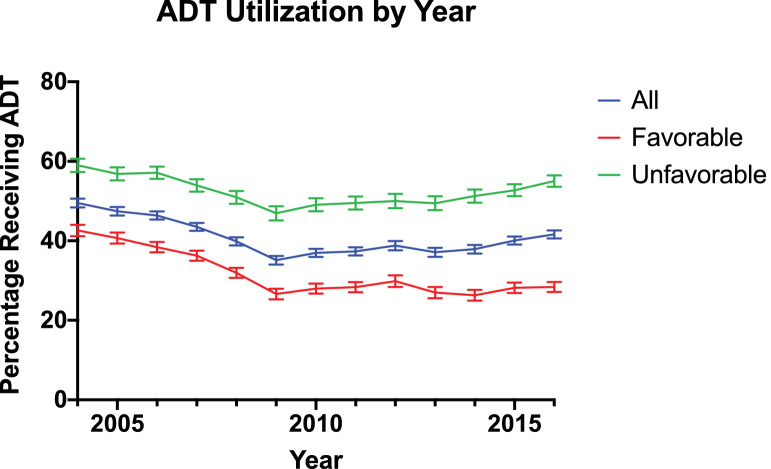
Demographic and clinical characteristics are presented in Table 1. In total, 108,185 men diagnosed with intermediate-risk prostate cancer underwent radiation therapy from 2004 to 2016. A flow diagram outlining the cohort selection is shown in Figure E1 in the Supplement. Of note, 5957 men with intermediate-risk prostate cancer who underwent radiation therapy who refused (4624) or had contraindications to (1333) ADT were excluded from the analysis. Of these men, 41.09% received ADT. The median ages of men who received and did not receive ADT were 69 and 68 years, respectively. Of patients with recorded treatment dates, ADT was initiated a median of 70 days before the start of radiation therapy, with an interquartile range of 107 days to 47 days before radiation therapy. White men composed 78.7% of the cohort, and Black men composed 17.2%. Approximately 85% of the cohort had a Charlson-Deyo Comorbidity Index score of 0. The median PSA level was 6.8 ng/mL. Among the 60,705 men with favorable intermediate-risk prostate cancer, ADT was used in 32.06%. Among the 47,480 men with unfavorable intermediate-risk prostate cancer, ADT was used in 52.64%. The trend of ADT use for the entire cohort and each subgroup from 2004 to 2016 is shown in Figure 1.
Table 1.
Demographic and clinical characteristics of men with intermediate-risk prostate cancer from 2004 to 2014 who underwent definitive radiation therapy with or without ADT
| Patient characteristic | All patients, No. (%) (N = 108,185) | No ADT, No. (%) (n = 63,731) | ADT, No. (%) (n = 44,454) | P |
|---|---|---|---|---|
| Year of diagnosis | ||||
| 2004 | 8086 (7.47) | 4081 (6.40) | 4005 (9.01) | <.001 |
| 2005 | 8360 (7.73) | 4393 (6.89) | 3967 (8.92) | |
| 2006 | 9156 (8.46) | 4911 (7.71) | 4245 (9.55) | |
| 2007 | 9593 (8.87) | 5418 (8.50) | 4175 (9.39) | |
| 2008 | 8916 (8.24) | 5361 (8.41) | 3555 (8.00) | |
| 2009 | 7387 (6.83) | 4792 (7.52) | 2595 (5.84) | |
| 2010 | 8377 (7.74) | 5279 (8.28) | 3098 (6.97) | |
| 2011 | 8475 (7.83) | 5311 (8.33) | 3164 (7.12) | |
| 2012 | 6767 (6.26) | 4141 (6.50) | 2626 (5.91) | |
| 2013 | 7046 (6.51) | 4430 (6.95) | 2616 (5.88) | |
| 2014 | 7647 (7.07) | 4748 (7.45) | 2899 (6.52) | |
| 2015 | 8926 (8.25) | 5350 (8.39) | 3576 (8.04) | |
| 2016 | 9449 (8.73) | 5516 (8.66) | 3933 (8.85) | |
| Age, y | ||||
| <60 | 15,366 (14.2) | 10,093 (15.8) | 5273 (11.9) | <.001 |
| 60 to <70 | 43,827 (40.5) | 26,848 (42.1) | 16,979 (38.2) | |
| 70 to <80 | 43,424 (40.1) | 23,870 (37.5) | 19,554 (44.0) | |
| 80 to 90 | 5568 (5.15) | 2920 (4.58) | 2648 (5.96) | |
| Gleason score | ||||
| 3 + 3 | 17,074 (15.8) | 11,565 (18.1) | 5509 (12.4) | <.001 |
| 3 + 4 | 62,150 (57.4) | 38,345 (60.2) | 23,805 (53.5) | |
| 4 + 3 | 28,961 (26.8) | 13,821 (21.7) | 15,140 (34.1) | |
| Median PSA level, ng/mL | 6.80 (5.00-10.3) | 6.40 (4.80-9.60) | 7.40 (5.20-11.2) | <.001 |
| PSA categories, ng/mL | ||||
| ≤4 | 12,255 (11.3) | 7736 (12.1) | 4519 (10.2) | <.001 |
| 4 to ≤10 | 67,389 (62.3) | 41,703 (65.4) | 25,686 (57.8) | |
| 10 to ≤20 | 28,541 (26.4) | 14,292 (22.4) | 14,249 (32.1) | |
| Clinical T stage | ||||
| T1a-T2a | 87,566 (80.9) | 53,329 (83.7) | 34,237 (77.0) | <.001 |
| T2b | 9333 (8.63) | 4746 (7.45) | 4587 (10.3) | |
| T2c | 11,286 (10.4) | 5656 (8.87) | 5630 (12.7) | |
| Charlson-Deyo Comorbidity Score | ||||
| 0 | 92,082 (85.1) | 54,298 (85.2) | 37,784 (85.0) | .206 |
| 1 | 12,757 (11.8) | 7521 (11.8) | 5236 (11.8) | |
| 2 | 2468 (2.28) | 1406 (2.21) | 1062 (2.39) | |
| ≥3 | 878 (0.81) | 506 (0.79) | 372 (0.84) | |
| Race | ||||
| White | 85,192 (78.7) | 50,281 (78.9) | 34911 (78.5) | .084 |
| Black | 18,591 (17.2) | 10,925 (17.1) | 7666 (17.2) | |
| Other | 4402 (4.07) | 2525 (3.96) | 1877 (4.22) | |
| Primary payer | ||||
| Medicare, Medicaid, or other government insurance | 69,052 (63.8) | 39,013 (61.2) | 30,039 (67.6) | <.001 |
| Private | 36,290 (33.5) | 23,056 (36.2) | 13,234 (29.8) | |
| Uninsured | 1367 (1.26) | 810 (1.27) | 557 (1.25) | |
| Unknown | 1476 (1.36) | 852 (1.34) | 624 (1.40) | |
| Median annual income, USD | ||||
| <38,000 | 20,453 (18.9) | 11,964 (18.8) | 8489 (19.1) | <.001 |
| 38,000-47,999 | 23,268 (21.5) | 13,745 (21.6) | 9523 (21.4) | |
| 8,000-62,999 | 24,979 (23.1) | 14,399 (22.6) | 10,580 (23.8) | |
| ≥63,000 | 39,485 (36.5) | 23,623 (37.1) | 15,862 (35.7) | |
| Education: High school diploma | ||||
| ≥21% | 21,359 (19.7) | 12,293 (19.3) | 9066 (20.4) | <.001 |
| 13%-20.9% | 27,651 (25.6) | 16,347 (25.6) | 11,304 (25.4) | |
| 7%-12.9% | 31,318 (28.9) | 18,307 (28.7) | 13,011 (29.3) | |
| <7% | 27,857 (25.7) | 16,784 (26.3) | 11,073 (24.9) | |
| Distance from treatment facility, miles | ||||
| ≤60 | 100,349 (92.8) | 58,343 (91.5) | 42,006 (94.5) | <.001 |
| 60-120 | 3894 (3.60) | 2527 (3.97) | 1367 (3.08) | |
| >120 | 3942 (3.64) | 2861 (4.49) | 1081 (2.43) | |
| Location type | ||||
| Metropolitan | 89,980 (83.2) | 53,296 (83.6) | 36,684 (82.5) | <.001 |
| Urban | 15,855 (14.7) | 9167 (14.4) | 6688 (15.0) | |
| Rural | 2350 (2.17) | 1268 (1.99) | 1082 (2.43) | |
| Facility type | ||||
| Community | 9819 (9.08) | 4992 (7.83) | 4827 (10.9) | <.001 |
| Comprehensive | 50,021 (46.2) | 28,544 (44.8) | 21,477 (48.3) | |
| Academic | 34,198 (31.6) | 21,678 (34.0) | 12,520 (28.2) | |
| Integrated | 14,147 (13.1) | 8517 (13.4) | 5630 (12.7) | |
| Facility location | ||||
| New England | 6862 (6.34) | 3369 (5.29) | 3493 (7.86) | <.001 |
| Mid-Atlantic | 18,385 (17.0) | 10,252 (16.1) | 8133 (18.3) | |
| South Atlantic | 27,332 (25.3) | 17,004 (26.7) | 10,328 (23.2) | |
| Central: East North | 19,811 (18.3) | 11,815 (18.5) | 7996 (18.0) | |
| Central: East South | 6735 (6.23) | 4189 (6.57) | 2546 (5.73) | |
| Central: West North | 7491 (6.92) | 3990 (6.26) | 3501 (7.88) | |
| Central: West South | 4466 (4.13) | 2568 (4.03) | 1898 (4.27) | |
| Mountain | 3794 (3.51) | 2321 (3.64) | 1473 (3.31) | |
| Pacific | 13,309 (12.3) | 8223 (12.9) | 5086 (11.4) | |
| Type of radiation therapy | ||||
| EBRT | 70,407 (65.1) | 38,202 (59.9) | 32,205 (72.4) | <.001 |
| BT | 23,680 (21.9) | 17,362 (27.2) | 6318 (14.2) | |
| Combination of EBRT and BT | 14,098 (13.0) | 8167 (12.8) | 5931 (13.3) | |
| Intermediate risk Stratification | ||||
| Favorable | 60,705 (56.1) | 41,244 (64.7) | 19,461 (43.8) | <.001 |
| Unfavorable | 47,480 (43.9) | 22,487 (35.3) | 24,993 (56.2) |
Abbreviations: ADT = androgen deprivation therapy; BT = brachytherapy; EBRT = external beam radiation therapy.
Fig. 1.
Trend in patients using androgen deprivation therapy as a percentage of all patients with intermediate-risk prostate cancer and subsets of patients with favorable intermediate-risk and unfavorable intermediate-risk prostate cancer from 2004 to 2016.
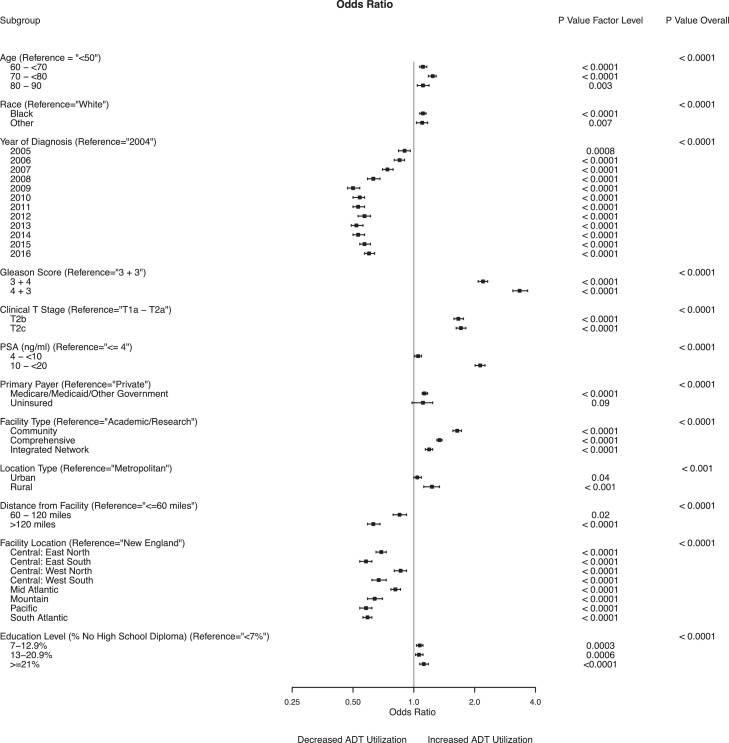
Multivariable analyses for men with intermediate-risk prostate cancer who received ADT are presented in Figure 2 and in Table E1 in the Supplement. Men with PSA values greater than 10 ng/mL (odds ratio [OR], 2.13; 95% CI, 2.01-2.25; P <.001) and PSA values of 4 to 10 ng/mL (OR, 1.05; 95% CI, 1.01-1.09; P = .02) had an increased likelihood of receiving ADT compared with men with PSA values ≤4 ng/mL. Men with a Gleason score of 3 + 4 = 7 (OR, 2.20; 95% CI, 2.08-2.32; P < .0001) and a Gleason score of 4 + 3 = 7 (OR, 3.36; 95% CI, 3.09-3.66; P < .0001) were more likely to receive ADT than were men with a Gleason score of 3 + 3 = 6. A higher clinical tumor stage was associated with use of ADT (T2b: OR, 1.66; 95% CI, 1.58-1.76; P < .0001; T2c: OR, 1.71; 95% CI, 1.62-1.81; P < .0001, compared with T1a-T2a).
Fig. 2.
Multivariable logistic regression model estimating use of androgen deprivation therapy among patients with intermediate-risk prostate cancer.
Black men had an increased likelihood of receiving ADT compared with White men (OR, 1.11; 95% CI, 1.07-1.15; P < .001). Older men had an increased likelihood of receiving ADT compared with men younger than 60 years (60 to <70 years: OR, 1.11; 95% CI, 1.07-1.16; P < .0001; 70 to <80 years: OR, 1.24; 95% CI, 1.18-1.29; P < .0001; 80-90 years: OR, 1.11; 95% CI, 1.04-1.19; P = .003).
Living more than 120 miles away from the treatment facility was associated with decreased ADT use compared with living less than 60 miles away (OR, 0.63; 95% CI, 0.59-0.68; P < .0001). Men whose treatment location was in the Mid-Atlantic (OR, 0.81; 95% CI, 0.77-0.86; P < .0001), Mountain (OR, 0.67; 95% CI, 0.59-0.70; P < .0001) or Pacific (OR, 0.58; 95% CI, 0.54-0.62; P < .0001) region were less likely to received ADT than were men with a New England facility location. Treatment in a community (OR, 1.64; 95% CI, 1.56-1.72; P < .001), comprehensive (OR, 1.34; 95% CI, 1.30-1.38; P < .0001), or integrated cancer center (OR, 1.19; 95% CI, 1.14-1.24; P < .0001) was associated with increased ADT use compared with treatment at an academic center. Men living in a rural area (OR, 1.23; 95% CI, 1.18-1.34; P < .0001) or an urban area (OR, 1.04; 95% CI, 1.00-1.09; P = .04) had an increased likelihood of receiving ADT compared with men living in a metropolitan area. Increased ADT use (OR, 1.12; 95% CI, 1.07-1.18; P < .001) was associated with a larger percentage of men without a high school diploma (>21% vs <7%).
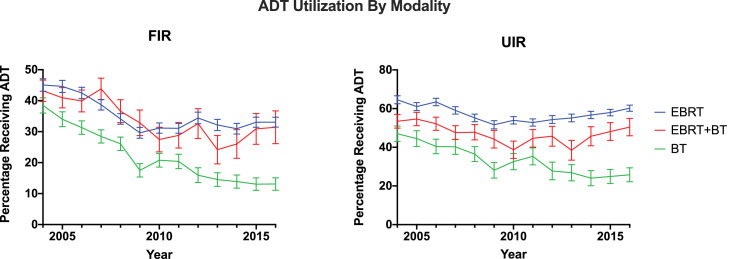
Characteristics of ADT use by radiation therapy modality are shown in Table 1. Of 70,407 men treated with external beam radiation therapy (EBRT) alone, the percentage of men who received ADT decreased from 54.13% in 2004 to 47.18% in 2016 (P < .0001). Of 14,098 men treated with EBRT and brachytherapy (EBRT + BT), the percentage of men who received ADT decreased from 48.22% in 2004 to 43.13% in 2016 (P < .0001). Of 23,680 men treated with BT alone, the percentage of men who received ADT decreased from 40.9% to 17.42% during the study period (P < .0001) (Fig 3). This consisted of a decrease from 38.4% to 13.1% for patients with favorable intermediate-risk prostate cancer and a decrease from 47.0% to 25.7% for patients with unfavorable intermediate-risk prostate cancer.
Fig. 3.
Trend in patients using androgen deprivation therapy as a percentage of patients receiving external beam radiation therapy (EBRT), EBRT with brachytherapy, or brachytherapy alone, grouped by favorable intermediate-risk and unfavorable intermediate-risk prostate cancer from 2004 to 2016.
Discussion
Using an oncology focused national database cohort of men with newly diagnosed intermediate-risk prostate cancer who had undergone radiation therapy, we provide a descriptive analysis of the largest and most recent study to date, to our knowledge, exploring trends and factors associated with the use of ADT. Recent data have suggested that men with favorable intermediate-risk prostate cancer do not benefit from the addition of ADT, whereas men with unfavorable intermediate-risk prostate cancer continue to show a failure-free survival benefit.4, 5, 6 Guidelines from the American Urologic Association, American Society for Radiation Oncology, and Society of Urologic Oncology now recommend informing men that favorable intermediate-risk prostate cancer can be treated with radiation therapy alone.7
The results of this study show decreasing use of ADT from 2004 to 2009 and increasing use of ADT from 2009 to 2016 for men with intermediate-risk prostate cancer who received radiation therapy. The observed increase in ADT use from 2009 to 2016 was primarily composed of an 8.1% absolute increase in ADT use among men with unfavorable intermediate-risk prostate cancer. In comparison, ADT use among patients with favorable intermediate-risk prostate cancer increased modestly by 1.8%. We identified multiple demographic and socioeconomic factors associated with ADT use. Notably, Black men were more likely to receive ADT than were White men, perhaps owing to concerns for increased disease aggressiveness or decreased survival in the Black population.8 This is consistent with findings demonstrating that Black men may be less likely to receive de-escalated treatment for prostate cancer, such as active surveillance for low-risk disease.9
The pattern of ADT use with respect to age is noteworthy. The use of ADT was associated with older ages compared with men younger than 60 years, who may derive the most benefit. There was an 11%, 24%, and 11% higher likelihood that men aged 60 to <70 years, 70 to <80 years, and 80 to 90 years, respectively, would receive ADT. Although our analysis excluded men who refused or had contraindications to ADT, they may reflect uncaptured patient preferences regarding the adverse effects of ADT (decreased libido, hot flashes, weight gain, cardiovascular risks, etc), notwithstanding its known survival benefits.
Most notably, men treated with BT had a marked decline in ADT use compared with men treated with EBRT. Men treated with EBRT + BT had a 5.3% absolute reduction in ADT use, in contrast to men treated with BT alone, who demonstrated a 23.5% absolute decrease in ADT use between 2004 and 2016. Multiple studies including the ASCENDE-RT randomized trial have demonstrated improved biochemical-free survival with dose-escalated therapy with BT in combination with EBRT.10, 11, 12, 13 Similarly, current evidence has demonstrated no benefit of adding ADT to BT alone for men with low-risk and favorable intermediate-risk prostate cancer.14, 15, 16 The results of this study support the rapid adoption of ADT de-escalation recommendations for men receiving BT alone. However, this decrease in ADT use was also demonstrated among patients with unfavorable intermediate-risk prostate cancer receiving BT alone, in contrast to patients receiving EBRT or EBRT + BT. These findings raise concern about potential undertreatment of the subset of patients with unfavorable intermediate-risk disease who receive BT alone. Furthermore, a recent systematic review of predominantly intermediate- and high-risk localized prostate cancer suggests that patients receiving EBRT + BT without ADT may have inferior overall survival compared with those receiving EBRT alone in combination with ADT.17
It is unclear why overall ADT use decreased from 2004 to 2009, although this may reflect multifactorial concerns regarding the morbidity of associated adverse effects, effects on quality of life, risk of cardiac toxic effects, or unclear therapeutic benefit.18, 19, 20, 21 The increased incorporation of ADT with definitive radiation therapy may reflect clinical adoption after seminal trials demonstrating overall survival benefit with short-term ADT before dose escalated radiation therapy. These trials include post hoc analysis of the RTOG 94 to 08 trial, which demonstrated improvement in overall survival with 4 months of ADT, with benefit predominantly in men with intermediate-risk prostate cancer.22 Similarly, long-term data from the Dana Farber Cancer Institute (DFCI) 95096 trial demonstrated an 8-year overall survival improvement of 13%, and the Trans Tasman Radiation Oncology Group 9601 trial demonstrated a 10-year all-cause mortality benefit of 13.2% with 6 months of ADT compared with radiation therapy alone.23,24 However, both of these trials included men with intermediate- and high-risk prostate cancer. Additionally, concerns regarding cardiotoxicity of ADT may have been mitigated by subsequent post hoc analyses of randomized ADT trials demonstrating no association with ADT and cardiovascular risk.25,26
The results of our analysis suggest that nearly 30% of men receiving radiation therapy for favorable intermediate-risk prostate cancer may be overtreated with ADT, and thus, this study has important implications for clinical practice. Conversely, 45% of men with unfavorable intermediate-risk prostate cancer do not receive ADT, which may affect survival. It is unclear why ADT use in this cohort is low, particularly given no significant differences in comorbidity index between men who receive ADT compared with those who do not. This may instead reflect uncaptured patient or physician preferences, rather than clinicopathologic features.
Limitations
The findings of this study are subject to the inherent biases of its retrospective nature. These data were derived from the NCDB registry, which comprehensively tabulates an estimated 70% of all new cancer diagnoses, thus limiting the scope of our study to approximately two-thirds of men diagnosed with intermediate-risk prostate cancer from 2004 to 2016. Despite the thorough data collected by the NCDB, it does not collect data on imaging that may influence clinical staging, biochemical progression-free survival, metastasis-free survival, prostate cancer–specific mortality, information on ADT duration, baseline genitourinary symptoms, compliance, or adverse effects of treatment. However, because the present study was not intended to report on survival or efficacy, this information would have added significant depth to the analyses but was not vital to our findings.
Conclusions
The findings of this study highlight that ADT use is variable in men undergoing radiation therapy for intermediate-risk prostate cancer, with data suggesting that several clinical and socioeconomic disparities influence its use. Ongoing review of practice patterns will be needed to assess adoption of clinical guidelines for ADT in intermediate-risk prostate cancer.
Footnotes
Sources of support: ViewRay, Lantheus, Boston Scientific, Genentech, Teleflex.
Disclosures: Dr Nagar reported serving on the ViewRay, Boston Scientific advisory board. All other authors have no disclosures to declare.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.100904.
Appendix. Supplementary materials
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Zumsteg ZS, Spratt DE, Daskivich TJ, et al. Effect of androgen deprivation on long-term outcomes of intermediate-risk prostate cancer stratified as favorable or unfavorable: A secondary analysis of the RTOG 9408 randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castle KO, Hoffman KE, Levy LB, et al. Is androgen deprivation therapy necessary in all intermediate-risk prostate cancer patients treated in the dose escalation era? Int J Radiat Oncol Biol Phys. 2013;85:693–699. doi: 10.1016/j.ijrobp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Edelman S, Liauw SL, Rossi PJ, et al. High-dose radiotherapy with or without androgen deprivation therapy for intermediate-risk prostate cancer: cancer control and toxicity outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1473–1479. doi: 10.1016/j.ijrobp.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Falchook AD, Basak R, Mohiuddin JJ, et al. Evaluation of the effectiveness of adding androgen deprivation to modern dose-escalated radiotherapy for men with favorable intermediate-risk prostate cancer. Cancer. 2016;22:2341–2349. doi: 10.1002/cncr.30049. [DOI] [PubMed] [Google Scholar]
- 7.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: Risk stratification, shared decision making, and care options. J Urol. 2018;199:683–690. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz K, Powell IJ, Underwood W, et al. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–1302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler S, Muralidhar V, Chavez J, et al. Active surveillance for low-risk prostate cancer in black patients. N Engl J Med. 2019;380:2070–2072. doi: 10.1056/NEJMc1900333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Shilkrut M, Merrick GS, McLaughlin PW, et al. The addition of low-dose-rate brachytherapy and androgen-deprivation therapy decreases biochemical failure and prostate cancer death compared with dose-escalated external-beam radiation therapy for high-risk prostate cancer. Cancer. 2013;119:681–690. doi: 10.1002/cncr.27784. [DOI] [PubMed] [Google Scholar]
- 12.Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for ocalized prostate cancer. Radiother Oncol. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 14.Potters L, Morgenstern C, Calugaru E, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2008;179:S20–S24. doi: 10.1016/j.juro.2008.03.133. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi T, Yorozu A, Saito S, et al. Outcomes following iodine-125 prostate brachytherapy with or without neoadjuvant androgen deprivation. Radiother Oncol. 2013;109:241–245. doi: 10.1016/j.radonc.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Morris WJ, Keyes M, Spadinger I, et al. Population-based 10-year oncologic outcomes after low-dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer. 2013;119:1537–1546. doi: 10.1002/cncr.27911. [DOI] [PubMed] [Google Scholar]
- 17.Jackson WC, Hartman HE, Dess RT, et al. Addition of androgen-deprivation therapy or brachytherapy boost to external beam radiotherapy for localized prostate cancer: A network meta-analysis of randomized trials. J Clin Oncol. 2020;38:3024–3031. doi: 10.1200/JCO.19.03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 19.Fowler FJ, McNaughton Collins M, Walker Corkery E, et al. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95:287–295. doi: 10.1002/cncr.10656. [DOI] [PubMed] [Google Scholar]
- 20.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 21.Green HJ, Pakenham KI, Headley BC, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: A randomized controlled trial. BJU Int. 2002;90:427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 23.D'Amico AV, Chen M-H, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 24.Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 25.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: Analysis of RTOG 92-02. Eur Urol. 2008;54:816–823. doi: 10.1016/j.eururo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.