Abstract
Background
Enlarging tuberculosis (TB) preventive treatment among at-risk populations is a critical component of the End TB Strategy. There is an urgent need to develop suitable latent tuberculosis infection (LTBI) testing and treatment tools according to the local TB epidemic and available resources worldwide.
Methods
Based on an open-label randomised controlled trial conducted since 2015 in China among rural residents aged 50–70 years with LTBI, the protective efficacy of a 6-week twice-weekly regimen of rifapentine plus isoniazid was further evaluated in a 5-year follow-up survey.
Results
1298 treated participants and 1151 untreated controls were included in the 5-year protective efficacy analysis. In the per-protocol analysis, the incidence rate was 0.49 (95% CI 0.30–0.67) per 100 person-years in the untreated control group and 0.19 (95% CI 0.07–0.32) per 100 person-years in the treated group; the protection rate was 61.22%. Subgroup analysis showed that the protection rate was 76.82% in the per-protocol analysis among participants with baseline interferon (IFN)-γ levels in the highest quartile (≥3.25 IU·mL−1). Multiple logistic regression analysis indicated that participants with baseline body mass index <18.5 kg·m−2 and with pulmonary fibrotic lesions had increased hazard of developing active disease with an adjusted hazard ratio (aHR) of 3.64 (95% CI 1.20–11.00) and 5.99 (95% CI 2.20–16.27), respectively. In addition, individuals with higher baseline IFN-γ levels showed an increased risk of TB occurrence (aHR 2.27, 95% CI 1.13–4.58).
Conclusions
Our findings suggest the 6-week twice-weekly regimen of rifapentine plus isoniazid for LTBI treatment might be an optional tool for TB control in the Chinese population.
Short abstract
A 6-week twice-weekly rifapentine plus isoniazid regimen showed a protective efficacy of >60% in 5 years, which indicated that preventive treatment based on a short-course regimen might be an optional tool for TB control in China. https://bit.ly/2Yeey7i
Introduction
It is estimated that about a quarter of the world's population is infected with Mycobacterium tuberculosis [1]. On average, 5–10% of those infected might develop active tuberculosis (TB) over the course of their lives, usually within the first 2 years after initial infection [2]. Comprehensive control strategies had been implemented to control the seedbeds of TB. Among them, TB preventive treatment was reported to reduce the risk of active TB development with an efficacy ranging from 60% to 90% [3]. Based on this, implementation of preventive treatment among individuals with latent tuberculosis infection (LTBI) under high risk of developing active disease has been recommended by the World Health Organization (WHO) as one of critical components of the End TB Strategy [4–6]. Currently, TB preventive treatment options can be broadly categorised into two types: monotherapy with isoniazid for at least 6 months or treatment with regimens containing isoniazid and a rifamycin (rifampicin or rifapentine). Isoniazid given for 6–9 months has been the preferred treatment regimen due to its low cost and proven efficacy. However, in consideration of the feasibility, resource requirements and acceptability to the treatment targets, short-course regimens with better tolerance and higher completion rates have been explored in the past decades. The 3-month weekly isoniazid plus rifapentine (3HP) regimen has been widely practised in several populations [7, 8]. At present, a 1-month regimen of rifapentine plus isoniazid (1HP) has been recommended for HIV infections by the WHO in the latest guidelines due to its equal protective efficacy and higher completion rate to 9 months compared with isoniazid alone [9, 10].
China is a country with a high burden for both TB and LTBI, and there is an urgent need to establish flexible preventive treatment policies to protect individuals at high risk of M. tuberculosis infection and active disease development [11, 12]. However, regimens suitable for the Chinese population based on domestic medicine have not been developed and evaluated in China through randomised controlled trials (RCTs). To explore short-course regimens for at-risk middle aged and elderly with LTBI in rural China, we conducted an open-label, pragmatic RCT in 2015. We initially wanted to evaluate two regimens, i.e. the WHO recommended 3HP and an innovative 2-month twice-weekly rifapentine plus isoniazid (2H2P2) regimen; however, the regimens were both modified due to an unexpected high frequency of side-effects simultaneously. Fortunately, the modified innovative 6-week twice-weekly regimen obtained good protective efficacy at the 2-year follow-up survey [13]. Nevertheless, since new infections are still common in rural areas in China, one of the biggest concerns about the implementation of LTBI treatment lies in re-infection, which would definitely influence the protective efficacy and shorten the protection period [14]. Thus, the aim of the present study was to further evaluate the 5-year protective efficacy of the innovative short-course regimen and to provide comprehensive estimation of the application of LTBI treatment in China and areas with similar situations.
Methods
Study design
This study was 5-year follow-up of an original open-label, pragmatic RCT aiming to explore the protective efficacy of short-course LTBI treatment regimens for at-risk middle aged and elderly in rural China. Detailed information of the original trial has been reported elsewhere [13]. Briefly, all participants aged 50–70 years with a QuantiFERON-TB Gold In-Tube (“QFT-GIT”; Qiagen, Germantown, MD, USA) positive result (a cut-off value of ≥0.35 IU·mL−1 was used as recommended by the manufacturer) and without current active TB at the baseline survey were included in the study. After randomisation, eligible participants were classified into three groups: group A (1284 for treatment with the 3HP regimen), group B (1299 for treatment with an innovative 2H2P2 regimen) and group C (1155 as untreated controls). Before the intervention, we pre-set early termination criteria based on the existing evidence for the 3HP regimen when the occurrence of hepatotoxicity (defined by aspartate transaminase/alanine transaminase >3× upper limit of normal (ULN) with symptoms or >5× ULN) was >1% [7]. The fact was that both regimens were terminated in advance during implementation (3HP was modified to 8 weeks and 2H2P2 was modified to 6 weeks). Thus, the protection rate of the modified regimens was evaluated and found to be 37% for the 8-week once-weekly regimen and 69% for the 6-week twice-weekly regimen at the 2-year follow-up investigation. Based on the results of the 2-year follow-up survey, the present study aimed to explore the long-term protection rate of the 6-week twice-weekly regimen at the 5-year follow-up investigation. The 8-week once-weekly regimen was dropped in this study due to its limited protective effect observed in the 2-year follow-up. All participants included in the study signed informed consent. The Ethics Committees of the Institute of Pathogen Biology and the Chinese Academy of Medical Sciences (Beijing, China) approved the study protocol (IPB-2015-5, IPB-2016-8 and IPB-2019-11).
Follow-up examinations and end-points
Between 2018 and 2020, follow-ups were carried out quarterly by trained interviewers through door-to-door or telephone survey for active case finding based on suspected symptoms screening. At the 5-year follow-up, between November and December 2020, participants were invited for a closing survey with investigation of suspected symptoms and digital chest radiography. All participants in the cohorts were scheduled to be seen for a 5-year closing visit unless they migrated out of the area, refused to be followed-up or died. All participants suspected of having TB because of clinical symptoms or radiographic abnormalities were referred for diagnosis according to the national guidelines. In addition, the national Tuberculosis Information Management System was also screened to find potential registered patients among our study participants.
The primary study end-points were microbiologically confirmed active pulmonary TB or clinically determined pulmonary TB. Individuals with positive results by sputum smear, culture and/or GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) were considered as microbiologically confirmed active TB. Response to empiric TB treatment among participants suspected as having TB but negative to microbiological tests was evaluated for a diagnosis of clinical TB by consensus from a panel of experts consisting of four radiologists, two clinical experts and one laboratory expert. TB diagnosis was made by the expert panel blinded to treatment assignment. The additional secondary end-point was death from any cause.
Statistical analysis
The frequencies of categorical variables in the study participants were compared between the groups using Pearson's Chi-squared test. The Kruskal–Wallis test was performed to assess differences in continuous variables. Distributions of baseline interferon (IFN)-γ levels were presented and compared using Wilcoxon rank sum tests. The incidence rates of active TB were assessed during 5 years after the completion of therapy. For those with relapse TB during follow-up, only the first diagnostic information was used. The time duration for each participant involved in the study was calculated based on the quarterly follow-up records. The intention-to-treat analysis included all enrolled eligible subjects in each group. The per-protocol analysis included all eligible enrolled subjects who completed the assigned study regimen (defined as completed ≥90% doses of modified therapy). In a subgroup analysis, all of the participants in each group were classified by the highest quartile of baseline IFN-γ levels. Subjects with missing data for baseline IFN-γ levels were excluded in the subgroup analysis. To identify potential variables related to active TB risk, Cox proportional hazards regression models were fitted to estimate adjusted hazard ratio (aHR) with 95% confidence interval. The other variables with p<0.05 (age, body mass index (BMI), baseline IFN-γ and presence of pulmonary fibrotic lesions) in the univariate model were also entered into the multivariable models. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Kaplan–Meier curve analysis was performed using Prism version 9 (GraphPad, San Diego, CA, USA).
Results
Characteristics of the study participants
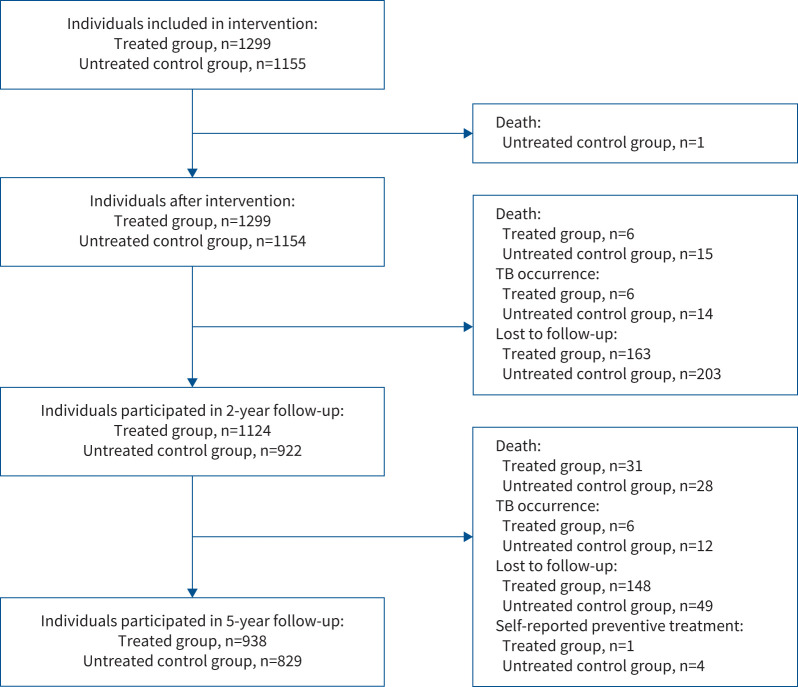
As shown in figure 1, a total of 1299 participants accepted treatment of the 6-week regimen and 1155 untreated controls were included in the original RCT. Among them, 938 (72.20%) and 829 (71.77%) completed the 5-year follow-up survey, respectively. Five participants were excluded because of self-reported preventive treatment after intervention performed by the study. Finally, a total of 1298 treated participants and 1151 untreated controls were included in the 5-year protective efficacy analysis. Table 1 shows the clinical and demographic characteristics of the population. Roughly, half of the treated and untreated participants were males. No significant difference was found between the two groups with respect to age, BMI, history of silicosis, smoking and alcohol drinking. Untreated participants showed a trend with a higher proportion of pulmonary fibrotic lesions found by chest radiography (p=0.015) and diabetes based on fasting blood glucose >7 mmol·L−1 (p=0.046) than treated participants.
FIGURE 1.
Flowchart of study participant intervention and follow-up. 1299 participants accepted treatment of the 6-week regimen and 1155 untreated controls were included in the original randomised controlled trial. 83.37% (2046 out of 2454) and 72.00% (1767 out of 2454) of the subjects completed the 2- and 5-year follow-up surveys, respectively. Five subjects (one in the treated group and four in the untreated control group) were excluded because of self-reported preventive treatment after intervention performed by the study. 1298 treated participants and 1151 untreated controls were included in the 5-year protective efficacy analysis.
TABLE 1.
Characteristics of the study participants included in the intention-to-treat analysis
| Treated group# (n=1298) | Untreated control group (n=1151) | p-value | |
| Age (years) | |||
| 50–55 | 317 (24.42) | 264 (22.94) | 0.637 |
| 56–60 | 343 (26.43) | 295 (25.63) | |
| 61–65 | 333 (25.65) | 299 (25.98) | |
| 66–70 | 305 (23.50) | 293 (25.46) | |
| Gender | |||
| Female | 590 (45.45) | 512 (44.48) | 0.630 |
| Male | 708 (54.55) | 639 (55.52) | |
| BMI (kg·m−2) | |||
| <18.5 | 542 (41.76) | 471 (40.92) | 0.755 |
| 18.5– <24 | 30 (2.31) | 22 (1.91) | |
| 24– <28 | 493 (37.98) | 458 (39.79) | |
| ≥28 | 233 (17.95) | 200 (17.38) | |
| Smoking | |||
| Never-smoker | 765 (58.94) | 691 (60.03) | 0.581 |
| Ever-smoker | 533 (41.06) | 460 (39.97) | |
| Presence of TB history | |||
| No | 1284 (98.92) | 1124 (97.65) | 0.015* |
| Yes | 14 (1.08) | 27 (2.35) | |
| Alcohol drinking | |||
| No | 901 (69.41) | 810 (70.37) | 0.606 |
| Yes | 397 (30.59) | 341 (29.63) | |
| Fasting blood glucose (mmol·L−1) | |||
| ≤7 | 1211 (93.30) | 1049 (91.14) | 0.046* |
| >7 | 87 (6.70) | 102 (8.86) | |
| History of silicosis | |||
| No | 1270 (97.84) | 1127 (97.91) | 0.902 |
| Yes | 28 (2.16) | 24 (2.09) |
Data are presented as n (%), unless otherwise stated. BMI: body mass index; TB: tuberculosis. #: completed 6 weeks of twice-weekly rifapentine plus isoniazid (both with a maximum dose of 600 mg). p-value by Chi-squared test. *: p<0.05.
Protective efficacy of the treatment regimen
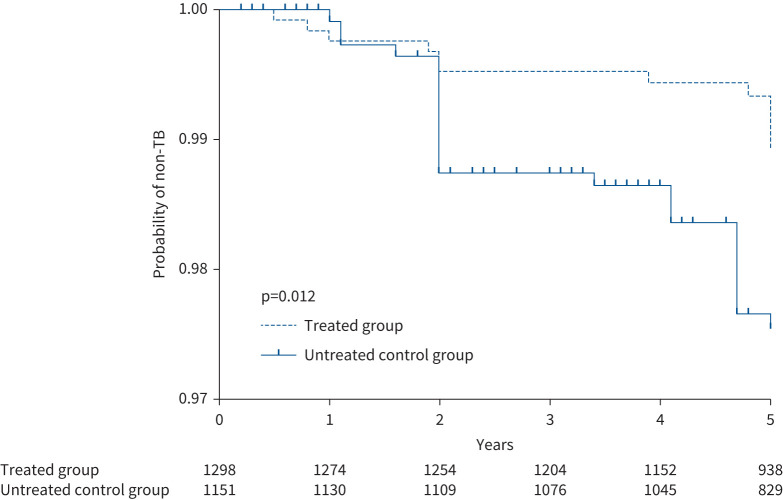
In total, 12 and 26 incident cases of active pulmonary TB were diagnosed for the treated and untreated groups during the 5-year follow-up post-intervention, respectively. Eight cases were confirmed microbiologically, while 30 cases were diagnosed clinically based on their suspected symptoms, abnormal chest radiography and positive responses to anti-TB treatment (for detailed information on the identified TB cases, see supplementary table S1). Kaplan–Meier curve analysis demonstrated that participants in the untreated group had a higher risk of developing active TB compared with the treated group (figure 2). As shown in supplementary figure S1, the treated individuals who completed the assigned study regimen had a lower risk of developing active TB (0.89% (nine out of 1013)) compared with those who did not complete the regimen (1.05% (three out of 285)), but the difference was statistically nonsignificant (p=0.706).
FIGURE 2.
Kaplan–Meier curve analysis of time to tuberculosis (TB) by study arm. Numbers of participants who remained without disease are listed by study arm. Most of the cases were identified in yearly examinations with chest radiography screening-based active case finding. The Kaplan–Meier curves demonstrate that participants in the untreated group had an increased risk of developing active TB compared with the treated control group.
In the intention-to-treat analysis (table 2), the cumulative incidence of active TB was 2.26% (95% CI 1.40–3.12%) in the untreated control group and 0.92% (95% CI 0.40–1.45%) in the treated group. The incidence rate of active TB was 0.49 (95% CI 0.30–0.67) per 100 person-years in the untreated control group and 0.20 (95% CI 0.09–0.32) per 100 person-years in the treated group. Compared with the untreated controls, the risk of active disease was decreased among treated individuals (aHR 0.41, 95% CI 0.20–0.84). In the per-protocol analysis, the cumulative incidence of active TB was 2.26% (95% CI 1.40–3.12%) in the untreated control group and 0.89% (95% CI 0.31–1.41%) in the treated group. The incidence rate of active TB was 0.49 (95% CI 0.30–0.67) per 100 person-years in the untreated control group and 0.19 (95% CI 0.07–0.32) per 100 person-years in the treated group. The 6-week twice-weekly regimen showed a protection rate of 61.22%.
TABLE 2.
Incidence of active tuberculosis in the study groups
| Treated group # | Untreated control group | |
| Intention-to-treat analysis | ||
| Subjects | 1298 | 1151 |
| Person-years | 5876 | 5337 |
| Incident cases | 12 | 26 |
| Cumulative incidence (% (95% CI)) | 0.92 (0.40–1.45) | 2.26 (1.40–3.12) |
| Protection rate¶ (%) | 59.29 | |
| Incidence rate per 100 person-years (95% CI) | 0.20 (0.09–0.32) | 0.49 (0.30–0.67) |
| Protection rate+ (%) | 59.18 | |
| Adjusted hazard ratio (95% CI)§ | 0.41 (0.20–0.84) | Reference |
| Per-protocol analysis | ||
| Subjects | 1012 | 1151 |
| Person-years | 4654 | 5337 |
| Incident cases | 9 | 26 |
| Cumulative incidence (% (95% CI)) | 0.89 (0.31–1.41) | 2.26 (1.40–3.12) |
| Protection rate¶ (%) | 60.62 | |
| Incidence rate per 100 person-years (95% CI) | 0.19 (0.07–0.32) | 0.49 (0.30–0.67) |
| Protection rate+ (%) | 61.22 | |
| Adjusted hazard ratio (95% CI)§ | 0.39 (0.17–0.88) | Reference |
Data are presented as n, unless otherwise stated. #: completed 6 weeks of twice-weekly rifapentine plus isoniazid (both with a maximum dose of 600 mg); ¶: calculated using the cumulative incidence; +: calculated using the incidence rate per 100 person-years; §: adjusted for age, body mass index, baseline interferon-γ and presence of pulmonary fibrotic lesions.
Protective efficacy of the treatment regimen among participants with baseline IFN-γ ≥3.25 IU·mL−1
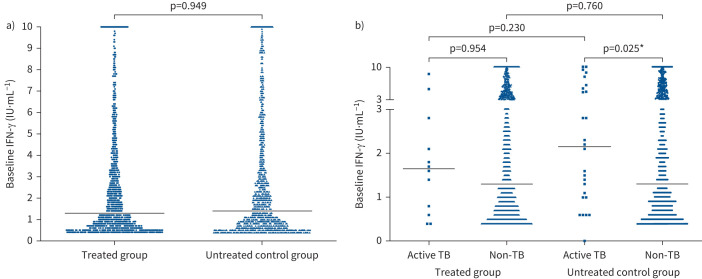
As shown in figure 3a, with respect to the QFT-GIT results, no significant difference was found for the median baseline levels of IFN-γ between the treated and untreated participants. For the untreated participants, the median baseline levels of IFN-γ were significantly higher among those who developed active TB than those who stayed healthy (figure 3b). Such a relation was also found in the treated group, although the difference was not statistically significant.
FIGURE 3.
Subgroup distributions and comparisons of interferon (IFN)-γ levels at baseline. a) Distribution of baseline IFN-γ levels between treated and untreated participants. b) Distribution of baseline IFN-γ levels between subjects with and without tuberculosis (TB) disease development classified by treatment. Horizontal lines represent median IFN-γ levels. Differences tested by Wilcoxon rank sum test. *: p<0.05.
The participants were classified into two subgroups by the highest quartile of baseline IFN-γ levels (table 3). For individuals with IFN-γ ≥3.25 IU·mL−1, in the per-protocol analysis, the incidence rate of active TB was 0.69 (95% CI 0.23–1.13) per 100 person-years in the untreated control group and 0.16 (95% CI 0.00–0.39) per 100 person-years in the treated group, with a protection rate of 76.82%. No significant difference was found for the major characteristics of the study participants between the groups as shown in supplementary table S2. In addition, participants who developed active TB showed a higher frequency of pulmonary fibrotic lesions than those who stayed healthy (supplementary table S3).
TABLE 3.
Subgroup analysis of active tuberculosis incidence classified into two subgroups by the 75% quartile of baseline interferon (IFN)-γ level (3.25 IU·mL−1)
| Baseline IFN-γ 0.35– <3.25 IU·mL−1 | Baseline IFN-γ ≥3.25 IU·mL−1 | |||
| Treated group # | Untreated control group | Treated group # | Untreated control group | |
| Intention-to-treat analysis | ||||
| Subjects | 961 | 861 | 337 | 288 |
| Person-years | 4322 | 4026 | 1554 | 1304 |
| Incident cases | 10 | 16 | 2 | 9 |
| Cumulative incidence (% (95% CI)) | 1.04 (0.40–1.68) | 1.86 (0.96–2.76) | 0.59 (0.00–1.41) | 3.13 (1.12–5.13) |
| Protection rate¶ (%) | 44.09 | 81.15 | ||
| Incidence rate per 100 person-years (95% CI) | 0.23 (0.08–0.37) | 0.40 (0.20–0.59) | 0.13 (0.00–0.31) | 0.69 (0.23–1.13) |
| Protection rate+ (%) | 42.50 | 81.16 | ||
| Adjusted hazard ratio (95% CI)§ | 0.50 (0.22–1.13) | Reference | 0.26 (0.05–1.25) | Reference |
| Per-protocol analysis | ||||
| Subjects | 748 | 861 | 264 | 288 |
| Person-years | 3021 | 4026 | 1233 | 1304 |
| Incident cases | 7 | 16 | 2 | 9 |
| Cumulative incidence (% (95% CI)) | 0.94 (0.24–1.63) | 1.86 (0.96–2.76) | 0.76 (0.00–1.80) | 3.13 (1.12–5.13) |
| Protection rate¶ (%) | 49.46 | 75.72 | ||
| Incidence rate per 100 person-years (95% CI) | 0.23 (0.06–0.40) | 0.40 (0.20–0.59) | 0.16 (0.00–0.39) | 0.69 (0.23–1.13) |
| Protectione rate+ (%) | 42.50 | 76.82 | ||
| Adjusted hazard ratio (95% CI)§ | 0.46 (0.18–1.15) | Reference | 0.33 (0.07–1.56) | Reference |
Data are presented as n, unless otherwise stated. Two subjects without baseline IFN-γ levels were treated as missing values in this analysis. #: completed 6 weeks of twice-weekly rifapentine plus isoniazid (both with a maximum dose of 600 mg); ¶: calculated using the cumulative incidence; +: calculated using the incidence rate per 100 person-years; §: adjusted for age, body mass index and presence of pulmonary fibrotic lesions.
Risk factors associated with active TB development among study participants
As shown in table 4, the incidence of active TB increased along with age in a dose–response relationship. The multiple logistic regression analysis indicted that participants with baseline BMI <18.5 kg·m−2 had a much higher hazard of developing active disease (aHR 3.64, 95% CI 1.20–11.00). Individuals with pulmonary fibrotic lesions identified by chest radiography were at a much higher risk of developing active TB compared with those with normal chest radiography (aHR 5.99, 95% CI 2.20–16.27). In addition, individuals with higher baseline IFN-γ levels had an increased risk of TB occurrence compared with those with lower baseline IFN-γ levels (aHR 2.27, 95% CI 1.13–4.58).
TABLE 4.
Risk factors related to the incidence of active tuberculosis (TB)
| Incidence of TB # | p-value | Adjusted HR¶ (95% CI) | |
| Age (years) | |||
| 50–55 | 2/581 (0.34) | <0.001* | Reference |
| 56–60 | 5/638 (0.78) | 1.56 (0.29–8.55) | |
| 61–65 | 12/632 (1.90) | 4.88 (1.09–21.89)* | |
| 66–70 | 19/598 (3.18) | 5.07 (1.13–22.62)* | |
| ptrend-value | <0.001* | ||
| Gender | |||
| Female | 12/1102 (1.09) | 0.094 | |
| Male | 26/1347 (1.93) | ||
| BMI (kg·m−2) | |||
| <18.5 | 4/52 (7.69) | <0.001* | 3.64 (1.20–11.00) |
| 18.5– <24 | 22/1013 (2.17) | Reference | |
| 24– <28 | 11/951 (1.16) | 0.63 (0.30–1.32) | |
| ≥28 | 1/433 (0.23) | 0.13 (0.02–1.00)* | |
| ptrend-value | <0.001* | ||
| Ever-smoker | |||
| No | 17/1456 (1.17) | 0.063 | |
| Yes | 21/993 (2.11) | ||
| Current alcohol drinking | |||
| No | 27/1711 (1.58) | 0.872 | |
| Yes | 11/738 (1.49) | ||
| With a history of silicosis | |||
| No | 37/2397 (1.54) | 0.827 | |
| Yes | 1/52 (1.92) | ||
| Chest radiography-identified prior TB | |||
| No | 33/2408 (1.37) | <0.001* | Reference |
| Yes | 5/41 (12.20) | 5.99 (2.20–16.27)* | |
| Fasting blood glucose (mmol·L−1) | |||
| ≤7 | 35/2260 (1.55) | 0.967 | |
| >7 | 3/189 (1.59) | ||
| Group | |||
| Treated group: completed the regimen | 9/1012 (0.89) | 0.028* | 0.40 (0.18–0.89)* |
| Treated group: did not complete the regimen | 3/286 (1.05) | 0.42 (0.12–1.50) | |
| Untreated control group | 26/1151 (2.26) | Reference | |
| Baseline IFN-γ level (IU·mL−1)+ | |||
| <1.34 | 12/1218 (0.99) | 0.034* | Reference |
| ≥1.34 | 25/1229 (2.03) | 2.27 (1.13–4.58)* |
Data are presented as n/N (%), unless otherwise stated. HR: hazard ratio; BMI: body mass index; IFN: interferon. #: the events in both groups were pooled for this analysis; ¶: all variables with p-values <0.05 in the univariate model were entered into the multivariate models; +: classified by median level of baseline IFN-γ. *: p<0.05.
Discussion
To the best of our knowledge, this is the first RCT performed in the Chinese population aiming to explore short-course regimens composed of domestic drugs for LTBI treatment. Our results showed that the 6-week twice-weekly isoniazid plus rifapentine regimen still had a protection rate of 61.22% at 5 years after the intervention. It indicated that under the current TB epidemic situation in China, preventive treatment based on a short-course regimen was an optional tool for TB control. Further analyses showed a higher protection rate of 76.82% among participants with higher baseline releasing level of IFN-γ, which provided important clues for further disclosing LTBI pathogenesis and developing precise intervention strategies.
Isoniazid prophylaxis has been reported to be effective in preventing TB in many different populations and under a variety of conditions, and the protective efficacy has been shown to persist for as long as 19 years in areas with a low TB burden [15]. Rifapentine is a rifamycin derivative with a long half-life and greater potency against M. tuberculosis than rifampin. Using rifapentine in combination with isoniazid provided an important opportunity for developing short-course and lower frequency regimens for LTBI treatment. Compared with the most recently reported short-course regimens [7, 9, 16], we developed a 6-week twice-weekly regimen showing comparable safety and completion rates. In addition, we were pleased to see that the regimen still showed a protective efficacy >60% at 5 years after the intervention. It is known that the duration of the protection period is affected by multiple factors, including the characteristics of the target population, local TB epidemic and TB control strategies. In high-burden areas like China with 0.8 million new TB cases annually, TB occurrence due to re-infection would definitely influence the protective efficacy and shorten the protection period. Our previous study suggested that the annual TB infection rate was ∼1.5% based on the persistent conversion of QFT-GIT testing in the rural community [14]. Under such circumstances, whether re-infection yielded an impact on the effect of preventive treatment has always been a hotly debated concern. In addition, although participants were randomly divided into treated and untreated groups with the main characteristics such as age and gender evenly distributed, the distribution of the other potential factors that might influence the occurrence of active TB was difficult to investigate, e.g. the onset of infection. As previously reported [17], parts of the baseline positives might be recently infected with a higher risk of developing active disease within the first 2 years of follow-up. Thus, an overlap observed in the Kaplan–Meyer curves in the first 2 years might be explained by such unevenly distributed factors between the two study groups. Considering that the frequency of self-reported history of close contact with active TB patients during the 5-year follow-up was not significantly different between the groups and no outbreak of TB was reported at the study site, the protective effect observed in our study should not be remarkably affected by the occurrence of new infections under the current local TB epidemic situation. Despite our results needing further verification in more populations and a longer follow-up period, they provide further evidence and confidence for exploring short-course or ultra-short-course LTBI preventive treatment regimens which might be more suitable for application in China and countries with a similar TB epidemic.
A positive relation was frequently observed between baseline IFN-γ levels and risk of TB progression [18, 19]. It is well accepted that the results of QFT-GIT cannot be used for assessing the risk of active disease development due to the low predictive value [20]. However, whether individuals with variant IFN-γ levels in QFT-GIT testing had different responses to preventive treatment has been rarely studied. Therefore, we further evaluated the protection rate among participants with the highest quartile of baseline IFN-γ levels in a subgroup analysis and the protective efficacy was found to be as high as 76.82%. The finding might be explained by two potential reasons. First, higher IFN-γ levels might reflect more active M. tuberculosis replication in the host and thus anti-bacterial effectiveness could be well presented by preventive treatment [21]. Second, individuals with higher IFN-γ levels might be recently infected with M. tuberculosis [22] and preventive treatment might be more effective for such high-risk individuals. Our present study could not provide positive evidence to support these speculations (supplementary table S4). In any case, the subgroup analysis was limited because it was not the original study objective. Therefore, it was difficult to draw a solid conclusion from this finding. Our results need to be verified by mechanistic research and observational studies with a larger sample size.
Consistent with a previous study [23], our results showed that the incidence rate of active pulmonary TB among participants with fibrotic lesions was higher than among participants with normal chest radiography findings. In addition, similar to the link between lower BMI and increased risk of active TB, our results showed that BMI <18.5 kg·m−2 was independently associated with the development of TB [24]. Our study also found that participants with baseline BMI ≥28 kg·m−2 had a much lower hazard of developing active disease (aHR 0.13, 95% CI 0.02–1.00), which was similar to previous epidemiological findings that obesity was associated with reduced risk of active TB in an inverse dose–response relationship [25–28]. Although currently we cannot define target populations by these potential risk factors, they still deserve our attention, especially when they appear together with other risk factors such as exposure to M. tuberculosis infection.
When interpreting the results of the study, several limitations should be kept in mind. First, study participants with pulmonary fibrotic lesions and diabetes mellitus based on fasting blood glucose >7 mmol·L−1 were not evenly distributed between the groups, which might influence the estimation of the protection rate because they are potential risk factors for disease development from LTBI [23, 29]. However, after excluding participants with pulmonary fibrotic lesions and diabetes mellitus, the protective efficacy of the 6-week regimen was still 50% (supplementary table S5). Second, as active case finding based on digital chest radiography and suspected symptoms investigation was used for tracking TB during follow-up, most of the incident cases (30 out of 38) were clinically diagnosed in this study. In order to minimise the potential misclassification bias, the clinical diagnosis was determined by an expert team, blinded to group assignment, based on responses to diagnostic anti-TB treatment. Third, as the original study (including the sample size) was designed for evaluation of the 2-year protection rate, the power to estimate the 5-year protective efficacy would not be sufficient. As we retrospectively calculated, the power of the current study to identify a 60% protection rate in 5 years was 76%. In addition, as has been previously discussed [13], both of the studied regimens were terminated in advance in this RCT and the group treated with the modified once-weekly regimen was dropped in the present study due to its low protective efficacy in the 2-year follow-up. Therefore, further studies with larger sample sizes are needed to verify our findings in different populations.
In conclusion, the 6-week twice-weekly rifapentine plus isoniazid regimen showed a protective efficacy >60% at 5 years after the intervention in a Chinese population with LTBI. Our findings provide more evidence and confidence to develop shorter and better-tolerated LTBI treatment regimens as potentially suitable tools for preventive treatment in China. However, the scale-up of LTBI treatment is a step-by-step process; besides suitable regimens, more innovative technologies, comprehensive strategies, resource input and government support are also needed.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables. ERJ-02359-2021.Supplement (305KB, pdf)
Supplementary figure S1. Kaplan–Meier curve of tuberculosis (TB) occurrence by completion of the regimen. Numbers of participants who remained disease free were listed by study arm. The Kaplan–Meier curve demonstrated that there was no significant difference in the risk of developing active TB between the participants who completed the regimen and who did not (p=0.706). ERJ-02359-2021.Figure (255KB, tif)
Shareable PDF
Acknowledgements
We thank Dingyong Sun (Center for Diseases Control and Prevention of Henan Province, Zhengzhou, China) for his support in study implementation. We thank Yu Ma and Zongde Zhang (Beijing Chest Hospital, Beijing, China) for their help on quality control of reading chest radiography. We thank all the investigators in the study team for their contributions.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00540-2022This article has supplementary material available from erj.ersjournals.com
This study is registered at the Chinese Clinical Trial Registry with identifier ChiCTR-IOR-15007202. The corresponding author can provide, upon request, individual participant data that underlie the results reported in this article or protocol after applying necessary measures to guarantee that no individual is identified or identifiable.
Author contributions: Lei Gao and Qi Jin designed the study. Lei Gao, Jianmin Liu, Shouguo Pan, Bin Zhang, Dakuan Wang, Zisen Liu and Jiaoxia Yan organised the implementation of the study. Henan Xin, Haoran Zhang, Xuefang Cao, Ying Du, Boxuan Feng, Ling Guan, Fei Shen, Yijun He, Yongpeng He and Zhusheng Quan carried out the epidemiological investigation and quality control. Xueling Guan interpreted the radiographs. Xuefang Cao, Henan Xin and Haoran Zhang performed data management and data analysis. Henan Xin, Xuefang Cao and Lei Gao wrote the report. All authors contributed to review and revision, and have seen and approved the final version of manuscript.
Conflict of interest: All co-authors disclose no conflicts of interest.
Support statement: This work was supported by the National Science and Technology Major Project of China (2017ZX10201302-009) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-013 and 2019-I2M-2-005). The funding sources were not involved in trial design, patient recruitment, data collection, analysis, interpretation or any aspect pertinent to the study. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . Global tuberculosis report 2020. 2020. www.who.int/publications/i/item/9789240013131 Date last accessed: 24 December 2020.
- 2.Hartman-Adams H, Clark K, Juckett G. Update on latent tuberculosis infection. Am Fam Physician 2014; 89: 889–896. [PubMed] [Google Scholar]
- 3.Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015; 372: 2127–2135. doi: 10.1056/NEJMra1405427 [DOI] [PubMed] [Google Scholar]
- 4.Uplekar M, Weil D, Lönnroth K, et al. WHO's new End TB Strategy. Lancet 2015; 385: 1799–1801. doi: 10.1016/S0140-6736(15)60570-0 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Implementing the End TB Strategy: the essentials. 2015. www.who.int/tb/publications/2015/end_tb_essential.pdf Date last accessed: 24 December 2020.
- 6.Lönnroth K, Migliori GB, Abubakar I, D, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 2015; 45: 928–952. doi: 10.1183/09031936.00214014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365: 2155–2166. doi: 10.1056/NEJMoa1104875 [DOI] [PubMed] [Google Scholar]
- 8.Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr 2015; 169: 247–255. doi: 10.1001/jamapediatrics.2014.3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380: 1001–1011. doi: 10.1056/NEJMoa1806808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. 2020. www.who.int/publications-detail/who-consolidated-guidelines-on-tuberculosis-module-1-prevention-tuberculosis-preventive-treatment Date last accessed: 24 March 2020.
- 11.Gao L, Li XW, Liu JM, et al. Incidence of active tuberculosis among individuals with latent tuberculosis infection in rural China: follow-up results of a population-based multicenter prospective cohort study. Lancet Infect Dis 2017; 17: 1053–1061. doi: 10.1016/S1473-3099(17)30402-4 [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Zhang H, Xin H, et al. Short-course regimens of rifapentine plus isoniazid to treat latent tuberculosis infection in older Chinese patients: a randomised controlled study. Eur Respir J 2018; 52: 1801470. doi: 10.1183/13993003.01470-2018 [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicenter, prospective cohort study. Lancet Infect Dis 2015; 15: 310–319. doi: 10.1016/S1473-3099(14)71085-0 [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Bai L, Liu J, et al. Annual risk of tuberculosis infection in rural China: a population-based prospective study. Eur Respir J 2016; 48: 168–178. doi: 10.1183/13993003.00235-2016 [DOI] [PubMed] [Google Scholar]
- 15.Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the Bethel isoniazid studies. Am Rev Respir Dis 1979; 119: 827–830. [DOI] [PubMed] [Google Scholar]
- 16.Ronald LA, FitzGerald JM, Bartlett-Esquilant G, et al. Treatment with isoniazid or rifampin for latent tuberculosis infection: population-based study of hepatotoxicity, completion and costs. Eur Respir J 2020; 55: 1902048. doi: 10.1183/13993003.02048-2019 [DOI] [PubMed] [Google Scholar]
- 17.Xin H, Zhang H, Yang S, et al. 5-year follow-up of active tuberculosis development from latent infection in rural China. Clin Infect Dis 2020; 70: 947–950. [DOI] [PubMed] [Google Scholar]
- 18.Winje BA, White R, Syre H, et al. Stratification by interferon-γ release assay level predicts risk of incident TB. Thorax 2018; 73: 652–661. doi: 10.1136/thoraxjnl-2017-211147 [DOI] [PubMed] [Google Scholar]
- 19.Xin H, Cao X, Zhang H, et al. Dynamic changes of interferon gamma release assay results with latent tuberculosis infection treatment. Clin Microbiol Infect 2020; 26: 1555.e1–1555.e7. doi: 10.1016/j.cmi.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 20.Abubakar I, Drobniewski F, Southern J, et al. Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis 2018; 18: 1077–1087. doi: 10.1016/S1473-3099(18)30355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang L, Shi R, Liu X, et al. Interferon-gamma response to the treatment of active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis 2017; 21: 1145–1149. doi: 10.5588/ijtld.16.0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Corral H, París SC, Marín ND, et al. IFN gamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One 2009; 4: e8257. doi: 10.1371/journal.pone.0008257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimouchi A, Ozasa K. [The incidence rate of active pulmonary tuberculosis among adult population with fibrotic lesions.] Kekkaku 2003; 78: 5–13. [PubMed] [Google Scholar]
- 24.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8: 286–298. [PubMed] [Google Scholar]
- 25.Leung CC, Lam TH, Chan WM, et al. Lower risk of tuberculosis in obesity. Arch Intern Med 2007; 167: 1297–1304. doi: 10.1001/archinte.167.12.1297 [DOI] [PubMed] [Google Scholar]
- 26.Lonnroth K, Williams BG, Cegielski P, et al. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39: 149–155. doi: 10.1093/ije/dyp308 [DOI] [PubMed] [Google Scholar]
- 27.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971–1992. Am J Epidemiol 2012; 176: 409–422. doi: 10.1093/aje/kws007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen YF, Hu HY, Lee YL, et al. Obesity/overweight reduces the risk of active tuberculosis: a nationwide population-based cohort study in Taiwan. Int J Obes 2017; 41: 971–975. doi: 10.1038/ijo.2017.64 [DOI] [PubMed] [Google Scholar]
- 29.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5: e152. doi: 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables. ERJ-02359-2021.Supplement (305KB, pdf)
Supplementary figure S1. Kaplan–Meier curve of tuberculosis (TB) occurrence by completion of the regimen. Numbers of participants who remained disease free were listed by study arm. The Kaplan–Meier curve demonstrated that there was no significant difference in the risk of developing active TB between the participants who completed the regimen and who did not (p=0.706). ERJ-02359-2021.Figure (255KB, tif)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02359-2021.Shareable (238.7KB, pdf)