Abstract
Purpose
Second primary (SP) neoplasms of the central nervous system (CNS) among cancer survivors are devastating but poorly understood processes. The absolute risk, or true incidence, of developing an SP CNS tumor among cancer survivors is not well characterized.
Methods and Materials
Patients diagnosed with cancer between 1975 and 2016 were queried using the Surveillance, Epidemiology, and End Results Program. Cumulative incidence rates (CIRs) were estimated using competitive risk analysis. The effects of covariates were assessed using multivariate competitive risk regression.
Results
More than 3.8 million patient records were extracted. The absolute risk of developing an SP CNS neoplasm at 25 years was highest among long-term survivors of CNS cancers (CIR, 6.6%). Cranial radiation increased the incidence of SP tumors in pediatric patients (25-year CIR, 5.7% vs 1.1%; P = .0012) but not adults (25-year CIR, 5.8% vs 5.0%; P = .66). Multivariate cumulative risk regression identified radiation among pediatric patients as the greatest risk for an increased CIR (subdistribution hazard ratio, 2.50; 95% CI, 1.86-3.38; P = 2e-9). Meningiomas (42.9% vs 24.1%; P = 2e-7) and glioblastomas (20.5% vs 14.5%; P = .046) represented a greater proportion of the SP CNS tumors in those who received cranial irradiation. The median age of an SP diagnosis was decreased among those who received prior radiation (41 years [interquartile range (IQR), 30-65 years] vs 49 years [IQR, 30-65 years]; P = 7e-5).
Conclusions
The risk of developing a second primary CNS neoplasm is elevated in patients with a prior CNS cancer independent of treatment history. The association between cranial radiation therapy and risk for subsequent cancers may be limited to the pediatric population.
Introduction
The improvement of diagnostic and treatment modalities has led to increased overall survival in a broad spectrum of cancers.1 Cancer survivors are at an increased risk of developing multiple morbidities, including second primary (SP) tumors, which are a major cause of mortality.2,3 Unlike recurrence or progression of the primary tumor, SP tumors arise from distinct carcinogenic processes. Neoplasms in the central nervous system (CNS) are rare but devastating presentations of SP tumors among cancer survivors that can develop on top of other long-term sequelae of cancer. Because these tumors are often resistant to conventional therapies and portend poor prognoses, the oncogenesis of CNS tumors warrants additional awareness.4
The etiology of SP CNS tumors is poorly understood, although previous reports have found associations with radiation therapy, genetic predispositions, and environmental factors.2,5, 6, 7 Many of these investigations were limited to studying childhood brain cancer survivors, who may not represent pan-cancer survivors. In treated pediatric populations, ionizing radiation was hypothesized to interfere with the ongoing developmental processes of the brain and contribute to secondary malignancies.2,8 However, the role of treatment-related carcinogenesis in adult survivors is not well elucidated. Furthermore, despite advances in our understanding of genetic and environmental risks of cancers, their effect on developing SP CNS tumors among cancer survivors is unclear.
In this study, we sought to quantify the absolute and relative risk of developing an SP CNS using registry data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program in a treatment-dependent and independent manner. We further assessed the association of prior radiation therapy among patients diagnosed with a primary CNS cancer with the risk of developing a second brain tumor and estimated the incidence rates of developing a subsequent CNS cancer.
Methods and Materials
Data source
This analysis was based on the SEER cancer registry program.9 We identified patients whose first primary cancer was diagnosed between 1975 and 2016 in 1 of 9 SEER registries. Both benign and malignant histologies were included. The primary site was determined based on the World Health Organization 2008 definition (ICD-O-3) codes. Endocrine, hematopoietic, and lymphoid cancers with sites within the CNS were considered primary CNS tumors. Among patients with multiple primary CNS tumors, only the first CNS tumor after the initial cancer diagnosis was examined. Latency was defined as the time between the first cancer diagnosis and the first subsequent CNS tumor. Cancers diagnosed within 2 months of the primary malignancy were excluded owing to the high likelihood of multiple primary tumors presenting synchronously rather than a second primary diagnosis.10, 11, 12 Patients were coded to have received radiation therapy or chemotherapy if they had known receipt of external beam radiation, isotopes, radioactive implants, or chemotherapy, as appropriate.
Second primary definition
SEER maintains strict coding rules regarding the definition of an SP tumor that are dependent on the behavior of the primary histology. Tumors described as metastases or extensions into contiguous sites are not considered SP tumors. Progression or recurrence of disease in similar ICD-O-3 topographic codes, such as developing a glioblastoma after a prior glial tumor, is not considered an SP. Similarly, neither recurrent nor metastatic disease would be considered an SP tumor. Neither timing nor laterality is used to determine multiple primary status in malignant CNS tumors, although laterality can be used to inform benign tumors. A detailed protocol of how SP CNS tumors are classified can be found in the SEER coding manual.13 Patients (n = 63) with an SP glial tumor of an identical histology as the primary tumor were excluded from analysis regardless of topography codes.
Statistical analysis
Individual case series were extracted using Multiple Primary Standardized Incidence Ratios (MP-SIR) SEER*Stat software, version 8.3.6 (National Cancer Institute).14 Long-term survivors excluded patients who were lost to follow-up or died in the 10-year period after their primary diagnosis. The incidence of SP CNS tumors was examined using competitive risk analysis where last follow-up and death without CNS SP malignancy were treated as noninformative and informative censors, respectively. Cumulative incidence rates (CIRs) were calculated using cumulative incidence functions (CIFs) and compared using Gray's test.15 In the interrogation of the effects of cranial radiation, groups were matched by age, sex, year of diagnosis, tumor location, histology, and receipt of chemotherapy using propensity score–based exact algorithms.16 Multivariate competitive risk regression using the Fine and Gray method was used to calculate the effect of covariates on the subdistribution or CIFs using all long-term CNS survivors.17 Frequency differences of categorical values and differences of continuous variables were assessed using Pearson's χ2d and the Wilcoxon rank-sum test, respectively. All tests were 2-sided with significance defined at P < .05. Statistical analyses and visualizations were performed using R software, version 3.6.1, and GraphPad Prism 8.
Results
Study population
More than 3.8 million case series were available from SEER 9 for analysis, including 1.2 million cases with long-term survival (Supplement 1). The median time-to-event (last follow-up, death, or SP CNS tumor) was 59 months (range, 2-503 months). Among patients who developed SP tumors (n = 13,657), 1690 developed within the first year after the index diagnosis. Primary sites were distributed among a variety of locations, with the most common being genitourinary (29.2%), breast (17.6%), gastrointestinal (17.4%), and lung (10.8%). Primary CNS tumors constituted 1.6% and 1.0% of all and long-term survivor diagnoses, respectively. Of all primary diagnoses, 55.6% had no known receipt of traditionally carcinogenic treatments, including radiation and chemotherapy.
Primary CNS cancers confer elevated risks of second primary CNS cancers
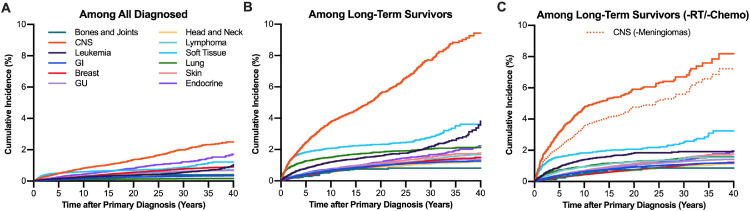
Rates of SP CNS tumors were compared following different primary cancer sites. Stratifying by primary cancer histology yielded diverging CIFs with CNS primary cancers portending the highest incidence of SP CNS tumors (Fig 1). The absolute risk of developing an SP CNS tumor at 25 years was highest among CNS (CIR, 1.66%), endocrine (CIR, 1.04%), and soft-tissue (CIR, 0.75%) cancers (Fig 1A and Supplement 2). Among long-term (>10-year) survivors, the CIR among CNS primaries at 10 and 25 years was 3.76% and 6.58%, respectively (Fig 1B, Supplement 2). When analyses excluded patients with known radiation or chemotherapy receipt and primary meningiomas, the high incidence of SPs among primary CNS cancers persisted (Fig 1B and 1C). The higher incidence of SP tumors of the same histology was not shared among all primary sites (Supplement 3).
Fig. 1.
Cumulative incidence of second primary CNS tumors among A, all survivors, B, long-term survivors, and C, long-term survivors with no history of radiation or chemotherapy. The group CNS (-meningiomas) refers to the incidence of second primary CNS tumors among nonmeningioma CNS primary tumors. Abbreviations: CNS = central nervous system; GI = gastrointestinal; GU = genitourinary; RT, radiation therapy.
Role of cranial radiation in the incidence of second primary malignancies
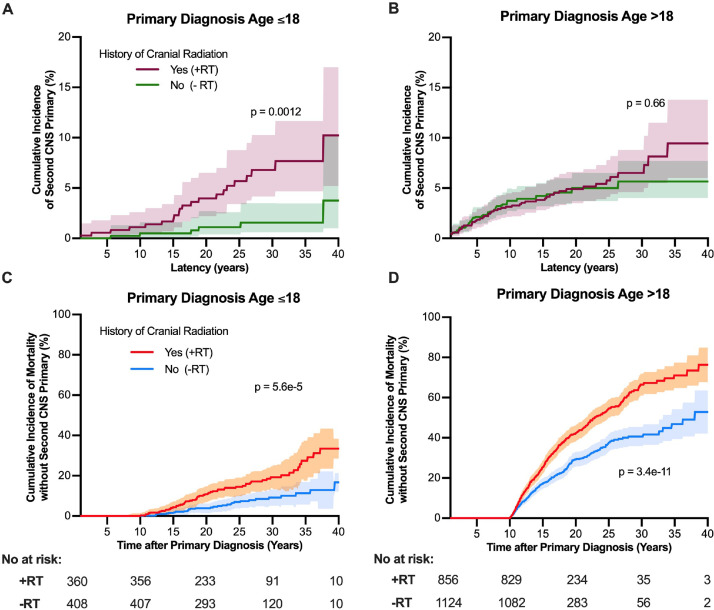
Among long-term survivors of CNS primary tumors (Supplement 4), CIFs were derived for groups matched by age, sex, year of diagnosis, tumor histology, tumor location, and receipt of chemotherapy. Cranial radiation was associated with a higher incidence of SP tumors in pediatric patients (25-year CIR, 5.7% vs 1.1%; P = .0012) but not adults (25-year CIR, 5.8% vs 5.0%; P = .66 [Fig 2A and 2B]). The CIR of mortality without developing an SP CNS tumor was higher among those who received prior radiation in both pediatric (P = 5.6e-5) and adult patients (P = 3.4e-11), which also reduced the persons at risk for developing an SP CNS tumor (Fig 2C and 2D).
Fig. 2.
Cumulative incidence of A and C, second primary CNS tumors and B and D, mortality among long-term survivors of CNS cancers by whether radiation was part of the primary treatment regimen. Groups were matched by age, sex, year of diagnosis, tumor location, histology, and receipt of chemotherapy using propensity score–based exact algorithms and stratified by age of initial diagnosis. Intervals reflect 95% confidence intervals of cumulative incidence functions. Abbreviations: CNS = central nervous system; RT, radiation therapy.
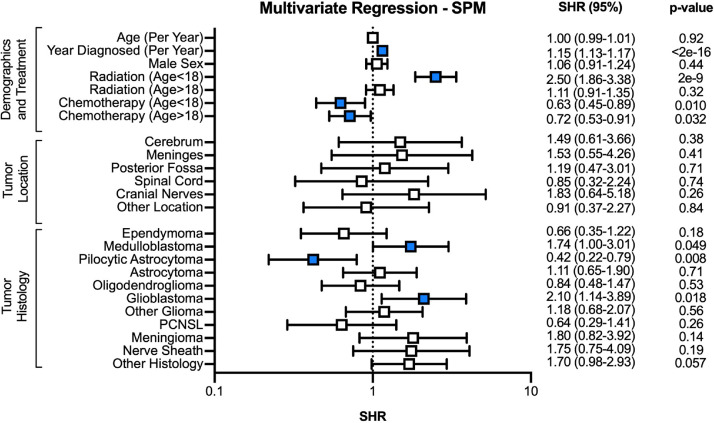
Multivariate cumulative risk regression on the CIF for developing an SP CNS tumor identified radiation among pediatric persons as a significant predictor (subdistribution hazard ratio [SHR], 2.50; 95% CI, 1.86-3.38; P = 2e-9 [Fig 3]). Radiation in adult patients did not result in increased CIRs (SHR, 1.11; 95% CI, 0.91-1.35; P = .32). Chemotherapy among both pediatric and adult patients was associated with decreased incidences of SP CNS tumors (SHR, 0.63 [95% CI, 0.45-0.89] and 0.72 [95% CI, 0.54-0.91], respectively). Primary diagnoses of pilocytic astrocytoma (SHR, 0.42; 95% CI, 0.22-0.79) were associated with lower CIRs, whereas glioblastoma (GBM; SHR, 2.10; 95% CI, 1.14-3.89) and medulloblastoma (SHR, 1.74; 95% CI, 1.00-3.01) increased the risk of SP CNS tumors. Both radiation and chemotherapy were associated with increased mortality without an SP tumor in the pediatric (SHR, 1.43 [95% CI, 1.22-1.67] and 1.54 [95% CI, 1.23-1.95], respectively) and adult population (SHR, 1.87 [95% CI, 1.72-2.04] and 1.13 [95% CI, 1.01-1.26], respectively [Supplement 5]). No significant interactions were detected between tumor histology and treatment receipt (data not shown).
Fig. 3.
Multivariate cumulative risk regression on risk of developing a second CNS tumor among long-term CNS cancer survivors. Abbreviations: CI = confidence interval; CNS = central nervous system; PCNSL = primary CNS lymphoma; SHR = subdistribution hazard ratio, SPM = second primary malignancy.
Characteristics of second primary malignancies after radiation
Second primary tumors among long-term surviving patients with and without prior cranial radiation differed in histology and age of diagnosis (Table 1). Meningiomas (42.9% vs 24.1%; P = 2e-7) and GBMs (20.5% vs 14.5%; P = .046) represented a greater proportion of the SP CNS tumors in those who received cranial irradiation. The enrichment of SP GBMs and meningiomas after radiation can be seen across multiple primaries (Supplement 6). Despite no significant differences in the age of primary diagnosis for patients who did and did not receive cranial radiation (median, 30 years [interquartile range (IQR), 14-42 years] vs 28 years [IQR, 11-47 years]; P = .72), SP CNS tumors in the setting of prior radiation developed at younger ages (median, 41 years [IQR, 30-54 years] vs 49 years [IQR, 30-65 years]; P = 7e-5). These differences were again seen in SP diagnoses of GBM (median, 45 years [IQR, 33-54 years] vs 63 years [IQR, 46-70 years]; P = 2e-5) and meningiomas (median, 40.5 years [IQR, 29-54 years] vs 61 years [IQR, 48-75 years]; P = 7e-13).
Table 1.
Distribution and age of diagnosis of second primary CNS tumors by whether radiation was part of the primary malignancy treatment course
| No. (%) |
Age of second primary diagnosis, median (1st to 3rd quantile), y |
|||||
|---|---|---|---|---|---|---|
| No radiation | Radiation | P value | No radiation | Radiation | P value | |
| Overall | 365 | 331 | – | 49 (30-65) | 41 (30-54) | 7.1e-5 |
| Ependymoma | 12 (3.3) | 6 (1.8) | .325 | 47 (28-64) | 43.5 (37-46) | 0.482 |
| Medulloblastoma | 7 (1.9) | 6 (1.8) | 1 | 21 (12-31) | 28 (19-50) | 0.295 |
| Pilocytic astrocytoma | 23 (6.3) | 2 (0.6) | 1.28e-4 | 20 (12-34) | 30 (22-38) | 0.581 |
| Astrocytoma | 35 (9.6) | 21 (6.3) | 0.152 | 49 (26-60) | 34 (18-44) | 0.054 |
| Oligodendroglioma | 33 (9.0) | 17 (5.1) | 0.065 | 39 (33-55) | 48 (43-51) | 0.412 |
| Glioblastoma | 53 (14.5) | 68 (20.5) | 0.046 | 63 (46-70) | 45 (33-54) | 1.55e-5 |
| Other glioma | 28 (7.7) | 21 (6.3) | 0.593 | 41 (24-54) | 34 (21-44) | 0.322 |
| Lymphoma | 4 (1.1) | 2 (0.6) | – | 82.5 (78-86) | 55.5 (48-63) | 0.133 |
| Meningioma | 88 (24.1) | 142 (42.9) | 2.19e-7 | 61 (48-75) | 40.5 (29-53) | 7.54e-13 |
| Nerve sheath | 27 (7.4) | 14 (4.2) | 0.107 | 40 (18-60) | 38.5 (31-63) | 0.492 |
| Other histology | 55 (15.1) | 32 (9.7) | 0.042 | 41 (21-58) | 46 (37-59) | 0.133 |
Abbreviation: CNS = central nervous system. Bold faced P-values indicate significance at P < .05.
Discussion
Using large-scale cancer registry data with long-term follow-up, we found that the risk of developing an SP CNS neoplasm was elevated in patients with a prior CNS cancer. Cranial irradiation was associated with a higher incidence rate of developing an SP CNS tumor in pediatric patients but not adults. As the therapeutic efficacy of radiation comes from the ability to induce irreparable DNA damage to tumor cells, developing brains may be more susceptible to off-target genomic insult.8,18 These findings align with previous reports of CNS radiation-associated tumors in the setting of pediatric atomic bomb exposure,19,20 tinea capitis management,6,21,22 and primary cancer treatment.23, 24, 25 Our estimated CIR of subsequent CNS tumors among radiated pediatric patients aligns with published estimates ranging from 1% to 28% at 20 years.26, 27, 28, 29 In contrast, adults have elevated rates of SP CNS tumors regardless of prior cranial radiation, likely driven by the higher incidence rates of brain tumors in the late adult to elderly period compared with early adulthood.30 The susceptibility to radiation decreases with increasing age of exposure,31,32 which may explain why adult patients treated with cranial radiation do not experience increased risks of brain tumors later in life. However, this observation may be limited by the typically shorter survival of adult patients. Although the role of chemotherapy in developing SP CNS tumors is controversial, we found that chemotherapy was associated with lower CIRs of subsequent CNS tumor. However, because chemotherapy was also associated with increased mortality, and thus a smaller proportion of at-risk survivors, we may not adequately capture the potential mutagenic effects of chemotherapy.
Tumors in the setting of prior radiation may represent unique disease processes. Glioblastomas, which are the most common primary malignant brain tumor, most frequently arise in the sixth decade of life.33 In contrast, low-grade gliomas such as oligodendrogliomas and astrocytomas tend to arise in midadulthood but can appear similarly on clinical and radiographic presentation as GBM.34 Therefore, the younger age of GBM diagnoses in those with a history of cranial radiation, with up to a quartile diagnosed under age 33, leads to a diagnostic challenge and warrants concern given the aggressive disease course of GBM. A similar enrichment for meningiomas was also seen after childhood exposure to radiation. Radiation-associated tumors were found to be more aggressive with higher recurrence rates than in comparable sporadic cases.35, 36, 37 Although the molecular pathogenesis of radiation-induced tumors is poorly understood, tumors arising in the setting of prior radiation may represent different natural histories and necessitate different treatment regimens than their sporadic counterparts.38,39
The increased absolute risk of developing an SP CNS tumor after prior CNS cancers, compared with other cancer sites, in the absence of radiation or chemotherapy highlights possible genetic or environmental predispositions to cancer. Our analysis estimates that nearly 9% of long-term survivors of primary CNS cancers will have developed a second CNS cancer 40 years later. An estimated 20% of children who develop an SP CNS tumor after a primary CNS tumor have a known tumor predisposition syndrome, such as Gorlin syndrome (nevoid basal cell carcinoma syndrome) or neurofibromatosis.40 In addition, there may be hereditary associations not captured by currently known predisposition syndromes. Brain tumors have also been shown to cluster within families, which may reflect environmental exposures and inheritance patterns not captured by predisposition syndromes.41, 42, 43, 44 Notably, patients who developed pilocytic astrocytomas had a lower risk of SP CNS tumors. Although the mechanisms of developing SP tumors were beyond the scope of this study, 1 potential explanation is that most pilocytic astrocytomas tend to occur in isolation rather than as part of a genetic syndrome45 As our ability to conduct genome- and epigenome-wide analysis improves, we may uncover more associations between genetic susceptibility and carcinogenesis.
Taken together, these findings may have implications on how to better care for and monitor long-term survivors of CNS cancers. Surveillance guidelines currently include recommendations for increased monitoring in the proximal postdiagnosis period given the high risk for tumor recurrence.46 A reconciliation of international guidelines to screen for second malignancies among adult survivors of pediatric, but not adult, brain tumors is in progress but includes recommendations that providers be aware of the risk of second CNS malignancies. However, CNS survivors diagnosed in adulthood have statically higher risks of developing an SP CNS tumor regardless of prior treatment exposure (25-year CIR, 5.0%-5.8%) and thus are inappropriately left out of most screening guidelines. Furthermore, radiation-associated tumors may have different natural histories: some studies have suggested radiation-induced CNS tumors have survival comparable with that of de novo tumors,27,47 whereas others have demonstrated worse outcomes.48,49 The high incidence of SP tumors among both pediatric and adult CNS cancer survivors warrants additional attention in survivorship studies and further investigation on the implications of differences in phenotypes of radiation-associated and de novo tumors.
Limitations
In our analysis, patients who died without developing an SP tumor were removed from the at-risk population and did not contribute to the cumulative incidence of SP CNS tumors. Therefore, our estimated incidence rate of SP CNS tumors after radiation may increase as survival improves. Furthermore, SEER combines patients with no and unknown receipt of radiation and chemotherapy. A recent SEER analysis cross-referenced SEER-Medicare information and found consistent treatment practices in the 2 databases, thus raising our confidence that the majority of patients with no definitive receipt did not receive therapy.50 Even though SEER maintains strict coding protocols, the contribution of data from multiple parties may lead to variations in data entry. Although it is possible that increased surveillance of the CNS may contribute to higher rates of SP CNS tumors, the finding that this predilection is not shared across other primary sites supports the notion that patients with a history of CNS cancers are at a much higher risk of developing an SP CNS tumor. Because SEER does not contain information on radiation plans, we could not ascertain the differential effects of dose, volume, or treatment modality on risk of SP CNS tumors. Similarly, we were also unable to account for genetic predispositions and cancer syndromes that may influence rates of SP CNS tumors. Despite these limitations, large registry data such as SEER are useful for evaluating the risk of rare processes that occur many years after the initial insult.
Conclusion
The risk of developing an SP CNS neoplasm is elevated in patients with a prior CNS cancer. The association between radiation therapy and risk for subsequent cancers may be limited to the pediatric population. However, because the incidence of CNS tumors is higher among all survivors of a prior CNS cancer, these survivors may benefit from long-term follow-up. The carcinogenesis of radiation-induced tumors may be unique, and treatment history should be taken into consideration when evaluating a new CNS lesion.
Footnotes
Presented at the American Society of Clinical Oncology 2020 Virtual Scientific Meeting (Abstract #1593).
Sources of support: This work had no specific funding.
Disclosures: Erik Sulman reports a relationship with Novocure Inc that includes: non-financial support, speaking and lecture fees, and travel reimbursement. Erik Sulman reports a relationship with Zai Lab that includes: speaking and lecture fees. Erik Sulman reports a relationship with Physician's Education Institution that includes: speaking and lecture fees. Erik Sulman reports a relationship with Karyopharm that includes: board membership. Erik Sulman reports a relationship with Society for Neuro-Oncology that includes: board membership.
Research data are available from the National Institutes of Health Surveillance, Epidemiology, and End Results database upon request.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.adro.2022.100969.
Appendix. Supplementary materials
References
- 1.Howlader N, Noone A, Krapcho M, et al. National Cancer Institute; Bethesda, MD: 2017. SEER Cancer Statistics Review, 1975-2014. [Google Scholar]
- 2.Marks AM, Packer RJ. A review of secondary central nervous system tumors after treatment of a primary pediatric malignancy. Semin Pediatr Neurol. 2012;19:43–48. doi: 10.1016/j.spen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Cai Y, Cao L, Bao X, et al. Second malignant neoplasms in childhood malignant brain tumour: A long-term population-based study. J Paediatr Child Health. 2012;48:990–996. doi: 10.1111/j.1440-1754.2012.02583.x. [DOI] [PubMed] [Google Scholar]
- 4.Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16:509–520. doi: 10.1038/s41571-019-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadetzki S, Flint-Richter P, Ben-Tal T, et al. Radiation-induced meningioma: A descriptive study of 253 cases. J Neurosurg. 2002;97:1078–1082. doi: 10.3171/jns.2002.97.5.1078. [DOI] [PubMed] [Google Scholar]
- 6.Ron E, Modan B, Boice JD, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 7.Little MP, Muirhead CR, Haylock RGE, et al. Relative risks of radiation-associated cancer: Comparison of second cancer in therapeutically irradiated populations with the Japanese atomic bomb survivors. Radiat Environ Biophys. 1999;38:267–283. doi: 10.1007/s004110050167. [DOI] [PubMed] [Google Scholar]
- 8.Kleinerman RA. Radiation-sensitive genetically susceptible pediatric sub-populations. Pediatr Radiol. 2009;39:S27–S31. doi: 10.1007/s00247-008-1015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anon. Surveillance, Epidemiology, and End Results Program. Overview of the SEER Program. Available at: https://seer.cancer.gov/about/overview.html. Accessed October 6, 2021.
- 10.Swaika A, Frank RD, Yang D, et al. Second primary acute lymphoblastic leukemia in adults: A SEER analysis of incidence and outcomes. Cancer Med. 2018;7:499. doi: 10.1002/cam4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146:265–272. doi: 10.1001/archdermatol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supramaniam R. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. J Epidemiol Community Heal. 2008;62:375–376. [Google Scholar]
- 13.Johnson CH, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards BK. National Cancer Institute Surveillance, Epidemiology, and End Results Program; Bethesda, MD: 2007. The 2007 Multiple Primary and Histology Coding Rules. January 1, 2007. [Google Scholar]
- 14.SEER*Stat Software. Version 8.3.6.1. National Cancer Institute, Surveillance, Epidemiology, and End Results Research Program. Available at: https://seer.cancer.gov/seerstat/. Accessed December 1, 2019.
- 15.Gray Robert J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Zhang Z. Propensity score method: A non-parametric technique to reduce model dependence. Ann Transl Med. 2017;5:7. doi: 10.21037/atm.2016.08.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Ivanov VN, Hei TK. Radiation-induced glioblastoma signaling cascade regulates viability, apoptosis and differentiation of neural stem cells (NSC) Apoptosis. 2014;19:1736–1754. doi: 10.1007/s10495-014-1040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozasa K. Epidemiological research on radiation-induced cancer in atomic bomb survivors. J Radiat Res. 2016;57:i112–i117. doi: 10.1093/jrr/rrw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shintani T, Hayakawa N, Kamada N. High incidence of meningioma in survivors of Hiroshima. Lancet. 1997;349:1369. doi: 10.1016/S0140-6736(05)63205-9. [DOI] [PubMed] [Google Scholar]
- 21.Sadetzki S, Chetrit A, Freedman L, et al. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163:424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 22.Modan B, Mart H, Baidatz D, et al. Radiation-induced head and neck tumours. Lancet. 1974;1:277–279. doi: 10.1016/s0140-6736(74)92592-6. [DOI] [PubMed] [Google Scholar]
- 23.Chojnacka M, Pędziwiatr K, Skowrónska-Gardas A, et al. Second brain tumors following central nervous system radiotherapy in childhood. Br J Radiol. 2014;87 doi: 10.1259/bjr.20140211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AJ, Frobisher C, Ellison DW, et al. Survival after second primary neoplasms of the brain or spinal cord in survivors of childhood cancer: Results from the British Childhood Cancer Survivor study. J Clin Oncol. 2009;27:5781–5787. doi: 10.1200/JCO.2009.22.4386. [DOI] [PubMed] [Google Scholar]
- 25.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong G, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the childhood cancer survivor study. J Natl Cancer Inst. 2009;101:948–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Wernicke AG. Risk and survival outcomes of radiation-induced CNS tumors. J Neurooncol. 2016;129:15–22. doi: 10.1007/s11060-016-2148-3. [DOI] [PubMed] [Google Scholar]
- 28.Walter AW, Hancock ML, Pui CH, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children's Research Hospital. J Clin Oncol. 1998;16 doi: 10.1200/JCO.1998.16.12.3761. 3761-377. [DOI] [PubMed] [Google Scholar]
- 29.Vinchon M, Leblond P, Caron S, et al. Radiation-induced tumors in children irradiated for brain tumor: A longitudinal study. Child's Nerv Syst. 2011;27:445–453. doi: 10.1007/s00381-011-1390-4. [DOI] [PubMed] [Google Scholar]
- 30.Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20 doi: 10.1093/neuonc/noy131. :iv1-iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smoll NR, Brady Z, Scurrah K, et al. Exposure to ionizing radiation and brain cancer incidence: The Life Span Study cohort. Cancer Epidemiol. 2016;42:60–65. doi: 10.1016/j.canep.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 33.AFa Tamimi, Juweid M. Glioblastoma. Codon Publications; Brisbane, Australia: 2017. Epidemiology and outcome of glioblastoma; pp. 143–153. [Google Scholar]
- 34.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 35.Shoshan Y, Chernova O, Jeun SS, et al. Radiation-induced meningioma: A distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59:614–620. doi: 10.1093/jnen/59.7.614. [DOI] [PubMed] [Google Scholar]
- 36.Rubinstein AB, Shalit MN, Cohen ML, et al. Radiation-induced cerebral meningioma: A recognizable entity. J Neurosurg. 1984;61:966–971. doi: 10.3171/jns.1984.61.5.0966. [DOI] [PubMed] [Google Scholar]
- 37.Izycka-Swieszewska E, Bien E, Stefanowicz J, et al. Malignant gliomas as second neoplasms in pediatric cancer survivors: Neuropathological study. Biomed Res Int. 2018;2018 doi: 10.1155/2018/4596812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rienstein S, Loven D, Israeli O, et al. Comparative genomic hybridization analysis of radiation-associated and sporadic meningiomas. Cancer Genet Cytogenet. 2001;131:135–140. doi: 10.1016/s0165-4608(01)00506-4. [DOI] [PubMed] [Google Scholar]
- 39.Braunstein S, Nakamura JL. Radiotherapy-induced malignancies: Review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol. 2013;3:73. doi: 10.3389/fonc.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsui K, Gajjar A, Li C, et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: Risk after modern multimodal therapy. Neuro Oncol. 2015;17:448–456. doi: 10.1093/neuonc/nou279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadetzki S, Bruchim R, Oberman B, et al. Description of selected characteristics of familial glioma patients—Results from the Gliogene Consortium. Eur J Cancer. 2013;49:1335–1345. doi: 10.1016/j.ejca.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz VY, Praska CE, Armstrong G, et al. Molecular subtyping of tumors from patients with familial glioma. Neuro Oncol. 2018;20:810–817. doi: 10.1093/neuonc/nox192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies 5 susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zakrzewski K, Jarzab M, Pfeifer A, et al. Transcriptional profiles of pilocytic astrocytoma are related to their three different locations, but not to radiological tumor features. BMC Cancer. 2015;15:1–16. doi: 10.1186/s12885-015-1810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janss AJ, Mazewski C, Patterson B. Guidelines for treatment and monitoring of adult survivors of pediatric brain tumors. Curr Treat Options Oncol. 2019;20(1):10. doi: 10.1007/s11864-019-0602-0. [DOI] [PubMed] [Google Scholar]
- 47.Bowers DC, Nathan PC, Constine L, et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. Lancet Oncol. 2013;14:e321–e328. doi: 10.1016/S1470-2045(13)70107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamanaka R, Hayano A, Kanayama T. Radiation-induced meningiomas: An exhaustive review of the literature. World Neurosurg. 2017;97:635–644. doi: 10.1016/j.wneu.2016.09.094. [DOI] [PubMed] [Google Scholar]
- 49.Yamanaka R, Hayano A, Kanayama T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg Rev. 2018;41:719–731. doi: 10.1007/s10143-016-0786-8. [DOI] [PubMed] [Google Scholar]
- 50.Morton LM, Dores GM, Schonfeld SJ, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5:318–325. doi: 10.1001/jamaoncol.2018.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.