Abstract
CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) have become ubiquitous approaches to control gene expression in bacteria due to their simple design and effectiveness. By regulating transcription of a target gene(s), CRISPRi/a can dynamically engineer cellular metabolism, implement transcriptional regulation circuitry, or elucidate genotype-phenotype relationships from smaller targeted libraries up to whole genome-wide libraries. While CRISPRi/a has been primarily established in the model bacteria Escherichia coli and Bacillus subtilis, a growing numbering of studies have demonstrated the extension of these tools to other species of bacteria (here broadly referred to as non-model bacteria). In this mini-review, we discuss the challenges that contribute to the slower creation of CRISPRi/a tools in diverse, non-model bacteria and summarize the current state of these approaches across bacterial phyla. We find that despite the potential difficulties in establishing novel CRISPRi/a in non-model microbes, over 190 recent examples across eight bacterial phyla have been reported in the literature. Most studies have focused on tool development or used these CRISPRi/a approaches to interrogate gene function, with fewer examples applying CRISPRi/a gene regulation for metabolic engineering or high-throughput screens and selections. To date, most CRISPRi/a reports have been developed for common strains of non-model bacterial species, suggesting barriers remain to establish these genetic tools in undomesticated bacteria. More efficient and generalizable methods will help realize the immense potential of programmable CRISPR-based transcriptional control in diverse bacteria.
Keywords: bacterial gene regulation, CRISPR interference (CRISPRi), CRISPR activation (CRISPRa), transcriptional interference, transcriptional activation, non-model bacteria, genome-wide library
Introduction
Since the development of CRISPR interference (CRISPRi) (Qi et al., 2013) and CRISPR activation (CRISPRa) (Bikard et al., 2013) in 2013, they have become efficient and prevalent tools for transcriptional regulation in bacteria. CRISPR-Cas originates as a form of prokaryotic immunity, with systems comprising one or more CRISPR-associated (Cas) proteins and a short guide RNA (gRNA) that complex together to target and cleave foreign DNA or RNA molecules, such as viruses (Nussenzweig and Marraffini, 2020). The gRNA leads the complex to target sequence via complementarity between the protospacer sequence of the gRNA and the target site on the DNA/RNA molecule. Various mechanisms exist to prevent cleavage of chromosomal DNA, which most often involves a protospacer adjacent motif (PAM) or equivalent next to the target site that is not present in the CRISPR arrays on the chromosome (Jackson et al., 2017).
Researchers developed CRISPRi technology by deactivating the nuclease activity of select Cas enzymes to create mutant dCas proteins that bind, but do not cleave, the DNA target (Qi et al., 2013). Most CRISPRi systems repress a gene’s expression through steric inhibition of RNA polymerase binding or extension (Qi et al., 2013), although some repress gene expression through RNA cleavage (Zhang K. et al., 2020; Rahman et al., 2021). Gene repression over 100-fold has been reported for several diverse CRISPRi tools and can approach near knockout levels of gene expression (Qi et al., 2013; Miao et al., 2019). Targeting a different sequence is easily achieved by changing the short protospacer sequence on the gRNA to bind a location within the promoter, untranslated region, or coding sequence of the target gene based on simple design rules (Qi et al., 2013; Zetsche et al., 2015; Zhang et al., 2017). Additionally, multiplexed gene repression can be achieved by simply expressing multiple gRNA within a cell (Qi et al., 2013; Zhang et al., 2017).
Shortly after the development of CRISPRi, researchers developed CRISPRa for bacterial transcriptional activation by combining the dCas protein with a transcriptional activator that recruits transcription machinery to the target gene’s promoter to increase gene expression (Bikard et al., 2013). The specific mechanism for transcriptional activation depends on the activator, which can be incorporated by directly fusing a transcriptional activator domain to the dCas protein (Bikard et al., 2013; Ho et al., 2020; Schilling et al., 2020), incorporating RNA scaffolds into the gRNA sequence to recruit activator domains to the dCas complex (Dong et al., 2018; Liu Y. et al., 2019; Fontana et al., 2020a), or using non-covalent protein-protein interaction domains to complex the transcriptional activator and dCas protein (Villegas Kcam et al., 2021; Villegas Kcam et al., 2022). Unlike CRISPRi, however, CRISPRa has complex design rules that often strongly depend on the CRISPRa technology (i.e., type of activation domain and approach to couple the activation domain and dCas complex) as wells as several other factors (Liu Y. et al., 2019; Fontana et al., 2020a; Ho et al., 2020; Villegas Kcam et al., 2021). These other considerations include the basal expression of the target gene and the location of the binding site for the CRISPRa complex, where activation is typically achieved in a narrow range upstream of the target gene’s promoter and the activation strength fluctuates sharply as the nucleotide position shifts. Combined with the PAM requirement for DNA binding, these requirements greatly restrict the available DNA target sites for effective gene activation, especially for endogenous genes. Due to these relatively stringent design rules for gene activation and often low (<10-fold) activation levels compared to CRISPRi repression (Bikard et al., 2013; Liu W. et al., 2019; Fontana et al., 2020a; Villegas Kcam et al., 2021), CRISPRa development has been slower in bacteria than in eukaryotes (Kampmann, 2018; Fontana et al., 2020b). Despite the current limitations of CRISPRa, however, the simplicity and inherent properties of CRISPRi/a gene regulation can provide strong transcriptional control of multiple genes simultaneously, making these approaches often easier and faster than traditional methods and allowing for dynamic transcriptional control.
CRISPR systems are classified into a variety of classes, types, subtypes, and variants, each with unique genes and properties (Koonin et al., 2017; Makarova et al., 2020). Many systems have been engineered to create effective CRISPRi/a tools. The first and most common tool is derived from the Type II Cas9 system, which comprises a single deactivated Cas9 (dCas9) protein and two small RNAs that create the gRNA (Qi et al., 2013). These two RNAs can be combined into a synthetic single guide RNA (sgRNA) for easier synthesis, but each sgRNA requires an independent promoter for expression (Jiang et al., 2015). Although many different dCas9 variants exist, the Streptococcus pyogenes dCas9 (Sp dCas9) system is the most common due to its short PAM sequence and strong transcription regulation abilities. Recently, tools derived from the Type V Cas12a (formerly Cpf1) system have been developed, which uses a single deactivated Cas12a (dCas12a) protein and one gRNA (Zetsche et al., 2015; Kim et al., 2017; Zhang et al., 2017; Miao et al., 2019). Unlike dCas9, dCas12a can process its gRNA from CRISPR arrays, providing easier multiplexed regulation (Fonfara et al., 2016; Zhang et al., 2017). Additionally, several studies have suggested that dCas12a variants are less toxic than dCas9 variants across different bacterial phyla (Liu W. et al., 2019; Knoot et al., 2020; Kuo et al., 2020), making them an attractive alternative to dCas9. The most common dCas12a variants used in bacteria are derived from Francisella tularensis subsp. novicida (Fn dCas12a) and Acidaminococcus sp. BV3L6 (As dCas12a). Several Type I CRISPRi/a tools have been designed, but due to the large number of genes in these systems, most tools are implemented by reprogramming the host species’ endogenous CRISPR system for gene repression (Luo et al., 2015; Xu et al., 2021; Villegas Kcam et al., 2022). Only a handful of CRISPRi tools from other systems have been reported for transcriptional regulation in bacteria, likely due to the novelty of the system (Rahman et al., 2021) or high cellular toxicity observed upon expression (Zhang K. et al., 2020).
Despite the unique traits and relevance of a vast diversity of bacteria, CRISPRi/a tools have been primarily developed in the model bacteria Escherichia coli and Bacillus subtilis. Yet, non-model bacteria (a broad definition of non-model, excluding E. coli and B. subtilis, is used here) offer great promise in research and industry spanning a wide range of medical, environmental, and biomanufacturing applications. For example, Streptomyces, Sorangium, and Photorhabdus spp. naturally produce bioactive secondary metabolites, such as antibiotics, and contain silent biosynthetic gene clusters with unknown and potentially useful products (Ye et al., 2019; Tian et al., 2020; Ke et al., 2021). Additionally, Rhodococcus and Corynebacterium spp. can produce valuable chemicals from cheap and simple feedstock and are tolerant to harsh conditions, making them ideal cell factories (Cleto et al., 2016; DeLorenzo et al., 2018). However, several conditions must be reached to successfully establish efficient CRISPRi/a tools in a non-model bacterium. In this mini-review, we detail these criteria, emphasizing the importance of characterized genetic parts to tightly control the expression of CRISPRi/a systems to limit potential toxicity while providing sufficient expression for effective transcriptional control. We demonstrate that despite the potential difficulties in creating these tools in non-model bacteria, they have been established across eight different bacterial phyla and have been used for a variety of applications, including high-throughput genome-wide selections. Finally, we highlight the current challenges to developing CRISPRi/a tools in non-model bacteria and novel species, which suggest directions for future progress.
Requirements and Challenges to Establish CRISPRi/a in Non-Model Bacteria
Several criteria must be met to successfully establish an effective CRISPRi/a tool in a non-model species or strain. First, the conditions for culturing, maintaining, and genetically manipulating the strain (often referred to as strain “domestication”) must be determined. For a phylogenetically similar strain to a previously established model bacteria, such as many Bacillus species (Zhan et al., 2020) and Enterobacteriaceae (Ho et al., 2020), suitable culture conditions may be similar to those previously determined. For novel or fastidious species, however, trial and error and patience may be required to determine appropriate culture conditions for growth and genetic manipulation, such as the obligate intracellular pathogen Chlamydia trachomatis (Ouellette, 2018). Additionally, introducing foreign DNA is often challenging for a non-model bacterium, as many are genetically recalcitrant, especially pathogens (Fernandes et al., 2021b) and novel strains (Zhao et al., 2020; Jin et al., 2022), and establishing a sufficient genetic transformation method can require significant effort. Additionally, care must be taken when introducing synthetic DNA to circumvent the bacterial host’s native immunity that may degrade foreign DNA, including restriction-modification and CRISPR systems (Marraffini and Sontheimer, 2008; Jin et al., 2022), such as by mimicking the recipient strain’s methylation patterns (Monk et al., 2015; Zhao et al., 2020). More discussion on the isolation and domestication of non-model bacteria can be found in other reviews (Vartoukian et al., 2010; Lewis et al., 2021; Riley and Guss, 2021).
Next, reliable genetic parts for the non-model bacterium are required to be able to express and tightly control the CRISPRi/a tool, including promoters, ribosome binding sites, terminators, and expression or integration vectors. For many non-model bacteria, especially novel species, these genetic part libraries are unavailable, and so, the necessary genetic parts must be created and characterized. In some cases, established genetic parts may be transferable from a model bacterium to a related species, such as promoters between Gram-positive bacteria (Liew et al., 2010). However, genetic parts often do not function equivalently between bacterial species or even strains (Tong et al., 2015; Leonard et al., 2018). Each CRISPRi/a component should be expressed using unique genetic parts to prevent repeated DNA sequences. Since dCas protein expression can elicit cytotoxicity, high strength promoters used for overexpression may not be optimal. If existing genetic parts are insufficient for a new bacterial species, identifying genetic regulatory elements from the endogenous genome provides an alternative to synthetic DNA design strategies (Fernandes et al., 2019). Libraries of genetic parts and inducible promoters are excellent tools to tune the expression of CRISPRi/a systems, and several studies have established such toolboxes in non-model bacteria to facilitate the development of genetic tools such as CRISPRi/a (Mimee et al., 2015; Leonard et al., 2018; Shin et al., 2019; Teh et al., 2019; Liow et al., 2020). These libraries and tunable parts are especially important to control the expression of the CRISPRi/a tool to minimize potential cellular toxicity and to precisely control transcriptional regulation (Qu et al., 2019; Bosch et al., 2021; Shabestary et al., 2021).
In the design of a synthetic CRISPRi/a system for a bacterium, consideration should be given to prevent interference with endogenous CRISPR systems and/or anti-CRISPR genes harbored on the strain’s genome. If the foreign and native CRISPR-Cas types are too similar, the introduction of the synthetic gRNA may induce cleavage of the host bacterium’s genome (via the catalytically active endogenous Cas enzyme) and can cause cell death in a DNA repair-deficient strain or undesired mutations if the strain has appropriate DNA repair pathways. This can be avoided by choosing a CRISPRi/a tool that does not share significant homology to any endogenous CRISPR-Cas. Native CRISPR-Cas systems can be predicted from the sequenced genome or proteome using computer software (Couvin et al., 2018; Chai et al., 2019), aiding in CRISPRi/a tool selection for novel strains. Alternatively, the native system can be engineered to create a CRISPRi/a tool via genetic manipulation, such as the deletion of the native cas2/3 or cas3 gene responsible for cleavage in Type I-F systems (Zheng et al., 2019; Qin et al., 2021; Xu et al., 2021) or mutating the native cas9 sequence for Type II systems (Shields et al., 2020; Dammann et al., 2021). Anti-CRISPR proteins, which inhibit CRISPR systems through a variety of mechanisms (Pawluk et al., 2018), may require deletion or disruption before a heterologous CRISPRi/a tool can be expressed (Xu et al., 2021). Online tools and databases are available to predict and describe anti-CRISPR proteins from protein sequences to help select an appropriate CRISPRi/a system (Wang et al., 2020; Wang et al., 2021a).
Finally, the CRISPRi/a components should be expressed at a level that provides adequate transcriptional repression or activation for the given application without significant cellular toxicity. Many studies have reported CRISPRi toxicity for diverse bacteria, while little is known about CRISPRa toxicity due to limited reports in the literature. These observed forms of toxicity include changes in cell morphology (Cho et al., 2018; Ouellette et al., 2021) and slower growth or complete growth inhibition (Rock et al., 2017; Yu et al., 2018; Wurihan et al., 2019; Zhang K. et al., 2020; Brito et al., 2020). To prevent toxicity, one can use a less toxic CRISPRi/a system for the host species (Rock et al., 2017; Zhao et al., 2019), or reduce the expression of the components by substituting genetic parts (Qu et al., 2019). Expansive libraries of genetic parts, including inducible and constitutive promoters, ribosome binding sites, and protein degradation tags, can be used to tune gene expression and characteristics of the CRISPRi/a tool (Depardieu and Bikard, 2020; Fleck and Grundner, 2021; Ouellette et al., 2021). However, the components cannot be expressed so low that it cannot effectively repress or activate the target gene(s), especially during multiplexed gene regulation that relies on a shared dCas protein pool for multiple gRNAs (Zhang and Voigt, 2018; Zhao et al., 2018). A careful balance is required to express the CRISPRi/a components.
Current CRISPRi/a Tools for Non-Model Bacteria
Different CRISPRi/a tools have been established in a range of bacteria that span many phyla and have been used for a variety of applications, as summarized here (Table 1). Overwhelmingly, these studies have utilized the Sp dCas9 CRISPRi system. More detailed information for each study can be found in Supplementary Table S1 (Supplementary Data Sheet 1).
TABLE 1.
CRISPRi/a studies in non-model bacteria and their key characteristics.
| Bacterium | Application | CRISPRi/a | CRISPR System(s) a | GW b | References c and type of study |
|---|---|---|---|---|---|
| Actinomycetota | |||||
| Bifidobacterium spp. | Probiotic | CRISPRi | As dCas12a | N | TD: Jin et al. (2022) |
| Corynebacterium glutamicum | Bioproduction | CRISPRa | Fn dCas12a- ω | N | TD: Liu et al. (2019a) |
| CRISPRi | Fn dCas12a | N | TD: Liu et al. (2019a); Li et al. (2020b); ME: Liu et al. (2019a); Li et al. (2020b); Huang et al. (2021) | ||
| Sp dCas9 | N | TD: Cleto et al. (2016); Zhang et al. (2016); Park et al. (2018); Gauttam et al. (2019); MGF: Li and Liu, (2017); Lee et al. (2018); ME: Cleto et al. (2016); Zhang et al. (2016); Park et al., 2018, 2019; Yoon and Woo, (2018); Gauttam et al. (2019) | |||
| N* | SS: Göttl et al. (2021) | ||||
| Rf Cas13d | N | TD: Zhang et al. (2020a) | |||
| Mycobacterium | |||||
| M. smegmatis, M. tuberculosis | Pathogen | CRISPRi | Fn dCas12a | N | TD: Fleck and Grundner, (2021) |
| M. smegmatis, M. tuberculosis, M. bovis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Choudhary et al. (2015); Singh et al. (2016); Xiao et al. (2019); Agarwal, (2020); Nadolinskaia et al. (2021); MGF: Thakur et al. (2016); Singh et al. (2017); Choudhary et al. (2019); Dutta et al. (2019); Agarwal, (2020); Lunge et al. (2020); Faulkner et al. (2021); Gani et al. (2021); Gibson et al. (2021) |
| M. smegmatis, M. tuberculosis | Pathogen | CRISPRi | Sth1 dCas9 | N | TD: Rock et al. (2017); Cheung et al. (2021); Judd et al. (2021); MGF: Baranowski et al. (2018); Landeta et al. (2019); Mai et al. (2019); McNeil and Cook, (2019); McNeil et al. (2020); McNeil et al. (2022); Randall et al. (2020); Brzostek et al. (2021); Quiñones-Garcia et al. (2021); Savková et al. (2021) |
| N* | SS: de Wet et al. (2020); McNeil et al. (2021) | ||||
| Y | SS: Bosch et al. (2021) | ||||
| M. tuberculosis | Pathogen | CRISPRi | Native Type III-A | Y | TD: Rahman et al. (2021); SS: Rahman et al. (2021) |
| Rhodococcus opacus | Bioproduction | CRISPRi | Sth1 dCas9 | N | TD: DeLorenzo et al., 2018, 2021; ME: DeLorenzo et al. (2018) |
| Saccharopolyspora erythraea | Bioproduction, bioresearch | CRISPRi | Sp dCas9 | N | ME: Liu et al. (2021b) |
| Streptomyces | |||||
| S. venezuelae | Bioproduction, bioresearch | CRISPRa | Sp dCas9-αNTD | N | TD: Ameruoso et al. (2021) |
| S. coelicolor | Bioproduction, bioresearch | CRISPRi | Fn dCas12a | N | TD: Li et al. (2018); MGF: Yan et al. (2022); ME: Liu et al. (2021c) |
| S. coelicolor, S. venezuelae, S. rapamycinicus, S. spp. | Bioproduction, bioresearch | CRISPRi | Sp dCas9 | N | TD: Tong et al. (2015); Tong et al. (2020); Zhao et al. (2018); Tian et al. (2020); Ameruoso et al. (2021); Wang et al. (2021b); ME: Tian et al. (2020); MGF: Ultee et al. (2020); Zhang et al. (2020b); Zhang et al. (2021); TRN: Tian et al. (2020) |
| Bacteroidetes | |||||
| Bacteroides thetaiotaomicron | Probiotic | CRISPRi | Sp dCas9 | N | TD: Mimee et al. (2015); TRN: Mimee et al. (2015); Taketani et al. (2020) |
| Bacteroides, Parabacteroides, Prevotella spp. | Probiotic | CRISPRi | As dCas12a | N | TD: Jin et al. (2022) |
| Chlamydiae | |||||
| Chlamydia trachomatis | Pathogen | CRISPRi | As dCas12a | N | TD: Ouellette et al. (2021) |
| Sa dCas9 | N | TD: Ouellette, (2018); Wurihan et al. (2019); Ouellette et al. (2021); MGF: Brockett et al. (2021) | |||
| Cyanobacteria | |||||
| Anabaena sp. PCC 7120 | Bioproduction, bioresearch | CRISPRi | Sp dCas9 | N | TD: Higo et al. (2018); Higo and Ehira, (2019); ME: Higo et al. (2018); Higo and Ehira, (2019); MGF: Higo et al. (2019) |
| Synechococcus sp. UTEX 2973 | Bioproduction | CRISPRi | Fn dCas12a | N | TD: Knoot et al. (2020); MGF: Knoot et al. (2020) |
| Synechococcus elongatus | Bioproduction | CRISPRi | Fn dCas12a | N | TD: Choi and Woo, (2020); ME: Choi and Woo, (2020) |
| Sp dCas9 | N | TD: Huang et al. (2016); ME: Huang et al. (2016); TRN: Lee and Woo, (2020) | |||
| Synechococcus sp. PCC 7002 | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Gordon et al. (2016); ME: Gordon et al. (2016) |
| Synechocystis sp. PCC 6803 | Bioproduction, bioresearch | CRISPRi | Fn dCas12a | N | TD: Liu et al. (2020a); MGF: Liu et al. (2020a) |
| Sp dCas9 | N | TD: Yao et al. (2016); Kirtania et al. (2019); MGF: Behler et al. (2018); Kaczmarzyk et al. (2018); Behle et al. (2021); 3; Santos et al. (2021); Shabestary et al. (2021); ME: Kaczmarzyk et al. (2018); Shabestary et al. (2018); Shabestary et al. (2021); Dietsch et al. (2021); Yunus et al. (2022) | |||
| Y | SS: Yao et al. (2020) | ||||
| Firmicutes | |||||
| Bacillus | |||||
| B. amyloliquefaciens | Bioproduction | CRISPRa | Sp dCas9-ω | N | TD: Zhao et al. (2020); ME: Zhao et al. (2020) |
| B. amyloliquefaciens, B. methanolicus, B. licheniformis | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Schultenkämper et al. (2019); Sha et al. (2020); Zhan et al. (2020); Zhao et al. (2020); MGF: Schultenkämper et al. (2019); Schultenkämper et al. (2021); ME: Sha et al. (2020); Zhan et al. (2020) |
| B. smithii | Bioproduction | CRISPRi | ThermodCas9 | N | TD: Mougiakos et al. (2017) |
| Clostridioides difficile | Pathogen | CRISPRi | Sp dCas9 | N | TD: Marreddy et al. (2019); Müh et al. (2019); MGF: Marreddy et al. (2019); Müh et al. (2019) |
| Clostridium | |||||
| C. sporogenes, C. spp. | Probiotic | CRISPRi | As dCas12a | N | TD: Jin et al. (2022) |
| C. ljungdahlii | Bioproduction | CRISPRi | Fn dCas12a | N | TD: Zhao et al. (2019); ME: Zhao et al. (2019) |
| C. autoethanogenum, C. acetobutylicum, C. beijerinckii, C. pasteurianum, C. cellulovorans, C. ljungdahlii | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Bruder et al. (2016); Li et al. (2016); Wang et al. (2016); Wen et al. (2017); Woolston et al. (2018); Fackler et al. (2021); ME: Wen et al. (2017); Woolston et al. (2018) |
| Enterococcus faecalis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019); Afonina et al. (2020); MGF: Afonina et al. (2020) |
| Eubacterium limosum | Bioproduction, probiotic | CRISPRi | Sp dCas9 | N | TD: Shin et al. (2019) |
| Hungateiclostridium thermocellum | Bioproduction | CRISPRi | ThermodCas9 | N | TD: Ganguly et al. (2020) |
| Lactiplantibacillus plantarum | Probiotic, bioproduction | CRISPRi | Sp dCas9 | N | TD: Myrbråten et al. (2019) |
| MGF: Myrbråten et al. (2019) | |||||
| Lactococcus lactis | Probiotic, bioproduction | CRISPRi | Sp dCas9 | N | TD: Berlec et al. (2018); Xiong et al. (2020) |
| Leuconostoc citreum | Probiotic | CRISPRi | Sp dCas9 | N | TD: Son et al. (2020) |
| ME: Son et al. (2020) | |||||
| Listeria monocytogenes | Pathogen | CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019) |
| Paenibacillus polymyxa | Bioproduction | CRISPRa | As dCas12a-SoxS | N | TD: Schilling et al. (2020); ME: Schilling et al. (2020) |
| Paenibacillus sonchi | Plant symbiote | CRISPRi | Sp dCas9 | N | TD: Brito et al. (2020) |
| Staphylococcus aureus, S. epidermidis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Chen et al. (2017); Dong et al. (2017); Zhao et al. (2017); Sato’o et al. (2018); Stamsås et al. (2018); Peters et al. (2019); Depardieu and Bikard, (2020); Jiang et al. (2020); Spoto et al. (2020); MGF: Wang and Nicholaou, (2017); Stamsås et al. (2018); Wu et al. (2019); Gelin et al. (2020); Mårli, (2020); Gallay et al. (2021); Myrbråten et al. (2021); Wang and Sun, (2021a) |
| Y | SS: Jiang et al. (2020); Mårli, (2020); Spoto et al. (2021) | ||||
| Streptococcus | |||||
| S. pneumoniae, S. salivarius | Pathogen | CRISPRi | Sp dCas9 | N | TD: Bikard et al. (2013); Liu et al. (2017); MGF: Domenech et al. (2018); Gallay et al. (2021); Knoops et al. (2022) |
| S. pneumoniae | Pathogen | CRISPRi | Sp dCas9 | N* | SS: Liu et al. (2017) |
| Y | SS: Dewachter et al. (2021); Gallay et al. (2021); Liu et al. (2021a); de Bakker et al. (2022) | ||||
| S. agalactiae | Pathogen | CRISPRi | Native dCas9 | N | TD: Dammann et al. (2021); MGF: Dammann et al. (2021) |
| S. mutans | Pathogen | CRISPRi | Native dCas9 | N* | TD: Shields et al. (2020); SS: Shields et al. (2020) |
| Proteobacteria | |||||
| Acidithiobacillus ferrooxidans | Bioresearch, bioremediation | CRISPRi | Sp dCas9 | N | TD: Yamada et al. (2022) |
| Acinetobacter baumannii, A. baylyi | Pathogen | CRISPRi | Sp dCas9 | N | TD: Geng et al. (2019); Peters et al. (2019); Bai et al. (2021); MGF: Bai et al. (2021); Colquhoun et al. (2021); Dai et al. (2021) |
| Aeromonas hydrophila | Bioproduction, bioresearch, bioremediation | CRISPRi | Sp dCas9 | N | TD: Wu et al. (2020); MGF: Wu et al. (2020) |
| Bartonella apis | Bee probiotic | CRISPRi | Sp dCas9 | N | TD: Leonard et al. (2018) |
| Burkholderia cenocepacia, B. multivorans, B.thailandensis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Hogan et al. (2019) |
| Caulobacter crescentus | Bioresearch | CRISPRi | Spa dCas9, Sth1 dCas9 | N | TD: Guzzo et al. (2020) |
| Sp dCas9 | N | TD: Irnov et al. (2017); MGF: Irnov et al. (2017); Werner et al. (2020) | |||
| Chromobacterium violaceum | Biorecovery | CRISPRi | Sp dCas9 | N | TD: Liow et al. (2020) |
| Enterobacter cloacae | Pathogen | CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019) |
| Gluconobacter oxydans | Bioproduction | CRISPRi | Native Type I-E | N | TD: Qin et al. (2021) |
| Halomonas sp. TD01 | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Tao et al. (2017); ME: Tao et al. (2017) |
| Klebsiella | |||||
| K. oxytoca | Pathogen | CRISPRa | Sp dxCas9 | N | TD: Liu et al. (2019b) |
| Sp dCas9-AsiA v2.1 | N | TD: Ho et al. (2020) | |||
| K. pneumoniae, K.oxytoca, K. aerogenes | Pathogen, bioproduction | CRISPRi | Sp dCas9 | N | TD: Wang et al. (2018a); Peters et al. (2019); Ho et al. (2020); ME: Wang et al. (2017); Wang et al. (2018a) |
| Komagataeibacter hansenii, K. xylinus | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Teh et al. (2019); Huang et al. (2020); MGF: Huang et al. (2020); ME: Huang et al. (2020) |
| Legionella pneumophila | Pathogen | CRISPRi | Sp dCas9 | N | TD: Ellis et al. (2021); MGF: Ellis et al. (2021) |
| Lysobacter enzymogenes | Bioproduction, bioresearch | CRISPRa | Sp dCas9-ω | N | TD: Yu et al. (2018); 11; ME: Yu et al. (2018); 11 |
| Methylorubrum extorquens | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Mo et al. (2020); MGF: Mo et al. (2020); ME: Mo et al. (2020) |
| Myxococcus xanthus | Bioproduction | CRISPRa | Sp dCas9-ω | N | TD: Peng et al. (2018); Wang et al. (2021c); MGF: Peng et al. (2018); Wang et al. (2021c); ME: Peng et al. (2018); Wang et al. (2021c) |
| Photorhabdus luminescens | Bioresearch | CRISPRa | Sp dCas9-ω | N | TD: Ke et al. (2021); MGF: Ke et al. (2021) |
| Proteus mirabilis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019) |
| Pseudomonas | |||||
| P. putida | Bioproduction, bioremediation | CRISPRa/i | Sp dCas9+MCP | N | TD: Kiattisewee et al. (2021); ME: Kiattisewee et al. (2021) |
| CRISPRi | Fn dCas12a | N | ME: Banerjee et al. (2020); Czajka et al. (2021) | ||
| P. putida, P. fluorescens | Bioproduction, plant symbiote, bioremediation | CRISPRi | Spa dCas9 | N | TD: Tan et al. (2018); MGF: Gauttam et al. (2021) |
| Sp dCas9 | N | TD: Sun et al. (2018); Noirot-Gros et al. (2019); Batianis et al. (2020); Kim et al. (2020); MGF: Noirot-Gros et al. (2019); ME: Kim et al. (2020); Kozaeva et al. (2021); Li and Ye, (2021); TRN: Liu et al. (2020b) | |||
| P. aeruginosa | Pathogen | CRISPRi | Spa dCas9 | N | TD: McMackin et al. (2019); Gauttam et al. (2021); MGF: McMackin et al. (2019); Gauttam et al. (2021) |
| Sp dCas9 | N | TD: Peters et al. (2019); Xiang et al. (2020); Stolle et al. (2021) | |||
| Sp dCas9, Sth1 dCas9 | N | TD: Qu et al. (2019); MGF: Qu et al. (2019) | |||
| Native Type I-F | N | TD: Xu et al. (2021) | |||
| Rhodobacter capsulatus | Bioproduction | CRISPRi | Fn dCas12a | N | TD: Zhang and Yuan, (2021) |
| Salmonella enterica | Pathogen | CRISPRa/i | Sp dCas9-ω | N | TD: Bhokisham et al. (2020); TRN: Bhokisham et al. (2020) |
| CRISPRa | Sp dCas9-AsiA v2.1 | N | TD: Ho et al. (2020) | ||
| CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019); Ho et al. (2020) | ||
| Ec Type I-E | N | TD: Rath et al. (2015) | |||
| Shewanella oneidensis | Bioproduction, bioresearch | CRISPRi | As dCas12a | N | TD: Li et al. (2020a); MGF: Li et al. (2020a) |
| CRISPRi | Sp dCas9 | N | TD: Cao et al. (2017); ME: Yi and Ng, (2021) | ||
| Sorangium cellulosum | Bioresearch | CRISPRa | Sp dCas9-VP64 | N | TD: Ye et al. (2019); MGF: Ye et al. (2019) |
| Vibrio casei | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Peters et al. (2019) |
| Vibrio natriegens | Bioproduction | CRISPRi | Sp dCas9 | Y | TD: Lee et al. (2019); SS: Lee et al. (2019) |
| Vibrio cholerae | Pathogen | CRISPRi | Sp dCas9 | N | TD: Caro et al. (2019); Wiles et al. (2020); MGF: Caro et al. (2019); Wiles et al. (2020) |
| Yersinia pestis | Pathogen | CRISPRi | Sp dCas9 | N | TD: Wang et al. (2019) |
| Zymomonas mobilis | Bioproduction | CRISPRi | Sp dCas9 | N | TD: Banta et al. (2020); MGF: Banta et al. (2020) |
| Native Type I-F | N | TD: Zheng et al. (2019) | |||
| Spirochaetes | |||||
| Borreliella burgdorferi | Pathogen | CRISPRi | Sp dCas9 | N | TD: Takacs et al. (2020) |
| Leptospira interrogans, L. biflexa, L. strain LGVF02 | Pathogen | CRISPRi | Sp dCas9 | N | TD: Fernandes et al. (2019); Fernandes et al. (2021a); Fernandes et al. (2021b); MGF: Fernandes et al. (2021b) |
| Tenericutes | |||||
| Mycoplasma pneumoniae, M. JCVI-syn1.0, M. JCVI-syn3.0 | Synthetic cells | CRISPRi | Sp dCas9 | N | TD: Mariscal et al. (2018) |
Acronyms for each CRISPR system can be found in Supplementary Table S2 (Supplementary Data Sheet 1).
Genome-wide (GW) classification for the relative size of the gRNA library: yes (Y) indicates a genome-wide library targeting >90% of coding genes on the genome; no (N) indicates a library of <50 target genes; and a smaller library targeting >50 genes but <90% of genome is indicated (N*).
Classifications for types of studies: tool development (TD), mapping gene function (MGF), metabolic engineering (ME), screens and/or selections (SS), transcriptional regulatory network (TRN).
Actinomycetota
CRISPRi has been well established in a wide range of Actinobacteria, including Mycobacteria, Streptomyces, and Corynebacterium, and has been used for metabolic engineering and the elucidation of gene functions in both small studies and genome-wide screens (Table 1). Additionally, several CRISPRi tools are commonly used in Mycobacteria (Choudhary et al., 2015; Rock et al., 2017; Agarwal, 2020; Fleck and Grundner, 2021) and Streptomycetes (Tong et al., 2015; Li et al., 2018; Zhao et al., 2018). CRISPRa has also been recently establish in Corynebacterium (Liu W. et al., 2019) and Streptomycetes (Ameruoso et al., 2021).
Cyanobacteria
CRISPRi/a is especially useful in cyanobacteria due to their polyploidal genomes (Kirtania et al., 2019). CRISPRi is relatively well-established in a wide range of cyanobacterial species, including those of research and industrial significance, and has been used for metabolic engineering, transcriptional regulatory networks, and the study of gene functions in small studies and a genome-wide screen/selection (Table 1). Many CRISPRi tools are available in cyanobacteria, each with their own characteristics (Gordon et al., 2016; Yao et al., 2016; Liu D. et al., 2020; Choi and Woo, 2020). CRISPRa has not yet been reported in cyanobacteria.
Firmicutes
CRISPRi is well-established in a wide range of Firmicutes, including Bacilli, Clostridia, Staphylococci, and Streptococci (Table 1). CRISPRi tools have been developed and used for metabolic engineering, elucidation of gene functions, and genome-wide screens and selections. CRISPRa has been reported in Bacillus amyloliquefaciens and Paenibacillus polymyxa in tool development work and some metabolic engineering applications (Schilling et al., 2020; Zhao et al., 2020).
Proteobacteria
CRISPRi and CRISPRa are well established in a wide variety of Proteobacteria, including Klebsiella, Salmonella, Pseudomonas, and Vibrio (Table 1). These tools have been developed and used for metabolic engineering, synthetic transcriptional regulatory networks, and mapping gene function using small gRNA sets and genome-wide screens and selections. Reports of CRISPRi are far more common than CRISPRa.
Other Bacterial Phyla
CRISPRi has also been reported in the phyla Chlamydiae, Tenericutes, Spirochaetes, and Bacteroidetes (Table 1). Although these reports have primarily been for tool development, some have used CRISPRi to investigate gene function (Fernandes et al., 2021b; Brockett et al., 2021) or create synthetic genetic circuits (Mimee et al., 2015; Taketani et al., 2020).
Applications of CRISPRi/a in Non-Model Bacteria
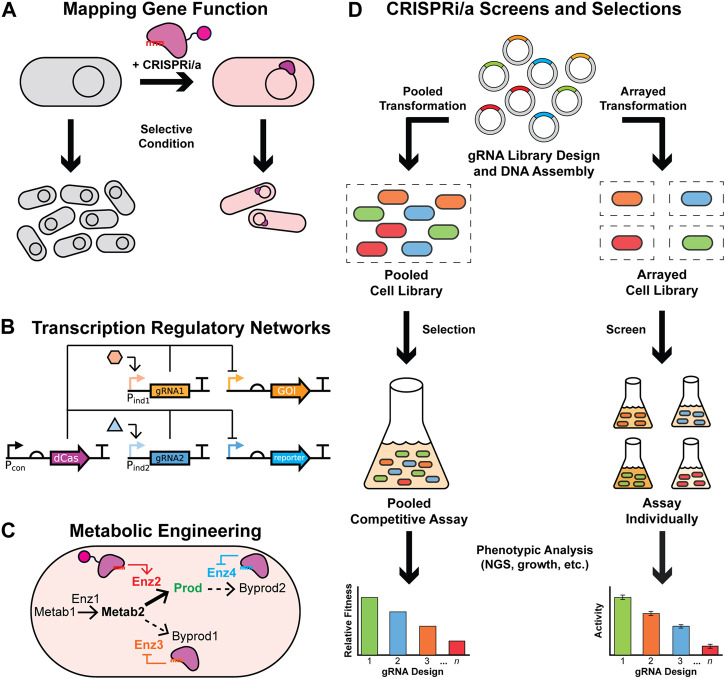
CRISPRi/a tools can be used for a variety of applications in non-model bacteria (Figure 1). The most common application is mapping a gene’s function by altering its gene expression and assaying cellular phenotypic change under some applied selective condition (Figure 1A). CRISPRi is particularly useful for investigating essential genes because its repression can be titrated to prevent full knockdown and cell death (Knoot et al., 2020; Bosch et al., 2021). Additionally, epistatic effects of multiple genes can easily be investigation by simply expressing multiple gRNA within the same cell (Ellis et al., 2021; McNeil et al., 2022). Although not as common as CRISPRi due to stricter design rules (Fontana et al., 2020a), CRISPRa can be used to induce expression of silent genes to investigate their functions and products, including entire silent biosynthetic gene clusters (Ke et al., 2021). Combined, these are the most common use of CRISPRi/a tools in non-model bacteria, with 80 reports across six phyla (Table 1) (Behler et al., 2018; Stamsås et al., 2018; Ke et al., 2021). The recent development of Mobile-CRISPRi (Peters et al., 2019), CRAGE-CRISPR (Ke et al., 2021), and a workflow for introducing genetic manipulation tools into non-model gut bacteria (Jin et al., 2022) will facilitate the expansion of CRISPRi/a tools into new species and strains, including recalcitrate pathogens and novel species without sequenced genomes.
FIGURE 1.
Applications of CRISPRi/a tools in bacteria. (A) CRISPRi/a tools can be used to study gene function by targeting a gene for repression or activation and assaying phenotypic differences under a given condition. (B) CRISPRi/a can control transcription regulatory networks through the expression of multiple guide RNAs and a single deactivated Cas (dCas) protein. By incorporating inducible promoters for the guide RNAs (gRNAs) and/or dCas protein, dynamic genetic circuits can be created that respond to multiple input stimuli to alter the expression of output genes, such as a gene of interest (GOI) and a reporter gene. (C) CRISPRi/a can be used for dynamic metabolic engineering by regulating gene expression to redirect carbon flux towards production of a desired biochemical, such as using CRISPRi to repress genes encoding competing enzymes (dashed arrows) while using CRISPRa to activate genes for the desired biochemical production (bold arrow). (D) CRISPRi/a has been used for high-throughput, large-scale screens and selections ranging from targeted sets of genes to genome-wide libraries. After designing and assembling the gRNA library for the given application, the library is transformed into bacteria that express the cognate dCas protein in either a mixed pooled (i.e., cells containing different gRNA(s) are mixed together) or an individual array format (i.e., cells containing different gRNA(s) are cultured separately). The resulting pooled cell library can be used in competitive selections for functional genomics analyses by applying selective conditions and measuring the relative gRNA fitness. An arrayed cell library can be assayed one-by-one to determine the relationship between strain activity (e.g. production or phenotype) and gene repression/activation.
Additionally, CRISPRi/a can be used to control transcription regulatory networks, such as genetic circuits, by designing and expressing gRNA to regulate the output promoter for each logic gate or node (Figure 1B). CRISPRi/a is especially effective for controlling complex synthetic transcription regulatory networks as the gRNA can be designed to target nearly any arbitrary sequence with an appropriate PAM (or equivalent) sequence (Taketani et al., 2020; Ellis et al., 2021). CRISPRi/a circuits can be fully synthetic and auxiliary to the native genetic regulatory networks, such as a heterologous sensor or multi-input circuit that senses and responds to external inputs in complex environments (Mimee et al., 2015; Taketani et al., 2020). Alternatively, CRISPRi/a can be interfaced with native gene regulatory systems to control the host’s metabolism in response to external stimuli, such as cell density, through either heterologous (Liu Y. et al., 2020) or even indigenous sensor systems (Tian et al., 2020). However, caution must be taken to prevent the expression of too many gRNA at once since they compete over the limited dCas protein resource and, thus, can decrease the repression of target genes (Del Vecchio et al., 2008; Li et al., 2018; Zhang and Voigt, 2018). Synthetic CRISPRi/a regulatory networks are rare in non-model bacteria, having been reported in only seven studies across four phyla, and primarily incorporate CRISPRi (Table 1). However, a single CRISPRa genetic circuit in Salmonella has been reported (Bhokisham et al., 2020).
CRISPRi/a tools have also been used to redirect carbon and energy flow for metabolic engineering in non-model bacteria (Figure 1C). CRISPRi is often used to repress a native gene(s), including essential genes, to redirect carbon flux towards a desired product (Wang et al., 2017; Shabestary et al., 2018) or bioactive molecule (Yu et al., 2018; Liu et al., 2021b). CRISPRa can be used to activate the desired metabolic pathway to increase biosynthesis of the desired product, such as an anti-cancer drug in a weakly-expressed biosynthetic gene cluster (Peng et al., 2018; Ye et al., 2019). In most examples, the CRISPRi/a components are constitutively expressed, yet some studies employ dynamic metabolic engineering strategies by utilizing inducible systems and/or genetically encoded biosensors to switch between cell growth and product biosynthesis states to improve production (Liu Y. et al., 2020; Tian et al., 2020; Shabestary et al., 2021). These tools can be used to tune endogenous metabolism and/or heterologous metabolic pathways (Peng et al., 2018; Banerjee et al., 2020). CRISPRi/a tools are most often combined with other metabolic engineering techniques, such as the deletion, overexpression, or mutation of select genes and optimization of medium, to further increase titers of the desired product (Park et al., 2019; Dietsch et al., 2021; Kozaeva et al., 2021).
Large-scale CRISPRi screens and selections have been developed to investigate genotype-phenotype relationships through gRNA fitness (Figure 1D). These assays can use small, targeted libraries, such as essential genes or genes in a metabolic pathway (Shields et al., 2020; Göttl et al., 2021), or large genome-wide libraries targeting nearly all genes in the bacterial genome (Lee et al., 2019; Jiang et al., 2020). Additionally, CRISPRi libraries can be constructed in two major forms—pooled libraries, where cells containing different gRNA are mixed during library construction (Bosch et al., 2021; Rahman et al., 2021), a strategy known as multiplexing, or arrayed libraries where different gRNA designs are constructed individually in different clonal populations, typically arrayed in microtiter plates (Liu et al., 2017; Göttl et al., 2021). Pooled competitive selections are more common due to the ease of DNA construction and analysis of large, genome-scale gRNA libraries with >10,000 designs by next-generation sequencing (Lee et al., 2019; Bosch et al., 2021). However, because all cells directly compete in pooled competitive growth assays, “cheaters” may arise that take advantage of different strain interactions, so the results of any individual gRNA design should be verified in isolation (Yao et al., 2020; Liu X. et al., 2021). Additionally, the results from these pooled CRISPRi screens or selections are specific to the gRNA design and not the target gene since confounding effects (i.e., off-target effects) could produce false positives or negatives, so careful design of gRNA libraries is vital (Cui et al., 2018; Wang T. et al., 2018). Genome-wide CRISPRi screens or selections are relatively uncommon (Table 1). While not demonstrated to date, genome-wide bacterial CRISPRa is theoretically possible, provided the design rules for activation are met (Fontana et al., 2020a).
Conclusion and Perspectives
CRISPRi has been established in non-model bacteria across eight phyla and applied from small, single gene functional studies to large genome-wide screens. The creation of new tools and protocols for introducing CRISPRi/a into non-model bacteria will facilitate the continuation of this rapid expansion. Several novel and exciting CRISPRa tools with greater activation and unique characteristics have been developed recently in both model and non-model bacteria, yet there remains a need for stronger and more versatile bacterial CRISPRa tools, especially for the activation of native genes. These bacterial CRISPRa tools have lagged behind the development of both eukaryotic CRISPRa tools and bacterial CRISPRi tools. However, the recent development of several new CRISPRa systems with less stringent design rules and higher levels of activation (>10-fold) shows great promise for effective, tailored gene activation in bacteria (Liu Y. et al., 2019; Fontana et al., 2020a; Ho et al., 2020; Villegas Kcam et al., 2021). These CRISPRa technologies were created using directed evolution and thorough tool design. Further improvements could be achieved by creating CRISPRa tools from CRISPR systems with more relaxed PAM requirements, directed evolution of CRISPRa components (activator domain, gRNA scaffold(s), and dCas protein) for greater activation, and high-throughput screening of gRNAs and promoters to uncover additional nuanced design rules for a given tool. CRISPRa has the potential to become a more effective and widely used tool for programmable gene activation in both model and non-model bacteria for a variety of industrial and research applications, such as metabolic engineering and elucidation of gene function. While many CRISPRi/a approaches in non-model bacteria have been established using genetic parts that are not well-defined or characterized, the creation of comprehensive genetic part toolboxes for these strains, which are vital for the rational design and precise control of CRISPRi/a tools, will accelerate further development and optimization of the tools. Finally, CRISPRi/a approaches have primarily been developed for more genetically tractable strains of non-model bacteria. There is a need for efficient workflows to domesticate and introduce CRISPRi/a tools to novel bacterial species and strains. Despite these current challenges, CRISPRi/a technology remains a versatile approach for programmable transcriptional regulation in non-model bacteria.
Acknowledgments
The authors would like to thank members of the Andrews research group for their discussions contributing to the manuscript.
Author Contributions
SC and LA conceived of the review and wrote the manuscript. All authors read and approved the manuscript.
Funding
This material is based upon work supported by the National Science Foundation under Grant No. DMR-1904901 to LA. Additional funding for this work was provided by startup funds to LA from the University of Massachusetts Amherst and a seed grant award from the UMass ADVANCE program funded by the National Science Foundation (awards #1824090 and #2136150). This work is also supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE- 1451512 to SC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgeed.2022.892304/full#supplementary-material
References
- Afonina I., Ong J., Chua J., Lu T., Kline K. A. (2020). Multiplex CRISPRi System Enables the Study of Stage-specific Biofilm Genetic Requirements in Enterococcus faecalis . mBio 11. 10.1128/mBio.01101-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N. (2020). Construction of a Novel CRISPRi-Based Tool for Silencing of Multiple Genes in Mycobacterium tuberculosis . Plasmid 110, 102515. 10.1016/j.plasmid.2020.102515 [DOI] [PubMed] [Google Scholar]
- Ameruoso A., Villegas Kcam M. C., Cohen K. P., Chappell J. (2021). Activating Natural Product Synthesis Using CRISPR Interference and Activation Systems in Streptomyces. bioRxiv. [Preprint]. 10.1101/2021.10.28.466254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Dai Y., Farinha A., Tang A. Y., Syal S., Vargas-Cuebas G., et al. (2021). Essential Gene Analysis in Acinetobacter Baumannii by High-Density Transposon Mutagenesis and CRISPR Interference. J. Bacteriol. 203, 1. 10.1128/JB.00565-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Eng T., Lau A. K., Sasaki Y., Wang B., Chen Y., et al. (2020). Genome-scale Metabolic Rewiring Improves Titers Rates and Yields of the Non-native Product Indigoidine at Scale. Nat. Commun. 11, 5385. 10.1038/s41467-020-19171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta A. B., Enright A. L., Siletti C., Peters J. M. (2020). A High-Efficacy CRISPR Interference System for Gene Function Discovery in Zymomonas Mobilis. Appl. Environ. Microbiol. 86, 1. 10.1128/AEM.01621-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski C., Welsh M. A., Sham L.-T., Eskandarian H. A., Lim H. C., Kieser K. J., et al. (2018). Maturing Mycobacterium Smegmatis Peptidoglycan Requires Non-canonical Crosslinks to Maintain Shape. eLife 7, e37516. 10.7554/eLife.37516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batianis C., Kozaeva E., Damalas S. G., Martín‐Pascual M., Volke D. C., Nikel P. I., et al. (2020). An Expanded CRISPRi Toolbox for Tunable Control of Gene Expression inPseudomonas Putida. Microb. Biotechnol. 13, 368–385. 10.1111/1751-7915.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behle A., Dietsch M., Goldschmidt L., Murugathas W., Brandt D., Busche T., et al. (2021). Uncoupling of the Diurnal Growth Program by Artificial Genome Relaxation in Synechocystis Sp. PCC 6803. bioRxiv. [Preprint], 453758. 10.1101/2021.07.26.453758 [DOI] [Google Scholar]
- Behler J., Sharma K., Reimann V., Wilde A., Urlaub H., Hess W. R. (2018). The Host-Encoded RNase E Endonuclease as the crRNA Maturation Enzyme in a CRISPR-Cas Subtype III-Bv System. Nat. Microbiol. 3, 367–377. 10.1038/s41564-017-0103-5 [DOI] [PubMed] [Google Scholar]
- Berlec A., Škrlec K., Kocjan J., Olenic M., Štrukelj B. (2018). Single Plasmid Systems for Inducible Dual Protein Expression and for CRISPR-Cas9/CRISPRi Gene Regulation in Lactic Acid Bacterium Lactococcus Lactis. Sci. Rep. 8, 1009. 10.1038/s41598-018-19402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhokisham N., VanArsdale E., Stephens K. T., Hauk P., Payne G. F., Bentley W. E. (2020). A Redox-Based Electrogenetic CRISPR System to Connect with and Control Biological Information Networks. Nat. Commun. 11, 2427. 10.1038/s41467-020-16249-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikard D., Jiang W., Samai P., Hochschild A., Zhang F., Marraffini L. A. (2013). Programmable Repression and Activation of Bacterial Gene Expression Using an Engineered CRISPR-Cas System. Nucleic Acids Res. 41, 7429–7437. 10.1093/nar/gkt520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B., DeJesus M. A., Poulton N. C., Zhang W., Engelhart C. A., Zaveri A., et al. (2021). Genome-wide Gene Expression Tuning Reveals Diverse Vulnerabilities of M. tuberculosis . Cell 184, 4579–4592. e24. 10.1016/j.cell.2021.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito L. F., Schultenkämper K., Passaglia L. M. P., Wendisch V. F. (2020). CRISPR Interference-Based Gene Repression in the Plant Growth Promoter Paenibacillus Sonchi Genomovar Riograndensis SBR5. Appl. Microbiol. Biotechnol. 104, 5095–5106. 10.1007/s00253-020-10571-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockett M. R., Lee J., Cox J. V., Liechti G. W., Ouellette S. P. (2021). A Dynamic, Ring-Forming Bactofilin Critical for Maintaining Cell Size in the Obligate Intracellular Bacterium Chlamydia trachomatis . Infect. Immun. 89, 1. 10.1128/IAI.00203-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder M. R., Pyne M. E., Moo-Young M., Chung D. A., Chou C. P. (2016). Extending CRISPR-Cas9 Technology from Genome Editing to Transcriptional Engineering in the Genus Clostridium. Appl. Environ. Microbiol. 82, 6109–6119. 10.1128/AEM.02128-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzostek A., Płociński P., Minias A., Ciszewska A., Gąsior F., Pawełczyk J., et al. (2021). Dissecting the RecA-(In)dependent Response to Mitomycin C in Mycobacterium tuberculosis Using Transcriptional Profiling and Proteomics Analyses. Cells 10, 1168. 10.3390/cells10051168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li X., Li F., Song H. (2017). CRISPRi-sRNA: Transcriptional-Translational Regulation of Extracellular Electron Transfer in Shewanella Oneidensis. ACS Synth. Biol. 6, 1679–1690. 10.1021/acssynbio.6b00374 [DOI] [PubMed] [Google Scholar]
- Caro F., Place N. M., Mekalanos J. J. (2019). Analysis of Lipoprotein Transport Depletion in Vibrio cholerae Using CRISPRi. Proc. Natl. Acad. Sci. U.S.A. 116, 17013–17022. 10.1073/pnas.1906158116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai G., Yu M., Jiang L., Duan Y., Huang J. (2019). HMMCAS: A Web Tool for the Identification and Domain Annotations of CAS Proteins. IEEE/ACM Trans. Comput. Biol. Bioinf. 16, 1313–1315. 10.1109/TCBB.2017.2665542 [DOI] [PubMed] [Google Scholar]
- Chen W., Zhang Y., Yeo W.-S., Bae T., Ji Q. (2017). Rapid and Efficient Genome Editing in Staphylococcus aureus by Using an Engineered CRISPR/Cas9 System. J. Am. Chem. Soc. 139, 3790–3795. 10.1021/jacs.6b13317 [DOI] [PubMed] [Google Scholar]
- Cheung C.-Y., McNeil M. B., Cook G. M. (2021). Utilization of CRISPR Interference to Investigate the Contribution of Genes to Pathogenesis in a Macrophage Model of Mycobacterium tuberculosis Infection. J. Antimicrob. Chemother. 77, 615–619. 10.1093/jac/dkab437 [DOI] [PubMed] [Google Scholar]
- Cho S., Choe D., Lee E., Kim S. C., Palsson B., Cho B.-K. (2018). High-Level dCas9 Expression Induces Abnormal Cell Morphology in Escherichia coli . ACS Synth. Biol. 7, 1085–1094. 10.1021/acssynbio.7b00462 [DOI] [PubMed] [Google Scholar]
- Choi S. Y., Woo H. M. (2020). CRISPRi-dCas12a: A dCas12a-Mediated CRISPR Interference for Repression of Multiple Genes and Metabolic Engineering in Cyanobacteria. ACS Synth. Biol. 9, 2351–2361. 10.1021/acssynbio.0c00091 [DOI] [PubMed] [Google Scholar]
- Choudhary E., Sharma R., Kumar Y., Agarwal N. (2019). Conditional Silencing by CRISPRi Reveals the Role of DNA Gyrase in Formation of Drug-Tolerant Persister Population in Mycobacterium tuberculosis . Front. Cell Infect. Microbiol. 9, 70. 10.3389/fcimb.2019.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary E., Thakur P., Pareek M., Agarwal N. (2015). Gene Silencing by CRISPR Interference in Mycobacteria. Nat. Commun. 6, 6267. 10.1038/ncomms7267 [DOI] [PubMed] [Google Scholar]
- Cleto S., Jensen J. V., Wendisch V. F., Lu T. K. (2016). Corynebacterium Glutamicum Metabolic Engineering with CRISPR Interference (CRISPRi). ACS Synth. Biol. 5, 375–385. 10.1021/acssynbio.5b00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun J. M., Farokhyfar M., Hutcheson A. R., Anderson A., Bethel C. R., Bonomo R. A., et al. (2021). OXA-23 β-Lactamase Overexpression in Acinetobacter Baumannii Drives Physiological Changes Resulting in New Genetic Vulnerabilities. mBio 12. 10.1128/mBio.03137-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvin D., Bernheim A., Toffano-Nioche C., Touchon M., Michalik J., Néron B., et al. (2018). CRISPRCasFinder, an Update of CRISRFinder, Includes a Portable Version, Enhanced Performance and Integrates Search for Cas Proteins. Nucleic Acids Res. 46, W246–W251. 10.1093/nar/gky425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Vigouroux A., Rousset F., Varet H., Khanna V., Bikard D. (2018). A CRISPRi Screen in E. coli Reveals Sequence-specific Toxicity of dCas9. Nat. Commun. 9, 1912. 10.1038/s41467-018-04209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajka J. J., Banerjee D., Eng T., Menasalvas J., Yan C., Munoz N. M., et al. (2021). Optimizing a High Performing Multiplex-CRISPRi P. Putida Strain with Integrated Metabolomics and 13C-Metabolic Flux Analyses. bioRxiv. [Preprint], 473729. 10.1101/2021.12.23.473729 [DOI] [Google Scholar]
- Dai Y., Pinedo V., Tang A. Y., Cava F., Geisinger E. (2021). A New Class of Cell Wall-Recycling L , D -Carboxypeptidase Determines β-Lactam Susceptibility and Morphogenesis in Acinetobacter Baumannii. mBio 12. 10.1128/mBio.02786-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann A. N., Chamby A. B., Catomeris A. J., Davidson K. M., Tettelin H., van Pijkeren J.-P., et al. (2021). Genome-Wide Fitness Analysis of Group B Streptococcus in Human Amniotic Fluid Reveals a Transcription Factor that Controls Multiple Virulence Traits. PLoS Pathog. 17, e1009116. 10.1371/journal.ppat.1009116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker V., Liu X., Bravo A. M., Veening J.-W. (2022). CRISPRi-seq for Genome-wide Fitness Quantification in Bacteria. Nat. Protoc. 17, 252–281. 10.1038/s41596-021-00639-6 [DOI] [PubMed] [Google Scholar]
- de Wet T. J., Winkler K. R., Mhlanga M., Mizrahi V., Warner D. F. (2020). Arrayed CRISPRi and Quantitative Imaging Describe the Morphotypic Landscape of Essential Mycobacterial Genes. eLife 9, e60083. 10.7554/eLife.60083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio D., Ninfa A. J., Sontag E. D. (2008). Modular Cell Biology: Retroactivity and Insulation. Mol. Syst. Biol. 4, 161. 10.1038/msb4100204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo D. M., Diao J., Carr R., Hu Y., Moon T. S. (2021). An Improved CRISPR Interference Tool to Engineer Rhodococcus Opacus. ACS Synth. Biol. 10, 786–798. 10.1021/acssynbio.0c00591 [DOI] [PubMed] [Google Scholar]
- DeLorenzo D. M., Rottinghaus A. G., Henson W. R., Moon T. S. (2018). Molecular Toolkit for Gene Expression Control and Genome Modification in Rhodococcus Opacus PD630. ACS Synth. Biol. 7, 727–738. 10.1021/acssynbio.7b00416 [DOI] [PubMed] [Google Scholar]
- Depardieu F., Bikard D. (2020). Gene Silencing with CRISPRi in Bacteria and Optimization of dCas9 Expression Levels. Methods 172, 61–75. 10.1016/j.ymeth.2019.07.024 [DOI] [PubMed] [Google Scholar]
- Dewachter L., Liu X., Dénéréaz J., de Bakker V., Costa C., Baldry M., et al. (2021). Amoxicillin-resistant Streptococcus Pneumoniae Can Be Resensitized by Targeting the Mevalonate Pathway as Indicated by sCRilecs-Seq. bioRxiv. [Preprint], 460059. 10.1101/2021.09.13.460059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietsch M., Behle A., Westhoff P., Axmann I. M. (2021). Metabolic Engineering of Synechocystis Sp. PCC 6803 for the Photoproduction of the Sesquiterpene Valencene. Metab. Eng. Commun. 13, e00178. 10.1016/j.mec.2021.e00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech A., Slager J., Veening J.-W. (2018). Antibiotic-Induced Cell Chaining Triggers Pneumococcal Competence by Reshaping Quorum Sensing to Autocrine-like Signaling. Cell Rep. 25, 2390–2400. e3. 10.1016/j.celrep.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Fontana J., Patel A., Carothers J. M., Zalatan J. G. (2018). Synthetic CRISPR-Cas Gene Activators for Transcriptional Reprogramming in Bacteria. Nat. Commun. 9, 2489. 10.1038/s41467-018-04901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Jin Y., Ming D., Li B., Dong H., Wang L., et al. (2017). CRISPR/dCas9-mediated Inhibition of Gene Expression in Staphylococcus aureus . J. Microbiol. Methods 139, 79–86. 10.1016/j.mimet.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Dutta A. K., Choudhary E., Wang X., Záhorszka M., Forbak M., Lohner P., et al. (2019). Trehalose Conjugation Enhances Toxicity of Photosensitizers against Mycobacteria. ACS Cent. Sci. 5, 644–650. 10.1021/acscentsci.8b00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis N. A., Kim B., Tung J., Machner M. P. (2021). A Multiplex CRISPR Interference Tool for Virulence Gene Interrogation in Legionella pneumophila . Commun. Biol. 4, 1–13. 10.1038/s42003-021-01672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler N., Heffernan J., Juminaga A., Doser D., Nagaraju S., Gonzalez-Garcia R. A., et al. (2021). Transcriptional Control of Clostridium Autoethanogenum Using CRISPRi. Synth. Biol. 6, ysab008. 10.1093/synbio/ysab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner V., Cox A. A., Goh S., van Bohemen A., Gibson A. J., Liebster O., et al. (2020). Re-sensitization of Mycobacterium Smegmatis to Rifampicin Using CRISPR Interference Demonstrates its Utility for the Study of Non-essential Drug Resistance Traits. Front. Microbiol. 11, 619427. 10.3389/fmicb.2020.619427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L. G. V., Guaman L. P., Vasconcellos S. A., Heinemann M. B., Picardeau M., Nascimento A. L. T. O. (2019). Gene Silencing Based on RNA-Guided Catalytically Inactive Cas9 (dCas9): a New Tool for Genetic Engineering in Leptospira. Sci. Rep. 9, 1839. 10.1038/s41598-018-37949-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L. G. V., Hornsby R. L., Nascimento A. L. T. O., Nally J. E. (2021a). Application of CRISPR Interference (CRISPRi) for Gene Silencing in Pathogenic Species of Leptospira. JoVE 174, e62631. 10.3791/62631 [DOI] [PubMed] [Google Scholar]
- Fernandes L. G. V., Hornsby R. L., Nascimento A. L. T. O., Nally J. E. (2021b). Genetic Manipulation of Pathogenic Leptospira: CRISPR Interference (CRISPRi)-Mediated Gene Silencing and Rapid Mutant Recovery at 37 °C. Sci. Rep. 11, 1768. 10.1038/s41598-021-81400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck N., Grundner C. (2021). A Cas12a-Based CRISPR Interference System for Multigene Regulation in Mycobacteria. J. Biol. Chem. 297, 100990. 10.1016/j.jbc.2021.100990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfara I., Richter H., Bratovič M., Le Rhun A., Charpentier E. (2016). The CRISPR-Associated DNA-Cleaving Enzyme Cpf1 Also Processes Precursor CRISPR RNA. Nature 532, 517–521. 10.1038/nature17945 [DOI] [PubMed] [Google Scholar]
- Fontana J., Dong C., Kiattisewee C., Chavali V. P., Tickman B. I., Carothers J. M., et al. (2020a). Effective CRISPRa-Mediated Control of Gene Expression in Bacteria Must Overcome Strict Target Site Requirements. Nat. Commun. 11, 1618. 10.1038/s41467-020-15454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J., Sparkman-Yager D., Zalatan J. G., Carothers J. M. (2020b). Challenges and Opportunities with CRISPR Activation in Bacteria for Data-Driven Metabolic Engineering. Curr. Opin. Biotechnol. 64, 190–198. 10.1016/j.copbio.2020.04.005 [DOI] [PubMed] [Google Scholar]
- Gallay C., Sanselicio S., Anderson M. E., Soh Y. M., Liu X., Stamsås G. A., et al. (2021). CcrZ Is a Pneumococcal Spatiotemporal Cell Cycle Regulator that Interacts with FtsZ and Controls DNA Replication by Modulating the Activity of DnaA. Nat. Microbiol. 6, 1175–1187. 10.1038/s41564-021-00949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly J., Martin‐Pascual M., Kranenburg R. (2020). CRISPR Interference (CRISPRi) as Transcriptional Repression Tool for Hungateiclostridium Thermocellum DSM 1313. Microb. Biotechnol. 13, 339–349. 10.1111/1751-7915.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani Z., Boradia V. M., Kumar A., Patidar A., Talukdar S., Choudhary E., et al. (2021). Mycobacterium tuberculosis Glyceraldehyde‐3‐phosphate Dehydrogenase Plays a Dual Role-As an Adhesin and as a Receptor for Plasmin(ogen). Cell. Microbiol. 23, e13311. 10.1111/cmi.13311 [DOI] [PubMed] [Google Scholar]
- Gauttam R., Mukhopadhyay A., Simmons B. A., Singer S. W. (2021). Development of Dual‐inducible Duet‐expression Vectors for Tunable Gene Expression Control and CRISPR Interference‐based Gene Repression in Pseudomonas Putida KT2440. Microb. Biotechnol. 14, 2659–2678. 10.1111/1751-7915.13832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauttam R., Seibold G. M., Mueller P., Weil T., Weiß T., Handrick R., et al. (2019). A Simple Dual-Inducible CRISPR Interference System for Multiple Gene Targeting in Corynebacterium Glutamicum. Plasmid 103, 25–35. 10.1016/j.plasmid.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Gelin M., Paoletti J., Nahori M.-A., Huteau V., Leseigneur C., Jouvion G., et al. (2020). From Substrate to Fragments to Inhibitor Active In Vivo against Staphylococcus aureus . ACS Infect. Dis. 6, 422–435. 10.1021/acsinfecdis.9b00368 [DOI] [PubMed] [Google Scholar]
- Geng P., Leonard S. P., Mishler D. M., Barrick J. E. (2019). Synthetic Genome Defenses against Selfish DNA Elements Stabilize Engineered Bacteria against Evolutionary Failure. ACS Synth. Biol. 8, 521–531. 10.1021/acssynbio.8b00426 [DOI] [PubMed] [Google Scholar]
- Gibson A. J., Passmore I. J., Faulkner V., Xia D., Nobeli I., Stiens J., et al. (2021). Probing Differences in Gene Essentiality between the Human and Animal Adapted Lineages of the Mycobacterium tuberculosis Complex Using TnSeq. Front. Vet. Sci. 8, 760717. 10.3389/fvets.2021.760717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G. C., Korosh T. C., Cameron J. C., Markley A. L., Begemann M. B., Pfleger B. F. (2016). CRISPR Interference as a Titratable, Trans-acting Regulatory Tool for Metabolic Engineering in the Cyanobacterium Synechococcus Sp. Strain PCC 7002. Metab. Eng. 38, 170–179. 10.1016/j.ymben.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttl V. L., Schmitt I., Braun K., Peters-Wendisch P., Wendisch V. F., Henke N. A. (2021). CRISPRi-Library-Guided Target Identification for Engineering Carotenoid Production by Corynebacterium Glutamicum. Microorganisms 9, 670. 10.3390/microorganisms9040670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo M., Castro L. K., Reisch C. R., Guo M. S., Laub M. T. (2020). A CRISPR Interference System for Efficient and Rapid Gene Knockdown in Caulobacter crescentus . mBio 11. 10.1128/mBio.02415-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo A., Ehira S. (2019). Spatiotemporal Gene Repression System in the Heterocyst-Forming Multicellular Cyanobacterium Anabaena Sp. PCC 7120. ACS Synth. Biol. 8, 641–646. 10.1021/acssynbio.8b00496 [DOI] [PubMed] [Google Scholar]
- Higo A., Isu A., Fukaya Y., Ehira S., Hisabori T. (2018). Application of CRISPR Interference for Metabolic Engineering of the Heterocyst-Forming Multicellular Cyanobacterium Anabaena Sp. PCC 7120. Plant Cell Physiology 59, 119–127. 10.1093/pcp/pcx166 [DOI] [PubMed] [Google Scholar]
- Higo A., Nishiyama E., Nakamura K., Hihara Y., Ehira S. (2019). cyAbrB Transcriptional Regulators as Safety Devices to Inhibit Heterocyst Differentiation in Anabaena Sp. Strain PCC 7120. J. Bacteriol. 201, 1. 10.1128/JB.00244-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H. I., Fang J. R., Cheung J., Wang H. H. (2020). Programmable CRISPR‐Cas Transcriptional Activation in Bacteria. Mol. Syst. Biol. 16, e9427. 10.15252/msb.20199427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan A. M., Rahman A. S. M. Z., Lightly T. J., Cardona S. T. (2019). A Broad-Host-Range CRISPRi Toolkit for Silencing Gene Expression in Burkholderia. ACS Synth. Biol. 8, 2372–2384. 10.1021/acssynbio.9b00232 [DOI] [PubMed] [Google Scholar]
- Huang C.-H., Shen C. R., Li H., Sung L.-Y., Wu M.-Y., Hu Y.-C. (2016). CRISPR Interference (CRISPRi) for Gene Regulation and Succinate Production in Cyanobacterium S. Elongatus PCC 7942. Microb. Cell Fact. 15, 196. 10.1186/s12934-016-0595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Chen J., Wang Y., Shi T., Ni X., Pu W., et al. (2021). Development of a Hyperosmotic Stress Inducible Gene Expression System by Engineering the MtrA/MtrB-dependent NCgl1418 Promoter in Corynebacterium Glutamicum. Front. Microbiol. 12. 10.3389/fmicb.2021.718511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Liu Q. J., Sun X. W., Li X. J., Liu M., Jia S. R., et al. (2020). Tailoring Bacterial Cellulose Structure through CRISPR Interference‐mediated Downregulation of galU in Komagataeibacter Xylinus CGMCC 2955. Biotechnol. Bioeng. 117, 2165–2176. 10.1002/bit.27351 [DOI] [PubMed] [Google Scholar]
- Irnov I., Wang Z., Jannetty N. D., Bustamante J. A., Rhee K. Y., Jacobs-Wagner C. (2017). Crosstalk between the Tricarboxylic Acid Cycle and Peptidoglycan Synthesis in Caulobacter crescentus through the Homeostatic Control of α-ketoglutarate. PLoS Genet. 13, e1006978. 10.1371/journal.pgen.1006978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. A., McKenzie R. E., Fagerlund R. D., Kieper S. N., Fineran P. C., Brouns S. J. J. (2017). CRISPR-cas: Adapting to Change. Science 356, eaal5056. 10.1126/science.aal5056 [DOI] [PubMed] [Google Scholar]
- Jiang F., Zhou K., Ma L., Gressel S., Doudna J. A. (2015). A Cas9-Guide RNA Complex Preorganized for Target DNA Recognition. Science 348, 1477–1481. 10.1126/science.aab1452 [DOI] [PubMed] [Google Scholar]
- Jiang W., Oikonomou P., Tavazoie S. (2020). Comprehensive Genome-wide Perturbations via CRISPR Adaptation Reveal Complex Genetics of Antibiotic Sensitivity. Cell 180, 1002–1017. e31. 10.1016/j.cell.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W.-B., Li T.-T., Huo D., Qu S., Li X. V., Arifuzzaman M., et al. (2022). Genetic Manipulation of Gut Microbes Enables Single-Gene Interrogation in a Complex Microbiome. Cell 185, 547–562. e22. 10.1016/j.cell.2021.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd J. A., Canestrari J., Clark R., Joseph A., Lapierre P., Lasek-Nesselquist E., et al. (2021). A Mycobacterial Systems Resource for the Research Community. mBio 12. 10.1128/mBio.02401-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarzyk D., Cengic I., Yao L., Hudson E. P. (2018). Diversion of the Long-Chain Acyl-ACP Pool in Synechocystis to Fatty Alcohols through CRISPRi Repression of the Essential Phosphate Acyltransferase PlsX. Metab. Eng. 45, 59–66. 10.1016/j.ymben.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Kampmann M. (2018). CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 13, 406–416. 10.1021/acschembio.7b00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J., Robinson D., Wu Z.-Y., Kuftin A., Louie K., Kosina S., et al. (2022). CRAGE-CRISPR Facilitates Rapid Activation of Secondary Metabolite Biosynthetic Gene Clusters in Bacteria. Cell Chem. Biol. 29, 696–710. 10.1016/j.chembiol.2021.08.009 [DOI] [PubMed] [Google Scholar]
- Kiattisewee C., Dong C., Fontana J., Sugianto W., Peralta-Yahya P., Carothers J. M., et al. (2021). Portable Bacterial CRISPR Transcriptional Activation Enables Metabolic Engineering in Pseudomonas Putida. Metab. Eng. 66, 283–295. 10.1016/j.ymben.2021.04.002 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Kim H., Ahn W.-C., Park K.-H., Woo E.-J., Lee D.-H., et al. (2017). Efficient Transcriptional Gene Repression by Type V-A CRISPR-Cpf1 from Eubacterium Eligens. ACS Synth. Biol. 6, 1273–1282. 10.1021/acssynbio.6b00368 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Yoon P. K., Kim S. J., Woo S. G., Rha E., Lee H., et al. (2020). CRISPR Interference‐mediated Gene Regulation in Pseudomonas Putida KT 2440. Microb. Biotechnol. 13, 210–221. 10.1111/1751-7915.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtania P., Hódi B., Mallick I., Vass I. Z., Fehér T., Vass I., et al. (2019). A Single Plasmid Based CRISPR Interference in Synechocystis 6803 - A Proof of Concept. PLOS ONE 14, e0225375. 10.1371/journal.pone.0225375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops A., Vande Capelle F., Fontaine L., Verhaegen M., Mignolet J., Goffin P., et al. (2022). The CovRS Environmental Sensor Directly Controls the ComRS Signaling System to Orchestrate Competence Bimodality in Salivarius Streptococci. mBio 13. 10.1128/mbio.03125-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoot C. J., Biswas S., Pakrasi H. B. (2020). Tunable Repression of Key Photosynthetic Processes Using Cas12a CRISPR Interference in the Fast-Growing Cyanobacterium Synechococcus Sp. UTEX 2973. ACS Synth. Biol. 9, 132–143. 10.1021/acssynbio.9b00417 [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Makarova K. S., Zhang F. (2017). Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 37, 67–78. 10.1016/j.mib.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaeva E., Volkova S., Matos M. R. A., Mezzina M. P., Wulff T., Volke D. C., et al. (2021). Model-guided Dynamic Control of Essential Metabolic Nodes Boosts Acetyl-Coenzyme A-dependent Bioproduction in Rewired Pseudomonas Putida. Metab. Eng. 67, 373–386. 10.1016/j.ymben.2021.07.014 [DOI] [PubMed] [Google Scholar]
- Kuo J., Yuan R., Sánchez C., Paulsson J., Silver P. A. (2020). Toward a Translationally Independent RNA-Based Synthetic Oscillator Using Deactivated CRISPR-Cas. Nucleic Acids Res. 48, 8165–8177. 10.1093/nar/gkaa557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeta C., McPartland L., Tran N. Q., Meehan B. M., Zhang Y., Tanweer Z., et al. (2019). Inhibition ofPseudomonas aeruginosaandMycobacterium Tuberculosisdisulfide Bond Forming Enzymes. Mol. Microbiol. 111, 918–937. 10.1111/mmi.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. H., Ostrov N., Wong B. G., Gold M. A., Khalil A. S., Church G. M. (2019). Functional Genomics of the Rapidly Replicating Bacterium Vibrio Natriegens by CRISPRi. Nat. Microbiol. 4, 1105–1113. 10.1038/s41564-019-0423-8 [DOI] [PubMed] [Google Scholar]
- Lee M., Woo H. M. (2020). A Logic NAND Gate for Controlling Gene Expression in a Circadian Rhythm in Cyanobacteria. ACS Synth. Biol. 9, 3210–3216. 10.1021/acssynbio.0c00455 [DOI] [PubMed] [Google Scholar]
- Lee S. S., Shin H., Jo S., Lee S.-M., Um Y., Woo H. M. (2018). Rapid Identification of Unknown Carboxyl Esterase Activity in Corynebacterium Glutamicum Using RNA-Guided CRISPR Interference. Enzyme Microb. Technol. 114, 63–68. 10.1016/j.enzmictec.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Leonard S. P., Perutka J., Powell J. E., Geng P., Richhart D. D., Byrom M., et al. (2018). Genetic Engineering of Bee Gut Microbiome Bacteria with a Toolkit for Modular Assembly of Broad-Host-Range Plasmids. ACS Synth. Biol. 7, 1279–1290. 10.1021/acssynbio.7b00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. H., Tahon G., Geesink P., Sousa D. Z., Ettema T. J. G. (2021). Innovations to Culturing the Uncultured Microbial Majority. Nat. Rev. Microbiol. 19, 225–240. 10.1038/s41579-020-00458-8 [DOI] [PubMed] [Google Scholar]
- Li J., Tang Q., Li Y., Fan Y.-Y., Li F.-H., Wu J.-H., et al. (2020a). Rediverting Electron Flux with an Engineered CRISPR-ddAsCpf1 System to Enhance the Pollutant Degradation Capacity of Shewanella Oneidensis. Environ. Sci. Technol. 54, 3599–3608. 10.1021/acs.est.9b06378 [DOI] [PubMed] [Google Scholar]
- Li J., Ye B.-C. (2021). Metabolic Engineering of Pseudomonas Putida KT2440 for High-Yield Production of Protocatechuic Acid. Bioresour. Technol. 319, 124239. 10.1016/j.biortech.2020.124239 [DOI] [PubMed] [Google Scholar]
- Li L., Wei K., Zheng G., Liu X., Chen S., Jiang W., et al. (2018). CRISPR-Cpf1-Assisted Multiplex Genome Editing and Transcriptional Repression in Streptomyces. Appl. Environ. Microbiol. 84, 1. 10.1128/AEM.00827-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chen J., Wang Y., Liu J., Huang J., Chen N., et al. (2020b). Efficient Multiplex Gene Repression by CRISPR-dCpf1 in Corynebacterium Glutamicum. Front. Bioeng. Biotechnol. 8, 357. 10.3389/fbioe.2020.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen J., Minton N. P., Zhang Y., Wen Z., Liu J., et al. (2016). CRISPR-based Genome Editing and Expression Control Systems inClostridium acetobutylicumandClostridium Beijerinckii. Biotechnol. J. 11, 961–972. 10.1002/biot.201600053 [DOI] [PubMed] [Google Scholar]
- Li Z., Liu J. Z. (2017). Transcriptomic Changes in Response to Putrescine Production in Metabolically Engineered Corynebacterium Glutamicum. Front. Microbiol. 8, 1987. 10.3389/fmicb.2017.01987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew A. T. F., Theis T., Jensen S. O., Garcia-Lara J., Foster S. J., Firth N., et al. (2011). A Simple Plasmid-Based System that Allows Rapid Generation of Tightly Controlled Gene Expression in Staphylococcus aureus . Microbiology 157, 666–676. 10.1099/mic.0.045146-0 [DOI] [PubMed] [Google Scholar]
- Liow L. T., Go M. K., Chang M. W., Yew W. S. (2020). Toolkit Development for Cyanogenic and Gold Biorecovery Chassis Chromobacterium Violaceum. ACS Synth. Biol. 9, 953–961. 10.1021/acssynbio.0c00064 [DOI] [PubMed] [Google Scholar]
- Liu D., Johnson V. M., Pakrasi H. B. (2020a). A Reversibly Induced CRISPRi System Targeting Photosystem II in the Cyanobacterium Synechocystis Sp. PCC 6803. ACS Synth. Biol. 9, 1441–1449. 10.1021/acssynbio.0c00106 [DOI] [PubMed] [Google Scholar]
- Liu W., Tang D., Wang H., Lian J., Huang L., Xu Z. (2019a). Combined Genome Editing and Transcriptional Repression for Metabolic Pathway Engineering in Corynebacterium Glutamicum Using a Catalytically Active Cas12a. Appl. Microbiol. Biotechnol. 103, 8911–8922. 10.1007/s00253-019-10118-4 [DOI] [PubMed] [Google Scholar]
- Liu X., Gallay C., Kjos M., Domenech A., Slager J., Kessel S. P., et al. (2017). High‐throughput CRISPRi Phenotyping Identifies New Essential Genes in Streptococcus Pneumoniae. Mol. Syst. Biol. 13, 931. 10.15252/msb.20167449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kimmey J. M., Matarazzo L., de Bakker V., Van Maele L., Sirard J.-C., et al. (2021a). Exploration of Bacterial Bottlenecks and Streptococcus Pneumoniae Pathogenesis by CRISPRi-Seq. Cell Host Microbe 29, 107–120. e6. 10.1016/j.chom.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen J., Crisante D., Jaramillo Lopez J. M., Mahadevan R. (2020b). Dynamic Cell Programming with Quorum Sensing-Controlled CRISPRi Circuit. ACS Synth. Biol. 9, 1284–1291. 10.1021/acssynbio.0c00148 [DOI] [PubMed] [Google Scholar]
- Liu Y., Khan S., Wu P., Li B., Liu L., Ni J., et al. (2021b). Uncovering and Engineering a Mini-Regulatory Network of the TetR-Family Regulator SACE_0303 for Yield Improvement of Erythromycin in Saccharopolyspora Erythraea. Front. Bioeng. Biotechnol. 9. 10.3389/fbioe.2021.692901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wan X., Wang B. (2019b). Engineered CRISPRa Enables Programmable Eukaryote-like Gene Activation in Bacteria. Nat. Commun. 10, 3693. 10.1038/s41467-019-11479-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang H., Li S., Zhang Y., Cheng X., Xiang W., et al. (2021c). Engineering of Primary Metabolic Pathways for Titer Improvement of Milbemycins in Streptomyces Bingchenggensis. Appl. Microbiol. Biotechnol. 105, 1875–1887. 10.1007/s00253-021-11164-7 [DOI] [PubMed] [Google Scholar]
- Lunge A., Gupta R., Choudhary E., Agarwal N. (2020). The Unfoldase ClpC1 of Mycobacterium tuberculosis Regulates the Expression of a Distinct Subset of Proteins Having Intrinsically Disordered Termini. J. Biol. Chem. 295, 9455–9473. 10.1074/jbc.RA120.013456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M. L., Mullis A. S., Leenay R. T., Beisel C. L. (2015). Repurposing Endogenous Type I CRISPR-Cas Systems for Programmable Gene Repression. Nucleic Acids Res. 43, 674–681. 10.1093/nar/gku971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J., Rao C., Watt J., Sun X., Lin C., Zhang L., et al. (2019). Mycobacterium tuberculosis 6C sRNA Binds Multiple mRNA Targets via C-Rich Loops Independent of RNA Chaperones. Nucleic Acids Res. 47, 4292–4307. 10.1093/nar/gkz149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova K. S., Wolf Y. I., Iranzo J., Shmakov S. A., Alkhnbashi O. S., Brouns S. J. J., et al. (2020). Evolutionary Classification of CRISPR-Cas Systems: a Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 18, 67–83. 10.1038/s41579-019-0299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscal A. M., Kakizawa S., Hsu J. Y., Tanaka K., González-González L., Broto A., et al. (2018). Tuning Gene Activity by Inducible and Targeted Regulation of Gene Expression in Minimal Bacterial Cells. ACS Synth. Biol. 7, 1538–1552. 10.1021/acssynbio.8b00028 [DOI] [PubMed] [Google Scholar]
- Mårli M. T. (2020). Using CRISPR Interference to Study Novel Biofilm-Associated Genes in Staphylococcus aureus . Available at: https://nmbu.brage.unit.no/nmbu-xmlui/handle/11250/2682085 (Accessed January 28, 2022).
- Marraffini L. A., Sontheimer E. J. (2008). CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting DNA. Science 322, 1843–1845. 10.1126/science.1165771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreddy R. K. R., Wu X., Sapkota M., Prior A. M., Jones J. A., Sun D., et al. (2019). The Fatty Acid Synthesis Protein Enoyl-ACP Reductase II (FabK) Is a Target for Narrow-Spectrum Antibacterials for Clostridium difficile Infection. ACS Infect. Dis. 5, 208–217. 10.1021/acsinfecdis.8b00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M. B., Cook G. M. (2019). Utilization of CRISPR Interference to Validate MmpL3 as a Drug Target in Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 63, 1. 10.1128/AAC.00629-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M. B., Keighley L. M., Cook J. R., Cheung C. Y., Cook G. M. (2021). CRISPR Interference Identifies Vulnerable Cellular Pathways with Bactericidal Phenotypes in Mycobacterium tuberculosis . Mol. Microbiol. 116, 1033–1043. 10.1111/mmi.14790 [DOI] [PubMed] [Google Scholar]
- McNeil M. B., Ryburn H. W. K., Harold L. K., Tirados J. F., Cook G. M. (2020). Transcriptional Inhibition of the F 1 F 0 -Type ATP Synthase Has Bactericidal Consequences on the Viability of Mycobacteria. Antimicrob. Agents Chemother. 64, 1. 10.1128/AAC.00492-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M. B., Ryburn H. W., Tirados J., Cheung C.-Y., Cook G. M. (2022). Multiplexed Transcriptional Repression Identifies a Network of Bactericidal Interactions between Mycobacterial Respiratory Complexes. iScience 25, 103573. 10.1016/j.isci.2021.103573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao C., Zhao H., Qian L., Lou C. (2019). Systematically Investigating the Key Features of the DNase Deactivated Cpf1 for Tunable Transcription Regulation in Prokaryotic Cells. Synthetic Syst. Biotechnol. 4, 1–9. 10.1016/j.synbio.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimee M., Tucker A. C., Voigt C. A., Lu T. K. (2015). Programming a Human Commensal Bacterium, Bacteroides Thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst. 1, 62–71. 10.1016/j.cels.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X.-H., Zhang H., Wang T.-M., Zhang C., Zhang C., Xing X.-H., et al. (2020). Establishment of CRISPR Interference in Methylorubrum Extorquens and Application of Rapidly Mining a New Phytoene Desaturase Involved in Carotenoid Biosynthesis. Appl. Microbiol. Biotechnol. 104, 4515–4532. 10.1007/s00253-020-10543-w [DOI] [PubMed] [Google Scholar]
- Monk I. R., Tree J. J., Howden B. P., Stinear T. P., Foster T. J. (2015). Complete Bypass of Restriction Systems for Major Staphylococcus aureus Lineages. mBio 6. 10.1128/mBio.00308-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougiakos I., Mohanraju P., Bosma E. F., Vrouwe V., Finger Bou M., Naduthodi M. I. S., et al. (2017). Characterizing a Thermostable Cas9 for Bacterial Genome Editing and Silencing. Nat. Commun. 8, 1647. 10.1038/s41467-017-01591-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müh U., Pannullo A. G., Weiss D. S., Ellermeier C. D. (2019). A Xylose-Inducible Expression System and a CRISPR Interference Plasmid for Targeted Knockdown of Gene Expression in Clostridioides Difficile. J. Bacteriol. 201, 1. 10.1128/JB.00711-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrbråten I. S., Stamsås G. A., Chan H., Angeles D. M., Knutsen T. M., Salehian Z., et al. (2021). SmdA Is a Novel Cell Morphology Determinant in Staphylococcus aureus . bioRxiv. [Preprint], 469651. 10.1101/2021.11.23.469651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrbråten I. S., Wiull K., Salehian Z., Håvarstein L. S., Straume D., Mathiesen G., et al. (2019). CRISPR Interference for Rapid Knockdown of Essential Cell Cycle Genes in Lactobacillus Plantarum. mSphere 4. 10.1128/mSphere.00007-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolinskaia N. I., Zamakhaev M. V., Shumkov M. S., Armianinova D. K., Karpov D. S., Goncharenko A. V. (2021). CRISPR Interference of Adenylate Cyclases from Mycobacterium tuberculosis . Appl. Biochem. Microbiol. 57, 421–425. 10.1134/S0003683821040128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot-Gros M.-F., Forrester S., Malato G., Larsen P. E., Noirot P. (2019). CRISPR Interference to Interrogate Genes that Control Biofilm Formation in Pseudomonas Fluorescens. Sci. Rep. 9, 15954. 10.1038/s41598-019-52400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig P. M., Marraffini L. A. (2020). Molecular Mechanisms of CRISPR-Cas Immunity in Bacteria. Annu. Rev. Genet. 54, 93–120. 10.1146/annurev-genet-022120-112523 [DOI] [PubMed] [Google Scholar]
- Ouellette S. P. (2018). Feasibility of a Conditional Knockout System for Chlamydia Based on CRISPR Interference. Front. Cell Infect. Microbiol. 8, 59. 10.3389/fcimb.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette S. P., Blay E. A., Hatch N. D., Fisher-Marvin L. A. (2021). CRISPR Interference to Inducibly Repress Gene Expression in Chlamydia trachomatis . Infect. Immun. 89, 1. 10.1128/IAI.00108-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Shin H., Lee S.-M., Um Y., Woo H. M. (2018). RNA-guided Single/double Gene Repressions in Corynebacterium Glutamicum Using an Efficient CRISPR Interference and its Application to Industrial Strain. Microb. Cell Fact. 17, 4. 10.1186/s12934-017-0843-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Yu B. J., Choi J.-i., Woo H. M. (2019). Heterologous Production of Squalene from Glucose in Engineered Corynebacterium Glutamicum Using Multiplex CRISPR Interference and High-Throughput Fermentation. J. Agric. Food Chem. 67, 308–319. 10.1021/acs.jafc.8b05818 [DOI] [PubMed] [Google Scholar]
- Pawluk A., Davidson A. R., Maxwell K. L. (2018). Anti-CRISPR: Discovery, Mechanism and Function. Nat. Rev. Microbiol. 16, 12–17. 10.1038/nrmicro.2017.120 [DOI] [PubMed] [Google Scholar]
- Peng R., Wang Y., Feng W.-w., Yue X.-j., Chen J.-h., Hu X.-z., et al. (2018). CRISPR/dCas9-mediated Transcriptional Improvement of the Biosynthetic Gene Cluster for the Epothilone Production in Myxococcus Xanthus. Microb. Cell Fact. 17, 15. 10.1186/s12934-018-0867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M., Koo B.-M., Patino R., Heussler G. E., Hearne C. C., Qu J., et al. (2019). Enabling Genetic Analysis of Diverse Bacteria with Mobile-CRISPRi. Nat. Microbiol. 4, 244–250. 10.1038/s41564-018-0327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L. S., Larson M. H., Gilbert L. A., Doudna J. A., Weissman J. S., Arkin A. P., et al. (2013). Repurposing CRISPR as an RNA-Guided Platform for Sequence-specific Control of Gene Expression. Cell 152, 1173–1183. 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z., Yang Y., Yu S., Liu L., Chen Y., Chen J., et al. (2021). Repurposing the Endogenous Type I-E CRISPR/Cas System for Gene Repression in Gluconobacter Oxydans WSH-003. ACS Synth. Biol. 10, 84–93. 10.1021/acssynbio.0c00456 [DOI] [PubMed] [Google Scholar]
- Qu J., Prasad N. K., Yu M. A., Chen S., Lyden A., Herrera N., et al. (2019). Modulating Pathogenesis with Mobile-CRISPRi. J. Bacteriol. 201. 10.1128/JB.00304-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Garcia S., Gilman R. H., Sheen P., Zimic M. (2021). Silencing of an Efflux Pump Coding Gene Decreases the Efflux Rate of Pyrazinoic Acid in Mycobacterium Smegmatis. bioRxiv. [Preprint], 466536. 10.1101/2021.10.29.466536 [DOI] [Google Scholar]
- Rahman K., Jamal M., Chen X., Zhou W., Yang B., Zou Y., et al. (2021). Reprogramming Mycobacterium tuberculosis CRISPR System for Gene Editing and Genome-wide RNA Interference Screening. Genomics, Proteomics Bioinforma. 2021, 1. 10.1016/j.gpb.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. E., Martini M. C., Zhou Y., Joubran S. R., Shell S. S. (2020). MamA Essentiality in Mycobacterium Smegmatis Is Explained by the Presence of an Apparent Cognate Restriction Endonuclease. BMC Res. Notes 13, 462. 10.1186/s13104-020-05302-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath D., Amlinger L., Hoekzema M., Devulapally P. R., Lundgren M. (2015). Efficient Programmable Gene Silencing by Cascade. Nucleic Acids Res. 43, 237–246. 10.1093/nar/gku1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. A., Guss A. M. (2021). Approaches to Genetic Tool Development for Rapid Domestication of Non-model Microorganisms. Biotechnol. Biofuels 14, 30. 10.1186/s13068-020-01872-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. M., Hopkins F. F., Chavez A., Diallo M., Chase M. R., Gerrick E. R., et al. (2017). Programmable Transcriptional Repression in Mycobacteria Using an Orthogonal CRISPR Interference Platform. Nat. Microbiol. 2, 1–9. 10.1038/nmicrobiol.2016.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Pacheco C. C., Yao L., Hudson E. P., Tamagnini P. (2021). CRISPRi as a Tool to Repress Multiple Copies of Extracellular Polymeric Substances (EPS)-Related Genes in the Cyanobacterium Synechocystis Sp. PCC 6803. Life 11, 1198. 10.3390/life11111198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato’o Y., Hisatsune J., Yu L., Sakuma T., Yamamoto T., Sugai M. (2018). Tailor-made Gene Silencing of Staphylococcus aureus Clinical Isolates by CRISPR Interference. PLOS ONE 13, e0185987. 10.1371/journal.pone.0185987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savková K., Huszár S., Baráth P., Pakanová Z., Kozmon S., Vancová M., et al. (2021). An ABC Transporter Wzm-Wzt Catalyzes Translocation of Lipid-Linked Galactan across the Plasma Membrane in Mycobacteria. Proc. Natl. Acad. Sci. U.S.A. 118. 10.1073/pnas.2023663118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C., Koffas M. A. G., Sieber V., Schmid J. (2020). Novel Prokaryotic CRISPR-Cas12a-Based Tool for Programmable Transcriptional Activation and Repression. ACS Synth. Biol. 9, 3353–3363. 10.1021/acssynbio.0c00424 [DOI] [PubMed] [Google Scholar]
- Schultenkämper K., Brito L. F., López M. G., Brautaset T., Wendisch V. F. (2019). Establishment and Application of CRISPR Interference to Affect Sporulation, Hydrogen Peroxide Detoxification, and Mannitol Catabolism in the Methylotrophic Thermophile Bacillus Methanolicus. Appl. Microbiol. Biotechnol. 103, 5879–5889. 10.1007/s00253-019-09907-8 [DOI] [PubMed] [Google Scholar]
- Schultenkämper K., Gütle D. D., López M. G., Keller L. B., Zhang L., Einsle O., et al. (2021). Interrogating the Role of the Two Distinct Fructose-Bisphosphate Aldolases of Bacillus Methanolicus by Site-Directed Mutagenesis of Key Amino Acids and Gene Repression by CRISPR Interference. Front. Microbiol. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Y., Qiu Y., Zhu Y., Sun T., Luo Z., Gao J., et al. (2020). CRISPRi-Based Dynamic Regulation of Hydrolase for the Synthesis of Poly-γ-Glutamic Acid with Variable Molecular Weights. ACS Synth. Biol. 9, 2450–2459. 10.1021/acssynbio.0c00207 [DOI] [PubMed] [Google Scholar]
- Shabestary K., Anfelt J., Ljungqvist E., Jahn M., Yao L., Hudson E. P. (2018). Targeted Repression of Essential Genes to Arrest Growth and Increase Carbon Partitioning and Biofuel Titers in Cyanobacteria. ACS Synth. Biol. 7, 1669–1675. 10.1021/acssynbio.8b00056 [DOI] [PubMed] [Google Scholar]
- Shabestary K., Hernández H. P., Miao R., Ljungqvist E., Hallman O., Sporre E., et al. (2021). Cycling between Growth and Production Phases Increases Cyanobacteria Bioproduction of Lactate. Metab. Eng. 68, 131–141. 10.1016/j.ymben.2021.09.010 [DOI] [PubMed] [Google Scholar]
- Shields R. C., Walker A. R., Maricic N., Chakraborty B., Underhill S. A. M., Burne R. A. (2020). Repurposing the Streptococcus Mutans CRISPR-Cas9 System to Understand Essential Gene Function. PLoS Pathog. 16, e1008344. 10.1371/journal.ppat.1008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kang S., Song Y., Jin S., Lee J. S., Lee J.-K., et al. (2019). Genome Engineering of Eubacterium Limosum Using Expanded Genetic Tools and the CRISPR-Cas9 System. ACS Synth. Biol. 8, 2059–2068. 10.1021/acssynbio.9b00150 [DOI] [PubMed] [Google Scholar]
- Singh A. K., Carette X., Potluri L.-P., Sharp J. D., Xu R., Prisic S., et al. (2016). Investigating Essential Gene Function inMycobacterium Tuberculosisusing an Efficient CRISPR Interference System. Nucleic Acids Res. 44, e143. 10.1093/nar/gkw625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. H., Jha B., Dwivedy A., Choudhary E., N A. G., Ashraf A., et al. (2017). Characterization of a Secretory Hydrolase from Mycobacterium tuberculosis Sheds Critical Insight into Host Lipid Utilization by M. tuberculosis . J. Biol. Chem. 292, 11326–11335. 10.1074/jbc.M117.794297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J., Jang S. H., Cha J. W., Jeong K. J. (2020). Development of CRISPR Interference (CRISPRi) Platform for Metabolic Engineering of Leuconostoc Citreum and its Application for Engineering Riboflavin Biosynthesis. Ijms 21, 5614. 10.3390/ijms21165614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoto M., Guan C., Fleming E., Oh J. (2020). A Universal, Genomewide GuideFinder for CRISPR/Cas9 Targeting in Microbial Genomes. mSphere 5. 10.1128/mSphere.00086-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoto M., Riera Puma J. P., Fleming E., Guan C., Nzutchi Y. O., Kim D., et al. (2021). Large-scale CRISPRi and Transcriptomics of Staphylococcus Epidermidis Identify Genetic Factors Implicated in Commensal-Pathogen Lifestyle Versatility. bioRxiv. [Preprint]. 10.1101/2021.04.29.442003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamsås G. A., Myrbråten I. S., Straume D., Salehian Z., Veening J. W., Håvarstein L. S., et al. (2018). CozEa and CozEb Play Overlapping and Essential Roles in Controlling Cell Division in Staphylococcus aureus . Mol. Microbiol. 109, 615–632. 10.1111/mmi.13999 [DOI] [PubMed] [Google Scholar]
- Stolle A.-S., Meader B. T., Toska J., Mekalanos J. J. (2021). Endogenous Membrane Stress Induces T6SS Activity in Pseudomonas aeruginosa . Proc. Natl. Acad. Sci. U.S.A. 118. 10.1073/pnas.2018365118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Wang Q., Jiang Y., Wen Z., Yang L., Wu J., et al. (2018). Genome Editing and Transcriptional Repression in Pseudomonas Putida KT2440 via the Type II CRISPR System. Microb. Cell Fact. 17, 41. 10.1186/s12934-018-0887-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs C. N., Scott M., Chang Y., Kloos Z. A., Irnov I., Rosa P. A., et al. (2021). A CRISPR Interference Platform for Selective Downregulation of Gene Expression in Borrelia Burgdorferi. Appl. Environ. Microbiol. 87, 1. 10.1128/AEM.02519-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani M., Zhang J., Zhang S., Triassi A. J., Huang Y.-J., Griffith L. G., et al. (2020). Genetic Circuit Design Automation for the Gut Resident Species Bacteroides Thetaiotaomicron. Nat. Biotechnol. 38, 962–969. 10.1038/s41587-020-0468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. Z., Reisch C. R., Prather K. L. J. (2018). A Robust CRISPR Interference Gene Repression System in Pseudomonas. J. Bacteriol. 200, 1. 10.1128/JB.00575-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W., Lv L., Chen G.-Q. (2017). Engineering Halomonas Species TD01 for Enhanced Polyhydroxyalkanoates Synthesis via CRISPRi. Microb. Cell Fact. 16, 48. 10.1186/s12934-017-0655-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh M. Y., Ooi K. H., Danny Teo S. X., Bin Mansoor M. E., Shaun Lim W. Z., Tan M. H. (2019). An Expanded Synthetic Biology Toolkit for Gene Expression Control in Acetobacteraceae. ACS Synth. Biol. 8, 708–723. 10.1021/acssynbio.8b00168 [DOI] [PubMed] [Google Scholar]
- Thakur P., Gantasala N. P., Choudhary E., Singh N., Abdin M. Z., Agarwal N. (2016). The Preprotein Translocase YidC Controls Respiratory Metabolism in Mycobacterium tuberculosis . Sci. Rep. 6, 24998. 10.1038/srep24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Yang G., Gu Y., Sun X., Lu Y., Jiang W. (2020). Developing an Endogenous Quorum-sensing Based CRISPRi Circuit for Autonomous and Tunable Dynamic Regulation of Multiple Targets in Streptomyces. Nucleic Acids Res. 48, 8188–8202. 10.1093/nar/gkaa602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Charusanti P., Zhang L., Weber T., Lee S. Y. (2015). CRISPR-Cas9 Based Engineering of Actinomycetal Genomes. ACS Synth. Biol. 4, 1020–1029. 10.1021/acssynbio.5b00038 [DOI] [PubMed] [Google Scholar]
- Tong Y., Whitford C. M., Blin K., Jørgensen T. S., Weber T., Lee S. Y. (2020). CRISPR-Cas9, CRISPRi and CRISPR-BEST-Mediated Genetic Manipulation in Streptomycetes. Nat. Protoc. 15, 2470–2502. 10.1038/s41596-020-0339-z [DOI] [PubMed] [Google Scholar]
- Ultee E., van der Aart L. T., Zhang L., van Dissel D., Diebolder C. A., van Wezel G. P., et al. (2020). Teichoic Acids Anchor Distinct Cell Wall Lamellae in an Apically Growing Bacterium. Commun. Biol. 3, 1–9. 10.1038/s42003-020-1038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartoukian S. R., Palmer R. M., Wade W. G. (2010). Strategies for Culture of 'unculturable' Bacteria. FEMS Microbiol. Lett. 309, no. 10.1111/j.1574-6968.2010.02000.x [DOI] [PubMed] [Google Scholar]
- Villegas Kcam M. C., Tsong A. J., Chappell J. (2022). Uncovering the Distinct Properties of a Bacterial Type I-E CRISPR Activation System. ACS Synth. Biol. 11, 1000–1003. 10.1021/acssynbio.1c00496 [DOI] [PubMed] [Google Scholar]
- Villegas Kcam M. C., Tsong A. J., Chappell J. (2021). Rational Engineering of a Modular Bacterial CRISPR-Cas Activation Platform with Expanded Target Range. Nucleic Acids Res. 49, 4793–4802. 10.1093/nar/gkab211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dai W., Li J., Li Q., Xie R., Zhang Y., et al. (2021a). AcrHub: an Integrative Hub for Investigating, Predicting and Mapping Anti-CRISPR Proteins. Nucleic Acids Res. 49, D630–D638. 10.1093/nar/gkaa951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dai W., Li J., Xie R., Dunstan R. A., Stubenrauch C., et al. (2020). PaCRISPR: a Server for Predicting and Visualizing Anti-CRISPR Proteins. Nucleic Acids Res. 48, W348–W357. 10.1093/nar/gkaa432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhao P., Li Y., Xu L., Tian P. (2018a). Engineering CRISPR Interference System in Klebsiella pneumoniae for Attenuating Lactic Acid Synthesis. Microb. Cell Fact. 17, 56. 10.1186/s12934-018-0903-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Nicholaou M. (2017). Suppression of Antimicrobial Resistance in MRSA Using CRISPR-dCas9. Clin. Lab. Sci. 30, 207–213. 10.29074/ascls.30.4.207 [DOI] [Google Scholar]
- Wang M., Liu L., Fan L., Tan T. (2017). CRISPRi Based System for Enhancing 1-butanol Production in Engineered Klebsiella pneumoniae . Process Biochem. 56, 139–146. 10.1016/j.procbio.2017.02.013 [DOI] [Google Scholar]
- Wang T., Guan C., Guo J., Liu B., Wu Y., Xie Z., et al. (2018b). Pooled CRISPR Interference Screening Enables Genome-Scale Functional Genomics Study in Bacteria with Superior Performance. Nat. Commun. 9, 2475. 10.1038/s41467-018-04899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wang M., Zhang Q., Cao S., Li X., Qi Z., et al. (2019). Reversible Gene Expression Control in Yersinia pestis by Using an Optimized CRISPR Interference System. Appl. Environ. Microbiol. 85, 1. 10.1128/AEM.00097-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun B. (2021). VraCP Regulates Cell Wall Metabolism and Antibiotic Resistance in Vancomycin-Intermediate Staphylococcus aureus Strain Mu50. J. Antimicrob. Chemother. 76, 1712–1723. 10.1093/jac/dkab113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fu Y., Wang M., Niu G. (2021b). Synthetic Cellobiose-Inducible Regulatory Systems Allow Tight and Dynamic Controls of Gene Expression in Streptomyces. ACS Synth. Biol. 10, 1956–1965. 10.1021/acssynbio.1c00152 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yue X., Yuan S., Hong Y., Hu W., Li Y. (2021c). Internal Promoters and Their Effects on the Transcription of Operon Genes for Epothilone Production in Myxococcus Xanthus. Front. Bioeng. Biotechnol. 9. 10.3389/fbioe.2021.758561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Z.-T., Seo S.-O., Lynn P., Lu T., Jin Y.-S., et al. (2016). Gene Transcription Repression inClostridium Beijerinckiiusing CRISPR-dCas9. Biotechnol. Bioeng. 113, 2739–2743. 10.1002/bit.26020 [DOI] [PubMed] [Google Scholar]
- Wen Z., Minton N. P., Zhang Y., Li Q., Liu J., Jiang Y., et al. (2017). Enhanced Solvent Production by Metabolic Engineering of a Twin-Clostridial Consortium. Metab. Eng. 39, 38–48. 10.1016/j.ymben.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Werner J. N., Shi H., Hsin J., Huang K. C., Gitai Z., Klein E. A. (2020). AimB Is a Small Protein Regulator of Cell Size and MreB Assembly. Biophysical J. 119, 593–604. 10.1016/j.bpj.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles T. J., Schlomann B. H., Wall E. S., Betancourt R., Parthasarathy R., Guillemin K. (2020). Swimming Motility of a Gut Bacterial Symbiont Promotes Resistance to Intestinal Expulsion and Enhances Inflammation. PLoS Biol. 18, e3000661. 10.1371/journal.pbio.3000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams McMackin E. A., Marsden A. E., Yahr T. L. (2019). H-NS Family Members MvaT and MvaU Regulate the Pseudomonas aeruginosa Type III Secretion System. J. Bacteriol. 201, 1. 10.1128/JB.00054-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolston B. M., Emerson D. F., Currie D. H., Stephanopoulos G. (2018). Rediverting Carbon Flux in Clostridium Ljungdahlii Using CRISPR Interference (CRISPRi). Metab. Eng. 48, 243–253. 10.1016/j.ymben.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Wu J., Cheng Z.-H., Min D., Cheng L., He R.-L., Liu D.-F., et al. (2020). CRISPRi System as an Efficient, Simple Platform for Rapid Identification of Genes Involved in Pollutant Transformation by Aeromonas Hydrophila. Environ. Sci. Technol. 54, 3306–3315. 10.1021/acs.est.9b07191 [DOI] [PubMed] [Google Scholar]
- Wu X., Zha J., Koffas M. A. G., Dordick J. S. (2019). ReducingStaphylococcus Aureusresistance to Lysostaphin Using CRISPR‐dCas9. Biotechnol. Bioeng. 116, 3149–3159. 10.1002/bit.27143 [DOI] [PubMed] [Google Scholar]
- Wurihan W., Huang Y., Weber A. M., Wu X., Fan H. (2019). Nonspecific Toxicities of Streptococcus Pyogenes and Staphylococcus aureus dCas9 in Chlamydia trachomatis . Pathogens Dis. 77, ftaa005. 10.1093/femspd/ftaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Qi F., Jiang L., Tan J., Deng C., Wei Z., et al. (2020). CRISPR‐dCas9‐mediated Knockdown of prtR , an Essential Gene in Pseudomonas aeruginosa . Lett. Appl. Microbiol. 71, 386–393. 10.1111/lam.13337 [DOI] [PubMed] [Google Scholar]
- Xiao J., Jia H., Pan L., Li Z., Lv L., Du B., et al. (2019). Application of the CRISPRi System to Repress sepF Expression in Mycobacterium Smegmatis. Infect. Genet. Evol. 72, 183–190. 10.1016/j.meegid.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Xiong Z.-Q., Wei Y.-Y., Kong L.-H., Song X., Yi H.-X., Ai L.-Z. (2020). Short Communication: An Inducible CRISPR/dCas9 Gene Repression System in Lactococcus Lactis. J. Dairy Sci. 103, 161–165. 10.3168/jds.2019-17346 [DOI] [PubMed] [Google Scholar]
- Xu Z., Li Y., Cao H., Si M., Zhang G., Woo P. C. Y., et al. (2021). A Transferrable and Integrative Type I-F Cascade for Heterologous Genome Editing and Transcription Modulation. Nucleic Acids Res. 49, e94. 10.1093/nar/gkab521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Suzuki Y., Kouzuma A., Watanabe K. (2022). Development of a CRISPR Interference System for Selective Gene Knockdown in Acidithiobacillus Ferrooxidans. J. Biosci. Bioeng. 133, 105–109. 10.1016/j.jbiosc.2021.10.012 [DOI] [PubMed] [Google Scholar]
- Yan Y.-S., Yang Y.-Q., Zou L.-S., Zhang L., Xia H.-Y. (2022). MilR3, a Unique SARP Family Pleiotropic Regulator in Streptomyces Bingchenggensis. Europepmc. [Preprint]. 10.21203/rs.3.rs-1248187/v1 [DOI] [PubMed] [Google Scholar]
- Yao L., Cengic I., Anfelt J., Hudson E. P. (2016). Multiple Gene Repression in Cyanobacteria Using CRISPRi. ACS Synth. Biol. 5, 207–212. 10.1021/acssynbio.5b00264 [DOI] [PubMed] [Google Scholar]
- Yao L., Shabestary K., Björk S. M., Asplund-Samuelsson J., Joensson H. N., Jahn M., et al. (2020). Pooled CRISPRi Screening of the Cyanobacterium Synechocystis Sp PCC 6803 for Enhanced Industrial Phenotypes. Nat. Commun. 11, 1666. 10.1038/s41467-020-15491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Liu T., Zhu M., Zhang W., Huang Z., Li S., et al. (2019). An Easy and Efficient Strategy for the Enhancement of Epothilone Production Mediated by TALE-TF and CRISPR/dcas9 Systems in Sorangium Cellulosum. Front. Bioeng. Biotechnol. 7, 334. 10.3389/fbioe.2019.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y.-C., Ng I.-S. (2021). Redirection of Metabolic Flux in Shewanella Oneidensis MR-1 by CRISPRi and Modular Design for 5-aminolevulinic Acid Production. Bioresour. Bioprocess. 8, 13. 10.1186/s40643-021-00366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Woo H. M. (2018). CRISPR Interference-Mediated Metabolic Engineering ofCorynebacterium Glutamicumfor Homo-Butyrate Production. Biotechnol. Bioeng. 115, 2067–2074. 10.1002/bit.26720 [DOI] [PubMed] [Google Scholar]
- Yu L., Su W., Fey P. D., Liu F., Du L. (2018). Yield Improvement of the Anti-MRSA Antibiotics WAP-8294A by CRISPR/dCas9 Combined with Refactoring Self-Protection Genes inLysobacter enzymogenesOH11. ACS Synth. Biol. 7, 258–266. 10.1021/acssynbio.7b00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus I. S., Anfelt J., Sporre E., Miao R., Hudson E. P., Jones P. R. (2022). Synthetic Metabolic Pathways for Conversion of CO2 into Secreted Short-To Medium-Chain Hydrocarbons Using Cyanobacteria. Metab. Eng. 72, 14–23. 10.1016/j.ymben.2022.01.017 [DOI] [PubMed] [Google Scholar]
- Zetsche B., Gootenberg J. S., Abudayyeh O. O., Slaymaker I. M., Makarova K. S., Essletzbichler P., et al. (2015). Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 163, 759–771. 10.1016/j.cell.2015.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Xu Y., Zheng P., He M., Sun S., Wang D., et al. (2020). Establishment and Application of Multiplexed CRISPR Interference System in Bacillus Licheniformis. Appl. Microbiol. Biotechnol. 104, 391–403. 10.1007/s00253-019-10230-5 [DOI] [PubMed] [Google Scholar]
- Zhang B., Liu Z.-Q., Liu C., Zheng Y.-G. (2016). Application of CRISPRi in Corynebacterium Glutamicum for Shikimic Acid Production. Biotechnol. Lett. 38, 2153–2161. 10.1007/s10529-016-2207-z [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang Z., Kang J., Chen J., Liu J., Gao N., et al. (2020a). CRISPR/Cas13d-Mediated Microbial RNA Knockdown. Front. Bioeng. Biotechnol. 8, 856. 10.3389/fbioe.2020.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ramijan K., Carrión V. J., van der Aart L. T., Willemse J., van Wezel G. P., et al. (2021). An Alternative and Conserved Cell Wall Enzyme that Can Substitute for the Lipid II Synthase MurG. mBio 12. 10.1128/mBio.03381-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Willemse J., Yagüe P., de Waal E., Claessen D., van Wezel G. P. (2020b). Branching of Sporogenic Aerial Hyphae in sflA and sflB Mutants of Streptomyces Coelicolor Correlates to Ectopic Localization of DivIVA and FtsZ in Time and Space. bioRxiv. [Preprint], 424426. 10.1101/2020.12.26.424426 [DOI] [Google Scholar]
- Zhang S., Voigt C. A. (2018). Engineered dCas9 with Reduced Toxicity in Bacteria: Implications for Genetic Circuit Design. Nucleic Acids Res. 46, 11115–11125. 10.1093/nar/gky884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang J., Cheng Q., Zheng X., Zhao G., Wang J. (2017). Multiplex Gene Regulation by CRISPR-ddCpf1. Cell Discov. 3, 1–9. 10.1038/celldisc.2017.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yuan J. (2021). CRISPR/Cas12a‐mediated Genome Engineering in the Photosynthetic Bacterium Rhodobacter Capsulatus. Microb. Biotechnol. 14, 2700–2710. 10.1111/1751-7915.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Shu X., Sun B. (2017). Construction of a Gene Knockdown System Based on Catalytically Inactive (“Dead”) Cas9 (dCas9) in Staphylococcus aureus . Appl. Environ. Microbiol. 83, 1. 10.1128/AEM.00291-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Liu Y., Zhang H., Chai C., Wang J., Jiang W., et al. (2019). CRISPR-Cas12a-Mediated Gene Deletion and Regulation in Clostridium Ljungdahlii and its Application in Carbon Flux Redirection in Synthesis Gas Fermentation. ACS Synth. Biol. 8, 2270–2279. 10.1021/acssynbio.9b00033 [DOI] [PubMed] [Google Scholar]
- Zhao X., Zheng H., Zhen J., Shu W., Yang S., Xu J., et al. (2020). Multiplex Genetic Engineering Improves Endogenous Expression of Mesophilic α-amylase Gene in a Wild Strain Bacillus Amyloliquefaciens 205. Int. J. Biol. Macromol. 165, 609–618. 10.1016/j.ijbiomac.2020.09.210 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li L., Zheng G., Jiang W., Deng Z., Wang Z., et al. (2018). CRISPR/dCas9-Mediated Multiplex Gene Repression inStreptomyces. Biotechnol. J. 13, 1800121. 10.1002/biot.201800121 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Han J., Wang B., Hu X., Li R., Shen W., et al. (2019). Characterization and Repurposing of the Endogenous Type I-F CRISPR-Cas System of Zymomonas Mobilis for Genome Engineering. Nucleic Acids Res. 47, 11461–11475. 10.1093/nar/gkz940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.