Abstract
Several studies, reviews and meta-analyses have documented that D-mannose use lowers the risk of recurrent urinary tract infections (UTI), but its role in the treatment of UTI/cystitis-related symptoms is unclear. In particular, no systematic review has analyzed the role of treatment with D-mannose in acute UTI/cystitis. In this paper, we systematically reviewed the published data on the effect of D-mannose, alone or in association with other compounds, on the typical symptoms of UTI/cystitis. PubMed/Medline and EMBASE databases were searched, from 1990 to January 2022, using combinations of the following keywords: ‘urinary tract infections’, ‘cystalgia’, ‘recurrent next urinary tract infection’, ‘cystitis’, ‘mannose’, ‘mannoside’, ‘D-mannose’, ‘bacteriuria’, ‘pyuria’, ‘pyelocystitis’ with the appropriate Boolean modifiers (Limits: Human, English, full article). Studies were selected for the systematic review if they were clinical studies and reported original data, the number of patients using D-mannose alone or in association with other treatments, and the number of patients with symptoms of UTI/cystitis at trial entry and after the follow-up period. A total of seven studies were identified. D-mannose was given alone in two studies, and was associated with cranberry extract, Morinda citrifolia fruit extract, pomegranate extract, fructo-oligosaccharides, lactobacilli, and N-acetylcysteine in the others. All studies reported that symptoms decreased after treatment with D-mannose. Despite the limitations of the studies, the consistent results observed among all studies give support to the general findings that D-mannose may be useful in the treatment of UTI/cystitis symptoms.
Keywords: D-mannose, urinary tract infection, cystitis, symptoms
1. Introduction
Urinary tract infections (UTI) are a common condition. Among them, cystitis is the most frequent disease. Treatment for UTI/cystitis ranges from over-the-counter medications to antibiotics if the cause is an infection. In such patients, antimicrobials are often inappropriately prescribed, and their use is associated with the selection of antimicrobial-resistant organisms colonizing or infecting the urinary tract (1).
The rise in antimicrobial resistant organisms is becoming a relevant clinical and public health issue. Worldwide, it has been estimated that ~10,000,000 deaths by 2050 will be attributable to antibiotic resistance (2).
Recent guidelines from the main international associations, such as the American and Canadian Urological Associations, and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (3), and the recent scientific literature (4,5) have underlined the importance of limited antibiotic use and of new research on molecules that interact with bacterial load or virulence mechanisms of uropathogens for the treatment of UTI.
Among these molecules, several studies have identified D-mannose (6-12), which is characterized by a non-pharmacological, non-metabolic, non-bacteriostatic or bactericidal, but biomechanical mechanism of action and does not affect antibiotic resistance (13).
2. Mechanism of action of D-mannose in the prevention and treatment of UTI
D-mannose is a monosaccharide naturally produced by the body from glucose. It is present in the body cells and in some foods. D-mannose differs from glucose by inversion of one of the four chiral centers of the molecule, precisely that on the carbon atom in the position 2. D-mannose is the ‘C-2 epimer’ of glucose (14,15).
At least 90% of ingested D-mannose is absorbed in the upper part of the intestine. Its peculiarity is that despite it being a simple molecule, this sugar is not metabolized by the organism. Consequently, it is not stored in the liver or other organs, but it is excreted unconverted into the urine via the kidneys. About 60 min after ingestion, it arrives unchanged in the urinary tract. D-mannose also has no effect on human metabolism after long-term use (16,17).
The most common agent of cystitis is the uropathogenic E. coli (UPEC). UPEC adheres to urothelial cells mainly through the interaction between FimH (a fimbrial adhesin) and mannosylated uroplakin proteins, which are the main determinants of the urovirulence of UPEC (2).
Several studies have shown that, in the urine, E. Coli attaches to D-mannose. This mechanism is based on the structural similarity between D-mannose and urothelial mannosylated receptors exposed by the epithelium of the urinary tract. Consequently, D-mannose prevents FimH-mediated bacterial adhesion to the bladder wall through a competitive inhibition mechanism (18).
Since the process of bacterial adhesion on the urothelial cell's surface is a determinant of the start of UTI, D-mannose was shown to be effective in treating UTIs caused by E. Coli (7,10). D-mannose exerts a urothelial barrier function, inhibiting the adhesion of bacteria to the urothelium. Binding free D-mannose, bacteria are blocked in the urine and then eliminated by the urinary tract (19). As a consequence of this mechanism, in vivo and in vitro studies have demonstrated that mannose-like molecules lower bacterial load 2-4 fold in the urinary tract and in the bladder (20).
This effect is also present in the case of concurrent antibiotic therapy. In fact, D-mannose has no bacteriostatic and/or bactericidal activity and does not modify the bacterial cell, thus it does not interfere with the action of antibiotics (2,21).
Furthermore, it has been suggested that the dosages of D-mannose used in clinical practice does not affect E. coli metabolism and growth and does not modify bacterial adhesiveness causing FimH variants. All these characteristics underline the fact that the long term use of D-mannose is safe (18).
Several studies, reviews and meta-analyses have shown that D-mannose use lowers the risk of recurrent (r)UTI (22,23). Less data has been published regarding the role of D-mannose in the treatment of UTI/cystitis related symptoms. In particular, to the best of our knowledge, no reviews have been published on the role of D-mannose in the treatment of acute UTI/cystitis, i.e., on the use of d-mannose not as prevention of recurrence but as treatment of acute symptoms.
In this paper, we have performed a systematic review of the available data on the effect of D-mannose on the typical symptoms of UTI/cystitis given alone or in association with other compounds.
3. Literature search methodology
Literature search
We searched PubMed (National Library of Medicine, Washington, DC) and EMBASE databases from 1990 to January 2022 using combinations of the key words: ‘mannose’, ‘mannoside’, ‘D-mannose’, ‘bacteriuria’, ‘pyuria’, ‘pyelocystitis’, ‘cystitis’, ‘urinary tract infections’, ‘cystalgia, recurrent next urinary tract infection’ with the appropriate Boolean modifiers (limits: full article, human, English). After the original search, we reviewed the reference lists of the identified articles to identify other pertinent studies.
Two authors reviewed the papers and independently identified the eligible articles for the systematic review and extracted the data. Any disagreement was solved after discussion with a third reviewer.
Studies were considered if they met all the following criteria: Clinical studies, studies reporting original data, studies reporting the number of patients using D-mannose alone or in association with other treatments, studies reporting number of patients with symptoms of UTI/cystitis at trial entry and after the follow-up period. Reviews, commentaries, and case reports were excluded.
This systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (24,25) and registered in the PROSPERO database (registration no. CRD42022303244).
Data extraction
A PICOS (Patient, Intervention, Comparator, Outcome, Study) structure was used for defining the study questions and the inclusion/exclusion criteria. The question was: ‘Is D-mannose effective in the treatment of symptoms of UTI/cystitis?’ (Table I).
Table I.
PICOS criteria for inclusion and exclusion of studies.
| Parameter | Inclusion criteria | Data extraction |
|---|---|---|
| Patient | Women with symptoms of low urinary tract infection/cystitis | Location, age, type of patients |
| Intervention | D-mannose | Dose and duration |
| Comparator | No treatment | Group definition |
| Outcome | Reduction of symptoms | Number of cases, type of assessment |
| Study | Cross-sectional, cohort, case–control studies, clinical trials | Type of study design |
For each study, the following information was extracted: First author's last name; year of publication; country of origin; design of the study; number of subjects treated with D-mannose; age if present; criteria for study entry; type or severity of symptoms at study entry and at follow up visit; type and dose of drug; and length of follow-up.
To evaluate the effect of D-mannose on the symptoms of UTI /cystitis, from the studies that presented data on long-term follow-up, we only considered information obtained during the evaluation of first symptoms after study entry.
Quality assessment
The quality of the studies included in the review was evaluated using the Newcastle-Ottawa scale (NOS) (26). Studies were evaluated according to three broad categories: Selection of study groups, comparability of study groups, and assessment of outcome (cohort studies) or ascertainment of exposure (case-control studies). The maximum score was 9.
For Randomized Controlled Trials (RCTs), the Revised Cochrane risk-of-bias tool for randomized trials was used (27).
4. Systematic review
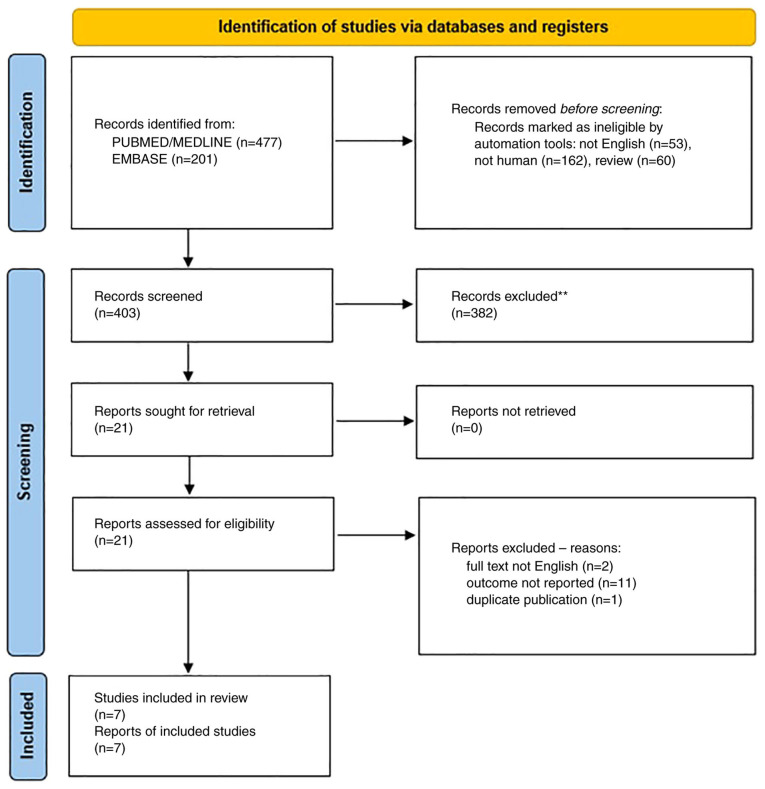
Our search retrieved 477 abstracts from PubMed/MEDLINE, and 201 from Embase (Fig. 1). After reviewing the abstracts, a total of 21 publications were identified that were fully read. Two studies were excluded as the full text was not in English (28,29), 11 studies (19,30-39) were excluded as they did not report the data on symptoms of UTI/cystitis, but only the risk of recurrent UTI or the frequency of UTI in patients submitted to urodynamics, and one (40) study was excluded as it reported a Visual Analogic Scale (VAS) evaluation of symptoms 12 months after study entry, but included women with rUTI without any specific indication of symptoms of UTI/cystitis at study entry.
Figure 1.
Flow chart of literature search.
A total of 7 studies were identified (6-12). Their primary methodological characteristics are presented in Table II. A total of four studies were prospective uncontrolled studies (7,8,10,11), one was a retrospective chart review case-controlled study (9) and two were randomized controlled trials (6,12). All but one of the studies (10), included only women. The sample size ranged from 33-93 subjects.
Table II.
Main characteristics of selected studies.
| First author, year | Study design | Country | Cohort | Inclusion criteria | Sample size, n | Drug doses | Control group | Follow-up | Mode of symptoms quantification | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Porru et al, 2014 | Randomized cross over trial | Italy | Women aged ≥18 years (range 22-54) | Acute symptomatic cystitis and history of rUTI | 46 | Oral D-mannose 1 g three times a day, for 2 weeks, and subsequently 1 g twice a day for 22 weeks | 5-day antibiotic therapy with TMP/SMX 160 mg/800 mg twice a day, followed by a single dose at bedtime for 1 week each month in the following 23 weeks | 24 weeks | VAS | (6) |
| Vicariotto, 2014 | Prospective uncontrolled | Italy | Premenopausal women aged >18 years | Acute uncomplicated cystitis diagnosed by urine dipstick testing and an evaluation of the presence of typical symptoms | 33 | 250 mg D-mannose/ 500 mg of a high PACs cranberry extract/2.5 billion live cells L. plantarum LP01/1 billion viable cells, L. paracasei LPC09/1 billion viable cells S. thermophilus ST10, 250 mg tara gum. 2 doses/day for 1 month, then 1 sachet/day for 1 month. | - | 30 weeks | UTI-Symptoms Assessment Questionnaire | (7) |
| Domenici et al, 2016 | Prospective uncontrolled | Italy | Women aged 18-65 years | Acute cystitis and/or history of rUTIs | 43 | D-mannose (1.5 g), sodium bicarbonate, sorbitol and silicon dioxide twice daily for 3 days and then once a day for 10 days. | - | 15 weeks | UTI-Symptoms Assessment Questionnaire | (8) |
| Marchiori and Zanello, 2017 | Retrospective case control study | Italy | Women with a diagnosis of breast cancer treated with aromatase inhibitors or tamoxifen or LHRH analogs | Women with rUTI complaining urogenital discomfort | 60 (Group 1 40 Group 2: 20) | D-mannose 500 mg, N-acetylcysteine 100 mg and Morinda citrifolia fruit extract 200 mg (NDM) 1 vial every 12 h for 60 days and then 1 vial every 24 h for 4 months, associated with antibiotic therapy (see Group 2) | Antibiotic therapy, depending on microbial sensitivity (Fosfomycin, 3 g per day for 2 days or nitrofurantoin 1 cprs 100 mg three times a day for 6 days or ciprofloxacin 1,000 RM or prulifloxacin 600 mg 1 cps/day for 6 days) | 2 months | Verbal rating scale ranking form 0 (absence of symptoms) to 4 (severe symptom) | (9) |
| Del Popolo and Nelli, 2018 | Prospective uncontrolled | Italy | Men and women attending a neuro-urologic clinic | Symptomatic UTI and history of rUTI | 78 patients (17 men) 39 patients had neurogenic bladder | 5-days regimen with a tid oral combination of 1,000 mg of D-mannose plus 200 mg of dry willow extract (salicin) followed by bid 7-days with 700 mg of D-mannose plus 50 mg (1x109 CFU) of Lactobacillus acidophilus (La-14). | - | 2 weeksa | VAS | (10) |
| Pugliese et al, 2020 | Prospective uncontrolled | Italy | Women (mean ± SD Age 38±11.2) | Women with urinary symptoms suggestive of UTI | 33 | D-mannose 2 g/fructo- oligosaccharide 1 g/pomegranate extract 250 mg (with 70% titration of ellagic acid 175 mg)/Lactobacillus plantarum (Lp115 ≥ 2 billion colony-forming unit) 2 times daily for 5 days and then once a day for 10 days | - | 15 days | Acute Cystitis Symptoms Score | (11) |
| Rădulescu et al, 2020 | Randomized control trial | Romania | Non-pregnant, healthy women aged 18-60 years (mean ± SD age 39.77±10.36 years) | Uncomplicated lower urinary tract infection | 93 | D-mannose 1 gr/400 mg cranberry extract for 7 days plus TMP-SMX | TMP-SMX alone for 7 days. | 7 days | 7 items questionnaire (dysuria, increased urinary frequency/ pollakiuria, urinary urgency, hematuria, hypogastric pain, lumbar pain, vesical tenesmus) and 3 degrees of intensity (absent, moderate, severe). | (12) |
aThe results at 12 weeks were not considered. LHRH, Luteinizing Hormone Releasing Hormone; PACs, proanthocyanidins; SMX, sulfamethoxazole; TID, twice daily; TMP, trimethoprim; UTI, urinary tract infection; rUTI, recurrent UTI; VAS, visual analogue scale.
With regard to the study drug, D-mannose was given alone in only two studies (6,8). In the study by Del Popolo and Nelli (10), D-mannose was given alongside dry willow extract (salicin) in the first phase of the study and with Lactobacillus acidophilus in the second phase. In the other studies D-mannose was given alongside several other compounds, including cranberry extract, Morinda citrifolia fruit extract, pomegranate extract, fructo-oligosaccharides, lactobacilli, and N-acetylcysteine.
Four studies evaluated the changes in symptoms in the short term (7-15 days) (8,10-12), one after 30 days (7), one after 60 days (9) and one after 24 weeks (6).
Quality of selected studies
Considering the observational studies using the NOS tool, study quality was constantly 5/9. There was the possibility that some evaluation using the NOS quality items was debatable (i.e., if the sample size was too little to control for important factors or if a not exposed cohort did exist). The two trials (6,12) had a low risk of bias according to the Cochrane risk of bias tool (Table III).
Table III.
Evaluation of the study quality according to the Newcastle-Ottawa Scale (cohort studies) or Cochrane risk of bias (randomized clinical trials).
| Cohort studya | Question # | Selection | Question # | Comparability | Question # | Outcome (Cohort studies) | Study quality | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Vicariotto, 2014 | 1 | * | 1 | - | 1 | - | 5/9 | (7) |
| 2 | - | 2 | - | 2 | * | |||
| 3 | * | 3 | * | |||||
| 4 | * | |||||||
| Domenici et al, 2016 | 1 | * | 1 | - | 1 | - | 5/9 | (8) |
| 2 | - | 2 | - | 2 | * | |||
| 3 | * | 3 | * | |||||
| 4 | * | |||||||
| Marchiori and Zanello, 2017 | 1 | * | 1 | - | 5/9 | (9) | ||
| 2 | - | 1 | - | 2 | * | |||
| 3 | * | 2 | - | 3 | * | |||
| 4 | * | |||||||
| Del Popolo and Nelli, 2018 | 1 | * | 1 | - | 5/9 | (10) | ||
| 2 | - | 1 | - | 2 | * | |||
| 3 | * | 2 | - | 3 | * | |||
| 4 | * | |||||||
| Pugliese et al, 2020 | 1 | * | 1 | 1 | - | 5/9 | (11) | |
| 2 | - | 2 | - | 2 | * | |||
| 3 | * | - | 3 | * | ||||
| 4 | * | |||||||
| Randomized clinical trialsb | Overall risk of bias | |||||||
| Porru et al, 2014 | Randomization: low risk | Low | (6) | |||||
| Assignment to intervention: low risk | ||||||||
| Adhering to intervention: low risk | ||||||||
| Missing outcome: low risk | ||||||||
| Measure of outcome: low risk | ||||||||
| Selection of results: low risk | ||||||||
| Rădulescu et al, 2020 | Randomization: some concerns | Low | (12) | |||||
| Assignment to intervention: some concerns | ||||||||
| Adhering to intervention: low risk | ||||||||
| Missing outcome: low risk | ||||||||
| Measure of outcome: low risk | ||||||||
| Selection of results: low risk | ||||||||
aThe Newcastle-Ottawa quality assessment scale was used for cohort studies, and the maximum score was 9. Most items were evaluated as ‘-’ due to the small sample size or lack of information on the cohort.
bFor the assessment of randomized controlled studies, the revised Cochrane risk of bias tool was used.
To facilitate the reading of the systematic review, we summarized the main results of the selected studies in Table IV.
Table IV.
Results of selected studiesk.
| Study, year | Methods for evaluation of the symptoms | Suprapubic pain | Dysuria | Frequent voiding | Urgency | Hematuria | Overall symptoms evaluation | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Porru et al, 2014 | VAS | (6) | ||||||
| Before D-mannose | 4.1 (1.1)c | 7.1 (1.1)b | 4.6 (1.1) | |||||
| After D-mannose | 2.2 (0.5)a | 4.7 (1.0)a | 2.6 (0.7)a | |||||
| Vicariotto, 2014 | UTI-SAQ | (7) | ||||||
| Baseline | 1.39 | 2.03 | 2.18 | 2.15 | 0.61 | |||
| Day 30 | 0.97a | 1.36a | 1.70a | 1.64a | 0.58 | |||
| Domenici et alb,h, 2016 | UTI-SAQ | (8) | ||||||
| Baseline | 1.47 (0.95) | 1.60 (±1.00) | 2.16 (1.52) | 1.73 (0.92) | 0.34 (0.90) | |||
| Day 15 | 0.15 (0.36)a | 0.31 (0.47)a | 0.60 (0.63)a | 0.23 (0.43)a | 0.10 (0.45) | |||
| Marchiori and Zanello, 2017 | VRS | (9) | ||||||
| Cases | ||||||||
| Baseline | 32.5% (13/40)e,j | 62.5% (25/40) | 37.5% (15/40)f | |||||
| 2 months | 25% (10/40) | 5% (2/40) | 0% (0/40) | |||||
| Control group | ||||||||
| Baseline | 50% (10/20) | 35% (7/20) | 20% (4/20) | |||||
| 2 months | 45% (9/20) | 25% (5/20) | 5% (1/20) | |||||
| Del Popolo and Nelli, 2020 | VAS | (10) | ||||||
| Neurogenic bladder group | ||||||||
| Baseline | 14.0 (2.6)d | 8.07 (1.70)h | ||||||
| 2 weeks | 6.9 (1.3)a | 4.74 (2.07)a | ||||||
| Non neurogenic bladder group | ||||||||
| Baseline | 15(3)b | 7.21 (1.9) | ||||||
| 2 weeks | 8(3)a | 3.74 (3.12)a | ||||||
| Pugliese et al, 2020 | ACSS | (11) | ||||||
| Baseline | 11.5i (95% CI, 10.5-12.6) | |||||||
| 15 days | 4.9a (95% CI 4.0-5.9) | |||||||
| Rădulescu et all, 2020 | 3 degrees | (12) | ||||||
| Cases | questionnaire | |||||||
| Baseline | 72.9 (35/48)g,j | 60.4 (29/48) | 85.4 (41/48) | 89.6% (43/48) | 10.4% (5/48) | |||
| 7 days | 2.1 (1/48) | 0% (0/48) | 2.1% (1/48) | 0% (0/48) | 0% (0/48) | |||
| Control group | ||||||||
| Baseline | 86.7% (39/45) | 55.6% (25/45) | 82.2% (37/45) | 80% (36/45) | 22.2% (10/45) | |||
| 7 days | 2.2% (1/45) | 6.7% (3/45) | 8.9% (4/45) | 4.4% (2/45) | 0% (0/45) |
aP<0.05.
bDomenici et al also reported a statistically significant reduction of back pain and nycturia.
cPain not otherwise specified.
dNumber of voiding events/24 h.
eBladder and urethral pain.
fImperious urination.
gVesical tenesmus.
hEvaluation of all symptoms.
iTypical symptoms score.
jModerate or severe/total subjects.
kUnless otherwise specified, data are presented as mean and standard deviation.
lRădulescu et al also reported a decrease of lumbar pain and hypogastric pain. UTI-SAQ, UTI-Symptoms Assessment Questionnaire; VRS, verbal rating scale; ACSS, Acute Cystitis Symptoms Score; CI, confidence interval; VAS, visual analogue scale.
Porru et al (6) conducted a randomized cross-over trial including women with an acute symptomatic UTI and three or more rUTIs during the 12 months before study entry. A total of 60 women were randomly divided into an antibiotic treatment with trimethoprim/sulfamethoxazole or oral D-mannose three times a day group, for 2 weeks (phase of the study considered in this review). The primary endpoint, which was out of the scope of this review, was the evaluation of the elapsed time to recurrence. We included the secondary endpoints, which were bladder pain (VASp) and urinary urgency (VASu). Mean VASp score, mean VASu score, and average number of 24-h voiding events decreased significantly after 2 weeks of treatment. Methods for taking into account the period effect were not clearly stated. The authors did not report any adverse events.
Vicariotto (7) reported the results of a small prospective observational study. Eligible for the study were 33 premenopausal, nonpregnant women diagnosed with acute uncomplicated cystitis. Patients were given a compound including D-mannose, cranberry dry extract, exopolysaccharides produced by Streptococcus thermophilus ST10, tara gum, Lactobacillus plantarum, and Lactobacillus paracasei, two doses per day for 1 month. At baseline and in the 30 day visits the following symptoms were evaluated: Dysuria, frequent voiding, urgency, and suprapubic pain. A statistically significant improvement was observed.
Domenici et al (8) reported an observational prospective study. A total of 43 women with acute cystitis were included. D-mannose was administered twice daily for 3 days and then once a day for 10 days. Patients' symptoms, the therapeutic effects and quality of life (QoL) were evaluated clinically using a validated questionnaire (UTISA) (41) The mean UTISA scores significantly improved for most symptoms between baseline and follow up visits.
Marchiori and Zanello (9) studied the effectiveness of D-mannose in association with N-acetylcysteine and Morinda citrifolia fruit extract (DNM) plus antibiotic therapy, in recurrent cystitis. A total of 60 women with breast cancer and recurrent cystitis were analyzed retrospectively. Of these, 40 patients received antibiotic therapy plus DNM and 20 women antibiotics alone for 6 months. After 2 months of study entry, women treated with DNM plus antibiotic therapy exhibited a more prominent improvement in urgency, frequency, urge, incontinence, bladder and urethral pain in comparison to women treated with antibiotics alone.
Del Popolo and Nelli (10) reported an observational uncontrolled prospective study including 85 patients (68 women and 17 men) with symptomatic UTI and a history of rUTI. A total of 78 patients received a 5-day regimen consisting of thrice daily oral D-mannose and dry willow extract (salicin), followed by a 7-day regimen of twice daily D-mannose and Lactobacillus acidophilus. Patients' symptoms were evaluated using a 3-day bladder diary and a VAS, 15 days after study entry. VAS scores decreased from 8.07±1.70 to 4.74±2.07 (P=0.001) in non-neurological patients (39 subjects, group A) and from 7.21±1.90 to 3.74±3.12 (P=0.001) in the neurological patients (39 subjects, group B). A significant reduction in daily frequency was noted in both groups, from 14±3 to 7±3 (P=0.001) in group A and from 15±3 to 8±3 (P=0.001) in group B.
D-mannose, pomegranate extract, prebiotics and probiotics were given twice daily for 5 days and then once a day for 10 days to 33 women (mean age 38.1±11.2 years) with urinary symptoms suggestive of an UTI, in a study conducted by Pugliese et al (11). Antibiotics were permitted on a clinical basis. Changes in patients' symptoms were evaluated using the Acute Cystitis Symptom Score (42,43) at baseline (T0), and 15 (T1) and 30 (T2) days later. For the purpose of this review, the results at T1 were considered. At T1, all or most of the symptoms disappeared in 10 women (30.3%), whereas they persisted or worsened in 7 patients (21.2%). Mean scores for typical symptoms (i.e. frequency, urgency, pain or burning with urination, pain in the suprapubic area, feeling of incomplete bladder emptying, and gross hematuria) decreased significantly from 11.5 [95% confidence interval (CI) 10.5-12.6)] to 4.9 (95% CI 4.0-5.9) (P<0.0001). For differential symptoms (i.e., lower back pain, vaginal discharge, urethral discharge, fever, and chills), mean scores decreased from 3.1 (95% CI 2.6-3.6) to 0.6 (95% CI 0.3-0.9) (P<0.0001). The QoL mean score also decreased from 7.2 (95% CI 6.7-7.7) to 4.0 (95% CI 3.3-4.6) (P<0.0001). Antibiotics were given to six patients. No adverse events were reported.
Rădulescu et al (12) reported a randomized study including 93 non-pregnant healthy women (mean age of 39.77±10.36 years) diagnosed with uncomplicated lower UTI. Patients were given antibiotics alone or in association with D-mannose plus cranberry extract for 7 days. Co-administration of D-mannose plus cranberry extract was not associated to statistically significant differences in symptoms, except for urinary urgency/pollakiuria (P=0.024).
5. Discussion
This systematic review is, to the best of our knowledge, the first attempt to systematically review the published data on the effect of D-mannose in the treatment of acute symptoms of UTI/cystitis. The results of this evaluation suggest that, in women with symptoms of UTI/cystitis, treatment with D-mannose alone or in association with other compounds is useful for lowering the intensity of symptoms both in the short and middle-term for all typical symptoms, except hematuria.
However, this finding has several limitations and should be considered cautiously. First, the limited data do not provide the opportunity for analysis in detail of the role of different doses of D-mannose or the effect of D-mannose alone or in association with other compounds. Furthermore, most data were derived from uncontrolled studies. In general, the findings from this analysis are based on an extremely limited number of studies with small sample sizes.
The populations included in the considered studies are markedly heterogeneous. For example, the study conducted by Del Popolo and Nelli (10) included men and women with neurological problems. Otherwise, the majority of studies included only women in the premenopausal period (6,7,11). Furthermore, as the methods of evaluation of symptoms differed, any comparison among studies of the magnitude of the effect of D-mannose is not feasible. Moreover, we also must take into account that the majority of included studies were conducted in Italy. That is, it is conceivable that only studies showing positive results were published in an international journal, whereas negative findings were instead published in local journals (44). Finally, limiting our analysis to publications in English language journals may have reduced the completeness of information, causing bias.
A strength of this analysis is that previous reviews and meta-analyses considered the role of D-mannose in the prevention of UTI recurrence (22,23), whereas we summarized the available evidence regarding its use in the treatment of UTI symptoms. However, from a biological point of view, our findings have a rationale, since D-mannose binds to the tip of type 1 pili and saturates adhesin FimH, blocking bacterial adhesion to the urothelium and the consequent urothelial invasion (45).
All these considerations were suggested to explain the well-documented role of D-mannose in lowering the risk of recurrent infections in women with rUTI. Less clear is the potential mechanism of D-mannose on UTI/cystitis symptoms. However, the fact that D-mannose interacts with bacteria to promote UPEC excretion may explain a faster resolution of symptoms (18).
We were not able to analyze the effect of D-mannose on different uropathogenetic agents separately. However, although type 1 fimbriae were extensively studied in E. coli, type 1 pili were documented in several other uropathogens commonly involved in rUTIs (23). Furthermore, recently, Zhang et al (46) suggested that D-mannose could play a role as an immune modulator. It was shown that prolonged exposure to D-mannose did not select FimH variants that modify bacterial adhesiveness after D-mannose removal, further indicating that it does not exert ‘antibiotic-like’ activity (21). Along this line, we have observed similar effects in short and middle-term treatments.
In conclusion, despite the limitations, consistent results among all studies give strong support to the general findings. Although the biological and clinical explanations of our results are not entirely clear, observational studies and clinical trials consistently suggest that D-mannose may be useful in the treatment of UTI/cystitis symptoms. Its non-pharmacological, non-metabolic, non-bacteriostatic or bactericidal, but biomechanical mechanism of action, and the fact that it does not affect antibiotic resistance may support the use of D-mannose in the treatment of UTI/cystitis.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Authors' contributions
FP and FF designed the study. FC, SC and GE searched the literature, selected the papers and extracted the data. SC and ER confirm the authenticity of all the raw data. FP and ER wrote the paper. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics (Basel) 2014;3:174–192. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarshar M, Behzadi P, Ambrosi C, Zagaglia C, Palamara AT, Scribano D. FimH and Anti-Adhesive therapeutics: A disarming strategy against uropathogens. Antibiotics (Basel) 2020;9(397) doi: 10.3390/antibiotics9070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman MR, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol. 2019;202:282–289. doi: 10.1097/JU.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 4.Taich L, Zhao H, Cordero C, Anger JT. New paradigms in the management of recurrent urinary tract infections. Curr Opin Urol. 2020;30:833–837. doi: 10.1097/MOU.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 5.Barea BM, Veeratterapillay R, Harding C. Nonantibiotic treatments for urinary cystitis: An update. Curr Opin Urol. 2020;30:845–852. doi: 10.1097/MOU.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 6.Porru D, Parmigiani A, Tinelli C, Barletta D, Choussos D, Di Franco C, Bobbi V, Bassi S, Miller O, Gardella B, et al. Oral D-mannose in recurrent urinary tract infections in women: A pilot study. J Clin Urol. 2014;7:208–213. [Google Scholar]
- 7.Vicariotto F. Effectiveness of an association of a cranberry dried extract, D-mannose and the three microorganisms Lactobacillus plantarum LP01, Lactobacillus. paracasei LPC09 in women affected by cystitis: A pilot study. J Clin Gastroenterol. 2014;48 (Suppl 1):S96–S101. doi: 10.1097/MCG.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 8.Domenici L, Monti M, Bracchi C, Giorgini M, Colagiovanni V, Muzii L, Benedetti*Panici P. D-mannose: A promising support for acute urinary tract infections in women. A pilot study. Eur Rev Med Pharmacol Sci. 2016;20:2920–2925. [PubMed] [Google Scholar]
- 9.Marchiori D, Zanello PP. Efficacy of N-acetylcysteine, D-mannose and morinda citrifolia to treat recurrent cystitis in breast cancer survivals. In Vivo. 2017;31:931–936. doi: 10.21873/invivo.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del*Popolo G, Nelli F. Recurrent bacterial symptomatic cystitis: A pilot study on a new natural option for treatment. Arch Ital Urol Androl. 2018;90:101–103. doi: 10.4081/aiua.2018.2.101. [DOI] [PubMed] [Google Scholar]
- 11.Pugliese D, Acampora A, Porreca A, Schips L, Cindolo L. Effectiveness of a novel oral combination of D-Mannose, pomegranate extract, prebiotics and probiotics in the treatment of acute cystitis in women. Arch Ital Urol Androl. 2020;92:34–38. doi: 10.4081/aiua.2020.1.34. [DOI] [PubMed] [Google Scholar]
- 12.Rădulescu D, David C, Turcu FL, Spătaru DM, Popescu P, Văcăroiu IA. Combination of cranberry extract and D-mannose-possible enhancer of uropathogen sensitivity to antibiotics in acute therapy of urinary tract infections: Results of a pilot study. Exp Ther Med. 2020;20:3399–3406. doi: 10.3892/etm.2020.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalewska-Piątek BM, Piątek RJ. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim Pol. 2019;66:129–138. doi: 10.18388/abp.2018_2787. [DOI] [PubMed] [Google Scholar]
- 14.Sharma V, Ichikawa M, Freeze HH. Mannose metabolism: More than meets the eye. Biochem Biophys Res Commun. 2014;453:220–228. doi: 10.1016/j.bbrc.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhardt RG, Calvin AD, Dodd EA. Taste-Structure correlation with alpha-D-Mannose and beta-D-Mannose. Science. 1962;135:367–368. doi: 10.1126/science.135.3501.367. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B. D -Mannose: Properties, production, and applications: An overview. Compr Rev Food Sci Food Saf. 2016;15:773–785. doi: 10.1111/1541-4337.12211. [DOI] [PubMed] [Google Scholar]
- 17.Alton G, Kjaergaard S, Etchison JR, Skovby F, Freeze HH. Oral ingestion of mannose elevates blood mannose levels: A first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem Mol Med. 1997;60:127–133. doi: 10.1006/bmme.1997.2574. [DOI] [PubMed] [Google Scholar]
- 18.Scaglione F, Musazzi UM, Minghetti P. Considerations on D-mannose mechanism of action and consequent classification of marketed healthcare products. Front Pharmacol. 2021;12(636377) doi: 10.3389/fphar.2021.636377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World J Urol. 2014;32:79–84. doi: 10.1007/s00345-013-1091-6. [DOI] [PubMed] [Google Scholar]
- 20.Klein T, Abgottspon D, Wittwer M, Rabbani S, Herold J, Jiang X, Kleeb S, Lüthi C, Scharenberg M, Bezençon J, et al. FimH antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J Med Chem. 2010;53:8627–8641. doi: 10.1021/jm101011y. [DOI] [PubMed] [Google Scholar]
- 21.Scribano D, Sarshar M, Prezioso C, Lucarelli M, Angeloni A, Zagaglia C, Palamara AT, Ambrosi C. D-Mannose treatment neither affects uropathogenic Escherichia coli properties nor induces stable fimH modifications. Molecules. 2020;25(316) doi: 10.3390/molecules25020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenger SM, Bradley MS, Thomas DA, Bertolet MH, Lowder JL, Sutcliffe S. D-Mannose vs other agents for recurrent urinary tract infection prevention in adult women: A systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:265.e1–265.e13. doi: 10.1016/j.ajog.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Nunzio C, Bartoletti R, Tubaro A, Simonato A, Ficarra V. Role of D-mannose in the prevention of recurrent uncomplicated cystitis: State of the art and future perspectives. Antibiotics (Basel) 2021;10(373) doi: 10.3390/antibiotics10040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6(e1000100) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(e1000097) doi: 10.1371/journal.pmed.1000097. PRISMA Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O'Connell D, Peterson J, Welch VL, et al. Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies in Meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, 2019. [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366(l4898) doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 28.Kuzmenko AV, Kuzmenko VV, Gyaurgiev TA. Use of D-mannose in the prevention of recurrent lower urinary tract infection in women. Urologiia. 2020;3:128–132. (In Russian) [PubMed] [Google Scholar]
- 29.Salinas-Casado J, Méndez-Rubio S, Esteban-Fuertes M, Gómez-Rodríguez A, Vírseda-Chamorro M, Luján-Galán M, Iglesias-García C, Rituman G. Large study (283 women) on the effectiveness of Manosar®: 2 g of d-mannose + 140 mg of proanthocyanidins (PAC), of prolonged release. Arch Esp Urol. 2020;73:491–498. (In English, Spanish) [PubMed] [Google Scholar]
- 30.Altarac S, Papeš D. Use of D-mannose in prophylaxis of recurrent urinary tract infections (UTIs) in women. BJU Int. 2014;113:9–10. doi: 10.1111/bju.12492. [DOI] [PubMed] [Google Scholar]
- 31.Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects. Mult Scler. 2012;18 [Google Scholar]
- 32.Efros M, Bromberg W, Cossu L, Nakeleski E, Katz AE. Novel concentrated cranberry liquid blend, UTI-STAT with proantinox, might help prevent recurrent urinary tract infections in women. Urology. 2010;76:841–845. doi: 10.1016/j.urology.2010.01.068. [DOI] [PubMed] [Google Scholar]
- 33.Genovese C, Davinelli S, Mangano K, Tempera G, Nicolosi D, Corsello S, Vergalito F, Tartaglia E, Scapagnini G, Di*Marco R. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. J Chemother. 2018;30:107–114. doi: 10.1080/1120009X.2017.1393587. [DOI] [PubMed] [Google Scholar]
- 34.Madhavan K, Rustagi S, Jena R, Singh UP, Ansari MS, Srivastava A, Kapoor R, Sureka SK. A prospective randomized study to define the role of low dose continuous prophylactic antibiotics and anti-adherence agents in altering the microbial colonization related to indwelling double-J stents. Asian J Urol. 2021;8:269–274. doi: 10.1016/j.ajur.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milandri R, Maltagliati M, Bocchialini T, Del Prete C, Bianchi G, Rocco BM, Micali S. Effectiveness of D-mannose, Hibiscus sabdariffa and Lactobacillus plantarum therapy in prevention of infectious events following urodynamic study. Urologia. 2019;86:122–125. doi: 10.1177/0391560318798291. [DOI] [PubMed] [Google Scholar]
- 36.Murina F, Vicariotto F, Lubrano C. Efficacy of an orally administered combination of Lactobacillus paracasei LC11, cranberry and D-mannose for the prevention of uncomplicated, recurrent urinary tract infections in women. Urologia. 2021;88:64–68. doi: 10.1177/0391560320957483. [DOI] [PubMed] [Google Scholar]
- 37.Palleschi G, Carbone A, Zanello PP, Mele R, Leto A, Fuschi A, Al Salhi Y, Velotti G, Al Rawashdah S, Coppola G, et al. Prospective study to compare antibiosis versus the association of N-acetylcysteine, D-mannose and Morinda citrifolia fruit extract in preventing urinary tract infections in patients submitted to urodynamic investigation. Arch Ital Urol Androl. 2017;89:45–50. doi: 10.4081/aiua.2017.1.45. [DOI] [PubMed] [Google Scholar]
- 38.Russo E, Montt Guevara M, Giannini A, Mannella P, Palla G, Caretto M, Pancetti F, Genazzani AD, Simoncini T. Cranberry, D-mannose and anti-inflammatory agents prevent lower urinary tract symptoms in women undergoing prolapse surgery. Climacteric. 2020;23:201–205. doi: 10.1080/13697137.2019.1679110. [DOI] [PubMed] [Google Scholar]
- 39.Phé V, Pakzad M, Haslam C, Gonzales G, Curtis C, Porter B, Chataway J, Panicker JN. Open label feasibility study evaluating D-mannose combined with home-based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol Urodyn. 2017;36:1770–1775. doi: 10.1002/nau.23173. [DOI] [PubMed] [Google Scholar]
- 40.Mainini G, Passaro M, Schiattarella A, Franciscis P, Donna MCD, Trezza G. Prevention and treatment of cystitis during menopause: Efficacy of a nutraceutical containing D-mannose, inulin, cranberry, bearberry, Olea europaea, Orthosiphon and Lactobacillus acidophilus. Prz Menopauzalny. 2020;19:130–134. doi: 10.5114/pm.2020.99567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): The UTI symptom assessment questionnaire. BJU Int. 2005;96:350–359. doi: 10.1111/j.1464-410X.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- 42.Alidjanov JF, Abdufattaev UA, Makhsudov SA, Pilatz A, Akilov FA, Naber KG, Wagenlehner FM. New self-reporting questionnaire to assess urinary tract infections and differential diagnosis: Acute cystitis symptom score. Urol Int. 2014;92:230–236. doi: 10.1159/000356177. [DOI] [PubMed] [Google Scholar]
- 43.Alidjanov JF, Naber KG, Abdufattaev UA, Pilatz A, Wagenlehner FM. Reliability of symptom-based diagnosis of uncomplicated cystitis. Urol Int. 2019;102:83–95. doi: 10.1159/000493509. [DOI] [PubMed] [Google Scholar]
- 44.Grégoire G, Derderian F, Le*Lorier J. Selecting the language of the publications included in a meta-analysis: Is there a tower of babel bias? J Clin Epidemiol. 1995;48:159–163. doi: 10.1016/0895-4356(94)00098-b. [DOI] [PubMed] [Google Scholar]
- 45.Bouckaert J, Berglund J, Schembri M, De Genst E, Cools L, Wuhrer M, Hung CS, Pinkner J, Slättegård R, Zavialov A, et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol Microbiol. 2005;55:441–455. doi: 10.1111/j.1365-2958.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Wang G, Lai F, Wu H. Structural characterization and immunomodulatory activity of a novel polysaccharide from lepidium meyenii. J Agric Food Chem. 2016;64:1921–1931. doi: 10.1021/acs.jafc.5b05610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.