Abstract
Background and Aim
This study aimed to clarify the efficacy and safety of oral semaglutide treatment in patients with non‐alcoholic fatty liver disease (NAFLD) complicated by type 2 diabetes mellitus (T2DM).
Methods
This was a single‐arm, open‐label pilot study. Sixteen patients with NAFLD who received oral semaglutide for T2DM were included in the analysis. Oral semaglutide was initiated at a dose of 3 mg once daily, and the dose was sequentially increased to 7 mg at 4 weeks and 14 mg at 8 weeks (maintenance dose) until the end of the 24‐week trial.
Results
Body weight and levels of liver‐related biochemistry, plasma glucose, and hemoglobin A1c decreased significantly from baseline to 12 weeks. These significant decreases were maintained until the end of the trial. Additionally, levels of the homeostasis model assessment‐insulin resistance and triglyceride significantly decreased at 24 weeks. Controlled attenuation parameter (CAP) values significantly decreased from baseline to 24 weeks. Changes in body weight were correlated with those in levels of alanine aminotransferase (r = 0.52) and CAP (r = 0.72). As for liver fibrosis markers, significant decreases from baseline to 24 weeks in levels of the fibrosis‐4 index, ferritin, and type IV collagen 7 s were found; however, the liver stiffness measurement did not significantly decrease. Most adverse events were grade 1–2 transient gastrointestinal disorders.
Conclusions
Oral semaglutide treatment in patients with NAFLD complicated by T2DM improved impaired liver function, hypertriglyceridemia, insulin resistance, and hepatic steatosis, as well as improving diabetic status and reducing body weight.
Keywords: controlled attenuation parameter, GLP‐1 receptor agonists, liver fibrosis, non‐alcoholic fatty liver disease, semaglutide
This is a single‐arm, open‐label pilot study. Oral semaglutide treatment for 24 weeks in patients with non‐alcoholic fatty liver disease complicated by type 2 diabetes improved impaired liver function, hypertriglyceridemia, insulin resistance, and hepatic steatosis, as well as improving diabetic status and reducing body weight.

Introduction
Non‐alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, accounting for approximately 25% of chronic liver disease cases worldwide. 1 , 2 , 3 Some patients with NAFLD exhibit liver necroinflammation and fibrosis, resulting in the development of liver cirrhosis and hepatocellular carcinoma. 4 NAFLD is a multifactorial disease mutually associated with metabolic syndrome. 5 Particularly, insulin resistance and type 2 diabetes mellitus (T2DM) induce NAFLD onset, deterioration of liver inflammation/fibrosis, and development of hepatocellular carcinoma. 6 , 7 Currently, there is no approved pharmacotherapy for NAFLD, although pioglitazone and vitamin E have been proposed as treatment options, given their histological improvement effects on NAFLD. In general, lifestyle modifications for weight reduction and treatment of extrahepatic comorbidities take precedence over direct NAFLD treatment. 8 , 9 , 10 Recently, anti‐diabetic drugs, such as glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) 11 , 12 , 13 and sodium/glucose cotransporter 2 inhibitors (SGLT2‐Is), 14 , 15 , 16 , 17 , 18 , 19 , 20 have been investigated as effective drugs for NAFLD treatment.
GLP‐1 RAs, including semaglutide, are anti‐diabetic drugs classified as incretin mimetics, which promote insulin secretion in a blood glucose‐dependent manner by acting on the pancreatic β cells and decrease blood glucose levels by suppressing glucagon secretion. Additionally, GLP‐1 RAs promote a feeling of fullness by activating GLP‐1 receptors in the hypothalamus, thus reducing appetite by delaying gastric emptying, leading to weight reduction. 21 Subcutaneous injection of GLP‐1 RAs has been reported effective in improving liver function and histology in patients with NAFLD. 11 , 12 , 13 However, the reluctance, on the part of patients and/or physicians, to use injectable products, as well as the time and effort required the patients to learn the injection procedures and the general lack of medical resources have hindered the broader adoption of GLP‐1 RA injection treatment in clinical practice. 22 In contrast, oral semaglutide, which is the only approved oral form of GLP‐1 RAs, can resolve the shortcomings of injectable products and, accordingly, may promote the adoption of GLP‐1 RA treatment. Oral semaglutide has been reported to facilitate blood glucose control and decrease body weight in patients with T2DM, as shown by the treatment outcomes of GLP‐1 RA injection. 23 However, there is, as yet, no published report on the efficacy and safety of oral semaglutide treatment in patients with NAFLD complicated by T2DM. Accordingly, this pilot study is the first to assess the efficacy and safety of 24‐week oral semaglutide treatment in patients with NAFLD complicated by T2DM.
Methods
Study design
This was a single‐arm, open‐label pilot study (UMIN registration no. 000045572). Among patients who visited Nippon Medical School Hospital (Tokyo, Japan), 17 consecutive patients with NAFLD received oral semaglutide treatment for the first time for T2DM with hemoglobin A1c (HbA1c) levels ≥6.2% despite dietary/exercise therapies and/or other antidiabetic drugs. NAFLD was diagnosed by (i) evidence of fat deposition on ultrasonography; (ii) daily alcohol consumption of <30 g for men and <20 g for women; and (iii) absence of other chronic liver diseases, such as viral hepatitis B or C, autoimmune hepatitis, primary biliary cholangitis, Wilson's disease, and hemochromatosis. The main exclusion criteria were as follows: (i) age <20 years; (ii) new administration of vitamin E or antidiabetic drugs within 12 weeks prior to oral semaglutide treatment; (iii) weight loss ≥5% within 12 weeks prior to oral semaglutide treatment; (iv) decompensated cirrhosis; and (v) pregnancy or lactation. The participants received oral semaglutide treatment over 24 weeks. Treatment was initiated at a dose of 3 mg once daily for the first 4 weeks, after which the dose was increased to 7 mg daily for the next 4 weeks, followed by an increase to 14 mg daily for another 4 weeks, all the while monitoring for any adverse events. The dose was then maintained at 14 mg daily for the remaining 12 weeks of the study. Three patients were switched from dipeptidyl peptidase‐4 inhibitors (DPP4‐Is) to oral semaglutide. For the other patients, no changes in antidiabetic or anti‐dyslipidemic medications including dosage were made during the observation period (Table S1, Supporting information). One of the 17 patients who received oral semaglutide treatment was lost to follow‐up (non‐adherence). The remaining 16 patients were included in this analysis. This study was conducted in accordance with the ethical guidelines of the 2013 Declaration of Helsinki and approved by the Ethics Committee of Nippon Medical School Hospital (approval number: B‐2021‐390). All participants provided written informed consent prior to their inclusion in the study. All treatments were provided as part of the routine care of the participants.
Clinical and laboratory data
Clinical and laboratory data were collected at the midpoint (12 weeks) and the conclusion (24 weeks) of the treatment. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Laboratory analyses included complete blood count, routine liver biochemistry (aspartate aminotransferase [AST], alanine aminotransferase [ALT], albumin, and gamma glutamyl transpeptidase [γ‐GTP]), kidney biochemistry (urea nitrogen, creatinine, and estimated glomerular filtration rate), fasting lipids (triglyceride, high‐density lipoprotein [HDL] cholesterol, and low‐density lipoprotein [LDL] cholesterol), diabetes‐related tests (fasting plasma glucose, HbA1c, and immunoreactive insulin), and uric acid. As an index of insulin resistance, the homeostasis model assessment‐insulin resistance (HOMA‐IR) was calculated as follows: fasting immunoreactive insulin (μU/mL) × fasting plasma glucose (mg/dL)/405. 24 Ferritin, 25 type IV collagen 7 s, 26 , 27 and Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein (WFA+‐M2BP) 27 , 28 , 29 were measured as liver fibrosis markers of NAFLD. The fibrosis‐4 (FIB‐4) index was calculated to estimate the degree of liver fibrosis, as previously reported. 30 , 31 Liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) were assessed through transient elastography, using FibroScan 502 equipped with the M‐probe (Echosens SA, Paris, France) at the initiation and 24 weeks of oral semaglutide treatment. One patient could not be measured due to severe obesity (Table S1).
Statistical analyses
Continuous variables are presented as medians and interquartile ranges (IQRs) in parentheses, whereas categorical variables are presented as numbers and percentages in parentheses. The kinetics of the aforementioned factors were examined using the Wilcoxon signed‐rank test. Correlations between continuous variables were analyzed using the Spearman's rank correlation coefficient. All statistical analyses were performed using the IBM SPSS version 17.0 (IBM Japan, Tokyo, Japan). The level of statistical significance was set at P < 0.05.
Results
Patient characteristics
The baseline characteristics of the 16 patients with NAFLD complicated by T2DM who received oral semaglutide for 24 weeks are summarized in Table 1. There were 9 men and 7 women, with a median age of 53 (IQR, 41–62) years. The median BMI value was 29.6 (27.4–33.7) kg/m2. The median AST, ALT, and γ‐GTP values were 40 (37–50) U/L, 67 (44–81) U/L, and 66 (55–114) U/L, respectively. The median HbA1c and HOMA‐IR values were 6.9% (6.6–7.3%) and 6.6 (4.3–11.2). Four of the 16 patients had histopathological evidence of NAFLD (steatosis in ≥5% of hepatocytes) by liver biopsy in addition to the diagnosis made by ultrasonography. Prior to semaglutide treatment, 11 patients (68.8%) received dietary and/or exercise therapies without antidiabetic drugs, whereas the remaining 5 (31.3%) received other antidiabetic drugs, including biguanides (n = 3), SGLT2‐Is (n = 1), DPP4‐Is (n = 3), and insulin (n = 2). The median values for fibrosis markers were as follows: 135.2 ng/mL for ferritin, 4.1 ng/mL for type IV collagen 7 s, 0.85 for WFA+‐M2BP, and 1.42 for FIB‐4 index. The median CAP and LSM values were 344 (312–354) dB/m and 5.5 (5.1–16.0) kPa, respectively. More detailed information regarding each patient is presented in Table S1.
Table 1.
Baseline characteristics of the 16 patients with NAFLD complicated by T2DM
| Factors | n = 16 |
|---|---|
| Age (years) | 53 (41–62) |
| Gender (men/women) | 9/7 |
| Body weight (kg) | 74.4 (72.0–86.9) |
| BMI (kg/m2) | 29.6 (27.4–33.7) |
| Platelets (×103/μL) | 231 (183–285) |
| AST (U/L) | 40 (37–50) |
| ALT (U/L) | 67 (44–81) |
| γ‐GTP (U/L) | 66 (55–114) |
| eGFR (mL/min/1.73m2) | 67 (61–94) |
| Uric acid (mg/dl) | 5.3 (5.0–6.9) |
| LDL cholesterol (mg/dL) | 101 (84–141) |
| HDL cholesterol (mg/dL) | 45 (43–51) |
| Triglyceride (mg/dL) | 175 (122–246) |
| Plasma glucose (mg/dL) | 123 (119–138) |
| HbA1c (%) | 6.9 (6.6–7.3) |
| Insulin (μU/mL) | 19.8 (15.4–32.8) |
| HOMA‐IR | 6.6 (4.3–11.2) |
| Method for the diagnosis of NAFLD | |
| Ultrasonography only | 12 (75.0%) |
| Histopathological diagnosis | 4 (25.0%) |
| Diabetes treatment prior to semaglutide | |
| Diet/exercise only (without antidiabetic drugs) | 11 (68.8%) |
| Anti‐diabetic drugs | 5 (31.3%) |
| Biguanides | 3 (18.8%) |
| SGLT2‐Is | 1 (6.3%) |
| DPP4‐Is | 3 (18.8%) |
| Insulin | 2 (12.5%) |
| Anti‐dyslipidemic drugs | 9 (56.3%) |
| Statins | 7 (43.8%) |
| Ferritin (ng/mL) | 135.2 (101.3–259.6) |
| Type IV collagen 7 s (ng/mL) | 4.1 (3.6–5.7) |
| WFA+‐M2BP (C.O.I.) | 0.85 (0.68–1.12) |
| FIB‐4 index | 1.42 (0.69–3.25) |
| CAP (dB/m) | 344 (312–354) |
| LSM (kPa) | 5.5 (5.1–16.0) |
Data are presented as numbers (percentages) or medians (interquartile ranges).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; DPP4‐Is, dipeptidyl peptidase‐4 inhibitors; eGFR, estimated glomerular filtration rate; FIB‐4, fibrosis‐4; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment‐insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; NAFLD, non‐alcoholic fatty liver disease; SGLT2‐Is, sodium glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; WFA+‐M2BP, Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein; γ‐GTP, gamma glutamyl transpeptidase.
Efficacy of oral semaglutide
The changes in each parameter, from baseline to 12 and 24 weeks of oral semaglutide treatment, are shown in Table 2, Figures 1, and 2. Significant decreases in body weight, BMI, liver‐related biochemistry (AST, ALT, and γ‐GTP), plasma glucose, and HbA1c were found at 12 weeks, compared with the baseline values. These significant reductions were maintained at 24 weeks. Significant reductions from baseline in HOMA‐IR were also found at 24 weeks. The platelet count significantly increased from baseline to 12 weeks, and this increase was maintained at 24 weeks. As for changes in fasting lipids, triglyceride levels decreased gradually, with a significant decrease from 175 (122–246) mg/dL at baseline to 128 (113–200) mg at 24 weeks. The median CAP values significantly decreased from 344 (312–354) dB/m at baseline to 279 (251–334) dB/m at week 24. Changes in body weight were correlated with those in ALT (r = 0.52, P < 0.05) and CAP (r = 0.72, P < 0.01) (Fig. 3).
Table 2.
Changes in clinical characteristics in the 16 patients who received oral semaglutide for 24 weeks
| Semaglutide therapy | |||||
|---|---|---|---|---|---|
| Baseline | 12 weeks | * P value | 24 weeks | * P value | |
| Body weight (kg) | 74.4 (72.0–86.9) | 72.5 (70.8–85.0) | <0.001 | 72.6 (67.8–83.5) | <0.001 |
| BMI (kg/m2) | 29.6 (27.4–33.7) | 28.7 (26.7–31.8) | <0.001 | 28.4 (25.4–31.2) | <0.001 |
| Platelets (×103/μL) | 231 (183–285) | 249 (206–280) | <0.05 | 238 (222–295) | <0.01 |
| AST (U/L) | 40 (37–50) | 32 (24–37) | <0.001 | 30 (22–34) | <0.001 |
| ALT (U/L) | 67 (44–81) | 42 (32–47) | < 0.01 | 34 (27–49) | <0.01 |
| γ‐GTP (U/L) | 66 (55–114) | 44 (37–72) | <0.001 | 41 (29–60) | <0.001 |
| eGFR (mL/min/1.73m2) | 67 (61–94) | 71 (60–88) | 0.44 | 72 (65–87) | 0.57 |
| Uric acid (mg/dL) | 5.3 (5.0–6.9) | 5.4 (4.8–6.4) | <0.05 | 5.9 (5.2–7.0) | 0.92 |
| LDL cholesterol (mg/dL) | 101 (84–141) | 80 (77–129) | <0.01 | 125 (75–136) | 0.11 |
| HDL cholesterol (mg/dL) | 45 (43–51) | 43 (39–50) | <0.05 | 47 (38–50) | 0.44 |
| Triglyceride (mg/dL) | 175 (122–246) | 150 (110–207) | 0.10 | 128 (113–200) | <0.05 |
| Plasma glucose (mg/dL) | 123 (119–138) | 102 (98–110) | <0.01 | 102 (93–106) | <0.01 |
| HbA1c (%) | 6.9 (6.6–7.3) | 6.0 (5.9–6.4) | <0.001 | 5.9 (5.6–6.1) | <0.001 |
| Insulin (μU/mL) | 19.8 (15.4–32.8) | 23.5 (14.4–45.3) | 0.15 | 15.6 (11.9–24.0) | <0.05 |
| HOMA‐IR | 6.6 (4.3–11.2) | 6.4 (4.2–11.4) | 0.96 | 4.3 (2.9–6.2) | <0.01 |
| Ferritin (ng/mL) | 135.2 (101.3–259.6) | — | — | 103.0 (56.3–235.2) | <0.01 |
| Type IV collagen 7 s (ng/mL) | 4.1 (3.6–5.7) | — | — | 3.5 (2.7–4.5) | <0.05 |
| WFA+‐M2BP (C.O.I) | 0.85 (0.68–1.12) | — | — | 0.69 (0.51–1.00) | 0.16 |
| FIB‐4 index | 1.42 (0.69–3.25) | — | — | 1.10 (0.53–2.03) | <0.01 |
| CAP (dB/m) | 344 (312–354) | — | — | 279 (251–334) | <0.01 |
| LSM (kPa) | 5.5 (5.1–16.0) | — | — | 6.5 (4.4–10.8) | 0.17 |
Data are presented as medians (interquartile ranges).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; eGFR, estimated glomerular filtration rate; FIB‐4, fibrosis‐4; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment‐insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; WFA+‐M2BP, Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein; γ‐GTP, gamma glutamyl transpeptidase.
Versus baseline.
Figure 1.
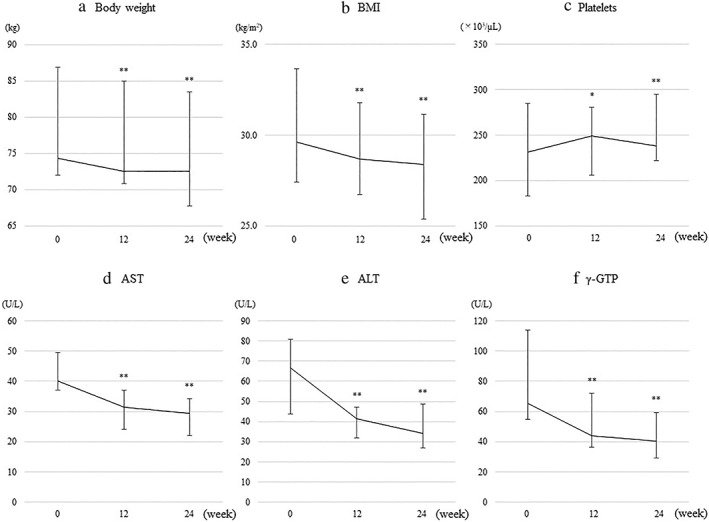
Changes from baseline in (a) body weight, (b) body mass index (BMI), (c) platelets, (d) aspartate aminotransferase (AST), (e) alanine aminotransferase (ALT), and (f) gamma glutamyl transpeptidase (γ‐GTP) in patients treated with oral semaglutide for 24 weeks. Error bars show the interquartile range. *P < 0.05, **P < 0.01 versus baseline.
Figure 2.
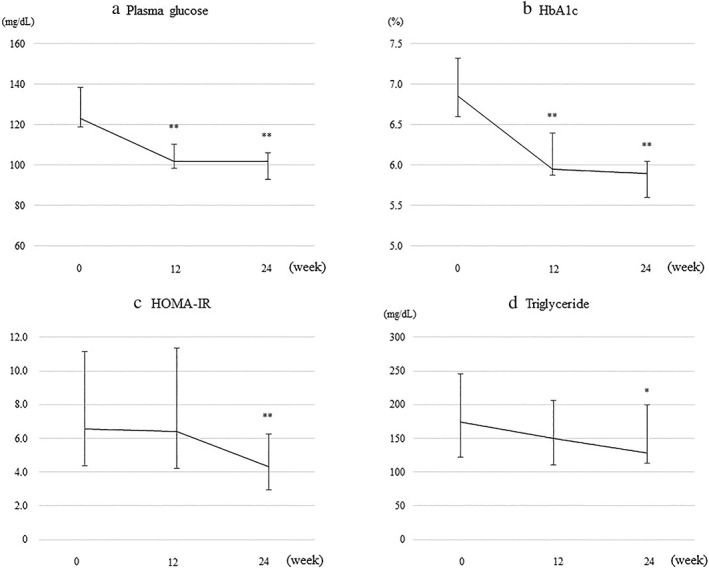
Changes from baseline in (a) plasma glucose, (b) hemoglobin A1c (HbA1c), (c) homeostasis model assessment‐insulin resistance (HOMA‐IR), and (d) triglyceride in patients treated with oral semaglutide for 24 weeks. Error bars show the interquartile range. *P < 0.05, **P < 0.01 versus baseline.
Figure 3.
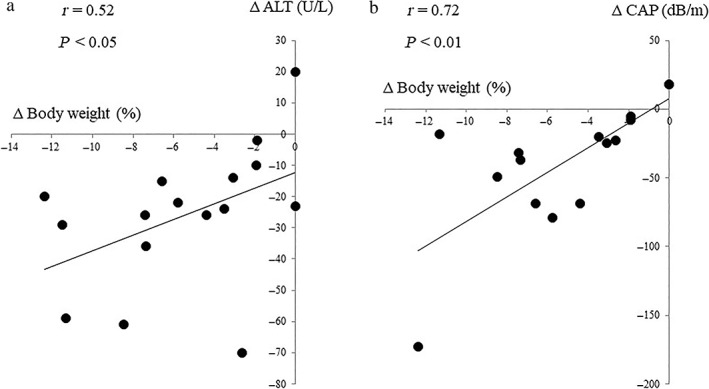
Correlation between changes in body weight from baseline to 24 weeks after oral semaglutide treatment and those in levels of (a) alanine aminotransferase (ALT) and (b) controlled attenuation parameter (CAP).
Anti‐fibrotic effect of oral semaglutide
As for changes in liver fibrosis markers (Table 2 and Fig. 4), the median levels of the FIB‐4 index, ferritin, and type IV collagen 7 s significantly decreased from 1.42, 135.2 ng/mL, and 4.1 ng/mL at baseline to 1.10, 103.0, and 3.5 ng/mL at 24 weeks, respectively. WFA+‐M2BP and LSM values showed no significant changes from baseline to 24 weeks of the treatment.
Figure 4.
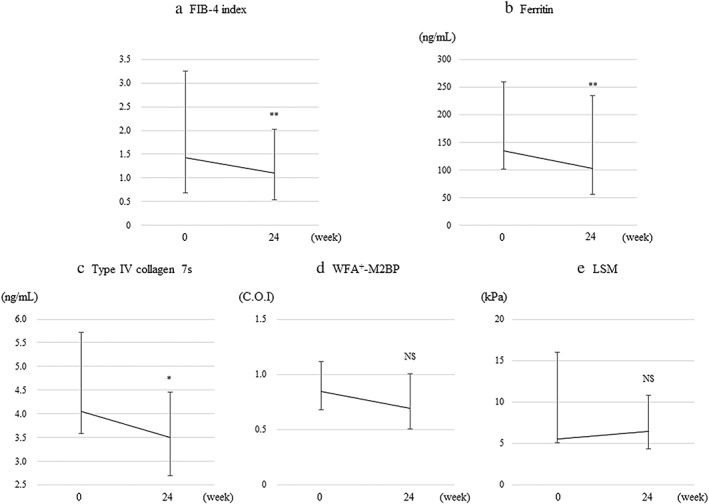
Changes from baseline to 24 weeks after oral semaglutide treatment in (a) fibrosis‐4 (FIB‐4) index, (b) ferritin, (c) type IV collagen 7 s, (d) Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein (WFA+‐M2B), and (e) liver stiffness measurement (LSM). Error bars indicate the interquartile ranges. **P < 0.01 versus baseline. *P < 0.05 versus baseline. NS; not significant.
Adverse events
Most adverse events were gastrointestinal disorders of grade 1 (mild) to grade 2 (moderate) severity and were transient (Table 3). Adverse events (nausea) led to drug dose reduction in three patients. One of the three patients had a temporary drug reduction (from a maintenance dose of 14–7 mg; 42 days), and the final maintenance dose of 14 mg was well tolerated. In the other two patients, nausea was controlled by reducing the maintenance dose to 3 mg. No grade 3 (severe) or higher adverse events or deaths occurred during the treatment period. In one case, semaglutide treatment was temporarily discontinued for 28 days because of urinary tract infection after bladder biopsy. No patients prematurely discontinued treatment due to semaglutide‐related adverse events.
Table 3.
Adverse events
| n = 16 | |
|---|---|
| Adverse event leading to discontinuation | 1 (6.3) † |
| Adverse events leading to dose reduction | 3 (18.8) ‡ |
| Grade 3–5 adverse event | 0 (0) |
| Grade 1–2 adverse events | |
| Nausea | 8 (50.0) |
| Dyspepsia | 3 (18.8) |
| Appetite loss | 2 (12.5) |
| Constipation | 2 (12.5) |
| Colonic hemorrhage | 1 (6.3) |
| Dizziness | 2 (12.5) |
| Urinary tract infection | 1 (6.3) |
Data are presented as numbers (percentages).
One patient of temporary discontinuation due to urinary tract infection following a bladder biopsy. Oral semaglutide treatment was temporarily discontinued for 28 days due to urinary tract infection following bladder biopsy, but resumed after recovery.
All adverse events leading to dose reduction were nausea.
Discussion
Placebo‐controlled trials of subcutaneous liraglutide and semaglutide showed the improvement effect of GLP‐1 RAs on liver histology in patients with NAFLD. 12 , 13 The LEAN study indicated that 9 of 23 (39%) patients with NASH who received subcutaneous liraglutide (1.8 mg daily) for 48 weeks had resolution of NASH, as confirmed by biopsy, and the resolution rate was significantly higher than that in the placebo group (2 of 22; 9%). 12 Another placebo‐controlled trial of 72‐week subcutaneous semaglutide, involving 320 patients with NASH, demonstrated that semaglutide reduced liver steatosis and inflammation without exacerbating liver fibrosis, leading to NASH resolution. 13 However, patients and physicians are generally reluctant to administer subcutaneous injections. In particular, antidiabetic drugs must be continuously administered over a long period in many patients; therefore, even when the frequency of administration is only once a week, the long‐term treatment is not desirable. 22 , 32 Oral semaglutide, which has recently become available in clinical practice, may facilitate the introduction of GLP‐1 RA treatment by compensating for this limitation of subcutaneous GLP‐1 RAs. In the research reported here—a single‐arm pilot study—we found that 24‐week oral semaglutide treatment in patients with NAFLD complicated by T2DM improved insulin resistance, diabetes mellitus, hypertriglyceridemia, impaired liver function, and hepatic steatosis, while also decreasing body weight. To our knowledge, this is the first report on the efficacy and safety of oral semaglutide treatment in patients with NAFLD complicated by T2DM.
In this study, we found significant correlations between changes in body weight and levels of ALT or CAP. These findings suggest that semaglutide may have indirect effects on hepatic steatosis and inflammation by improving obesity and insulin resistance, which are the key drivers of NAFLD onset and progression. Preclinical studies have shown the direct effects of GLP‐1 RAs on liver function, such as suppression of de novo lipogenesis, 33 promotion of fatty acid β‐oxidation, improvement of insulin signals, 34 and anti‐inflammatory actions. 35 These metabolic modulations may contribute to the improvement or reduction of the pathogenesis of NAFLD. The study included mostly obese patients with severe insulin resistance. Therefore, further studies are needed to determine the efficacy of oral semaglutide in lean/nonobese NAFLD, which is relatively common in Japanese patients. 36 , 37
A consensus regarding the influence of GLP‐1 RAs on liver fibrosis in patients with NAFLD has not yet been reached. The above‐referenced placebo‐controlled trials of subcutaneous liraglutide 12 and semaglutide 13 demonstrated that GLP‐1 RAs have inhibitory effects on the exacerbation of liver fibrosis but do not result in significant improvement in this disease. Similarly, in the current study, we failed to determine the improvement effect of oral semaglutide on liver fibrosis. However, the following reasons should be considered: (i) histological evaluation by liver biopsy was not conducted, (ii) evaluation of liver fibrosis using a noninvasive test (NIT) was difficult due to a small number of registered patients with advanced fibrosis, and (iii) the treatment period was too short (24 weeks) to verify the effect of oral semaglutide on liver fibrosis.
Liver biopsy is the gold standard for evaluating liver fibrosis; however, it has several limitations, such as invasiveness, risk of complications, and sampling errors. Additionally, it is difficult to detect a one‐stage improvement in liver fibrosis using the current semiquantitative staging systems. A long‐term observation period (probably several years) is required to detect significant improvement of fibrosis. 13 In this study, significant decreases in quantitative NIT parameters, such as ferritin, type IV collagen 7 s, and FIB‐4 index, at 24 weeks of oral semaglutide treatment may reflect a slight improvement in liver fibrosis, which cannot be found in liver biopsy specimens or using the histological semiquantitative staging systems. Although an improvement in the FIB‐4 index may be attributable to the normalization of AST and ALT levels, a significant increase in the platelet count suggests that a decrease in the FIB‐4 index could indicate an improvement in liver fibrosis. The FIB4‐index is associated with extrahepatic complications, such as cardiovascular events and non‐liver cancers, which comprise a greater proportion of deaths. 38 , 39 , 40 GLP‐1 RA treatment may prevent these complications and improve the prognosis of patients with NAFLD. In this study, we found no significant changes in LSM values after oral semaglutide treatment. Therefore, a large‐scale, long‐term study is required to investigate whether GLP‐1 RA treatment improves liver fibrosis and extrahepatic complications and, consequently, the prognosis in patients with NAFLD complicated by T2DM.
There are some limitations in the current study. First, considering that this was a single‐arm, open‐label study, assessing the effect of significant potential confounding factors, such as calorie intake and physical activity, could not be performed. Second, as described above, the study was conducted on a small number of patients with a wide range of LSM from low to very high, and the duration of treatment was limited, in examining the effect of semaglutide on liver fibrosis. Third, there were some patients with very high LSM or severe obesity in the study, and it is possible that LSM and CAP might not be accurately measured in these cases using the FibroScan 502 with the M‐probe, as indeed one could not be measured due to severe obesity.
In conclusion, the 24‐week oral semaglutide treatment was effective and safe in patients with NAFLD complicated by T2DM. Oral semaglutide treatment significantly improved impaired liver function, hypertriglyceridemia, insulin resistance, and hepatic steatosis, as well as improving diabetic status and reducing body weight. Although this pilot study had numerous limitations, the results suggest a potential benefit of semaglutide in the treatment of liver fibrosis, and further investigation is warranted.
Supporting information
Table S1. Baseline characteristics of each patient with NAFLD complicated by T2DM.
Acknowledgments
The authors wish to thank all medical doctors from all institutions who were involved in this study.
Declaration of conflict of interest: The authors have no potential conflicts of interest to declare concerning the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Masanori Atsukawa, upon reasonable request.
References
- 1. Younossi Z, Anstee QM, Marietti M et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018; 15: 1120. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 3. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia—as common and important as in the West. Nat. Rev. Gastroenterol. Hepatol. 2013; 10: 307–18. [DOI] [PubMed] [Google Scholar]
- 4. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006; 43: S99–S112. [DOI] [PubMed] [Google Scholar]
- 5. Lonardo A, Nascimbeni F, Targher G et al. AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig. Liver Dis. 2017; 49: 471–83. [DOI] [PubMed] [Google Scholar]
- 6. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011; 34: 274–85. [DOI] [PubMed] [Google Scholar]
- 7. Loomba R, Abraham M, Unalp A et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012; 56: 943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67: 328–57. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J. Hepatol. 2016; 64: 1388–402. [DOI] [PubMed] [Google Scholar]
- 10. Tokushige K, Ikejima K, Ono M et al. Evidence‐based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol. Res. 2021; 51: 1013–25. [DOI] [PubMed] [Google Scholar]
- 11. Eguchi Y, Kitajima Y, Hyogo H et al. Pilot study of liraglutide effects in non‐alcoholic steatohepatitis and non‐alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN‐J). Hepatol. Res. 2015; 45: 269–78. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong MJ, Gaunt P, Aithal GP et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016; 387: 679–90. [DOI] [PubMed] [Google Scholar]
- 13. Newsome PN, Buchholtz K, Cusi K et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021; 384: 1113–24. [DOI] [PubMed] [Google Scholar]
- 14. Akuta N, Watanabe C, Kawamura Y et al. Effects of a sodium‐glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: preliminary prospective study based on serial liver biopsies. Hepatol. Commun. 2017; 1: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takahashi H, Kessoku T, Kawanaka M et al. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol. Commun. 2022; 6: 120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arai T, Atsukawa M, Tsubota A et al. Effect of sodium‐glucose cotransporter 2 inhibitor in patients with non‐alcoholic fatty liver disease and type 2 diabetes mellitus: a propensity score‐matched analysis of real‐world data. Ther. Adv. Endocrinol. Metab. 2021; 12: 20420188211000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seko Y, Sumida Y, Tanaka S et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017; 47: 1072–8. [DOI] [PubMed] [Google Scholar]
- 18. Sumida Y, Murotani K, Saito M et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective, single‐arm trial (LEAD trial). Hepatol. Res. 2019; 49: 64–71. [DOI] [PubMed] [Google Scholar]
- 19. Phrueksotsai S, Pinyopornpanish K, Euathrongchit J et al. The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non‐alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021; 36: 2952–9. [DOI] [PubMed] [Google Scholar]
- 20. Sinha B, Datta D, Ghosal S. Meta‐analysis of the effects of sodium glucose cotransporter 2 inhibitors in non‐alcoholic fatty liver disease patients with type 2 diabetes. JGH Open. 2020; 5: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology. 2007; 132: 2131–57. [DOI] [PubMed] [Google Scholar]
- 22. Matza LS, Curtis SE, Jordan JB, Adetunji O, Martin SA, Boye KS. Physician perceptions of GLP‐1 receptor agonists in the UK. Curr. Med. Res. Opin. 2016; 32: 857–64. [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen MF. The development of oral semaglutide, an oral GLP‐1 analog, for the treatment of type 2 diabetes. Diabetol. Int. 2020; 11: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–19. [DOI] [PubMed] [Google Scholar]
- 25. Manousou P, Kalambokis G, Grillo F et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non‐alcoholic fatty liver disease patients. Liver Int. 2011; 31: 730–9. [DOI] [PubMed] [Google Scholar]
- 26. Yoneda M, Mawatari H, Fujita K et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J. Gastroenterol. 2007; 42: 375–81. [DOI] [PubMed] [Google Scholar]
- 27. Ogawa Y, Honda Y, Kessoku T et al. Wisteria floribunda agglutinin‐positive Mac‐2‐binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non‐alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2018; 33: 1795–803. [DOI] [PubMed] [Google Scholar]
- 28. Abe M, Miyake T, Kuno A et al. Association between Wisteria floribunda agglutinin‐positive Mac‐2 binding protein and the fibrosis stage of non‐alcoholic fatty liver disease. J. Gastroenterol. 2015; 50: 776–84. [DOI] [PubMed] [Google Scholar]
- 29. Atsukawa M, Tsubota A, Okubo T et al. Serum Wisteria floribunda agglutinin‐positive Mac‐2 binding protein more reliably distinguishes liver fibrosis stages in non‐alcoholic fatty liver disease than serum Mac‐2 binding protein. Hepatol. Res. 2018; 48: 424–32. [DOI] [PubMed] [Google Scholar]
- 30. Vallet‐Pichard A, Mallet V, Nalpas B et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection: comparison with liver biopsy and fibrotest. Hepatology. 2007; 46: 32–6. [DOI] [PubMed] [Google Scholar]
- 31. Sumida Y, Yoneda M, Hyogo H et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drucker DJ. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020; 19: 277–89. [DOI] [PubMed] [Google Scholar]
- 33. Ben‐Shlomo S, Zvibel I, Shnell M et al. Glucagon‐like peptide‐1 reduces hepatic lipogenesis via activation of AMP‐activated protein kinase. J. Hepatol. 2011; 54: 1214–23. [DOI] [PubMed] [Google Scholar]
- 34. Svegliati‐Baroni G, Saccomanno S, Rychlicki C et al. Glucagon‐like peptide‐1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high‐fat diet in nonalcoholic steatohepatitis. Liver Int. 2011; 31: 1285–97. [DOI] [PubMed] [Google Scholar]
- 35. Rakipovski G, Rolin B, Nøhr J et al. The GLP‐1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE −/− and LDLr −/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018; 3: 844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim D, Kim WR. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 2017; 15: 474–85. [DOI] [PubMed] [Google Scholar]
- 37. Ito T, Ishigami M, Zou B et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta‐analysis with individual and forecasting analysis, 1995‐2040. Hepatol. Int. 2021; 15: 366–79. [DOI] [PubMed] [Google Scholar]
- 38. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013; 57: 1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tada T, Kumada T, Toyoda H et al. Progression of liver fibrosis is associated with non‐liver‐related mortality in patients with nonalcoholic fatty liver disease. Hepatol. Commun. 2017; 1: 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim GA, Lee HC, Choe J et al. Association between non‐alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018; 68: 140–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of each patient with NAFLD complicated by T2DM.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Masanori Atsukawa, upon reasonable request.


