Abstract
Background
With the emergence of resistance to front-line antimalarials, there is an urgent need to develop new medicines, including those targeting sexual development. This study aimed to assess the activity of a panel of phosphatase inhibitors against the sexual development of Plasmodium berghei and evaluate their potential as transmission-blocking agents.
Methods
Twenty-five compounds were screened for transmission-blocking activity in vitro using the P. berghei ookinete culture assay. The inhibitory effects on male gametogenesis, gamete-ookinete, and zygote-ookinete formation were evaluated. The transmission-blocking activity of two compounds was evaluated using an in vivo mosquito feeding assay. Their cytotoxic effects were assessed on the human cell line HepG2.
Results
Twelve compounds inhibited P. berghei ookinete formation with an IC50 < 10 μM. Two compounds, BVT-948 and alexidine dihydrochloride, significantly inhibited different developmental stages from gametogenesis through ookinete maturation. They also showed a substantial in vivo transmission-blocking activity by the mosquito feeding assay.
Conclusions
Some phosphatase inhibitors effectively inhibited Plasmodium sexual development and exhibited evident transmission-blocking activity, suggesting that phosphatases are valid targets for antimalarial development.
Keywords: Malaria, Plasmodium berghei, Transmission, Drug, Phosphatase
Graphical abstract
Highlights
-
•
BVT-948 and alexidine·2HCl inhibit sexual developmental stages of Plasmodium.
-
•
BVT-948 and alexidine·2HCl show a substantial transmission-blocking activity.
-
•
BVT-948 and alexidine·2HCl show a relatively low cytotoxicity to HepG2 cells.
-
•
Phosphatases are valid targets for antimalarial development.
1. Introduction
Despite substantial efforts to control and eradicate malaria, it remains a significant public health threat, responsible for 241 million cases and 627,000 deaths worldwide in 2020 alone, according to the World Health Organization. While effective vaccines are anticipated, antimalarials are still the primary means to reduce the burden of malaria infection in humans (WHO, 2016). However, the emergence and spread of parasites resistant to front-line antimalarials have compromised the effectiveness of drug treatment, jeopardized malaria elimination, and necessitated the search for new effective antimalarials with novel modes of action (Hamilton et al., 2019).
Malaria parasites have a complex life cycle, requiring both vertebrate and mosquito hosts (Cowman et al., 2016; Shaw et al., 2022; Sinden, 2015). During its development in human erythrocytes, most parasites undergo an asexual replication cycle, causing the repetitive febrile episodes characteristic of the disease. A minor fraction of the parasites initiates sexual development to produce gametocytes, which are obligative for transmission to anopheline mosquitoes. Once ingested by blood-feeding Anopheles mosquitoes, female and male gametocytes undergo gametogenesis to produce gametes in the mosquito midgut, which are fertilized to form zygotes. Within 24 h, zygotes transform into motile ookinetes, which penetrate the midgut epithelium and eventually settle underneath the basal lamina to start sporogonic development.
For decades, most antimalarials have traditionally targeted the pathogenic asexual blood stages within infected erythrocytes while virtually neglecting the sexual stages (Hanboonkunupakarn and White, 2022; Reader et al., 2021). Until recently, the malaria elimination agenda has desired increased research on drugs and vaccines that block the transmission of the parasites to vectors (Delves et al., 2012; Plouffe et al., 2016). Transmission-blocking drugs include compounds targeting gametocytes in the blood circulation of the human host, early sporogonic stages (gametes, zygotes, ookinetes) developing in the mosquito blood meal, and the mosquito vector itself (e.g., the endectocide ivermectin) (Birkholtz et al., 2022). Such drugs aim to reduce both the prevalence of infected mosquitoes in a population and the parasite burden in individual infected insects, thus decreasing the exposure of a human population to potentially infectious mosquitoes. Due to the underlying biological differences between asexual and sexual stages, most currently used antimalarials are ineffective against Plasmodium gametocytes (Plouffe et al., 2016; Reader et al., 2021).
The reversible protein phosphorylation, catalyzed by the opposing actions of protein kinases and phosphatases, plays an obligatory role in regulating cell processes such as proliferation, differentiation, migration, and maintenance of homeostasis (Humphrey et al., 2015). The human malaria parasite P. falciparum encodes 80–100 protein kinases (Talevich et al., 2012; Ward et al., 2004). Kinome-wide functional studies conducted in P. falciparum and the murine parasite P. berghei have identified kinases essential for erythrocytic schizogony and sexual development (Solyakov et al., 2011; Tewari et al., 2010). In comparison, the protein phosphatome in Plasmodium is much smaller than the kinome, with nearly 30 protein phosphatases (Guttery et al., 2014; Pandey et al., 2014). They are divided into four families: serine/threonine protein phosphatases (PPPs), metal ion-dependent protein phosphatases (PPMs), protein tyrosine phosphatases (PTPs), and NLI-linked protein phosphatases (NIFs) (Kutuzov and Andreeva, 2008; Moorhead et al., 2009; Wilkes and Doerig, 2008). Functional studies of the phosphatome in P. berghei identified six protein phosphatases required for development in mosquitoes (Guttery et al., 2014). For instance, deletion of PP1 and PP5 of the PPP family inhibited oocyst and male gamete formation, respectively (Hollin et al., 2019; Zhu et al., 2019).
Protein phosphatases have increasingly been considered “druggable” targets (De Munter et al., 2013; Vainonen et al., 2021). For example, cyclosporin A inhibits calcineurin activity and works as an immunosuppressor in renal and liver transplantation as well as other immune regulatory dysfunctional disease therapies (Faulds et al., 1993). Pimecrolimus is used to manage psoriasis, eczema, and other inflammatory skin diseases (Ashcroft et al., 2007; Ayer and Young, 2013). The identification of protein kinases and phosphatases that are essential for Plasmodium sexual development suggests that they may be viable targets for transmission-blocking drugs. Herein, we performed a phenotype screen of a protein phosphatase inhibitor library on sexual development using the rodent malaria parasite P. berghei. Screening of 25 phosphatase inhibitors resulted in the discovery of BVT-948 and alexidine dihydrochloride with micromolar (μM) parasiticidal activity in sexual stage parasites. This study validated protein phosphatases as novel targets for developing antimalarial drugs with transmission-blocking activity.
2. Materials and methods
2.1. Chemicals and compounds
Phosphatase inhibitors were selected from the Screen-Well® Phosphatase Inhibitor library (Enzo Life Sciences, NY, USA) for the initial screening of their transmission-blocking activity (Appendix A, Appendix A; Appendix A, Appendix A). The compounds were dissolved in dimethyl sulfoxide (DMSO) as 10 mM stock solutions. For further analysis, BVT-948 and alexidine dihydrochloride were purchased from MedChemExpress (MCE, NJ, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively, dissolved in DMSO as 40 mM stocks, and stored at −80 °C. The final concentrations for experiments were prepared by diluting stock with the culture medium. All compounds used in the transmission-blocking assay had less than 0.1% of the final solvent concentration. 0.1% DMSO in culture medium and PBS solution were used as the negative control. Dihydroartemisinin (DHA), cycloheximide (CH), and atovaquone (ATV) were obtained from MCE and used as positive controls, as they are known inhibitors of relevant parasite stages (Azevedo et al., 2017; Saenz et al., 2013).
2.2. Mice, mosquitoes, and parasites
Six to eight-week-old female BALB/c mice weighing 18–25 g were purchased from Beijing Animal Institute (Beijing, China). Adult female Anopheles stephensi mosquitoes (Hor strain) were used for the in vivo transmission-blocking assays. Mosquitoes were reared in an insectary at 20 ± 2 °C, 12 h light/dark cycle and 50–80% relative humidity, and fed 10% (w/v) glucose solution soaked in cotton balls. The murine malaria parasite P. berghei ANKA strain 2.34 was maintained by serial passage and employed in transmission-blocking experiments (Braks et al., 2006). In short, 5 × 106 parasite-infected erythrocytes were inoculated into naive mice through an intravenous (i.v.) route three days before each experiment. All animal experiments complied with the animal ethics committee of China Medical University.
2.3. Microgametogenesis assay
A standard ookinete medium (RPMI 1640 medium supplemented with 20% (v/v) heat-inactivated fetal calf serum, 50 mg/L penicillin, and 50 mg/L streptomycin, pH 8.0) was used for in vitro gametocyte activation and ookinete culture. Exflagellation centers of male gametocytes were quantified as described (Guttery et al., 2014). In brief, 10 μL of tail blood from infected mice were added to 40 μL of standard ookinete medium containing designated concentrations of drugs. Fifteen minutes after culturing at 25 °C, 1.5 μL of the culture was placed on a coverslip and analyzed under a light microscope at 100 × magnification. An exflagellation center is defined as an exflagellating male gametocyte interacting with at least four red blood cells (RBCs).
2.4. In vitro ookinete culture assay
In vitro ookinete formation was quantified as described (Tewari et al., 2010). Briefly, 10 μL of tail blood from infected mice were added to 90 μL of standard ookinete medium containing either solvent only or drugs and incubation at 19 °C for 24 h. For in vitro ookinete inhibitory assay and concentration dependence assay, designated concentrations of drugs were added to the culture medium before adding the tail blood of infected mice and incubated throughout the culture period. To evaluate the activity of the drugs on gamete-ookinete or zygote-ookinete conversion, the ookinete culture was incubated at 25 °C for 15 min or 19 °C for 2 h to allow the formation of gametes or zygotes (Appendix A, Appendix A). Then, the compounds at the designated concentrations were added to the culture medium, mixed, and incubated at 19 °C until 24 h. For the time dependence assay, the inhibitors were added to the culture medium before adding the infected mouse blood. The culture was incubated at 19 °C for different times (1–24 h) before the drugs were washed out and a fresh drug-free medium was replenished. The ookinete culture was continued until 24 h (Appendix A, Appendix A).
At 24 h of the ookinete culture, 1 μL of the culture mixture was placed on a slide, and the cells were fixed with 4% paraformaldehyde. After washing, samples were blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS, pH 7.4) for 1 h at room temperature and probed with the mouse anti-Pbs21 (1:1000) for 1 h. Cells were washed with PBS and incubated with Alexa Fluor(R) 594 goat anti-mouse IgG (Molecular Probes, 1:1000) for 1 h. Following three PBS washes, ookinetes in 1 μL culture were counted under a Nikon Upright E800 fluorescence microscope at 100 × magnification.
2.5. Mosquito feeding experiment
Mice were infected with P. berghei three days before mosquito feeding. The test compounds A3 and F1, the positive control drug ATV, negative control PBS and solvent were injected into mice through the tail vein 10 min before feeding. The A3 and F1 doses corresponded to 10 times the IC50 values determined from in vitro ookinete formation assay, assuming a 1.8 mL peripheral blood volume for 25 g body weight of mice. The dose of A3 was 0.19 mg/kg body weight, and F1 was 0.23 mg/kg body weight. The inhibitors or drugs were diluted in saline to a total volume of 200 μL. Four-day-old female A. stephensi mosquitoes (∼100/mouse) starved for 6 h were allowed to feed on infected mice for 30 min. Unfed mosquitoes were then removed, and fed mosquitoes were maintained at 19–22 °C in 50–80% relative humidity. Ten days after feeding, up to 30 mosquitoes were dissected in each group. The midguts of mosquitoes were removed and stained with 0.5% mercurochrome (Sigma-Aldrich). Oocysts were counted to determine the prevalence (proportion of infected mosquitoes) and intensity of infection (number of oocysts per positive midgut).
2.6. Toxicity assays
The HepG2 hepatocellular carcinoma cell line was propagated in high-glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were maintained at 37 °C in a 5% CO2 chamber. Twenty-four hours before drug addition, cells were seeded in 96-well plates at a density of 5000 cells/well. Cells were incubated with 2-fold serial dilutions of BVT-948 and alexidine dihydrochloride (19.5–100 μM/mL) for 24 h. Control wells contained media with an equivalent concentration of DMSO. Then, 20 μL of 0.5% MTT solution (Solarbio) was added to the wells, and plates were further incubated at 37 °C for 4 h. After removing the medium from wells, 150 μL of DMSO were added to each well to dissolve formazan. The plates were read using a Varioskan Flash (Thermo Scientific) with excitation/emission wavelengths set at 544/590 nm. Three biological replicates were performed with three technical replicates per compound.
2.7. Statistical methods
Statistical comparison between groups (exflagellation center and ookinete number) was performed by Student's t-test using the GraphPad Prism software. The intensity of mosquito infection (oocysts/midgut) was analyzed using the Mann-Whitney U test, while infection prevalence was analyzed by Fisher's exact test using SPSS version 21.0. The half-maximal inhibitory or effective concentration (IC50 or EC50) was calculated by nonlinear regression from the sigmoidal dose-response curves using GraphPad Prism software (version 8.0). All data were from three independent experiments.
3. Results
3.1. Identification of compounds inhibiting ookinete formation
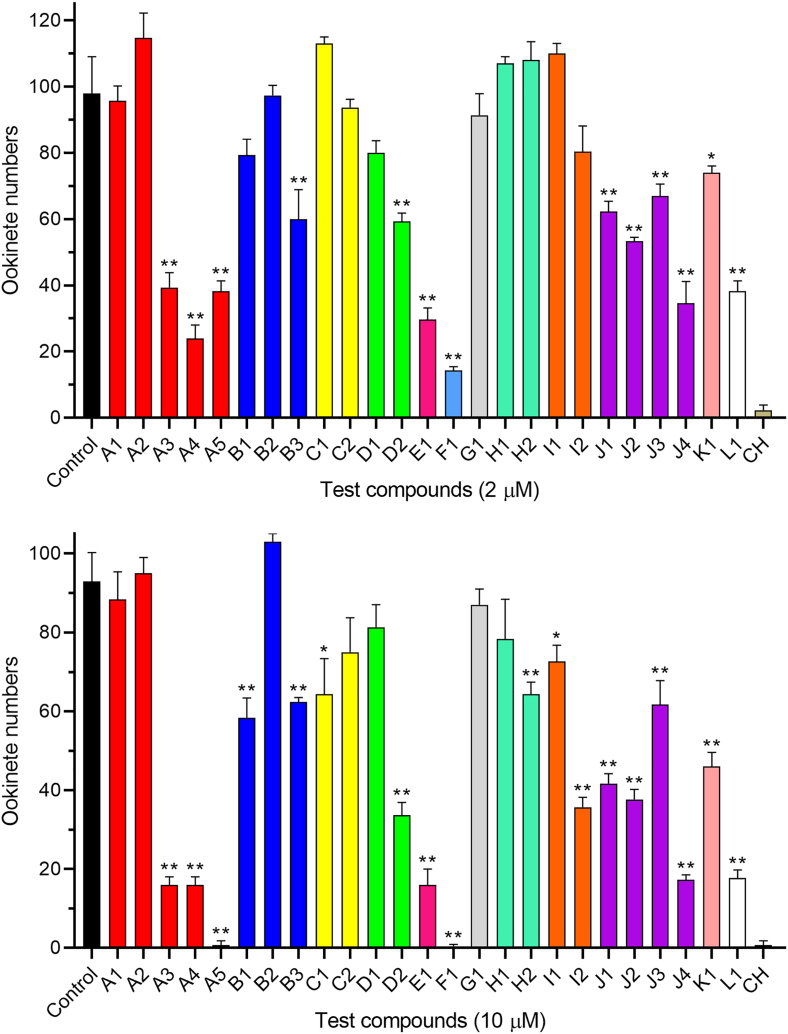
Since signal transduction plays a critical role in Plasmodium sexual development, we wanted to identify phosphatase inhibitors that may disrupt signaling during this process as future candidates to block malaria transmission. We screened a compound library of 25 inhibitors of mammalian phosphatases for their inhibitory effects on ookinete formation at a final concentration of 2 μM and 10 μM (Fig. 1, Table 1). At 2 μM, 13 compounds significantly inhibited ookinete formation, with seven (A3, A4, A5, E1, F1, J4, and L1) showing >50% inhibition. When drug concentration was increased to 10 μM, additional five compounds displayed a significant inhibitory effect on ookinete formation. At 10 μM, seven compounds (A3, A4, A5, E1, F1, J4, and L1) inhibited ookinete formation by more than 80%; two inhibitors, A5 (alendronate sodium hydrate) and F1 (alexidine dihydrochloride), completely blocked ookinete formation.
Fig. 1.
Evaluation of the inhibitory effect on ookinete formation of 25 phosphatase inhibitors. Numbers of ookinetes in 1 μL of ookinete culture 24 h after incubation with 2 μM and 10 μM of the compounds. Data are presented as the mean ± SD from 3 experiments. * indicates P < 0.05, and ** indicates P < 0.01 for comparison with the control. CH, cycloheximide.
Table 1.
Names, codes, targets and inhibitory actions of phosphatase inhibitors used in this study*.
3.2. Identification of compounds with male gametocytocidal activity
To identify the steps of sexual development at which the compounds are effective, we chose the 12 compounds showing more than 50% inhibition on ookinete formation at 10 μM to investigate whether they could inhibit gametogenesis of male gametocytes using the formation of exflagellation centers as a quantitative indicator. The results showed that all 12 compounds significantly inhibited exflagellation at both 2 and 10 μM concentrations (Fig. 2A and B, Table 1). The inhibitory effect was concentration-dependent for most compounds, with a stronger effect at 10 μM.
Fig. 2.
Evaluation of 12 compounds on exflagellation, gamete-ookinete conversion, and zygote-ookinete conversion in P. berghei. (A, B) Numbers of exflagellation centers of male gametocytes in 10 microscopic fields under a 100 × objective 15 min after incubation with the compounds at 2 μM (A) and 10 μM (B). (C, D) Numbers of ookinetes in 1 μL of ookinete culture 24 h after culture. The inhibitors were administered at 2 μM (C) and 10 μM (D) after gametogenesis induction at 25 °C for 15 min. (E, F) Numbers of ookinetes in 1 μL of ookinete culture 24 h after culture. The inhibitors were administered at 2 μM (E) and 10 μM (F) after culture at 19 °C for 2 h. Data are presented as the mean ± SD from 3 experiments. * and ** indicates P < 0.05 and P < 0.01, respectively, for comparison with the control group (t-test). CH, cycloheximide.
3.3. Activity of compounds on gamete-ookinete and zygote-ookinete conversion
We then evaluated the inhibitory effect of the compounds on gamete-to-ookinete conversion. After in vitro gametocyte activation at 25 °C for 15 min, compounds were added to the ookinete cultures at a final concentration of either 2 or 10 μM and maintained during the subsequent ookinete culture (Appendix A, Appendix A). At 2 μM, only three compounds, A3, F1, and K1, exhibited apparent inhibition of ookinete formation (Fig. 2C, Table 1). At 10 μM, nine compounds (A3, A4, A5, E1, F1, I2, J2, K1, and L1) showed significant inhibition of ookinete formation, with A3 and F1 having the highest activity (Fig. 2D, Table 1).
To assess the effects of the compounds on zygote-to-ookinete conversion, we cultured parasites at 19 °C for 2 h to allow the formation of zygotes and then added the compound solutions in the culture, which were maintained for an additional 22 h (Appendix A, Appendix A). Only A3 and F1 showed significant inhibition of ookinete conversion at both concentrations (Fig. 2E and F, Table 1).
These results indicated that compounds A3, A4, A5, E1, F1, I2, J2, K1, and L1 inhibited the fertilization process, while A3 and F1 further blocked the zygote-to-ookinete development.
3.4. Stage-specific activities of A3 and F1
The inhibitory effects of A3 (BVT-948) and F1 (alexidine dihydrochloride) on different developmental stages of ookinete formation prompted us to perform a time-course study to investigate the potential stage-specific activities of these two compounds. We performed ookinete culture in the presence of the compounds for different lengths of time and assessed the efficiency of ookinete formation (Appendix A, Appendix A). Incubation with A3 at 2 μM for less than 2 h did not inhibit ookinete formation. As the incubation time increased from 6 h to 18 h, the inhibition rate increased and almost plateaued at 18 h (∼60%) (Fig. 3A). A similar trend was observed for A3 at 10 μM, though incubation for 1–2 h at the beginning of the culture still inhibited ookinete formation by ∼20% (Fig. 3B), suggesting the activity of A3 on gametogenesis and fertilization.
Fig. 3.
Evaluation of the time dependence of A3 and F1 for inhibiting ookinete development. The compounds were incubated for 1–24 h before washing out from the culture medium and the ookinete formation at 24 h was determined. (A) A3, at 2 μM; (B) A3 at 10 μM; (C) F1 at 2 μM; (D) F1 at 10 μM. Data are presented as the mean ± SD from three experiments.
Compared with A3, F1 displayed a greater activity. Ookinete culture in the presence of 2 μM of F1 for 1–2 h inhibited ookinete formation by more than 50%, while the inhibitory effect reached the maximum by 6 h (Fig. 3C). At 10 μM, F1 exhibited almost maximum inhibition of ookinete formation (∼90%) even after 1 h exposure (Fig. 3D). These results showed that both A3 and F1 had inhibitory activity on zygote formation and subsequent ookinete development.
3.5. Potency and toxicity of A3 and F1
With the presence of the compounds throughout the 24 h ookinete culture, we determined the IC50 values for A3 and F1 for the inhibition of ookinete formation, which were 1.11 μM and 0.56 μM, respectively (Fig. 4A).
Fig. 4.
The activity of the compounds for blocking ookinete formation and toxicity in hepatocytes. (A) Dose-response curve of A3 and F1 for inhibiting ookinete formation. The compounds were included in ookinete culture for 24 h. (B) Dose-dependent survival of HepG2 cells after incubation with different concentrations of A3 and F1. IC50 and EC50 were calculated by GraphPad Prism 8.0. Data were presented as the mean ± SD from three experiments.
We used the HepG2 human hepatocellular carcinoma cell line to evaluate the in vitro toxicity of A3 and F1. Dose-response experiments showed EC50 values of 39.8 μM and 14.0 μM for A3 and F1, respectively (Fig. 4B), resulting in a selectivity index of 36 for A3 and 25 for F1.
3.6. Evaluation of the transmission-blocking activity of A3 and F1
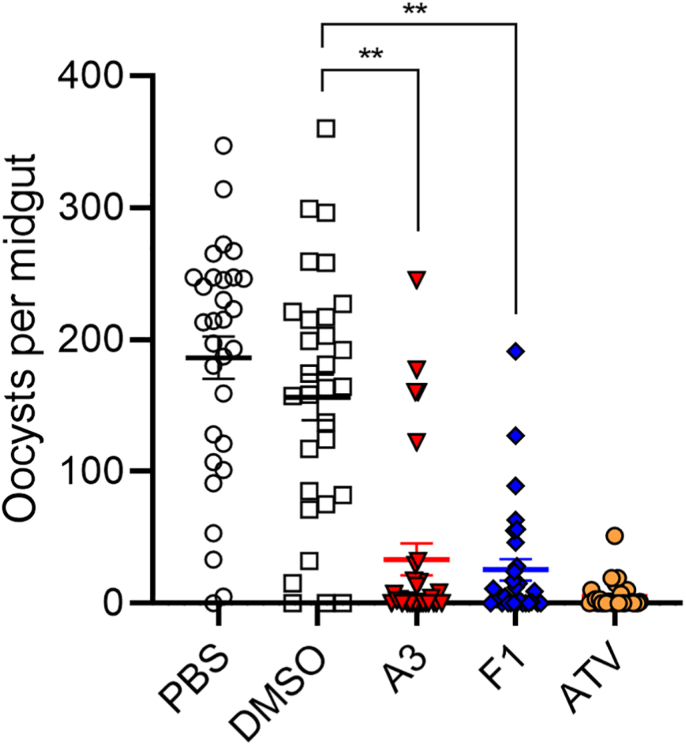
We further evaluated the transmission-blocking activity of the two compounds using the direct mosquito feeding assay. A3 and F1 were injected into P. berghei-infected BALb/c mice through the tail vein 10 min before mosquito feeding. The dose used for A3 and F1 was 0.19 and 0.23 mg/kg body weight, respectively. At 14 days after feeding, mosquitoes were dissected to quantify midgut infection. As shown in Fig. 5 and Table 2, A3 and F1 showed significant inhibitory effects on oocyst numbers and mosquito infection. With A3, the mosquito infection prevalence was reduced by 43.3%, and the oocyst density was reduced by 65.9%. Compared to control, the mosquito infection prevalence and oocyst density in the F1 group were reduced by 36.7% and 76.9%, respectively.
Fig. 5.
The transmission-blocking activity of A3 and F1 assessed by mosquito feeding assays. The inhibitors were injected into P. berghei-infected mice, which were fed to mosquitoes 10 min later. Oocyst numbers per mosquito midgut were counted. ** indicates P < 0.01 (t-test) for comparison with the vehicle control group (DMSO).
Table 2.
Evaluation of the in vivo transmission-blocking activity of A3 and F1.
| PBS | DMSO | A3 | F1 | ATQ | |
|---|---|---|---|---|---|
| Mosquitoes Infected/Dissected | 29/30 | 27/30 | 17/30 | 19/30 | 10/30 |
| Prevalence of infection (%)a | 96.7 | 90.0 | 56.7 | 63.3 | 33.3 |
| Reduction in prevalence (%)b | 43.3 | 36.7 | 66.7 | ||
| Oocyst intensity (mean ± SEM)c | 193 ± 16.2 | 173 ± 17.3 | 59 ± 12.1 | 40 ± 8.0 | 15 ± 1.9 |
| Reduction in oocyst intensity (%)d | 65.9 | 76.9 | 91.3 |
Drug-administrated infected mice were fed to mosquitoes. The prevalence of infection and oocyst density were compared with the negative control group (vehicle).
Prevalence of the mosquito infection = infected number/dissected number × 100%.
Reduction of prevalence = % prevalenceControl - % prevalenceDrug.
The number of oocysts per midgut.
Reduction in oocysts density = (mean oocyst densityControl – mean oocyst densityDrugs)/mean oocyst densityControl × 100%.
4. Discussion
In this work, we explored the potential of phosphatase inhibitors as malaria transmission-blocking agents and performed screening of a pilot library of 25 human phosphatase inhibitors. We found that 18 compounds significantly inhibited in vitro ookinete formation, with 12 of them showing more than 50% inhibition. Further testing of the 12 compounds showed that all possessed activity in blocking male gametogenesis, and two of them, BVT-948 (A3) and alexidine dihydrochloride (F1), also had inhibitory activity on zygote to ookinete conversion. Both A3 and F1 had <2 μM IC50 values for inhibiting in vitro ookinete formation and also possessed considerable transmission-blocking and transmission-reducing activities in mosquito-feeding assays. At doses as low as 0.2 mg/kg, A3 and F1 could reduce oocyst density by ∼50%.
BVT-948 is a non-specific PTP inhibitor that irreversibly inhibits the activity of several PTPs in vitro. BVT-948 could antagonize the RIG-I receptor and, to some extent, regulate autoimmune dysfunction (Rawling et al., 2020). BVT-948 has also been found to have anti-tumor effects by inhibiting tumor growth and blocking invasion (Choi et al., 2020; Hwang et al., 2013). Alexidine dihydrochloride is a bisbiguanide compound commonly used as an oral disinfectant and in contact lens solutions. Alexidine dihydrochloride binds to various ligands, such as phospholipase B, protein-tyrosine phosphatase mitochondrial 1 (PTPMT1), lipopolysaccharide, hexokinase, and vacuolar ATPase (Chan et al., 2012; Doughty-Shenton et al., 2010; Eltahan et al., 2019; Jagtap et al., 2018; Soares et al., 2010). It possesses antimicrobial, anti-inflammatory, anticancer, and anti-osteolytic properties (Bao et al., 2021; Chan et al., 2012; Eltahan et al., 2019; Jagtap et al., 2018; Soares et al., 2010).
The protein phosphatome is highly conserved between P. falciparum and P. berghei with only three phosphatases without direct orthologs-PPM10 in P. falciparum and PTP2 and NIF1 in P. berghei (Guttery et al., 2014). Among them, PTP1, PTP2, YVH1, PRL, and PTPLA are expressed in gametocytes, with PTP1 being female-specific (Guttery et al., 2014). PTP1, PTP2, and PTPLA are also expressed in P. berghei ookinetes. Thus, the two inhibitors may target some of these PTPs to inhibit gametocyte to ookinete development. Since PTP1, PTP2 and PTPLA were found to be dispensable for asexual and sexual development (Guttery et al., 2014), it is also possible that additional targets are present during gametocyte to ookinete development. Yvh1 and prl are essential for Plasmodium asexual development (Guttery et al., 2014; Kumar et al., 2004), but their roles in sexual development are unknown. Future studies using a promoter swap strategy to down-regulate YVH1/PRL expression in sexual stages (Pandey et al., 2020) may reveal their roles in sexual development and determine if they are critical targets of the phosphatase inhibitors. Since BVT-948 and alexidine dihydrochloride have more than one target in mammalian systems (Liljebris et al., 2004; Mamouei et al., 2018), it is possible that they may possess additional targets other than phosphatases in Plasmodium sexual stages. Further, despite the conservation in protein phosphatome between P. falciparum and P. berghei, it remains to be tested whether compounds with transmission-blocking activity act similarly on P. falciparum.
With the expression of several phosphatases in asexual blood stages, these phosphatase inhibitors may also possess schizonticidal activities, which need to be explored. If the compound inhibits the same phosphatases expressed in both asexual and sexual stages, it would be prone to resistance development. Conversely, if the inhibitor targets different phosphatases differentially expressed in asexual and sexual stages, resistance development is expected to be slow since resistance in asexual stages may not be easily transmitted. Given the relatively narrow range between the IC50s of the two compounds and the toxicity in human hepatocytes (selectivity index <50 × ), future structure-activity relationship studies are needed to increase the potency of the compounds on parasites and reduce their toxicity in human cells (Jin et al., 2016). Future studies should also consider the pharmacokinetic property of the compound, given the requirement for a relatively long half-life in humans in order to produce sufficient transmission-blocking activity. Thus, these chemical scaffolds may serve as the starting point for developing phosphatase-based antimalarials that are effective against multiple parasite stages.
Declaration of interest
None.
Authors’ contributions
EL, YC and LC conceived of the study and helped to design the study and draft the manuscript. XJ carried out the function studies, statistical analysis and drafted the manuscript. FL carried out the classification and compound information generalization. XJ, FL, JB carried out the activity studies. YZ, and JB carried out the cell toxicity assay and statistical analysis. All authors contributed to the writing of the manuscript.
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01AI150553 and U19AI089672), and National Science Foundation of China (81429004 and 81760367).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.06.003.
Contributor Information
Yaming Cao, Email: ymcao@cmu.edu.cn.
Enjie Luo, Email: ejluo@cmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ashcroft D.M., Chen L.C., Garside R., Stein K., Williams H.C. Cochrane Database Syst Rev; CD005500: 2007. Topical Pimecrolimus for Eczema. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer J., Young H.S. Pimecrolimus for psoriasis. Expet Opin. Pharmacother. 2013;14:767–774. doi: 10.1517/14656566.2013.775247. [DOI] [PubMed] [Google Scholar]
- Azevedo R., Markovic M., Machado M., Franke-Fayard B., Mendes A.M., Prudencio M. Bioluminescence method for in vitro screening of Plasmodium transmission-blocking compounds. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02699-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M.H., Yang C., Tse A.P., Wei L., Lee D., Zhang M.S., Goh C.C., Chiu D.K., Yuen V.W., Law C.T., Chin W.C., Chui N.N., Wong B.P., Chan C.Y., Ng I.O., Chung C.Y., Wong C.M., Wong C.C. Genome-wide CRISPR-Cas9 knockout library screening identified PTPMT1 in cardiolipin synthesis is crucial to survival in hypoxia in liver cancer. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108676. [DOI] [PubMed] [Google Scholar]
- Birkholtz L.M., Alano P., Leroy D. Transmission-blocking drugs for malaria elimination. Trends Parasitol. 2022;38:390–403. doi: 10.1016/j.pt.2022.01.011. [DOI] [PubMed] [Google Scholar]
- Braks J.A., Franke-Fayard B., Kroeze H., Janse C.J., Waters A.P. Development and application of a positive-negative selectable marker system for use in reverse genetics in Plasmodium. Nucleic Acids Res. 2006;34:e39. doi: 10.1093/nar/gnj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.Y., Prudom C., Raines S.M., Charkhzarrin S., Melman S.D., De Haro L.P., Allen C., Lee S.A., Sklar L.A., Parra K.J. Inhibitors of V-ATPase proton transport reveal uncoupling functions of tether linking cytosolic and membrane domains of V0 subunit a (Vph1p) J. Biol. Chem. 2012;287:10236–10250. doi: 10.1074/jbc.M111.321133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.K., Kim Y.K., Park S.W., Lee H., Lee S., Kim S.A., Kim S.J., Lee J., Kim W., Min S.H., Yu J.H. The histone lysine methyltransferase SETD8 regulates angiogenesis through HES-1 in human umbilical vein endothelial cells. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-69103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A.F., Healer J., Marapana D., Marsh K. Malaria: biology and disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- De Munter S., Kohn M., Bollen M. Challenges and opportunities in the development of protein phosphatase-directed therapeutics. ACS Chem. Biol. 2013;8:36–45. doi: 10.1021/cb300597g. [DOI] [PubMed] [Google Scholar]
- Delves M., Plouffe D., Scheurer C., Meister S., Wittlin S., Winzeler E.A., Sinden R.E., Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty-Shenton D., Joseph J.D., Zhang J., Pagliarini D.J., Kim Y., Lu D., Dixon J.E., Casey P.J. Pharmacological targeting of the mitochondrial phosphatase PTPMT1. J. Pharmacol. Exp. Therapeut. 2010;333:584–592. doi: 10.1124/jpet.109.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltahan R., Guo F., Zhang H., Zhu G. The action of the hexokinase inhibitor 2-deoxy-d-glucose on cryptosporidium parvum and the discovery of activities against the parasite hexokinase from marketed drugs. J. Eukaryot. Microbiol. 2019;66:460–468. doi: 10.1111/jeu.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds D., Goa K.L., Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45:953–1040. doi: 10.2165/00003495-199345060-00007. [DOI] [PubMed] [Google Scholar]
- Guttery D.S., Poulin B., Ramaprasad A., Wall R.J., Ferguson D.J., Brady D., Patzewitz E.M., Whipple S., Straschil U., Wright M.H., Mohamed A.M., Radhakrishnan A., Arold S.T., Tate E.W., Holder A.A., Wickstead B., Pain A., Tewari R. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe. 2014;16:128–140. doi: 10.1016/j.chom.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.L., Amato R., van der Pluijm R.W., Jacob C.G., Quang H.H., Thuy-Nhien N.T., Hien T.T., Hongvanthong B., Chindavongsa K., Mayxay M., Huy R., Leang R., Huch C., Dysoley L., Amaratunga C., Suon S., Fairhurst R.M., Tripura R., Peto T.J., Sovann Y., Jittamala P., Hanboonkunupakarn B., Pukrittayakamee S., Chau N.H., Imwong M., Dhorda M., Vongpromek R., Chan X.H.S., Maude R.J., Pearson R.D., Nguyen T., Rockett K., Drury E., Goncalves S., White N.J., Day N.P., Kwiatkowski D.P., Dondorp A.M., Miotto O. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect. Dis. 2019;19:943–951. doi: 10.1016/S1473-3099(19)30392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanboonkunupakarn B., White N.J. Advances and roadblocks in the treatment of malaria. Br. J. Clin. Pharmacol. 2022;88:374–382. doi: 10.1111/bcp.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollin T., De Witte C., Freville A., Guerrera I.C., Chhuon C., Saliou J.M., Herbert F., Pierrot C., Khalife J. Essential role of GEXP15, a specific Protein Phosphatase type 1 partner, in Plasmodium berghei in asexual erythrocytic proliferation and transmission. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S.J., James D.E., Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol. Metabol. 2015;26:676–687. doi: 10.1016/j.tem.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Hwang B.M., Chae H.S., Jeong Y.J., Lee Y.R., Noh E.M., Youn H.Z., Jung S.H., Yu H.N., Chung E.Y., Kim J.S. Protein tyrosine phosphatase controls breast cancer invasion through the expression of matrix metalloproteinase-9. BMB Rep. 2013;46:533–538. doi: 10.5483/BMBRep.2013.46.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap P., Mishra R., Khanna S., Kumari P., Mittal B., Kashyap H.K., Gupta S. Mechanistic evaluation of lipopolysaccharide-alexidine interaction using spectroscopic and in silico approaches. ACS Infect. Dis. 2018;4:1546–1552. doi: 10.1021/acsinfecdis.8b00087. [DOI] [PubMed] [Google Scholar]
- Jin Y., Khadka D.B., Cho W.J. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin. Ther. Pat. 2016;26:229–243. doi: 10.1517/13543776.2016.1118060. [DOI] [PubMed] [Google Scholar]
- Kumar R., Musiyenko A., Cioffi E., Oldenburg A., Adams B., Bitko V., Krishna S.S., Barik S. A zinc-binding dual-specificity YVH1 phosphatase in the malaria parasite, Plasmodium falciparum, and its interaction with the nuclear protein, pescadillo. Mol. Biochem. Parasitol. 2004;133:297–310. doi: 10.1016/j.molbiopara.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kutuzov M.A., Andreeva A.V. Protein Ser/Thr phosphatases of parasitic protozoa. Mol. Biochem. Parasitol. 2008;161:81–90. doi: 10.1016/j.molbiopara.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Liljebris C., Baranczewski P., Bjorkstrand E., Bystrom S., Lundgren B., Tjernberg A., Warolen M., James S.R. Oxidation of protein tyrosine phosphatases as a pharmaceutical mechanism of action: a study using 4-hydroxy-3,3-dimethyl-2H-benzo[g]indole-2,5(3H)-dione. J. Pharmacol. Exp. Therapeut. 2004;309:711–719. doi: 10.1124/jpet.103.062745. [DOI] [PubMed] [Google Scholar]
- Mamouei Z., Alqarihi A., Singh S., Xu S., Mansour M.K., Ibrahim A.S., Uppuluri P. Alexidine dihydrochloride has broad-spectrum activities against diverse fungal pathogens. mSphere. 2018;3 doi: 10.1128/mSphere.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead G.B., De Wever V., Templeton G., Kerk D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009;417:401–409. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- Pandey R., Abel S., Boucher M., Wall R.J., Zeeshan M., Rea E., Freville A., Lu X.M., Brady D., Daniel E., Stanway R.R., Wheatley S., Batugedara G., Hollin T., Bottrill A.R., Gupta D., Holder A.A., Le Roch K.G., Tewari R. Plasmodium condensin core subunits SMC2/SMC4 mediate atypical mitosis and are essential for parasite proliferation and transmission. Cell Rep. 2020;30:1883–1897 e1886. doi: 10.1016/j.celrep.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R., Mohmmed A., Pierrot C., Khalife J., Malhotra P., Gupta D. Genome wide in silico analysis of Plasmodium falciparum phosphatome. BMC Genom. 2014;15:1024. doi: 10.1186/1471-2164-15-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe D.M., Wree M., Du A.Y., Meister S., Li F., Patra K., Lubar A., Okitsu S.L., Flannery E.L., Kato N., Tanaseichuk O., Comer E., Zhou B., Kuhen K., Zhou Y., Leroy D., Schreiber S.L., Scherer C.A., Vinetz J., Winzeler E.A. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe. 2016;19:114–126. doi: 10.1016/j.chom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling D.C., Jagdmann G.E., Jr., Potapova O., Pyle A.M. Small-molecule antagonists of the RIG-I innate immune receptor. ACS Chem. Biol. 2020;15:311–317. doi: 10.1021/acschembio.9b00810. [DOI] [PubMed] [Google Scholar]
- Reader J., van der Watt M.E., Taylor D., Le Manach C., Mittal N., Ottilie S., Theron A., Moyo P., Erlank E., Nardini L., Venter N., Lauterbach S., Bezuidenhout B., Horatscheck A., van Heerden A., Spillman N.J., Cowell A.N., Connacher J., Opperman D., Orchard L.M., Llinas M., Istvan E.S., Goldberg D.E., Boyle G.A., Calvo D., Mancama D., Coetzer T.L., Winzeler E.A., Duffy J., Koekemoer L.L., Basarab G., Chibale K., Birkholtz L.M. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021;12:269. doi: 10.1038/s41467-020-20629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz F.E., Lacrue A.N., Cross R.M., Maignan J.R., Udenze K.O., Manetsch R., Kyle D.E. 4-(1H)-Quinolones and 1,2,3,4-Tetrahydroacridin-9(10H)-ones prevent the transmission of Plasmodium falciparum to Anopheles freeborni. Antimicrob. Agents Chemother. 2013;57:6187–6195. doi: 10.1128/AAC.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W.R., Marcenac P., Catteruccia F. Plasmodium development in Anopheles: a tale of shared resources. Trends Parasitol. 2022;38:124–135. doi: 10.1016/j.pt.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R.E. The cell biology of malaria infection of mosquito: advances and opportunities. Cell Microbiol. 2015;17:451–466. doi: 10.1111/cmi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D.A., de Andrade R.V., Silva S.S., Bocca A.L., Soares Felipe S.M., Petrofeza S. Extracellular Paracoccidioides brasiliensis phospholipase B involvement in alveolar macrophage interaction. BMC Microbiol. 2010;10:241. doi: 10.1186/1471-2180-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solyakov L., Halbert J., Alam M.M., Semblat J.P., Dorin-Semblat D., Reininger L., Bottrill A.R., Mistry S., Abdi A., Fennell C., Holland Z., Demarta C., Bouza Y., Sicard A., Nivez M.P., Eschenlauer S., Lama T., Thomas D.C., Sharma P., Agarwal S., Kern S., Pradel G., Graciotti M., Tobin A.B., Doerig C. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun. 2011;2:565. doi: 10.1038/ncomms1558. [DOI] [PubMed] [Google Scholar]
- Talevich E., Tobin A.B., Kannan N., Doerig C. An evolutionary perspective on the kinome of malaria parasites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2607–2618. doi: 10.1098/rstb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R., Straschil U., Bateman A., Bohme U., Cherevach I., Gong P., Pain A., Billker O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8:377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainonen J.P., Momeny M., Westermarck J. Druggable cancer phosphatases. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abe2967. [DOI] [PubMed] [Google Scholar]
- Ward P., Equinet L., Packer J., Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genom. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2016. Global Technical Strategy for Malaria 2016-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes J.M., Doerig C. The protein-phosphatome of the human malaria parasite Plasmodium falciparum. BMC Genom. 2008;9:412. doi: 10.1186/1471-2164-9-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Sun L., He Y., Wei H., Hong M., Liu F., Liu Q., Cao Y., Cui L. Plasmodium berghei serine/threonine protein phosphatase PP5 plays a critical role in male gamete fertility. Int. J. Parasitol. 2019;49:685–695. doi: 10.1016/j.ijpara.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.